Prolotherapy as a Regenerative Treatment in the Management of Chronic Low Back Pain: A Systematic Review
Abstract
1. Introduction
1.1. Definition of Chronic Low Back Pain (CLBP)
1.2. Pathophysiological Mechanisms and Etiology of CLBP
1.3. Epidemiology and Public Health Impact of CLBP
1.4. Risk Factors for CLBP
1.5. Prolotherapy
1.6. Mechanisms of Prolotherapy
1.7. Therapeutic Uses of Prolotherapy (Dextrose Prolotherapy)
1.8. Prolotherapy (Dextrose Prolotherapy) in CLBP
1.9. Aim of the Review
2. Materials and Methods
2.1. Inclusion Criteria
- −
- Original studies (randomized controlled trials, cohort studies, experimental studies, or observational designs) evaluating the effects of prolotherapy (dextrose prolotherapy) in patients with CLBP;
- −
- Studies assessing outcomes related to pain intensity, lumbar mobility, or quality of life following prolotherapy (dextrose prolotherapy);
- −
- Studies involving adult or elderly participants of any age, no age restriction applied;
- −
- Studies including CLBP of mechanical cause, degenerative (facet joint degeneration, lumbar spinal stenosis), disc herniation—regardless of surgical status, failed back surgery syndrome, sacroiliac joint dysfunction, non-specific causes;
- −
- Studies published in English;
- −
- Studies published within the last 10 years.
2.2. Exclusion Criteria
- −
- Systematic reviews, meta-analyses, case reports, editorials, letters to the editor, conference abstracts, and book chapters;
- −
- Studies involving patients with inflammatory, infectious, or neoplastic or post-traumatic causes of low back pain;
- −
- Studies focused on pediatric populations;
- −
- Animal studies or preclinical research without clinical application;
- −
- Studies published in languages other than English;
- −
- Studies published before 2015.
3. Results
3.1. Short Description of the Included Studies
| Authors and Publication Year/Country | Pathology | Study Design | Sample Size | Intervention Protocols | Outcome Measures Related to Pain Reduction and Lumbar Function | No of Reff. |
|---|---|---|---|---|---|---|
