Anatomical Reasons for an Impaired Internal Jugular Flow
Abstract
1. Introduction
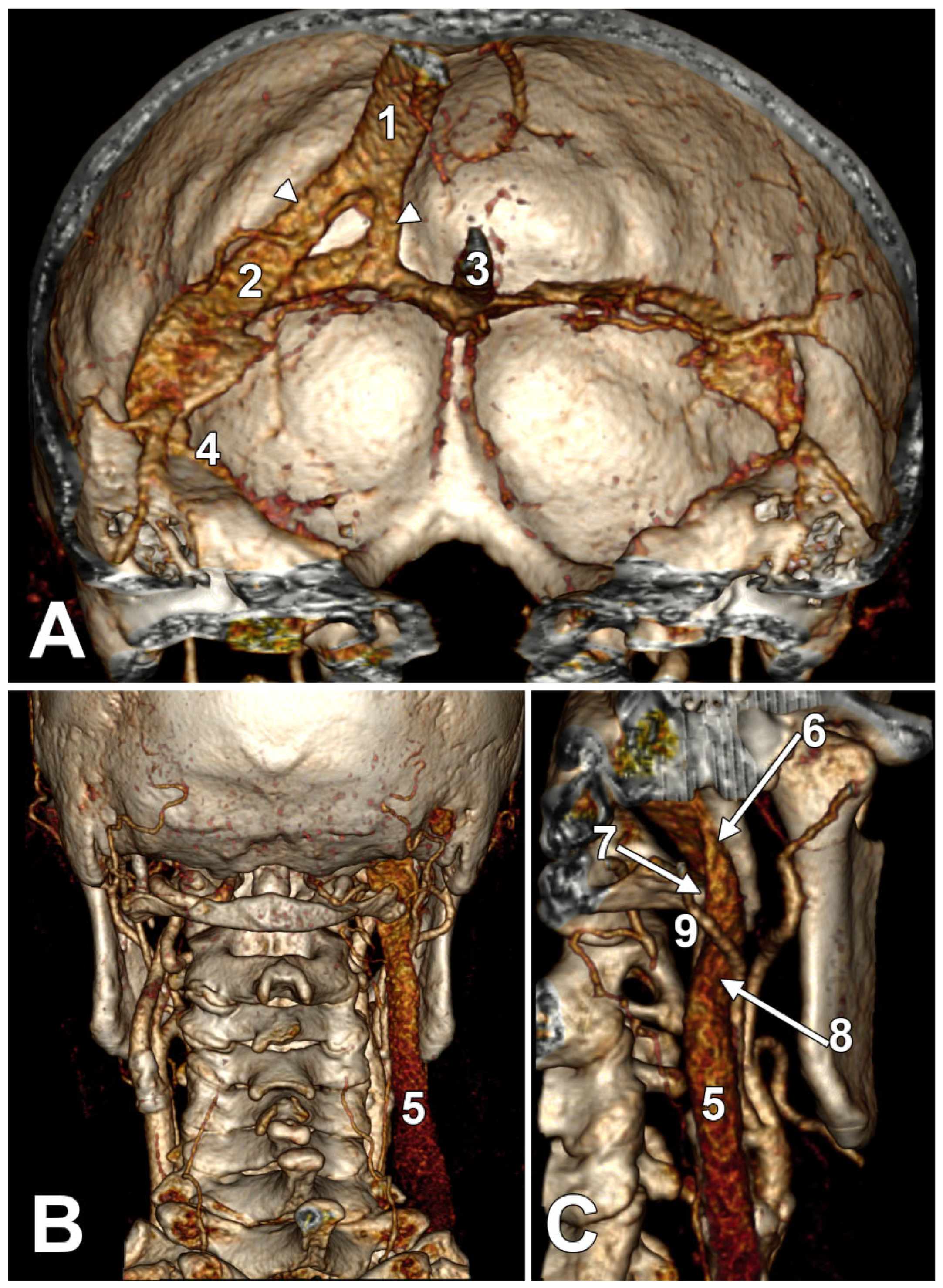
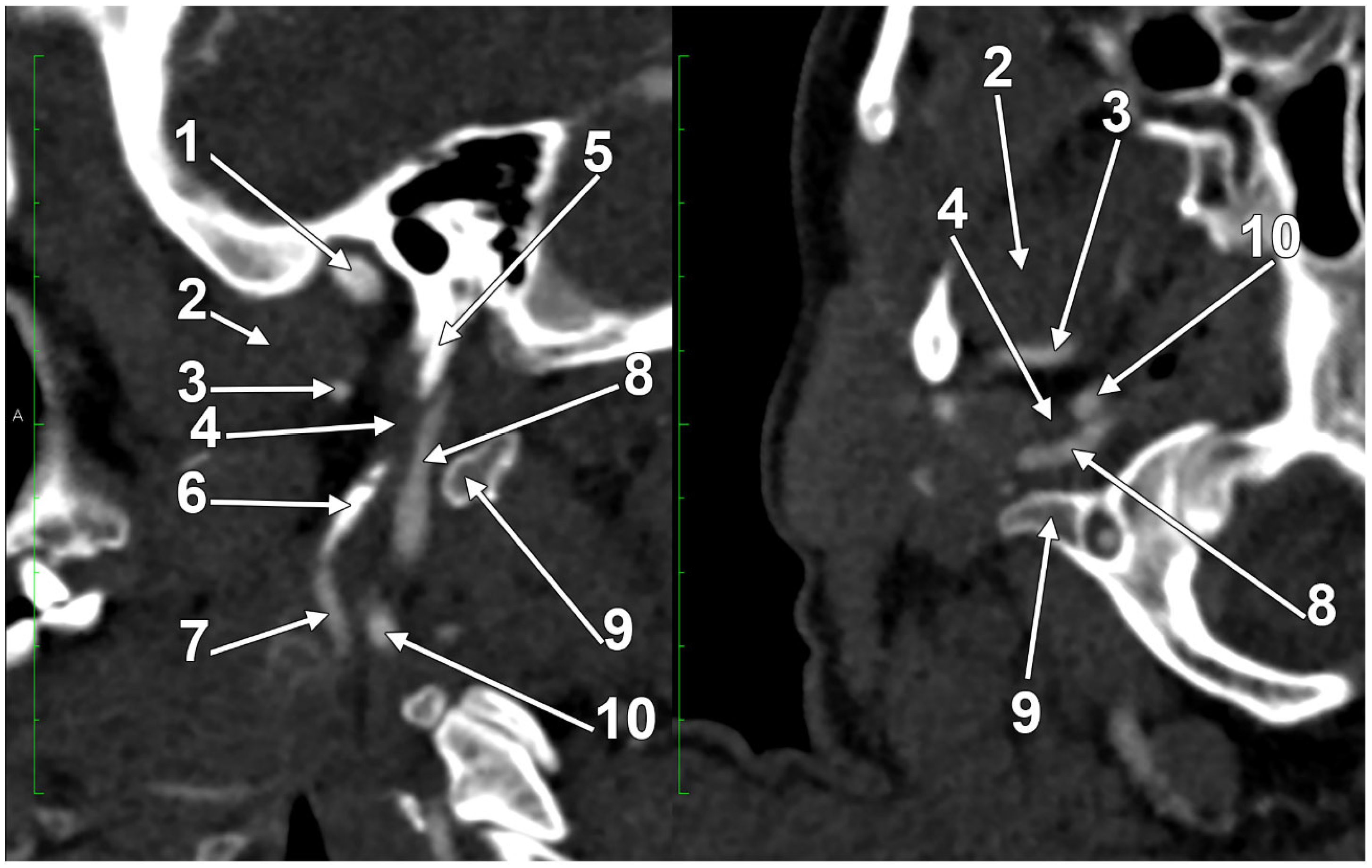
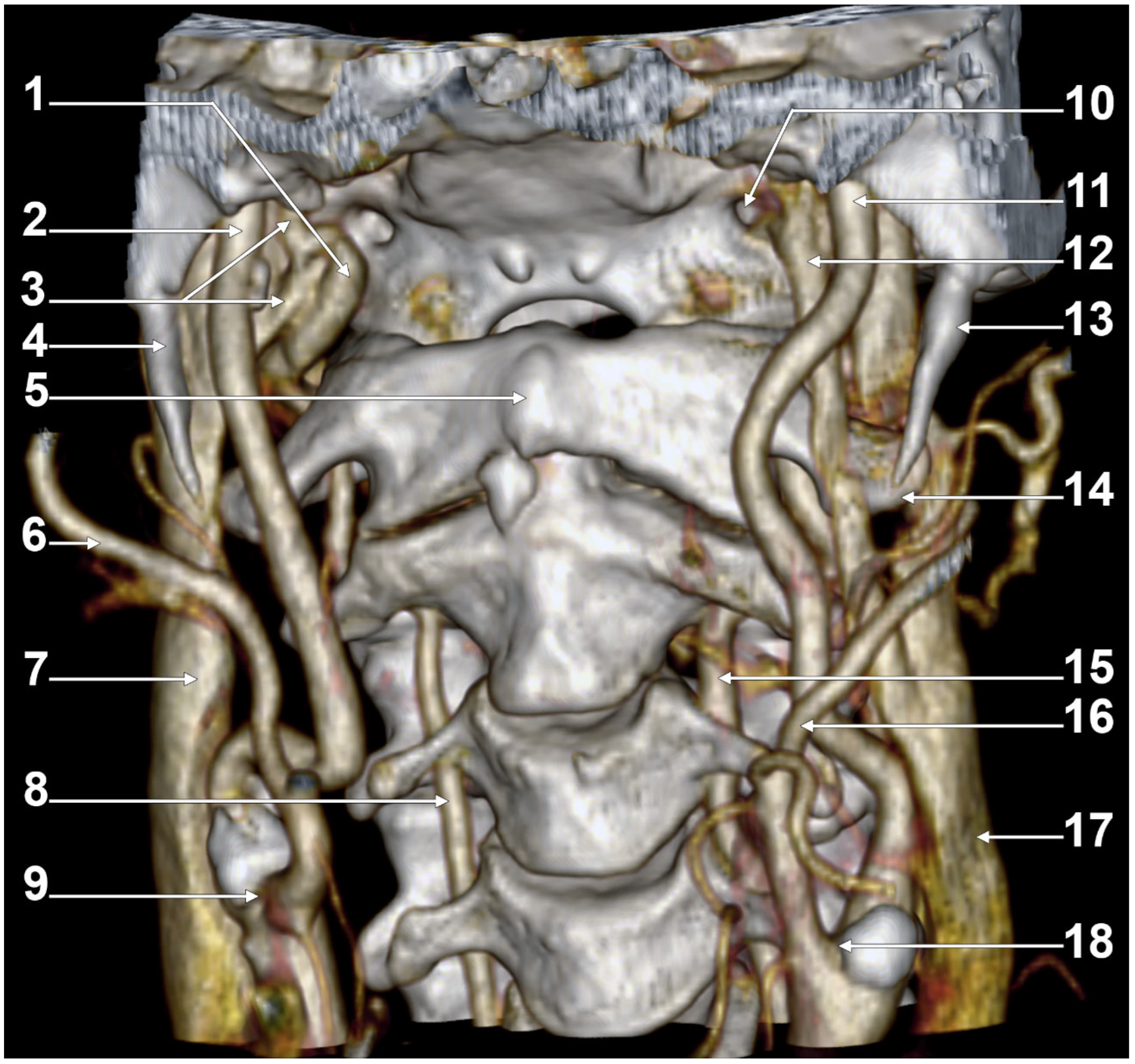
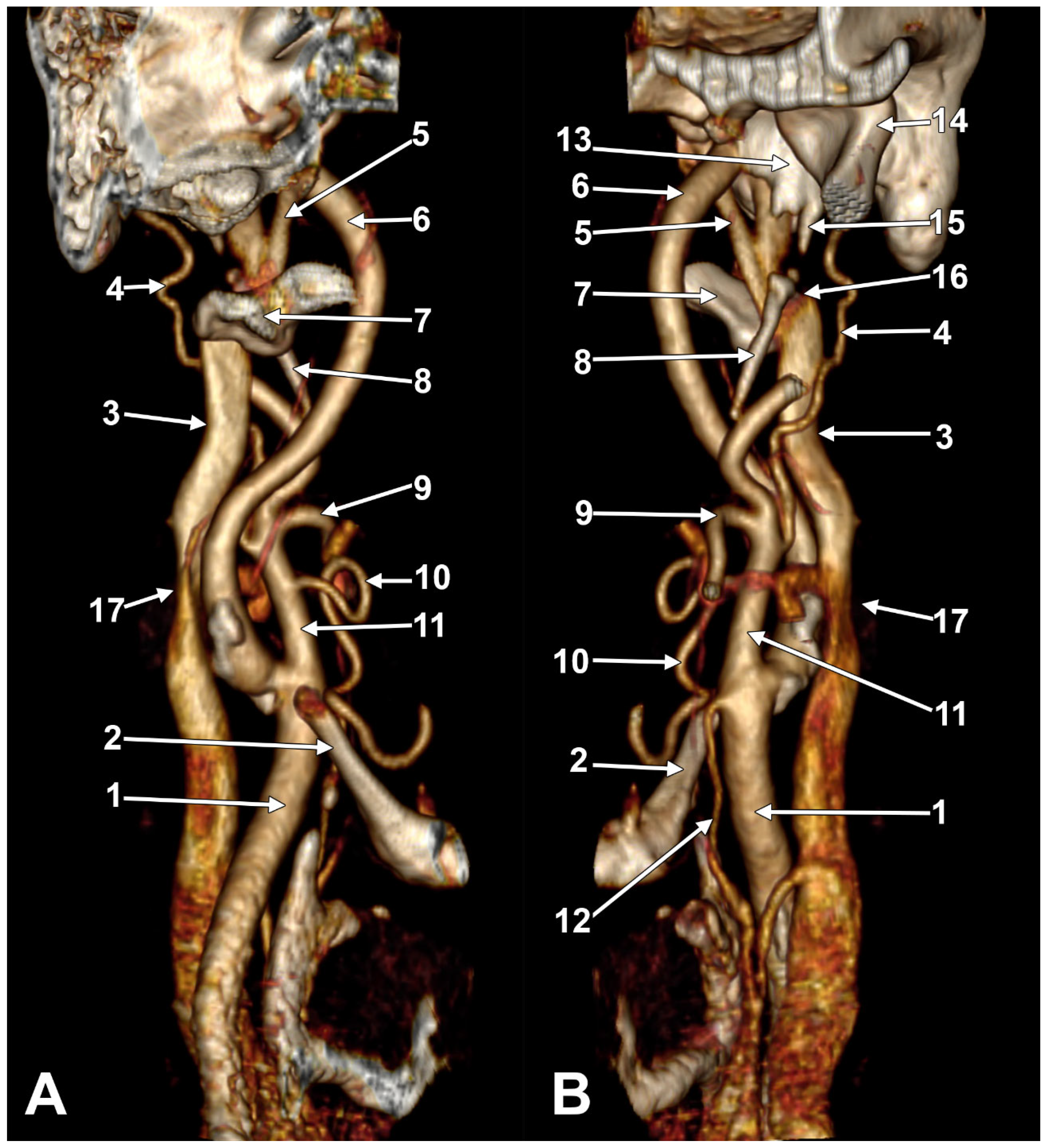
2. Normal Anatomy of the Internal Jugular Vein
2.1. The Segments of the Internal Jugular Vein
2.2. The Internal Jugular Vein’s Valves
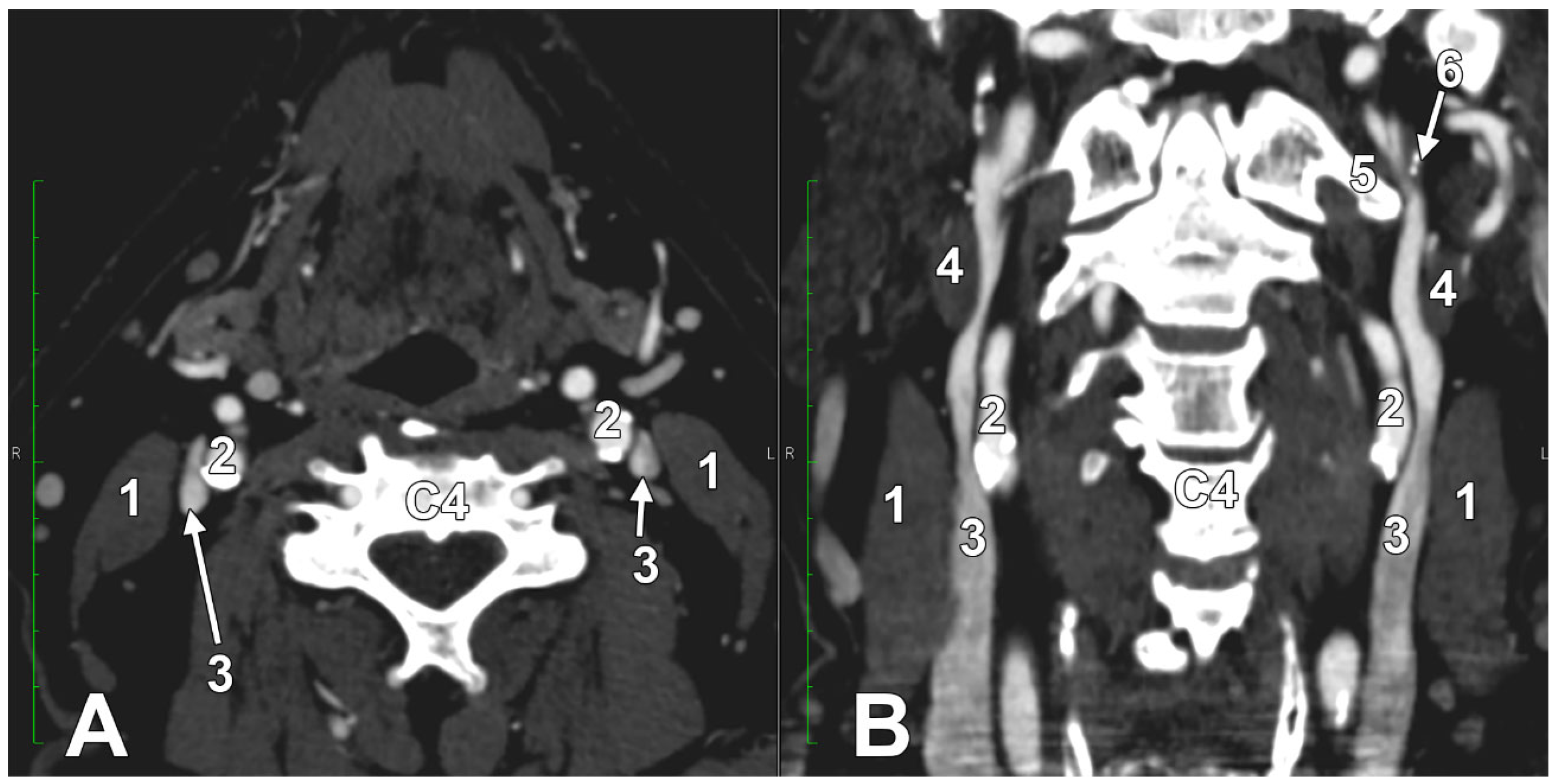
2.3. The Dominant Internal Jugular Vein
2.4. The “Tunnel” of the Internal Jugular Vein at the Level of the Transverse Process of the Atlas
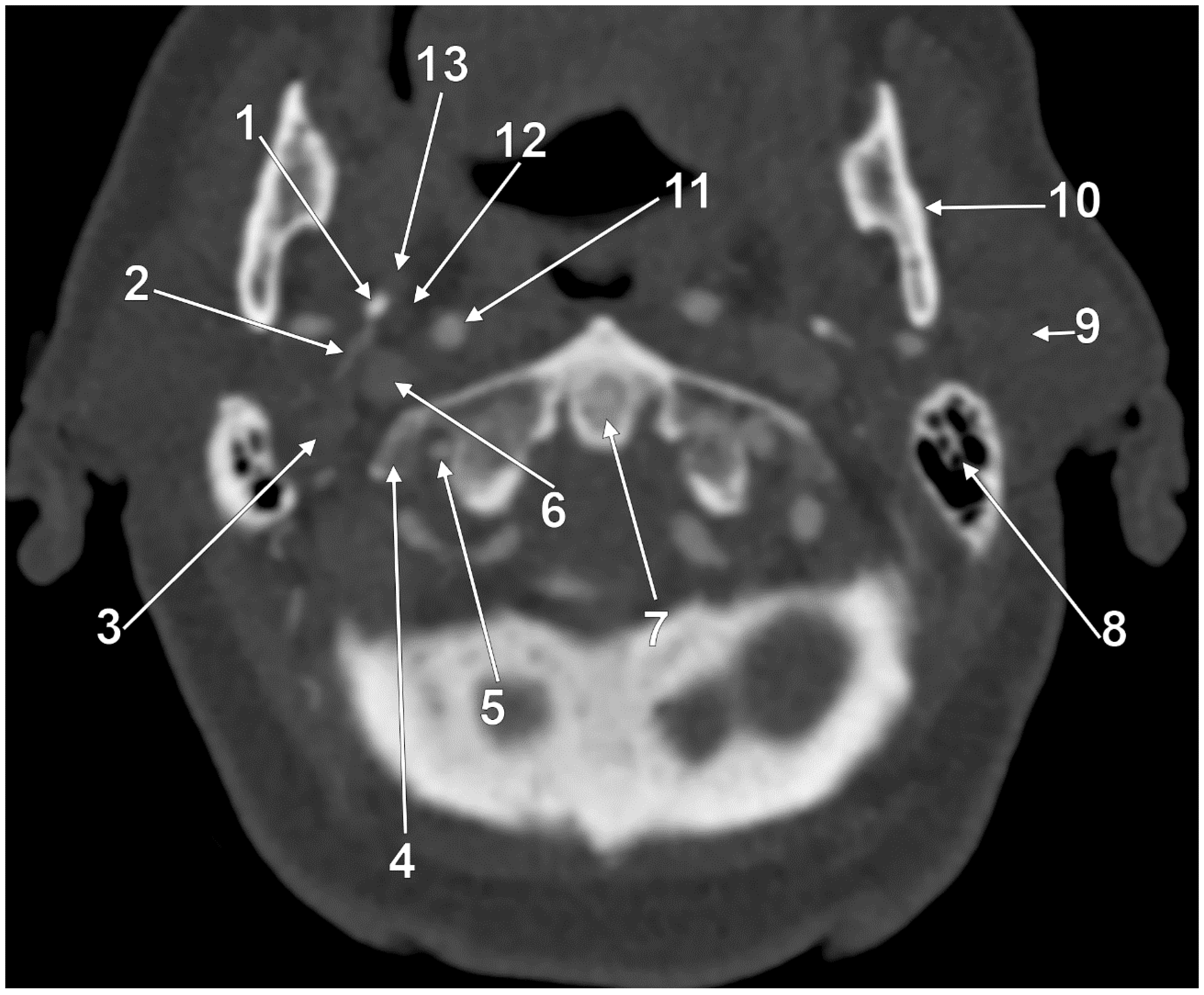
3. Anatomical Variations of the Internal Jugular Vein
| Jugular Bulb Variant | Definition | Clinical Significance | Prevalence/Range | References |
|---|---|---|---|---|
| High JB | JB apex abnormally high vs. otic landmarks (e.g., reaches/traverses IAC floor; above round window/basal turn/superior annulus). Morphology: “waist-like” JB (vs. finger-like diverticulum). | Can narrow Trautmann’s triangle; bleeding risk in otologic/skull-base work; common cause of pulsatile tinnitus. | Prevalence varies by definition/method, ranging from ~0% to 46% across studies. | [57,58,59,60,61,62,63] |
| Dehiscent JB | JB extends into the middle ear via a dehiscent sigmoid plate; mucosa only (no complete bony cover). | Injury-prone during ear surgery; haemorrhage; associations with thin carotid canal wall/dehiscence. | Reported ~1–7.5%; side bias often right-dominant. | [63,64,65,66,67,68] |
| JB Diverticulum | True outpouching from the JB dome (often finger-like) projecting superiorly/medially/posteriorly within the petrous bone. | Pulsatile tinnitus; conductive/±sensorineural loss; vertigo; can complicate middle ear/skull-base routes. | Reported from ~0.8% up to ~8% (method/definition dependent). | [58,60,62,63,67,69,70] |
| Hypoplastic JB | Proposed threshold: ≤5 mm max diameter. | May reflect reduced venous pathway calibre; may be consistent in measurement plane/modality. | Not standardised; threshold proposed to normalise reporting. | [71,72,73] |
| Hyperplastic JB | Proposed threshold: ≥15 mm max diameter. | Consider high-flow states/bone disorders; evaluate adjacent bony walls. | Not standardised; noted with achondroplasia/Paget’s disease, etc. | [71,72,74] |
| Topographic Variants | Type 1: none; Type 2: below PSC; Type 3: between PSC and IAC; Type 4: above IAC; “A” intact bone, “B” dehiscent. | Guides risk mapping near PSC/IAC; flags dehiscence (“B”) zones. | Classification, no pooled prevalence. | [69,75,76] |
| Condylar Jugular Diverticulum | Actually an enlarged condylar canal communicating with JB; best seen on posterior coronal images (maintains calibre to condylar fossa). | Prevents mislabelling as JBD and unnecessary intervention. | — | [60,70,77] |
Absence of the Internal Jugular Vein: Clinical Rarity, Causes, and Implications
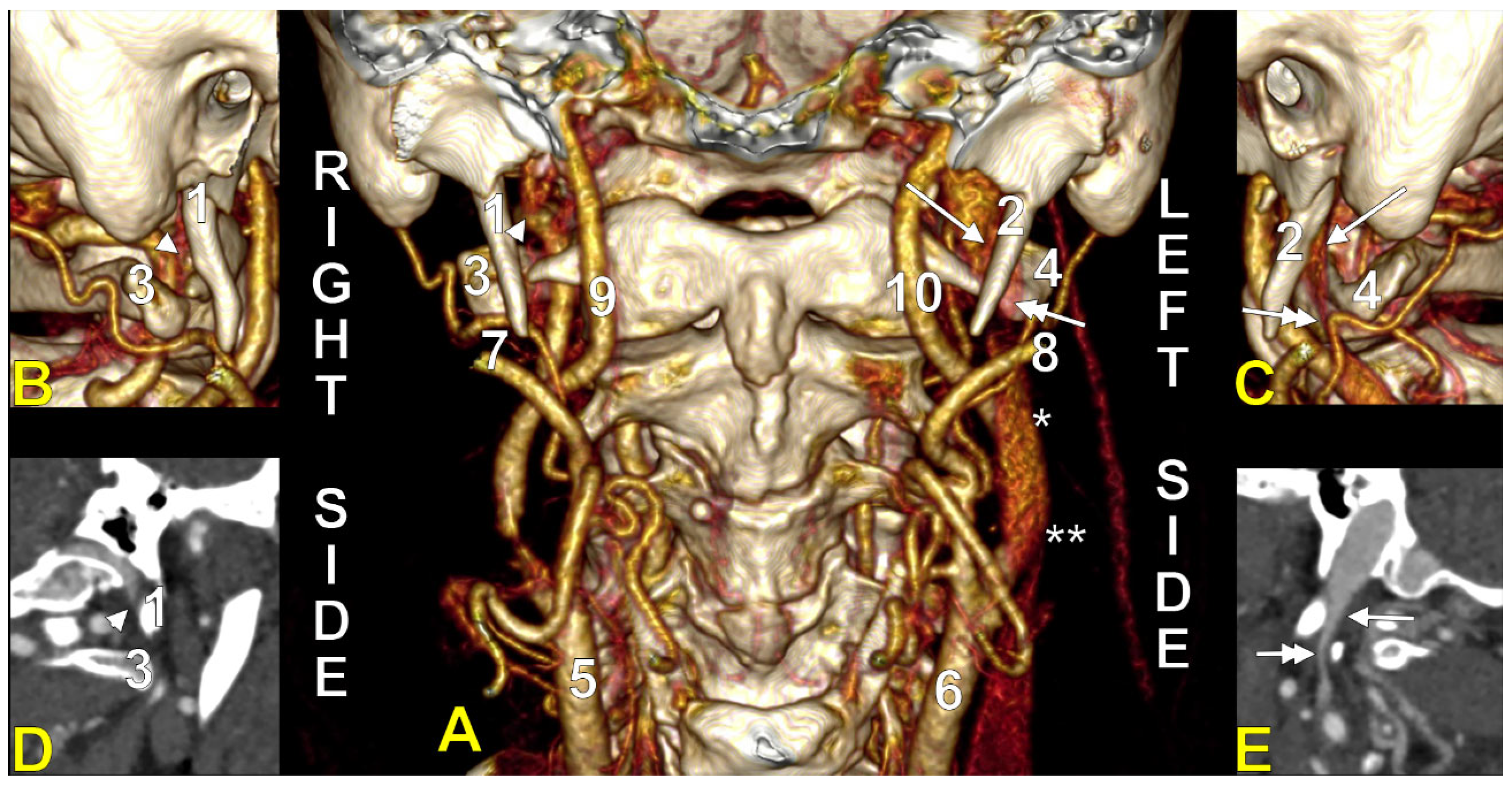
4. The Styloid Process and the Stylohyoid Chain
| Type/Pattern | Description | Prevalence/Notes | Citations |
|---|---|---|---|
| normal styloid process | typical length (<30 mm), no calcification | most common (74.97–86.5%) | [103,104] |
| elongated styloid process | length > 30 mm | 7.1–25.03% | [99,103,104] |
| calcified stylohyoid ligament | partial or total ossification of the ligament | 2.2% (partial more common than total) | [99,103] |
| segmented (pseudo-articulated) type | SHC appears as multiple ossified segments | 3.89–4.39% | [104] |
| absent stylohyoid chain | SHC not visible or absent | 2.8% | [103] |
The Length of the Styloid Process
5. The Nutcracker Syndromes
6. Eagle’s Syndromes
7. Compressions of the Internal Jugular Vein
| Cause/Intervention | Main Outcome/Symptom | Reference(s) |
|---|---|---|
| Styloid process/C1 vertebra | Headache, tinnitus, insomnia | [45,128,133,147,148] |
| Muscular/osseous structures | Atypical facial pain, hypertension | [147,149] |
| Subcutaneous emphysema/tumour | Vein narrowing, variable symptoms | [150,151] |
| Compression collar (protective) | Reduced brain/axonal injury | [30,31,152,153,154] |
| Condition/Cancer Type | Mechanism of IJV Compression | Clinical Consequence | Citations |
|---|---|---|---|
| Thyroid cancer | Lymph node metastasis | Occlusion/thrombosis | [151,169] |
| Lymphoma (e.g., Grey Zone Lymphoma) | Mediastinal/cervical lymphadenopathy | Thrombosis, SVC syndrome | [170] |
| Gastric/prostate carcinoma | Metastatic lymphadenopathy | Thrombosis, embolic events | [171,172] |
A Long Inferior Petrosal Sinus May Also Enter into the Nutcracker
8. Clinical Anatomy
8.1. Cannulation of the Internal Jugular Vein
8.2. Stenosis of the Internal Jugular Vein
Multiple Stenotic Segments of the Internal Jugular Vein
8.3. Thrombosis of the Internal Jugular Vein
8.4. Solving the Decompression of the Internal Jugular Vein
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCA | common carotid artery |
| CT | computed tomography |
| EA | Eagle’s syndrome |
| ECA | external carotid artery |
| ESP | elongated styloid process |
| HJB | high jugular bulb |
| IAC | internal acoustic canal |
| ICA | internal carotid artery |
| IJV | internal jugular vein |
| IPS | inferior petrosal sinus |
| JB | jugular bulb |
| JBD | jugular bulb diverticulum |
| JNS | jugular nutcracker syndrome |
| OA | occipital artery |
| OMH | omohyoid muscle |
| PAA | posterior auricular artery |
| PSC | posterior semicircular canal |
| SCM | sternocleidomastoid muscle |
| SHC | stylohyoid complex |
| SP | styloid process |
References
- Bandlamuri, S.; Khan, A.S.; Bialowas, C. Surgical approach to internal and external jugular venous agenesis: Case report. Surg. Radiol. Anat. 2023, 45, 989–993. [Google Scholar] [CrossRef]
- Asouhidou, I.; Natsis, K.; Asteri, T.; Sountoulides, P.; Vlasis, K.; Tsikaras, P. Anatomical variation of left internal jugular vein: Clinical significance for an anaesthesiologist. Eur. J. Anaesthesiol. 2008, 25, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Nomura, T.; Mizuno, Y.; Miyashita, T.; Goto, T. Pre-anesthetic ultrasonographic assessment of the internal jugular vein for prediction of hypotension during the induction of general anesthesia. J. Anesth. 2019, 33, 612–619. [Google Scholar] [CrossRef]
- Helwani, M.; Saied, N.; Ikeda, S. Accuracy of Anatomical Landmarks in Locating the Internal Jugular Vein Cannulation Site among Different Levels of Anesthesia Trainees. J. Educ. Perioper. Med. 2008, 10, E050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ding, J.; Asmaro, K.; Pan, L.; Ya, J.; Yang, Q.; Fan, C.; Ding, Y.; Ji, X.; Meng, R. Clinical Characteristics and Neuroimaging Findings in Internal Jugular Venous Outflow Disturbance. Thromb. Haemost. 2019, 119, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Seoane, E.; Rhoton, A.L., Jr. Compression of the internal jugular vein by the transverse process of the atlas as the cause of cerebellar hemorrhage after supratentorial craniotomy. Surg. Neurol. 1999, 51, 500–505. [Google Scholar] [CrossRef]