| Liza Maniquis-Smigel et al., 2017 [59] Unites States of America | CLBP with radiation to buttock/leg, including -Lumbar spinal stenosis (34%) -Lumbar radiculopathy (26%) -Nonspecific low back pain (26%) -Peripheral neuropathy (6%) -Failed back surgery (11%) | Randomized Double-Blind Controlled Trial | Of 56 eligible patients, 19 declined and 2 were excluded (due to substantial cramping pain before receiving the full 10 mL injection), leaving 35 participants (19 dextrose, 16 saline). | Intervention: Single caudal epidural injection of 10 mL 5% dextrose. Control: Single caudal epidural injection of 10 mL 0.9% saline. Technique: Vertical caudal approach under epidurography guidance (25 G needle). | Outcome measures: -Change in a numerical rating scale (NRS) pain score (0–10). Follow-up: Assessments at 15 min, 2 h, 4 h, 48 h, 2 weeks post-injection. Key Findings: -Epidural 5% dextrose is a rapid-acting, safe neurogenic analgesic for short-term CLBP management. | 50 |
| Özlem Köroğlu et al., 2019 [60] Turkey | Radicular Low Back Pain due to lumbar disc herniation | Retrospective study | Total: 40 patients 20 participants: Prolotherapy only, 20 participants: Prolotherapy + Physical Therapy. | -5% Dextrose prolotherapy injections at iliolumbar/transverse ligament insertions and facet joints -3 injection sessions at 4-week intervals Group 1: Prolotherapy only. Group 2: Prolotherapy + Physical Therapy: Transcutaneous Electrical Nerve Stimulation (TENS), infrared, stretching exercises; 15 sessions. -NSAIDs prohibited (only paracetamol allowed). | Outcome measures: -Visual Analog Scale (VAS) for pain intensity. -Oswestry Disability Index (ODI) for functional disability. -36-Item Short Form Survey (SF-36) for quality of life. Follow-up: Assessments at baseline, 3, 12, and 52 weeks. Key Findings: -5% dextrose prolotherapy significantly reduces pain and disability in chronic radicular low back pain from lumbar disc herniation, with effects lasting up to 1 year. -Adding physical therapy did not enhance outcomes, supporting prolotherapy as a practical primary treatment. | 21 |