- Tudose, R.C.; Rusu, M.C.; Triantafyllou, G.; Piagkou, M.; Toader, C.; Radoi, P.M. Anatomical Variations of the Jugular Bulb: A Critical and Comprehensive Review. Medicina 2024, 60, 1408. [Google Scholar] [CrossRef]
- Matsushima, K.; Funaki, T.; Komune, N.; Kiyosue, H.; Kawashima, M.; Rhoton, A.L., Jr. Microsurgical anatomy of the lateral condylar vein and its clinical significance. Neurosurgery 2015, 11 (Suppl. 2), 135–145, discussion 145–146. [Google Scholar] [CrossRef]
- Spittau, B.; Millan, D.S.; El-Sherifi, S.; Hader, C.; Singh, T.P.; Motschall, E.; Vach, W.; Urbach, H.; Meckel, S. Dural arteriovenous fistulas of the hypoglossal canal: Systematic review on imaging anatomy, clinical findings, and endovascular management. J. Neurosurg. 2015, 122, 883–903. [Google Scholar] [CrossRef]
- Takahashi, S.; Sakuma, I.; Omachi, K.; Otani, T.; Tomura, N.; Watarai, J.; Mizoi, K. Craniocervical junction venous anatomy around the suboccipital cavernous sinus: Evaluation by MR imaging. Eur. Radiol. 2005, 15, 1694–1700. [Google Scholar] [CrossRef]
- Yamada, H.; Mizutani, K.; Akiyama, T.; Toda, M. Extracranial prevertebral venous network of the craniocervical junction: CT-digital subtraction venography analysis. Neuroradiology 2022, 64, 2227–2233. [Google Scholar] [CrossRef]
- Takemoto, K.; Tateshima, S.; Rastogi, S.; Gonzalez, N.; Jahan, R.; Duckwiler, G.; Vinuela, F. Onyx embolization of anterior condylar confluence dural arteriovenous fistula. BMJ Case Rep. 2013, 6, e13. [Google Scholar] [CrossRef]
- Mortazavi, M.M.; Tubbs, R.S.; Riech, S.; Verma, K.; Shoja, M.M.; Zurada, A.; Benninger, B.; Loukas, M.; Cohen Gadol, A.A. Anatomy and pathology of the cranial emissary veins: A review with surgical implications. Neurosurgery 2012, 70, 1312–1318, discussion 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Lassalle, S.L.; Serrano Bernardez, V.C.; Cifone, T.; Rimoldi, S.; Benitez, N.; Diaz, F.D.; Garay, V.; Bendersky, M. Compartmentalization of the human cephalic parapharyngeal space: A scoping review. Surg. Radiol. Anat. 2025, 47, 189. [Google Scholar] [CrossRef] [PubMed]
- Buch, K.; Groller, R.; Nadgir, R.N.; Fujita, A.; Qureshi, M.M.; Sakai, O. Variability in the Cross-Sectional Area and Narrowing of the Internal Jugular Vein in Patients without Multiple Sclerosis. AJR Am. J. Roentgenol. 2016, 206, 1082–1086. [Google Scholar] [CrossRef]
- Mumtaz, S.; Singh, M. Surgical review of the anatomical variations of the internal jugular vein: An update for head and neck surgeons. Ann. R. Coll. Surg. Engl. 2019, 101, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bazaral, M.; Harlan, S. Ultrasonographic anatomy of the internal jugular vein relevant to percutaneous cannulation. Crit. Care Med. 1981, 9, 307–310. [Google Scholar] [CrossRef]
- Qin, X.H.; Zhang, H.; Mi, W.D. Anatomic relationship of the internal jugular vein and the common carotid artery in Chinese people. Chin. Med. J. 2010, 123, 3226–3230. [Google Scholar]
- Simka, M. Obstructive Malformations of the Internal Jugular Vein. In Vascular Malformations; Khanna, A.K., Tiwary, S.K., Eds.; Springer: Singapore, 2021; pp. 295–303. [Google Scholar]
- Mancini, M.; Lanzillo, R.; Liuzzi, R.; Di Donato, O.; Ragucci, M.; Monti, S.; Salvatore, E.; Morra, V.B.; Salvatore, M. Internal jugular vein blood flow in multiple sclerosis patients and matched controls. PLoS ONE 2014, 9, e92730. [Google Scholar] [CrossRef]
- Valdueza, J.M.; von Munster, T.; Hoffman, O.; Schreiber, S.; Einhaupl, K.M. Postural dependency of the cerebral venous outflow. Lancet 2000, 355, 200–201. [Google Scholar] [CrossRef]
- van Zandwijk, J.K.; Kuijer, K.M.; Stassen, C.M.; Ten Haken, B.; Simonis, F.F.J. Internal Jugular Vein Geometry Under Multiple Inclination Angles with 3D Low-Field MRI in Healthy Volunteers. J. Magn. Reson. Imaging 2022, 56, 1302–1308. [Google Scholar] [CrossRef]
- Giordano, C.R.; Murtagh, K.R.; Mills, J.; Deitte, L.A.; Rice, M.J.; Tighe, P.J. Locating the optimal internal jugular target site for central venous line placement. J. Clin. Anesth. 2016, 33, 198–202. [Google Scholar] [CrossRef]
- Ishizuka, M.; Nagata, H.; Takagi, K.; Kubota, K. Right internal jugular vein is recommended for central venous catheterization. J. Investig. Surg. 2010, 23, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Lobato, E.B.; Sulek, C.A.; Moody, R.L.; Morey, T.E. Cross-sectional area of the right and left internal jugular veins. J. Cardiothorac. Vasc. Anesth. 1999, 13, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.J.; Sutherland, R.; Scott, D.H. The effect of position and different manoeuvres on internal jugular vein diameter size. Acta Anaesthesiol. Scand. 1994, 38, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, P.; London, N.R., Jr.; Xu, H.; Chen, X.; Carrau, R.L. Relevance of the Internal Jugular Vein for Surgery in the Upper Parapharyngeal Space. Ear Nose Throat J. 2023. [Google Scholar] [CrossRef]
- Pagano, S.; Ricciuti, V.; Mancini, F.; Barbieri, F.R.; Chegai, F.; Marini, A.; Marruzzo, D.; Paracino, R.; Ricciuti, R.A. Eagle syndrome: An updated review. Surg. Neurol. Int. 2023, 14, 389. [Google Scholar] [CrossRef]
- Dinsmore, M.; Hajat, Z.; Brenna, C.T.; Fisher, J.; Venkatraghavan, L. Effect of a neck collar on brain turgor: A potential role in preventing concussions? Br. J. Sports Med. 2022, 56, 605–607. [Google Scholar] [CrossRef]
- Sindelar, B.; Bailes, J.; Sherman, S.; Finan, J.; Stone, J.; Lee, J.; Ahmadian, S.; Zhou, Y.; Patel, V.; Smith, D. Effect of Internal Jugular Vein Compression on Intracranial Hemorrhage in a Porcine Controlled Cortical Impact Model. J. Neurotrauma 2017, 34, 1703–1709. [Google Scholar] [CrossRef]
- Sindelar, B.; Shinners, M.; Sherman, S.; Novak, K.; Erickson, K.; Patel, V.; Kubilis, P.; Smith, D.; Finan, J.; Bailes, J.E. Internal Jugular Vein Compression: A Novel Approach to Mitigate Blast Induced Hearing Injury. Otol. Neurotol. 2017, 38, 591–598. [Google Scholar] [CrossRef]
- Fargen, K.M.; Midtlien, J.P.; Belanger, K.; Hepworth, E.J.; Hui, F.K. The Promise, Mystery, and Perils of Stenting for Symptomatic Internal Jugular Vein Stenosis: A Case Series. Neurosurgery 2024, 95, 400–407. [Google Scholar] [CrossRef]
- Cha, Y.H.; Gharib, M.; Chan, K.; Karam, J. Sternocleidomastoid Omohyoid Entrapment of the Internal Jugular Vein Causing Vertigo and Headaches. Clin. Otolaryngol. 2025, 50, 918–923. [Google Scholar] [CrossRef]
- Cha, Y.-H.; Chan, K.; Gharib, M.; Karam, J. Jugular Variant Bow-Hunter’s Syndrome from Sternocleidomastoid Entrapment of the Internal Jugular Vein (P11-10.001). Neurology 2024, 102, 6341. [Google Scholar] [CrossRef]
- Kosnik, N.; Kowalski, T.; Lorenz, L.; Valacer, M.; Sakthi-Velavan, S. Anatomical review of internal jugular vein cannulation. Folia Morphol. 2024, 83, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, X.; Jia, Y.; Wang, Y.; Ji, X.; Meng, R. A hemodynamic screening tool for symptomatic internal jugular vein stenosis in J3 segment. Phlebology 2025, 40, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, S.; d’Alessandro, A.; Niglio, T.; Bruno, A.; Bernardo, B.; Mandolesi, D.; Caroli, A.; d’Alessandro, A.; Fedele, F. Jugular diameter and venous reflux. Ann. Ital. Chir. 2016, 87, 129–137. [Google Scholar]
- Scerrati, A.; Menegatti, E.; Zamboni, M.; Malagoni, A.M.; Tessari, M.; Galeotti, R.; Zamboni, P. Internal Jugular Vein Thrombosis: Etiology, Symptomatology, Diagnosis and Current Treatment. Diagnostics 2021, 11, 378. [Google Scholar] [CrossRef]
- Manta, M.D.; Rusu, M.C.; Hostiuc, S.; Tudose, R.C.; Manta, B.A.; Jianu, A.M. The vertical topography of the carotid bifurcation—Original study and review. Surg. Radiol. Anat. 2024, 46, 1253–1263. [Google Scholar] [CrossRef]
- Ding, J.Y.; Zhou, D.; Pan, L.Q.; Ya, J.Y.; Liu, C.; Yan, F.; Fan, C.Q.; Ding, Y.C.; Ji, X.M.; Meng, R. Cervical spondylotic internal jugular venous compression syndrome. CNS Neurosci. Ther. 2020, 26, 47–54. [Google Scholar] [CrossRef]
- Zivadinov, R.; Chung, C.P. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med. 2013, 11, 260. [Google Scholar] [CrossRef]
- Franklin, K.J. Valves in Veins: An Historical Survey. Proc. R. Soc. Med. 1927, 21, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Schell, R.M.; Cole, D.J. Cerebral monitoring: Jugular venous oximetry. Anesth. Analg. 2000, 90, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Saifi, R.; Augarde, R.; Prin, S.; Schmitt, J.M.; Page, B.; Pipien, I.; Jardin, F. The Internal jugular veins are asymmetric. Usefulness of ultrasound before catheterization. Intensive Care Med. 2001, 27, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.V.; Boxerman, J.L.; Davis, L.M.; Haas, R.A.; Rogg, J.M. Incidence of extrinsic compression of the internal jugular vein in unselected patients undergoing CT angiography. AJNR Am. J. Neuroradiol. 2012, 33, 1247–1250. [Google Scholar] [CrossRef]
- Calota, R.N.; Rusu, M.C.; Vrapciu, A.D. The External Carotid Artery and the Styloid Process. Curr. Health Sci. J. 2024, 50, 232–236. [Google Scholar]
- Rusu, M.C.; Dumitru, C.C.; Tudose, R.C. Aberrant courses of the occipital artery. Surg. Radiol. Anat. 2025, 47, 135. [Google Scholar] [CrossRef]
- Paturet, G. Traite D’anatomie Humaine; Masson et Cie: Paris, France, 1964. [Google Scholar]
- Contrera, K.J.; Aygun, N.; Ward, B.K.; Gooi, Z.; Richmon, J.D. Internal jugular vein duplication and fenestration: Case series and literature review. Laryngoscope 2016, 126, 1585–1588. [Google Scholar] [CrossRef]
- Nova-Baeza, P.; Valenzuela-Fuenzalida, J.J.; Valdivia-Arroyo, R.; Becerra-Rodriguez, E.S.; Escalona-Manzo, C.; Castano-Gallego, Y.T.; Luque-Bernal, R.M.; Oyanedel-Amaro, G.; Suazo-Santibanez, A.; Orellana-Donoso, M.; et al. Systematic Review and Meta-Analysis of Internal Jugular Vein Variants and Their Relationship to Clinical Implications in the Head and Neck. Diagnostics 2024, 14, 2765. [Google Scholar] [CrossRef]
- Li, M.; Gao, X.; Rajah, G.B.; Liang, J.; Chen, J.; Yan, F.; Bao, Y.; Jiao, L.; Zhang, H.; Ding, Y.; et al. Styloidectomy and Venous Stenting for Treatment of Styloid-Induced Internal Jugular Vein Stenosis: A Case Report and Literature Review. World Neurosurg. 2019, 130, 129–132. [Google Scholar] [CrossRef]
- Bergman, R.; Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rusu, M.C.; Vrapciu, A.D.; Popescu, S.A. High bilateral fenestration of the internal jugular vein. Surg. Radiol. Anat. 2022, 44, 703–708. [Google Scholar] [CrossRef]
- Majeed, T.A.; Deshpande, R.K.; Upadhaya, S.; Deshmukh, S.A. Agenesis of internal jugular vein a cause for concern. Indian J. Surg. Oncol. 2010, 1, 341–342. [Google Scholar] [CrossRef][Green Version]
- Waltner, J.G. Anatomic variations of the lateral and sigmoid sinuses. Arch. Otolaryngol. 1944, 39, 307–312. [Google Scholar] [CrossRef]
- Laff, H. Unilateral absence of sigmoid sinus. Arch. Otolaryngol. 1930, 11, 151–157. [Google Scholar] [CrossRef]
- Graham, M.D. The jugular bulb. Its anatomic and clinical considerations in contemporary otology. Arch. Otolaryngol. 1975, 101, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Bilgen, C.; Kirazli, T.; Ogut, F.; Totan, S. Jugular bulb diverticula: Clinical and radiologic aspects. Otolaryngol. Head Neck Surg. 2003, 128, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.R.; Eubig, J.; Winata, L.S.; Pramanik, B.K.; Merchant, S.N.; Lalwani, A.K. Prevalence of jugular bulb abnormalities and resultant inner ear dehiscence: A histopathologic and radiologic study. Otolaryngol. Head Neck Surg. 2012, 147, 750–756. [Google Scholar] [CrossRef]
- Wadin, K.; Wilbrand, H. The jugular bulb diverticulum. A radioanatomic investigation. Acta Radiol. Diagn. 1986, 27, 395–401. [Google Scholar] [CrossRef]
- Wadin, K.; Thomander, L.; Wilbrand, H. Effects of a high jugular fossa and jugular bulb diverticulum on the inner ear. A clinical and radiologic investigation. Acta Radiol. Diagn. 1986, 27, 629–636. [Google Scholar] [CrossRef]
- Jahrsdoerfer, R.A.; Cail, W.S.; Cantrell, R.W. Endolymphatic duct obstruction from a jugular bulb diverticulum. Ann. Otol. Rhinol. Laryngol. 1981, 90, 619–623. [Google Scholar] [CrossRef]
- Atilla, S.; Akpek, S.; Uslu, S.; Ilgit, E.T.; Isik, S. Computed tomographic evaluation of surgically significant vascular variations related with the temporal bone. Eur. J. Radiol. 1995, 20, 52–56. [Google Scholar] [CrossRef]
- Ball, M.; Elloy, M.; Vaidhyanath, R.; Pau, H. Beware the silent presentation of a high and dehiscent jugular bulb in the external ear canal. J. Laryngol. Otol. 2010, 124, 790–792. [Google Scholar] [CrossRef]
- Atmaca, S.; Elmali, M.; Kucuk, H. High and dehiscent jugular bulb: Clear and present danger during middle ear surgery. Surg. Radiol. Anat. 2014, 36, 369–374. [Google Scholar] [CrossRef]
- Sayit, A.T.; Gunbey, H.P.; Fethallah, B.; Gunbey, E.; Karabulut, E. Radiological and audiometric evaluation of high jugular bulb and dehiscent high jugular bulb. J. Laryngol. Otol. 2016, 130, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Koesling, S.; Kunkel, P.; Schul, T. Vascular anomalies, sutures and small canals of the temporal bone on axial CT. Eur. J. Radiol. 2005, 54, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, Y.; Wang, H.; Li, C.; Wu, Y.; Shi, H.; Yin, S.; Chen, Z. Prevalence of High Jugular Bulb across Different Stages of Adulthood in A Chinese Population. Aging Dis. 2020, 11, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Manjila, S.; Bazil, T.; Kay, M.; Udayasankar, U.K.; Semaan, M. Jugular bulb and skull base pathologies: Proposal for a novel classification system for jugular bulb positions and microsurgical implications. Neurosurg. Focus 2018, 45, E5. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Mallio, C.A.; Quattrocchi, C.C. Right Condylar Jugular Diverticulum: Contrast-enhanced Computed Tomography Findings of a Rare Anatomical Variant of Jugular Bulb. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 2257–2259. [Google Scholar] [CrossRef]