| Timur Yildirim et al., 2021 [61] Turkey | CLBP (mechanical origin) | Retrospective Comparative Study | Total: 178 patients Group 1: 91 participants—Facet joint injection Group 2: 87 participants—Prolotherapy | Facet Joint Injection: 20 mg methylprednisolone + 2–4 mL 0.25% bupivacaine per single-level facet joint. Prolotherapy: 5 mL 25% dextrose solution injected into the facet joint capsule per single level. | Outcome measures: -Visual Analog Scale (VAS) measured at baseline, day 1, day 15, and month 3. -Lumbar Function: Oswestry Disability Index (ODI) was measured at baseline and month 3. Key Findings: -Facet injection reduced VAS more effectively at day 1 (p < 0.001). -Prolotherapy showed superior VAS reduction at month 3 (p < 0.001). -ODI scores were higher (worse function) in the prolotherapy group than the corticosteroid group at month 3 (p < 0.001). | 15 |
| Gholamreza Raissi et al., 2022 [62] Iran | Sacroiliac joint dysfunction (SIJD) as an etiology of low back pain (LBP) | Randomized double-blind clinical trial (RCT) | Total: 40 patients, 2 patients withdrew from each group for personal reasons Group 1:18 participants—dextrose prolotherapy; Group 2: 18 participants—corticosteroid. | Prolotherapy Group: Single ultrasound-guided injection of 2.5 mL 20% dextrose solution. Corticosteroid Group: Single ultrasound-guided injection of 2.5 mL triamcinolone (40 mg/mL). Technique: -Approach: Inferomedial (1 inch medial and below Posterior Superior Iliac Spine); -Following the intra-articular injection of 2 mL of 2.5% bupivacaine; -Guidance: Real-time ultrasound (transverse to sacral hiatus); -Needle: 22-gauge spinal needle. | Outcome measures: -Visual Analog Scale (VAS) measured at Baseline, 2 weeks, 8 weeks, and 36 weeks; -Dallas Pain Questionnaire (DPQ) measured at Baseline, 2 weeks, and 8 weeks. Key Findings: VAS: -Significant pain reduction in both groups at all timepoints, no difference between groups; DPQ: -Corticosteroid group: Significant functional improvement at 2 and 8 weeks; -Dextrose group: Non-significant improvement. | 20 |
| Carl PC Chen et al., 2023 [63] China | Lumbar Spinal Stenosis (LSS) | Observational Retrospective | Total: 211 patients Group 1: 104 participants— mechanical needling + sterile water; Group 2: 107 participants— polotherapy: Dextrose 5% in Water (D5W). | Group 1: ultrasound-guided (USG) mechanical needling + sterile water injection into facet joints, medial branches, and multifidus muscles (4 weekly sessions; 1 mL/site, 3 mL/level, total 12 mL/session); Group 2: Single USG injection