- Tudose, R.C.; Rusu, M.C.; Triantafyllou, G.; Piagkou, M.; Moraru, L.; Dumitru, C.C. Jugular bulb anatomical variations and pneumatization patterns: A comprehensive CBCT analysis. Surg. Radiol. Anat. 2024, 46, 1001–1013. [Google Scholar] [CrossRef]
- Walsh, R.M.; Lannigan, F.J.; McGlashan, J.A.; Bowdler, D.A. Jugular bulb phlebectasia. Int. J. Pediatr. Otorhinolaryngol. 1993, 25, 249–254. [Google Scholar] [CrossRef]
- Nager, G.T.; Stein, S.A.; Dorst, J.P.; Holliday, M.J.; Kennedy, D.W.; Diehn, K.W.; Jabs, E.W. Sclerosteosis involving the temporal bone: Clinical and radiologic aspects. Am. J. Otolaryngol. 1983, 4, 1–17. [Google Scholar] [CrossRef]
- Amroliwalla, F.K.; Bennett, R.M. An Unusual Complication of Paget’s Disease. J. R. Army Med. Corps. 1971, 117, 20. [Google Scholar] [CrossRef]
- Vachata, P.; Petrovicky, P.; Sames, M. An anatomical and radiological study of the high jugular bulb on high-resolution CT scans and alcohol-fixed skulls of adults. J. Clin. Neurosci. 2010, 17, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Juelke, E.; Butzer, T.; Yacoub, A.; Wimmer, W.; Caversaccio, M.; Anschuetz, L. Assessment of jugular bulb variability based on 3D surface models: Quantitative measurements and surgical implications. Surg. Radiol. Anat. 2023, 45, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, K.; Cure, J.K.; Harnsberger, H.R. Condylar jugular diverticulum. J. Comput. Assist. Tomogr. 2009, 33, 309–311. [Google Scholar] [CrossRef]
- Rusu, M.C.; Tudose, R.C.; Vrapciu, A.D.; Butucescu, M. Details of rare and novel anatomical variations in a case with bilateral long styloid processes. Rom. J. Morphol. Embryol. 2025, 66, 399–403. [Google Scholar] [CrossRef]
- Manta, M.D.; Jianu, A.M.; Rusu, M.C.; Popescu, S.A. Launay’s External Carotid Vein. Medicina 2021, 57, 985. [Google Scholar] [CrossRef]
- Hage, N.; Kappagantu, K.M.; Singh, N.K.; Ramamourthy, B. Anomalous Posterior Branching of the Internal Jugular Vein: A Report of Two Patients. Int. J. Oral. Maxillofac. Surg. 2024, 53, 547–550. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, J.; Bai, C.; Ding, Y.; Ji, X.; Meng, R. Clinical Classification and Collateral Circulation in Chronic Cerebrospinal Venous Insufficiency. Front. Neurol. 2020, 11, 913. [Google Scholar] [CrossRef]
- Karim, N.K.A.; Zain, N.H.M. An Incidental Finding of Internal Jugular Vein Ectasia on Cervical Spine Magnetic Resonance Imaging. Mal. J. Med. Health Sci. 2018, 14, 82–84. [Google Scholar]
- Kong, K.; Jian, C.; Ji, R.; Irwin, M.G. Management of an incidental finding of right internal jugular vein agenesis. Hong Kong Med. J. 2017, 23, 414–415. [Google Scholar] [CrossRef][Green Version]
- Essafti, M.; Belhadj, A.; Qamouss, Y.; Rokhsi, R.; Aissaoui, Y. Agenesis of the left internal jugular vein: An unusual finding during an ultra-sound guided central venous catheterization. J. Clin. Anesth. 2018, 44, 87–88. [Google Scholar] [CrossRef]
- Filograna, L.; Calcagni, A.; Rossi, G.; Di Stefano, C.; Beninati, E.; Collura, A.; Floris, R. Internal jugular vein agenesis: A rare vascular abnormality and incidental finding. A case of internal jugular vein agenesis in a 52-years old male. Radiol. Case Rep. 2019, 14, 452–455. [Google Scholar] [CrossRef]
- Kayiran, O.; Calli, C.; Emre, A.; Soy, F.K. Congenital agenesis of the internal jugular vein: An extremely rare anomaly. Case Rep. Surg. 2015, 2015, 637067. [Google Scholar] [CrossRef]
- Rewari, V.; Chandran, R.; Ramachandran, R.; Trikha, A. Absent internal jugular vein: Another case for ultrasound guided vascular access. Indian J. Crit. Care Med. 2015, 19, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.A.; Colak, Y.Z.; Kacmaz, O.; Kolu, M.; Toprak, H.I. Determination of Absence of Right Internal Jugular Vein During Ultrasonographic Guided Central Venous Cannulation. Turk. J. Anaesthesiol. Reanim. 2017, 45, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; García-Muro, C.; Alatorre-Jiménez, M.A.; López-García, C.A.; Marín-Medina, A. Thrombosis of the internal jugular vein, a rare entity: A case report and brief review of the literature. J. Acute Dis. 2023, 12, 80–82. [Google Scholar] [CrossRef]
- Luis, S.A.A.; Garcia, L.; Isabel, G.G.M.; Tejera, H.A.; Loro, P.J.; Fernandez, A.M. Right jugular vein agenesis. a challenging case for experienced endocrine surgeons. Endocr. Abstr. Biosci. 2024, 99, EP966. [Google Scholar] [CrossRef]
- Miller, B.R. Absence of a right internal jugular vein detected by ultrasound imaging. Paediatr. Anaesth. 2011, 21, 91. [Google Scholar] [CrossRef]
- Amin, M.A.; Nahin, S.; Hawlader, M.D.H. Persistent headaches sometimes concern incidental findings: A rare case of internal jugular vein agenesis in a 32-year-old man. Clin. Case Rep. 2022, 10, e6423. [Google Scholar] [CrossRef]
- Kurbanova, A.; Polat Balkan, E.; Incebeyaz, B.; Aksoy, S.; Orhan, K. Retrospective evaluation of ponticulus posticus prevalence, sella turcica types, and stylohyoid complex calcifications in a group of Turkish population. Anat. Sci. Int. 2025, 100, 54–63. [Google Scholar] [CrossRef]
- Andrei, F.; Motoc, A.G.; Didilescu, A.C.; Rusu, M.C. A 3D cone beam computed tomography study of the styloid process of the temporal bone. Folia Morphol. 2013, 72, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, P.; Gronkiewicz, S.; Solinski, D.; Pers, A.; Lachowski, K.; Domagala, Z. A case of elongated styloid process in a modern-age skull from Puerto Cabello, Venezuela. Folia Morphol. 2015, 74, 475–478. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, B.S.; de Bakker, H.M.; Soerdjbalie-Maikoe, V.; Dikkers, F.G. Variants of the hyoid-larynx complex, with implications for forensic science and consequence for the diagnosis of Eagle’s syndrome. Sci. Rep. 2019, 9, 15950. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Tudose, R.C.; Vrapciu, A.D.; Popescu, Ş.A. Lowered hyoid bone overlapping the thyroid cartilage in CT angiograms. Surg. Radiol. Anat. 2024, 46, 333–339. [Google Scholar] [CrossRef]
- Ghosh, L.M.; Dubey, S.P. The syndrome of elongated styloid process. Auris Nasus Larynx 1999, 26, 169–175. [Google Scholar] [CrossRef]
- Guimaraes, A.C.; Pozza, D.H.; Guimaraes, A.S. Prevalence of morphological and structural changes in the stylohyoid chain. J. Clin. Exp. Dent. 2020, 12, e1027–e1032. [Google Scholar] [CrossRef]
- Sultan, S.; Acharya, Y.; Soliman, O.; Hynes, N. Stylohyoid Eagle syndrome and EXTracranial INternal Carotid arTery pseudoaneurysms (EXTINCT) with internal jugular vein nutcracker syndrome: A challenging clinical scenario. BMJ Case Rep. 2022, 15, e249558. [Google Scholar] [CrossRef]
- Guimarães, S.M.R.; Carvalho, A.C.P.; Guimarães, J.P.; Gomes, M.B.; Cardoso, M.d.M.M.; Reis, H.N. Prevalence of morphological alterations of the styloid process in patients with temporomandibular joint disorder. Radiol. Bras. 2006, 39, 407–411. [Google Scholar] [CrossRef]
- Langlais, R.P.; Langland, O.E.; Nortjé, C. Diagnostic Imaging of the Jaws; Williams & Wilkins: Baltimore, MD, USA, 1995. [Google Scholar]
- Altındağ, A.; Eren, H.; Küçükkalem, M.; Altındağ, Ö. Prevalence and pattern of stylohyoid chain complex on panoramic radiographs: A retrospective study. Clin. Exp. Health Sci. 2022, 12, 906–912. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Paschopoulos, I.; Duparc, F.; Tsakotos, G.; Papadopoulos-Manolarakis, P.; Piagkou, M. The Anatomy of the Stylohyoid Chain: A Systematic Review with Meta-Analysis. Diagnostics 2025, 15, 925. [Google Scholar] [CrossRef]
- Balcioglu, H.A.; Kilic, C.; Akyol, M.; Ozan, H.; Kokten, G. Length of the styloid process and anatomical implications for Eagle’s syndrome. Folia Morphol. 2009, 68, 265–270. [Google Scholar]
- Onbas, O.; Kantarci, M.; Murat Karasen, R.; Durur, I.; Cinar Basekim, C.; Alper, F.; Okur, A. Angulation, length, and morphology of the styloid process of the temporal bone analyzed by multidetector computed tomography. Acta Radiol. 2005, 46, 881–886. [Google Scholar] [CrossRef]
- Jung, T.; Tschernitschek, H.; Hippen, H.; Schneider, B.; Borchers, L. Elongated styloid process: When is it really elongated? Dentomaxillofac Radiol. 2004, 33, 119–124. [Google Scholar] [CrossRef]
- Munoz-Leija, M.A.; Ordonez Rivas, F.O.; Barrera-Flores, F.J.; Trevino-Gonzalez, J.L.; Pinales-Razo, R.; Guzman-Lopez, S.; Elizondo-Omana, R.E.; Quiroga-Garza, A. A proposed extension to the elongated styloid process definition: A morphological study with high-resolution tomography computer. Morphologie 2020, 104, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, G.; Zangrossi, P.; Flacco, M.E.; Di Domenico, G.; Nastro Siniscalchi, E.; De Ponte, F.S.; Maugeri, R.; De Bonis, P.; Cavallo, M.A.; Zamboni, P.; et al. Styloid Jugular Nutcracker: The Possible Role of the Styloid Process Spatial Orientation-A Preliminary Morphometric Computed Study. Diagnostics 2023, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Mantovani, G.; Cavallo, M.A.; Scerrati, A.; De Bonis, P. Neurosurgical implications of the Jugular Vein Nutcracker. Veins Lymphat. 2023, 12, 11892-1–11892-10. [Google Scholar] [CrossRef]
- Dashti, S.R.; Nakaji, P.; Hu, Y.C.; Frei, D.F.; Abla, A.A.; Yao, T.; Fiorella, D. Styloidogenic jugular venous compression syndrome: Diagnosis and treatment: Case report. Neurosurgery 2012, 70, E795–E799. [Google Scholar] [CrossRef]
- Zhang, F.L.; Zhou, H.W.; Guo, Z.N.; Yang, Y. Eagle Syndrome as a Cause of Cerebral Venous Sinus Thrombosis. Can. J. Neurol. Sci. 2019, 46, 344–345. [Google Scholar] [CrossRef]
- Alpoz, E.; Akar, G.C.; Celik, S.; Govsa, F.; Lomcali, G. Prevalence and pattern of stylohyoid chain complex patterns detected by panoramic radiographs among Turkish population. Surg. Radiol. Anat. 2014, 36, 39–46. [Google Scholar] [CrossRef]
- Penfold, D.; Leslie, S.W.; Lotfollahzadeh, S. Nutcracker Syndrome and Left Renal Vein Entrapment; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Damen, N.S.; Hostiuc, S.; Jianu, A.M.; Manta, B.A.; Rusu, M.C.; Dobra, M.A. Anatomical variants of the retroaortic left renal vein. Ann. Anat. 2024, 251, 152170. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Chen, S.; Tian, L.; Li, D.; Li, M.; Jin, W.; Zhang, H. Nutcracker syndrome—how well do we know it? Urology 2014, 83, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Eagle, W.W. Elongated styloid processes: Report of two cases. Arch. Otolaryngol. 1937, 25, 584–587. [Google Scholar] [CrossRef]
- Eagle, W.W. Elongated styloid process; further observations and a new syndrome. Arch. Otolaryngol. 1948, 47, 630–640. [Google Scholar] [CrossRef]
- Badhey, A.; Jategaonkar, A.; Anglin Kovacs, A.J.; Kadakia, S.; De Deyn, P.P.; Ducic, Y.; Schantz, S.; Shin, E. Eagle syndrome: A comprehensive review. Clin. Neurol. Neurosurg. 2017, 159, 34–38. [Google Scholar] [CrossRef]
- Patil, S.; Ghosh, S.; Vasudeva, N. Morphometric study of the styloid process of temporal bone. J. Clin. Diagn. Res. 2014, 8, AC04–AC06. [Google Scholar] [CrossRef]
- Tadjer, J.; Bejot, Y. Vascular variant of Eagle syndrome: A review. Front. Neurol. 2024, 15, 1463275. [Google Scholar] [CrossRef]
- Farina, R.; Foti, P.V.; Pennisi, I.; Conti, A.; Meli, G.A.; Vasile, T.; Gozzo, C.; Tallamona, E.; Ini, C.; Palmucci, S.; et al. Stylo-Jugular Venous Compression Syndrome: Lessons Based on a Case Report. Am. J. Case Rep. 2021, 22, e932035. [Google Scholar] [CrossRef]
- Suzuki, Y.; Toma, N.; Kuroda, Y.; Miura, Y.; Shiba, M.; Yasuda, R.; Suzuki, H. Dural Arteriovenous Fistula Formation as Eagle Jugular Syndrome: A Case Report and Literature Review. World Neurosurg. 2020, 144, 154–161. [Google Scholar] [CrossRef]
- Zamboni, P.; Scerrati, A.; Menegatti, E.; Galeotti, R.; Lapparelli, M.; Traina, L.; Tessari, M.; Ciorba, A.; De Bonis, P.; Pelucchi, S. The eagle jugular syndrome. BMC Neurol. 2019, 19, 333. [Google Scholar] [CrossRef]
- Woo, H.G.; Ryu, J.; Kim, E.J.; Lee, K.M. Styloidogenic jugular venous compression syndrome as a source of cerebral venous sinus thrombosis. Quant. Imaging Med. Surg. 2022, 12, 4316–4319. [Google Scholar] [CrossRef]
- Mejia-Vergara, A.J.; Sultan, W.; Kostas, A.; Mulholland, C.B.; Sadun, A. Styloidogenic Jugular Venous Compression Syndrome with Papilloedema: Case Report and Review of the Literature. Neuroophthalmology 2022, 46, 54–58. [Google Scholar] [CrossRef]
- Mooney, J.; Lepard, J.; Akbari, S.H.A.; Johnston, J.M. Styloidogenic jugular venous compression syndrome: A case report and review of the literature. Childs Nerv. Syst. 2020, 36, 3135–3139. [Google Scholar] [CrossRef]
- Pokeerbux, M.R.; Delmaire, C.; Morell-Dubois, S.; Demondion, X.; Lambert, M. Styloidogenic compression of the internal jugular vein, a new venous entrapment syndrome? Vasc. Med. 2020, 25, 378–380. [Google Scholar] [CrossRef]
- Zhao, X.; Cavallo, C.; Hlubek, R.J.; Mooney, M.A.; Belykh, E.; Gandhi, S.; Moreira, L.B.; Lei, T.; Albuquerque, F.C.; Preul, M.C.; et al. Styloidogenic Jugular Venous Compression Syndrome: Clinical Features and Case Series. Oper. Neurosurg. 2019, 17, 554–561. [Google Scholar] [CrossRef]