of 5% dextrose water into the multifidus muscles. | Outcome measures: -VAS for low back pain (0–10); -VAS for leg/radicular pain (0–10); -Walking distance (meters before calf pain). Follow-up: Assessments at Pre-injection, immediately post-injection, 1 week, 1 month, 3 months, 6 months. Key Findings: -Mechanical needling + sterile water: Significantly reduced back/leg pain (VAS) and increased walking distance vs. baseline at 1, 3, and 6 months; -Prolotherapy (D5W): Moderate pain reduction and improved walking distance only at 1 week and 1 month. Effects diminished by 3 months (VAS/walking distance reverted to baseline). | 20 |
| Jose Alberto Pereira Pires et al., 2023 [64] Brazil | CLBP, non-traumatic, unresponsive to physical therapy. Facet joint degeneration confirmed. | Randomized, blinded clinical trial. | Total: 40 patients, 2 lost to follow-up Group 1: 19 participants—hypertonic glucose Group 2: 19 participants—conservative group | Hypertonic glucose Group: Single facet joint injection of 75% hypertonic glucose (1 mL/kg) + lifestyle modifications; Conservative Group: Clinical management (diet/lifestyle changes) only. | Outcome measures: -Visual Analog Scale (VAS) for pain reduction, -Roland–Morris Disability Questionnaire for lumbar function. Follow-up: assessments at 1, 3, and 6 months. Key Findings: -Both groups showed significant improvement in VAS and Roland–Morris scores in dynamics; -The authors concluded that prolotherapy did not outperform conservative care. | 37 |
| Yasmine Ragab Elsayed Mohamed et al., 2024 [65] Egypt | Failed Back Surgery Syndrome (FBSS) with CLBP | Prospective, randomized, double-blinded clinical trial | Total: 90 patients, 7 lost to follow-up Group 1: 40 participants—Prolotherapy; Group 2: 43 participants—Steroid. | Prolotherapy Group: US + fluoroscopy-guided caudal epidural injection of: -5 mL bupivacaine 0.5%; -4 mL Omnipaque contrast (350 mg/mL); -10 mL dextrose 25%; -6 mL distilled water (Total volume: 25 mL); Steroid Group: US + fluoroscopy-guided caudal epidural injection of: -1 mL methylprednisolone (40 mg/mL); -4 mL Omnipaque contrast (350 mg/mL); -5 mL bupivacaine 0.5%; -15 mL distilled water (Total volume: 25 mL). | Outcome measures: -Visual Analog Scale (VAS) for