- Cantin, L.M.; Suazo Galdames, I. Eagle syndrome or stylohyoid syndrome? Neurologia 2011, 26, 254. [Google Scholar] [CrossRef] [PubMed]
- Camarda, A.J.; Deschamps, C.; Forest, D., II. Stylohyoid chain ossification: A discussion of etiology. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Luginbuhl, A.; Finden, S.; Curry, J.M.; Cognetti, D.M. Styloid/C1 transverse process juxtaposition as a cause of Eagle’s syndrome. Head Neck 2015, 37, E153–E156. [Google Scholar] [CrossRef]
- Scerrati, A.; Norri, N.; Mongardi, L.; Dones, F.; Ricciardi, L.; Trevisi, G.; Menegatti, E.; Zamboni, P.; Cavallo, M.A.; De Bonis, P. Styloidogenic-cervical spondylotic internal jugular venous compression, a vascular disease related to several clinical neurological manifestations: Diagnosis and treatment-a comprehensive literature review. Ann. Transl. Med. 2021, 9, 718. [Google Scholar] [CrossRef]
- Gitomer, S.A.; Giannoni, C.M.; Canadas, K.T. Pediatric lymphedema caused by diffuse cervical lymphadenopathy: A case report and review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 67–70. [Google Scholar] [CrossRef]
- Guan, J.; Song, S.; Wang, W.; Ji, X.; Meng, R. Cerebral venous sinus thrombosis due to external compression of internal jugular vein. J. Int. Med. Res. 2021, 49, 3000605211006609. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, Z. Intracranial hypertension due to bilateral internal jugular venous occlusion in eagle syndrome: A case report. Heliyon 2023, 9, e16188. [Google Scholar] [CrossRef]
- Gianesini, S.; Menegatti, E.; Mascoli, F.; Salvi, F.; Bastianello, S.; Zamboni, P. The omohyoid muscle entrapment of the internal jugular vein. A still unclear pathogenetic mechanism. Phlebology 2014, 29, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Tudose, R.C.; Vrapciu, A.D.; Toader, C.; Popescu, S.A. Anatomical Variations of the External Jugular Vein: A Pictorial and Critical Review. Medicina 2023, 59, 622. [Google Scholar] [CrossRef] [PubMed]
- San Millan Ruiz, D.; Gailloud, P.; Rufenacht, D.A.; Delavelle, J.; Henry, F.; Fasel, J.H. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am. J. Neuroradiol. 2002, 23, 1500–1508. [Google Scholar] [PubMed]
- Reis, C.V.; Deshmukh, V.; Zabramski, J.M.; Crusius, M.; Desmukh, P.; Spetzler, R.F.; Preul, M.C. Anatomy of the mastoid emissary vein and venous system of the posterior neck region: Neurosurgical implications. Neurosurgery 2007, 61, 193–200, discussion 201. [Google Scholar] [CrossRef]
- Mandolesi, S.; d’Alessandro, A.; Ciccone, M.M.; Zito, A.; Manconi, E.; Niglio, T.; Orsini, A.; Mandolesi, D.; d’Alessandro, A.; Fedele, F. Analysis of patients with chronic cerebro-spinal venous insufficiency and multiple sclerosis: Identification of parameters of clinical severity. Veins Lymphat. 2015, 4, 4570. [Google Scholar] [CrossRef]
- Piraino, D.; Garofalo, G.; Faletra, A.; Messina, A. The omohyoid and sternocleidomastoid muscles entrapment of the internal jugular vein: Which role in Mèniére disease patients? Treatment perspective description. Veins Lymphat. 2018, 7, 7760. [Google Scholar] [CrossRef]
- Simka, M.; Majewski, E.; Fortuna, M.; Zaniewski, M. Internal jugular vein entrapment in a multiple sclerosis patient. Case Rep. Surg. 2012, 2012, 293568. [Google Scholar] [CrossRef]
- Monnier, H.; Owashi, K.; Liu, P.; Metanbou, S.; Capel, C.; Baledent, O. Quantification of the Dynamics of the Vascular Flows in the Cerebral Arterial and Venous Trees. Biomedicines 2025, 13, 1106. [Google Scholar] [CrossRef]
- Das, K.K.; Mehrotra, A.; Sahu, R.N.; Srivastava, A.K.; Jaiswal, A.K.; Behari, S. Unilateral lateral mass hypertrophy: An extremely rare congenital anomaly of atlas. J. Craniovertebral Junction Spine 2013, 4, 73–75. [Google Scholar] [CrossRef]
- Onishi, E.; Sakamoto, A.; Murata, S.; Nakamura, S.; Matsushita, M. Unilateral atlantal lateral mass hypertrophy associated with atlanto-occipital fusion. Eur. Spine J. 2013, 22 (Suppl. 3), S429–S433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozkara, B.B.; Karabacak, M.; Aygun, A.A. External musculoskeletal compression of the internal jugular vein: A systematic review. Acta Phlebol. 2022, 23, 43–51. [Google Scholar] [CrossRef]
- Bolognese, P.A.; Caton, M.T.; Ruhoy, I.S.; Bloom, S.; Amaru, J.N.; Biggins, J.B.; Nayyar, N.S. A case report on symptomatic internal jugular venous compression: Clinical and radiographic improvement with bilateral C1 tubercle resection. Med. Res. Arch. 2024, 12, 5415. [Google Scholar] [CrossRef]
- Oushy, S.; Wald, J.T.; Janus, J.; Fulgham, J.R.; Lanzino, G. Dynamic Internal Jugular Vein Compression by Hypertrophic Hyoid Bone: Management and Outcomes. Cureus 2020, 12, e7445. [Google Scholar] [CrossRef]
- Ichiyama, S.; Nomura, O.; Ishizawa, Y. Disruption of the internal jugular vein by subcutaneous emphysema. BMJ Case Rep. 2023, 16, e253066. [Google Scholar] [CrossRef]
- Hartl, D.M.; Zafereo, M.E.; Kowalski, L.P.; Randolph, G.W.; Olsen, K.D.; Fernandez-Alvarez, V.; Nixon, I.J.; Shaha, A.R.; Angelos, P.; Shah, J.P.; et al. Occlusion of the internal jugular vein in differentiated thyroid carcinoma: Causes and diagnosis. Eur. J. Surg. Oncol. 2021, 47, 1552–1557. [Google Scholar] [CrossRef]
- Smith, D.W.; Bailes, J.E.; Fisher, J.A.; Robles, J.; Turner, R.C.; Mills, J.D. Internal jugular vein compression mitigates traumatic axonal injury in a rat model by reducing the intracranial slosh effect. Neurosurgery 2012, 70, 740–746. [Google Scholar] [CrossRef]
- Mannix, R.; Morriss, N.J.; Conley, G.M.; Meehan, W.P., 3rd; Nedder, A.; Qiu, J.; Float, J.; DiCesare, C.A.; Myer, G.D. Internal Jugular Vein Compression Collar Mitigates Histopathological Alterations after Closed Head Rotational Head Impact in Swine: A Pilot Study. Neuroscience 2020, 437, 132–144. [Google Scholar] [CrossRef]
- Delgadillo, B.E.; Montz, F.; Ward, B., Jr.; Herson, A.B.; Toldi, J.P. Current Evidence for the Use of Jugular Vein Compression Collars in Sport: A Systematic Review. Curr. Sports Med. Rep. 2025, 24, 18–27. [Google Scholar] [CrossRef]
- Yeoh, T.Y.; Venkatraghavan, L.; Fisher, J.A.; Meineri, M. Internal jugular vein blood flow in the upright position during external compression and increased central venous pressure: An ultrasound study in healthy volunteers. Can. J. Anaesth. 2017, 64, 854–859. [Google Scholar] [CrossRef]
- Yang, K.; Shah, K.; Begley, S.L.; Prashant, G.; White, T.; Costantino, P.; Patsalides, A.; Lo, S.L.; Dehdashti, A.R. Extreme lateral infracondylar approach for internal jugular vein compression syndrome: A case series with preliminary clinical outcomes. Acta Neurochir. 2023, 165, 3445–3454. [Google Scholar] [CrossRef]
- Fritch, C.; Voronovich, Z.; Carlson, A.P. C1 Transverse Process Resection for Management of Jugular Stenosis. Oper. Neurosurg. 2020, 19, E209–E213. [Google Scholar] [CrossRef]