pain; -Oswestry Disability Index (ODI); -McGill Pain Questionnaire (MPQ); Follow-up: Assessments at 2, 4, 6, 8 weeks, 3 and 6 months post-injection. Key Findings: -No significant difference was observed between groups during the early post-injection phase (2–6 weeks); -Steroids provide superior long-term relief ( > 6 weeks); -Both have comparable safety. | 13 |
3.2. Structured Synthesis of Results
3.2.1. Targeted Pathologies
3.2.2. Control Group
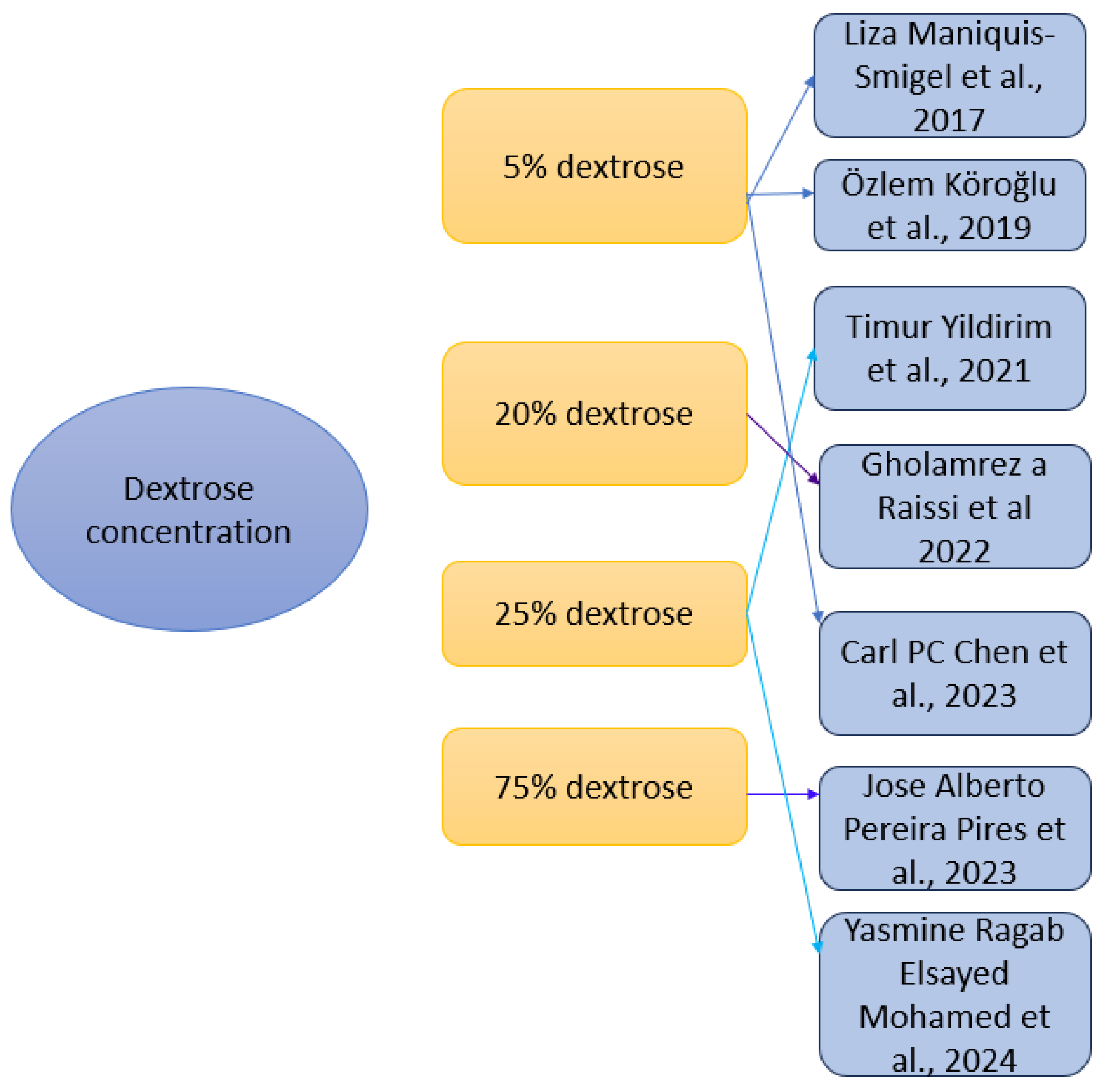
3.2.3. Dextrose Prolotherapy Concentration and Injection Protocols
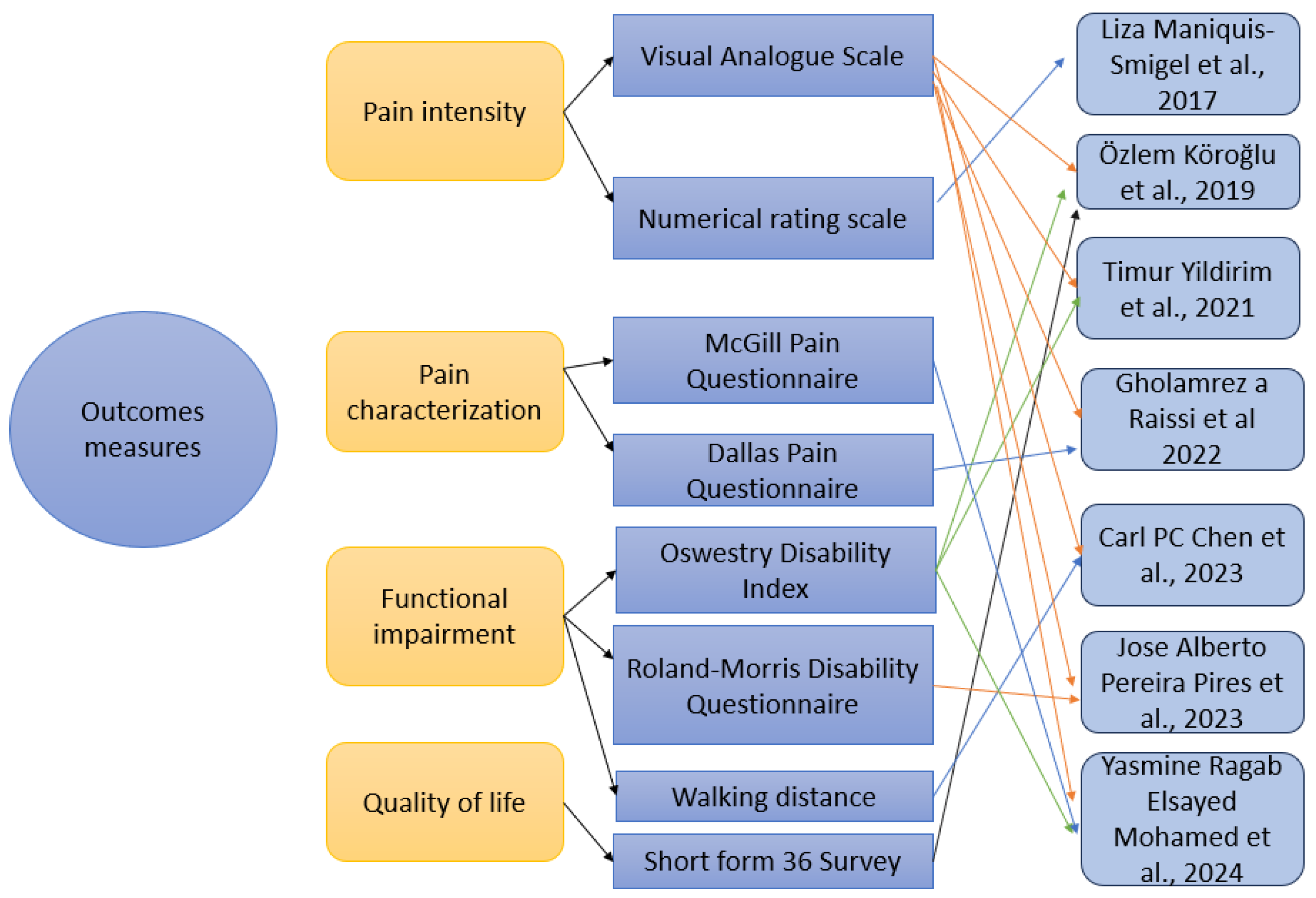
3.2.4. Outcome Measures
3.2.5. Follow-Up Duration
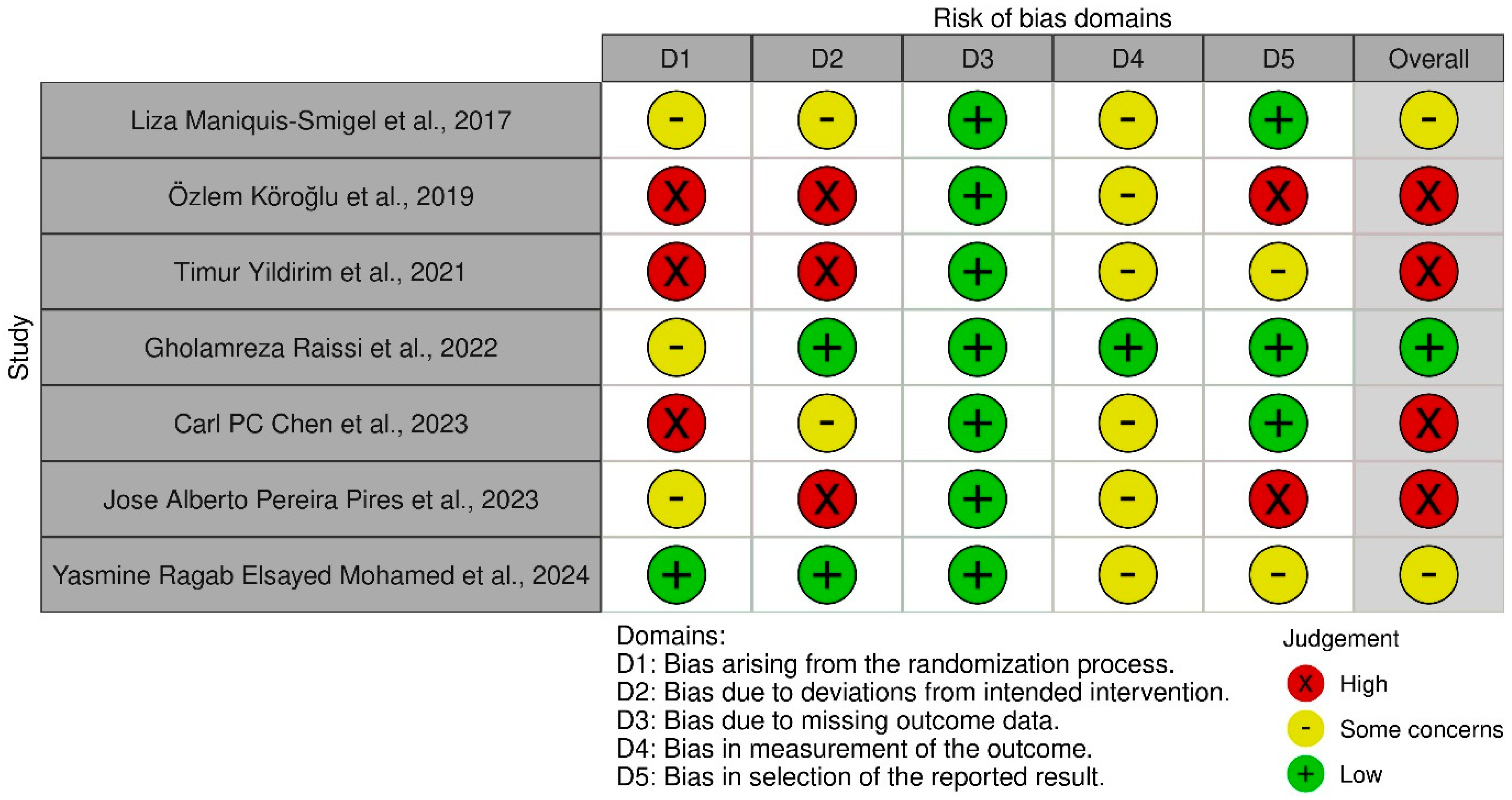
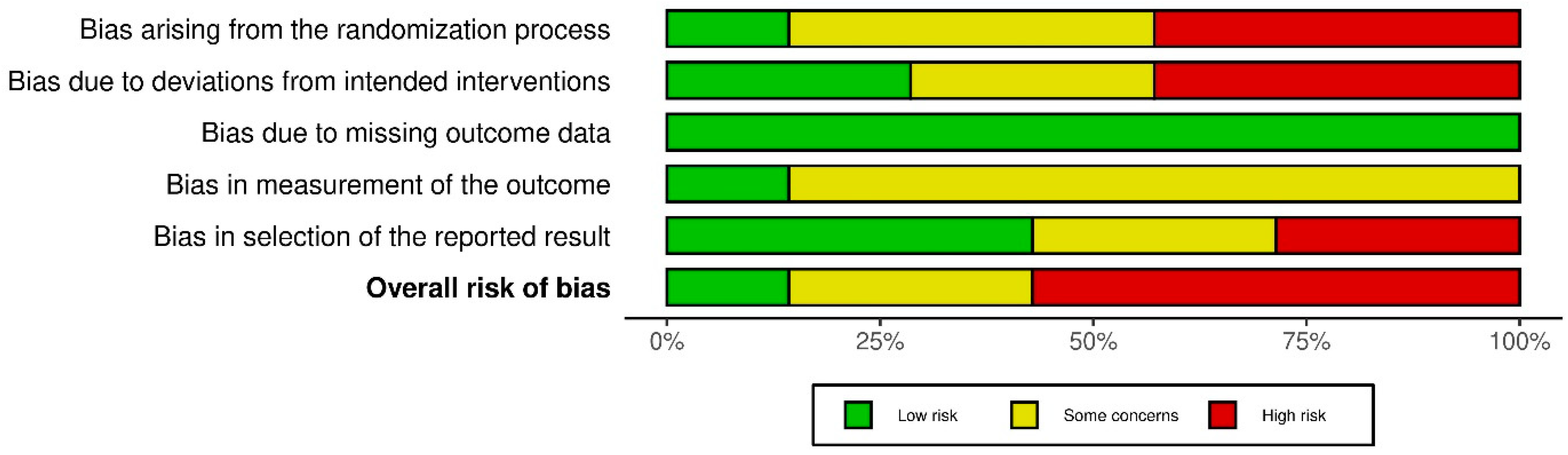
3.3. Risk Bias
4. Discussion
4.1. Diversity of Methodology (Substances, Concentration)
4.2. Anatomical Approach
4.3. Using Imaging Guidance
4.4. Number of Sessions for Injection
4.5. Control Groups
4.6. Scale-Based Assessment
4.7. Clinical Significance
4.8. Functional Outcomes Versus Pain Outcomes
4.9. Impact of Dextrose Concentration
4.10. Anatomical Target and Guidance Modality
4.11. Summary of Outcomes According to Intervention Parameters
4.11.1. Dextrose Concentration
4.11.2. Injection Frequency
4.11.3. Anatomical Target of Injection Appears to Play a Critical Role in Treatment Outcomes
4.11.4. Guidance Modality of the Procedure Significantly Influenced Treatment Consistency and Effectiveness
| Parameter | Category | Outcome Summary | Studies |
|---|---|---|---|
| Dextrose concentration | Low (5%) | Rapid analgesia, sustained effects with repeated sessions | Maniquis-Smigel et al., 2017 [59]; Köroğlu et al., 2019 [60] |
| Intermediate (20–25%) | Variable outcomes: superior to corticosteroids at 25% concentration [61], versus inferior to corticosteroids at 20% concentration [65] | Yildirim et al., 2021 [61], Mohamed et al. [65] | |
| High (75%) | No added benefit over conservative care | Pires et al., 2023 [64] | |
| Injection Frequency | Single session | Short-term relief only | Maniquis-Smigel et al., 2017 [59]; Yildirim et al., 2021; Chen et al., 2023 [63] |
| Multiple sessions | Sustained improvements in pain and function | Köroğlu et al., 2019 [60] | |