- Succop, B., Jr.; Thompson, N.J.; Dedmon, M.M.; Gelinne, A.; Selleck, A.; Reed, S.; Sindelar, M.B.D. Noninvasive Treatment of Venous Pulsatile Tinnitus with an Internal Jugular Vein Compression Collar. Laryngoscope 2024, 134, 3342–3348. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Yuan, W.; Barber Foss, K.D.; Smith, D.; Altaye, M.; Reches, A.; Leach, J.; Kiefer, A.W.; Khoury, J.C.; Weiss, M.; et al. The Effects of External Jugular Compression Applied during Head Impact Exposure on Longitudinal Changes in Brain Neuroanatomical and Neurophysiological Biomarkers: A Preliminary Investigation. Front. Neurol. 2016, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Hatt, A.; Cheng, S.; Tan, K.; Sinkus, R.; Bilston, L.E. MR Elastography Can Be Used to Measure Brain Stiffness Changes as a Result of Altered Cranial Venous Drainage During Jugular Compression. AJNR Am. J. Neuroradiol. 2015, 36, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Diekfuss, J.A.; Yuan, W.; Barber Foss, K.D.; Dudley, J.A.; DiCesare, C.A.; Reddington, D.L.; Zhong, W.; Nissen, K.S.; Shafer, J.L.; Leach, J.L.; et al. The effects of internal jugular vein compression for modulating and preserving white matter following a season of American tackle football: A prospective longitudinal evaluation of differential head impact exposure. J. Neurosci. Res. 2021, 99, 423–445. [Google Scholar] [CrossRef]
- Myer, G.D.; Barber Foss, K.; Thomas, S.; Galloway, R.; DiCesare, C.A.; Dudley, J.; Gadd, B.; Leach, J.; Smith, D.; Gubanich, P.; et al. Altered brain microstructure in association with repetitive subconcussive head impacts and the potential protective effect of jugular vein compression: A longitudinal study of female soccer athletes. Br. J. Sports Med. 2019, 53, 1539–1551. [Google Scholar] [CrossRef]
- Brieck, K.A.; Brieck, Z.J.; Ashby, J.A.; Phelps, O.C.; Cernak, I. A Narrative Review of the Effects of Internal Jugular Vein Compression on Brain Structure and Function During Periods of Head Impact. Cureus 2025, 17, e77625. [Google Scholar] [CrossRef]
- Yuan, W.; Diekfuss, J.A.; Barber Foss, K.D.; Dudley, J.A.; Leach, J.L.; Narad, M.E.; DiCesare, C.A.; Bonnette, S.; Epstein, J.N.; Logan, K.; et al. High School Sports-Related Concussion and the Effect of a Jugular Vein Compression Collar: A Prospective Longitudinal Investigation of Neuroimaging and Neurofunctional Outcomes. J. Neurotrauma 2021, 38, 2811–2821. [Google Scholar] [CrossRef]
- Nonaka, T.; Sakata, K.; Abe, T.; Hattori, G.; Orito, K.; Miyagi, N.; Tokutomi, T.; Morioka, M. The eagle jugular syndrome as the cause of delayed intracranial hemorrhage after microvascular decompression for hemifacial spasm: A case report. Surg. Neurol. Int. 2021, 12, 584. [Google Scholar] [CrossRef]
- Li, M.; Su, C.; Fan, C.; Chan, C.C.; Bai, C.; Meng, R. Internal jugular vein stenosis induced by tortuous internal carotid artery compression: Two case reports and literature review. J. Int. Med. Res. 2019, 47, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Han, A.Y.; Lentz, J.F.; Kuan, E.C.; Araya, H.H.; Kamgar, M. A Case of Reactive Cervical Lymphadenopathy with Fat Necrosis Impinging on Adjacent Vascular Structures. Case Rep. Otolaryngol. 2016, 2016, 6019501. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Ding, J.; Da, Z.; Sun, J.; Liu, C.; Pan, L.; Ya, J.; Wang, Z.; Guan, J.; Jin, K.; et al. Probable risk factors of internal jugular vein stenosis in Chinese patients-A real-world cohort study. Clin. Neurol. Neurosurg. 2020, 191, 105678. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.J.; Wang, J.L.; Liu, L.H.; Zhao, S.R.; Yu, S.J.; Yang, B.B.; Xu, Q.L.; Li, J.K.; Wang, S.R. Internal Jugular Vein Thrombosis After Microwave Ablation of Cervical Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Case Report. Front. Endocrinol. 2022, 13, 792715. [Google Scholar] [CrossRef]
- Weeraddana, P.; Nepal, N.; Saleh, M.; Sandeep, F.; Weerasooriya, N.; Dandwani, M.; Ma, E. Internal Jugular Venous Thrombosis with Superior Vena Cava Syndrome: A Rare First Presentation of Gray Zone Lymphoma. Cureus 2023, 15, e37096. [Google Scholar] [CrossRef]
- Illuminati, G.; Pasqua, R.; Nardi, P.; Fratini, C.; Minni, A.; Giordano, C. Resection for Internal Jugular Vein Thrombosis and Cervical Lymph Nodes’ Involvement from Gastric Cancer. Anticancer. Res. 2020, 40, 2889–2893. [Google Scholar] [CrossRef]
- Bandara, A.R.; Wimalarathna, H.; Kalupahana, R.; Gunathilake, S.S. Internal jugular venous thrombosis due to Trousseau’s syndrome as the presenting feature of metastatic prostate carcinoma: A case report. J. Med. Case Rep. 2016, 10, 104. [Google Scholar] [CrossRef]
- Paulsen, F.; Tillmann, B.; Christofides, C.; Richter, W.; Koebke, J. Curving and looping of the internal carotid artery in relation to the pharynx: Frequency, embryology and clinical implications. J. Anat. 2000, 197 Pt 3, 373–381. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Mao, W.; He, T.; Xiong, Z. Anatomical relationship between the omohyoid muscle and the internal jugular vein on ultrasound guidance. BMC Anesthesiol. 2022, 22, 181. [Google Scholar] [CrossRef]
- De Bonis, P.; Menegatti, E.; Cavallo, M.A.; Sisini, F.; Trapella, G.; Scerrati, A.; Zamboni, P. JEDI (jugular entrapment, dilated ventricles, intracranial hypertension) syndrome: A new clinical entity? A case report. Acta Neurochir. 2019, 161, 1367–1370. [Google Scholar] [CrossRef]
- Patra, P.; Gunness, T.K.; Robert, R.; Rogez, J.M.; Heloury, Y.; Le Hur, P.A.; Leborgne, J.; Laude, M.; Barbin, J.Y. Physiologic variations of the internal jugular vein surface, role of the omohyoid muscle, a preliminary echographic study. Surg. Radiol. Anat. 1988, 10, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Frau, G.N.; Pagani, R.; Maronato, F.; Agarwal, N.; Contarino, C.; Toro, E.F. The role of omohyoid muscle entrapment of the internal jugular vein and is surgical transection in Ménière’s disease and other inner ear disorders. Veins Lymphat. 2019, 8, 35. [Google Scholar] [CrossRef]
- Brinjikji, W.; Graffeo, C.S.; Perry, A.; Zimmerman, T.; Janus, J.R.; Morris, P.P.; Cascino, G.D.; Lanzino, G. Moving target: Transient rotational stenosis precipitating jugular bow hunter’s syndrome. J. Neurointerv. Surg. 2017, 9, e28. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.M.; Griessenauer, C.J.; Krishnamurthy, S.; Verma, K.; Loukas, M.; Tubbs, R.S. The inferior petrosal sinus: A comprehensive review with emphasis on clinical implications. Childs Nerv. Syst. 2014, 30, 831–834. [Google Scholar] [CrossRef]
- Mitsuhashi, Y.; Nishio, A.; Kawahara, S.; Ichinose, T.; Yamauchi, S.; Naruse, H.; Matsuoka, Y.; Ohata, K.; Hara, M. Morphologic evaluation of the caudal end of the inferior petrosal sinus using 3D rotational venography. AJNR Am. J. Neuroradiol. 2007, 28, 1179–1184. [Google Scholar] [CrossRef]