| Anatomical target | Facet joint/sacroiliac joint | Favorable outcomes | Yildirim et al., 2021 [61]; Raissi et al., 2022 [62] |
| Epidural | Rapid but short-term improvement | Maniquis-Smigel et al., 2017 [59], Mohamed et al. [65] | |
| Multiple targets | Modest to sustained improvement | Köroğlu et al., 2019 [60], Chen et al., 2023 [63] | |
| Guidance modality | Image-guided (ultrasound and/or fluoroscopy) | Consistent and lasting outcomes | Raissi et al., 2022 [62]; Chen et al., 2023 [63]; Mohamed et al., 2024 [65] |
| Anatomical land-mark guided | Variable outcomes, potential inaccuracy | Köroğlu et al., 2019 [60]; Yildirim et al., 2021 [61] |
4.12. Summary of Findings and Certainty of Evidence (GRADE Assessment)
4.13. Limitations of the Evidence Included in the Review
4.14. Limitations of the Review Processes Used
4.15. Implications of the Results for Practice, Policy, and Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLBP | Chronic low back pain |
| D5W | Dextrose 5% in Water |
| DPQ | Dallas Pain Questionnaire |
| FBSS | Failed Back Surgery Syndrome |
| GBD | Global Burden of Disease |
| LBP | Low back pain |
| LSS | Lumbar Spinal Stenosis |
| mL | milliliter |
| MPQ | McGill Pain Questionnaire |
| NRS | numerical rating scale |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| ODI | Oswestry Disability Index |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized double-blind clinical trial |
| RoB 2 | Risk of Bias 2 |
| SF-36 | 36-Item Short Form Survey |
| SI | sacroiliac |
| SIJD | Sacroiliac joint dysfunction |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| USA | The United States of America |
| USG | Ultrasound-guided |
| US | Ultrasound |
| VAS | Visual Analog Scale |
| YLDs | years lived with disability |
References
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform Soc. Eur. Sect. Cerv. Spine Res. Soc. 2006, 15 (Suppl. S2), S192–S300. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Centre (UK). Low Back Pain and Sciatica in Over 16s: Assessment and Management [Internet] (National Institute for Health and Care Excellence: Guidelines); National Institute for Health and Care Excellence (NICE): London, UK, 2016. Available online: http://www.ncbi.nlm.nih.gov/books/NBK401577/ (accessed on 5 July 2025).
- Qaseem, A.; Wilt, T.J.; McLean, R.M.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians; Denberg, T.D.; Barry, M.J.; Boyd, C.; Chow, R.D.; Fitterman, N.; et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 514–530. [Google Scholar] [CrossRef]
- Adams, M.A.; Dolan, P. Intervertebral disc degeneration: Evidence for two distinct phenotypes. J. Anat. 2012, 221, 497–506. [Google Scholar] [CrossRef]
- DePalma, M.J.; Ketchum, J.M.; Saullo, T. What Is the Source of Chronic Low Back Pain and Does Age Play a Role? Pain Med. 2011, 12, 224–233. [Google Scholar] [CrossRef]
- Suri, P.; Hunter, D.J.; Rainville, J.; Guermazi, A.; Katz, J.N. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthr. Cartil. 2013, 21, 1199–1206. [Google Scholar] [CrossRef]
- Kreiner, D.S.; Hwang, S.W.; Easa, J.E.; Resnick, D.K.; Baisden, J.L.; Bess, S.; Cho, C.H.; DePalma, M.J.; Dougherty, P.; Fernand, R.; et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. Off. J. N. Am. Spine Soc. 2014, 14, 180–191. [Google Scholar] [CrossRef]
- Koes, B.W.; van Tulder, M.W.; Peul, W.C. Diagnosis and treatment of sciatica. BMJ 2007, 334, 1313–1317. [Google Scholar] [CrossRef]
- Vleeming, A.; Schuenke, M.D.; Masi, A.T.; Carreiro, J.E.; Danneels, L.; Willard, F.H. The sacroiliac joint: An overview of its anatomy, function and potential clinical implications. J. Anat. 2012, 221, 537–567. [Google Scholar] [CrossRef]
- Kalichman, L.; Cole, R.; Kim, D.H.; Li, L.; Suri, P.; Guermazi, A.; Hunter, D.J. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J. Off. J. N. Am. Spine Soc. 2009, 9, 545–550. [Google Scholar] [CrossRef]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T.; Shekelle, P.; Owens, D.K. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med. 2007, 147, 478–491. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef]
- Smart, K.M.; Blake, C.; Staines, A.; Thacker, M.; Doody, C. Mechanisms-based classifications of musculoskeletal pain: Part 1 of 3: Symptoms and signs of central sensitisation in patients with low back (±leg) pain. Man. Ther. 2012, 17, 336–444. [Google Scholar] [CrossRef] [PubMed]
- Schuttert, I.; Timmerman, H.; Petersen, K.K.; McPhee, M.E.; Arendt-Nielsen, L.; Reneman, M.F.; Wolff, A.P. The Definition, Assessment, and Prevalence of (Human Assumed) Central Sensitisation in Patients with Chronic Low Back Pain: A Systematic Review. J. Clin. Med. 2021, 10, 5931. [Google Scholar] [CrossRef]
- GBD 2021 Low Back Pain Collaborators. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R.A. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef]
- Gorasso, V.; Van der Heyden, J.; De Pauw, R.; Pelgrims, I.; De Clercq, E.M.; De Ridder, K.; Vandevijvere, S.; Vansteelandt, S.; Vaes, B.; De Smedt, D.; et al. The health and economic burden of musculoskeletal disorders in Belgium from 2013 to 2018. Popul. Health Metr. 2023, 21, 4. [Google Scholar] [CrossRef]
- da Silva, T.; Mills, K.; Brown, B.T.; Herbert, R.D.; Maher, C.G.; Hancock, M.J. Risk of Recurrence of Low Back Pain: A Systematic Review. J. Orthop. Sports Phys. Ther. 2017, 47, 305–513. [Google Scholar] [CrossRef]
- Heneweer, H.; Staes, F.; Aufdemkampe, G.; van Rijn, M.; Vanhees, L. Physical activity and low back pain: A systematic review of recent literature. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform Soc. Eur. Sect. Cerv. Spine Res. Soc. 2011, 20, 826–845. [Google Scholar] [CrossRef]
- Samartzis, D.; Karppinen, J.; Mok, F.; Fong, D.Y.T.; Luk, K.D.K.; Cheung, K.M.C. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J. Bone Joint Surg. Am. 2011, 93, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Wáng, Y.X.J.; Wáng, J.Q.; Káplár, Z. Increased low back pain prevalence in females than in males after menopause age: Evidences based on synthetic literature review. Quant Imaging Med. Surg. 2016, 6, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Chen, S.; Klyne, D.M.; Harrich, D.; Ding, W.; Yang, S.; Han, F.Y. Low back pain and osteoarthritis pain: A perspective of estrogen. Bone Res. 2023, 11, 42. [Google Scholar] [CrossRef]