- Zhang, W.G.; Ye, Y.Y.; Chen, J.H.; Chen, R.; Kuang, L.Q.; Li, X. Imaging study of the long extracranial extension of the inferior petrosal sinus with MSCT. Clin. Anat. 2010, 23, 160–167. [Google Scholar] [CrossRef]
- Gailloud, P.; Fasel, J.H.; Muster, M.; Desarzens, F.; Ruefenacht, D.A. Termination of the inferior petrosal sinus: An anatomical variant. Clin. Anat. 1997, 10, 92–96. [Google Scholar] [CrossRef]
- Benndorf, G.; Campi, A. Aberrant inferior petrosal sinus: Unusual transvenous approach to the cavernous sinus. Neuroradiology 2002, 44, 158–163. [Google Scholar] [CrossRef]
- Shukla, I.; Agarwal, A.; Changanath Kader, R. Internal Jugular Vein Duplication: A series of Seven Cases and Review of Literature. Iran. J. Otorhinolaryngol. 2025, 37, 91–94. [Google Scholar]
- Ibrahim, B.; Berania, I.; Moubayed, S.P.; Christopoulos, A.; Ayad, T. Internal Jugular Vein Duplication: Anatomic Relationship with the Spinal Accessory Nerve. J. Oral. Maxillofac. Surg. 2016, 74, 1502.e1–1502.e4. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Vassiou, K.; Olewnik, L.; Duparc, F.; Tsakotos, G.; Vlychou, M.; Zielinska, N.; Piagkou, M. Internal Jugular Vein Fenestration and Duplication. J. Craniofac Surg. 2024, 36, 1790–1792. [Google Scholar] [CrossRef]
- Wang, X.; Peng, L.; Guo, H.; Hernesniemi, J.; Xiong, X.; Andrade-Barazarte, H.; Qian, R. Internal Jugular Vein Fenestration and Duplication: Anatomical Findings, Prevalence, and Literature Review. Front. Surg. 2020, 7, 593367. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Tortori-Donati, P. Internal jugular vein phlebectasia and duplication: Case report with magnetic resonance angiography features. Pediatr. Radiol. 2001, 31, 134. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Sureka, K.; Satheesh, G.; Venugopalan, V.; Durairaj, D. Duplication of internal jugular vein—An incidental finding. Oral Oncol. Rep. 2025, 13, 100699. [Google Scholar] [CrossRef]
- Prades, J.M.; Timoshenko, A.; Dumollard, J.M.; Durand, M.; Merzougui, N.; Martin, C. High duplication of the internal jugular vein: Clinical incidence in the adult and surgical consequences, a report of three clinical cases. Surg. Radiol. Anat. 2002, 24, 129–132. [Google Scholar] [CrossRef]
- Greene, P.F. Internal jugular vein compression: A benign entity or an underappreciated phenomenon? eNeurologicalSci 2025, 40, 100577. [Google Scholar] [CrossRef]
- Motoyama, Y.; Sasaki, H.; Nakajima, T.; Hayami, H.; Matsuoka, R.; Fukutome, K.; Tei, R.; Shin, Y.; Aketa, S. Eagle jugular syndrome accompanied by de novo brainstem cavernous malformation: A case-based systematic review. Acta Neurochir. 2024, 166, 20. [Google Scholar] [CrossRef]
- Frydrychowski, A.F.; Winklewski, P.J.; Guminski, W. Influence of acute jugular vein compression on the cerebral blood flow velocity, pial artery pulsation and width of subarachnoid space in humans. PLoS ONE 2012, 7, e48245. [Google Scholar] [CrossRef]
- Bosnjak, R.; Kordas, M. Circulatory effects of internal jugular vein compression: A computer simulation study. Med. Biol. Eng. Comput. 2002, 40, 423–431. [Google Scholar] [CrossRef]
- Potts, D.G.; Deonarine, V. Effect of positional changes and jugular vein compression on the pressure gradient across the arachnoid villi and granulations of the dog. J. Neurosurg. 1973, 38, 722–728. [Google Scholar] [CrossRef]
- Yoon, H.K.; Lee, H.K.; Jeon, Y.T.; Hwang, J.W.; Lim, S.M.; Park, H.P. Clinical significance of the cross-sectional area of the internal jugular vein. J. Cardiothorac. Vasc. Anesth. 2013, 27, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Lorchirachoonkul, T.; Ti, L.K.; Manohara, S.; Lye, S.T.; Tan, S.A.; Shen, L.; Kang, D.S. Anatomical variations of the internal jugular vein: Implications for successful cannulation and risk of carotid artery puncture. Singap. Med. J. 2012, 53, 325–328. [Google Scholar]
- Sznajder, J.I.; Zveibil, F.R.; Bitterman, H.; Weiner, P.; Bursztein, S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch. Intern. Med. 1986, 146, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.L.; Keshava, S.N.; Lea, M. Anatomical variations of the internal jugular veins and their relationship to the carotid arteries: A CT evaluation. Australas. Radiol. 2006, 50, 314–318. [Google Scholar] [CrossRef]
- Denys, B.G.; Uretsky, B.F. Anatomical variations of internal jugular vein location: Impact on central venous access. Crit. Care Med. 1991, 19, 1516–1519. [Google Scholar] [CrossRef]
- Wang, M.; Wu, X.; Lan, D.; Zhou, D.; Ding, Y.; Ji, X.; Meng, R. Differentiation between anatomical slenderness and acquired stenosis of the internal jugular veins. CNS Neurosci. Ther. 2022, 28, 1849–1860. [Google Scholar] [CrossRef]
- Fargen, K.M.; Midtlien, J.P.; Margraf, C.; Kiritsis, N.R.; Chang, E.; Hui, F. Dynamic internal jugular vein venography: A descriptive study in 89 patients with suspected cerebral venous outflow disorders. J. Neurointerv. Surg. 2025, 17, 646–652. [Google Scholar] [CrossRef]
- Siniscalchi, E.N.; Raffa, G.; Germanò, A.; De Ponte, F.S. Is eagle jugular syndrome an underestimated potentially life-threatening disease? Can. J. Neurol. Sci. 2020, 47, 581–582. [Google Scholar] [CrossRef]
- Petersingham, G.; Shrestha, N.; Elliott, M.; Allan, R.S.; Parker, G.; Camp, L.V.; Rao, P.J. Invasive surgical management of cervical internal jugular venous compression: A literature review. J. Clin. Neurosci. 2025, 137, 111304. [Google Scholar] [CrossRef]
- Werheim, E.; Sokol, Z.; Brown, D.; Oselkin, M. Eagle syndrome causing cerebral sinus hypertension: Case report. Radiol. Case Rep. 2023, 18, 2758–2762. [Google Scholar] [CrossRef]
- Pang, S.; Kolarich, A.R.; Brinjikji, W.; Nakaji, P.; Hepworth, E.; Hui, F. Interventional and surgical management of internal jugular venous stenosis: A narrative review. J. Neurointerv. Surg. 2022, 14, 503–507. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dincă, V.; Ionescu, P.; Tudose, R.C.; Munteanu, M.; Vrapciu, A.D.; Rusu, M.C. Anatomical Reasons for an Impaired Internal Jugular Flow. Medicina 2025, 61, 1627. https://doi.org/10.3390/medicina61091627
Dincă V, Ionescu P, Tudose RC, Munteanu M, Vrapciu AD, Rusu MC. Anatomical Reasons for an Impaired Internal Jugular Flow. Medicina. 2025; 61(9):1627. https://doi.org/10.3390/medicina61091627
Chicago/Turabian StyleDincă, Viviana, Paris Ionescu, Răzvan Costin Tudose, Mădălin Munteanu, Alexandra Diana Vrapciu, and Mugurel Constantin Rusu. 2025. "Anatomical Reasons for an Impaired Internal Jugular Flow" Medicina 61, no. 9: 1627. https://doi.org/10.3390/medicina61091627
APA StyleDincă, V., Ionescu, P., Tudose, R. C., Munteanu, M., Vrapciu, A. D., & Rusu, M. C. (2025). Anatomical Reasons for an Impaired Internal Jugular Flow. Medicina, 61(9), 1627. https://doi.org/10.3390/medicina61091627