- Jegnie, M.; Afework, M. Prevalence of Self-Reported Work-Related Lower Back Pain and Its Associated Factors in Ethiopia: A Systematic Review and Meta-Analysis. J. Environ. Public Health 2021, 2021, 6633271. [Google Scholar] [CrossRef]
- Lucha-López, M.O.; Hidalgo-García, C.; Monti-Ballano, S.; Márquez-Gonzalvo, S.; Ferrández-Laliena, L.; Müller-Thyssen-Uriarte, J.; Lucha-López, A.C. Body Mass Index and Its Influence on Chronic Low Back Pain in the Spanish Population: A Secondary Analysis from the European Health Survey (2020). Biomedicines 2023, 11, 2175. [Google Scholar] [CrossRef]
- Akmal, M.; Kesani, A.; Anand, B.; Singh, A.; Wiseman, M.; Goodship, A. Effect of nicotine on spinal disc cells: A cellular mechanism for disc degeneration. Spine 2004, 29, 568–575. [Google Scholar] [CrossRef]
- Kett, A.R.; Sichting, F.; Milani, T.L. The Effect of Sitting Posture and Postural Activity on Low Back Muscle Stiffness. Biomechanics 2021, 1, 214–224. [Google Scholar] [CrossRef]
- Baradaran Mahdavi, S.; Riahi, R.; Vahdatpour, B.; Kelishadi, R. Association between sedentary behavior and low back pain; A systematic review and meta-analysis. Health Promot. Perspect. 2021, 11, 393–410. [Google Scholar] [CrossRef]
- Pincus, T.; Burton, A.K.; Vogel, S.; Field, A.P. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 2002, 27, E109–E120. [Google Scholar] [CrossRef]
- Ikeda, T.; Sugiyama, K.; Aida, J.; Tsuboya, T.; Watabiki, N.; Kondo, K.; Osaka, K. Socioeconomic inequalities in low back pain among older people: The JAGES cross-sectional study. Int. J. Equity Health 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wei, Z.; Xu, T.; Gamber, M.; Han, J.; Jiang, Y.; Li, J.; Yang, D.; Sun, W. The association between the socioeconomic status and body pain: A nationwide survey. Medicine 2020, 99, e19454. [Google Scholar] [CrossRef]
- Rabago, D.; Slattengren, A.; Zgierska, A. Prolotherapy in primary care practice. Prim. Care 2010, 37, 65–80. [Google Scholar] [CrossRef]
- Hackett, G.S. Ligament and Tendon Relaxation, 3rd ed.; Charles C Thomas Pub Ltd.: Illinois, IL, USA, 1958; 151p. [Google Scholar]
- Hauser, R.A.; Lackner, J.B.; Steilen-Matias, D.; Harris, D.K. A Systematic Review of Dextrose Prolotherapy for Chronic Musculoskeletal Pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 139–159. [Google Scholar] [CrossRef]
- Jensen, K.T.; Rabago, D.P.; Best, T.M.; Patterson, J.J.; Vanderby, R. Early inflammatory response of knee ligaments to prolotherapy in a rat model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 816–823. [Google Scholar] [CrossRef]
- Zhao, A.T.; Caballero, C.J.; Nguyen, L.T.; Vienne, H.C.; Lee, C.; Kaye, A.D. A Comprehensive Update of Prolotherapy in the Management of Osteoarthritis of the Knee. Orthop. Rev. 2022, 14, 33921. [Google Scholar] [CrossRef]
- Siadat, A.H.; Isseroff, R.R. Prolotherapy: Potential for the Treatment of Chronic Wounds? Adv. Wound Care. 2019, 8, 160–167. [Google Scholar] [CrossRef]
- Sit, R.W.S.; Wu, R.W.K.; Rabago, D.; Reeves, K.D.; Chan, D.C.C.; Yip, B.H.K.; Chung, V.C.H.; Wong, S.Y.S. Efficacy of Intra-Articular Hypertonic Dextrose (Prolotherapy) for Knee Osteoarthritis: A Randomized Controlled Trial. Ann. Fam. Med. 2020, 18, 235–342. [Google Scholar] [CrossRef]
- Scarpone, M.; Rabago, D.P.; Zgierska, A.; Arbogast, G.; Snell, E. The efficacy of prolotherapy for lateral epicondylosis: A pilot study. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2008, 18, 248–254. [Google Scholar] [CrossRef]
- Rabago, D.; Zgierska, A.; Fortney, L.; Kijowski, R.; Mundt, M.; Ryan, M.; Grettie, J.; Patterson, J.J. Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: Results of a single-arm uncontrolled study with 1-year follow-up. J. Altern. Complement. Med. 2012, 18, 408–414. [Google Scholar] [CrossRef]
- Dean, B.J.F.; Lostis, E.; Oakley, T.; Rombach, I.; Morrey, M.E.; Carr, A.J. The risks and benefits of glucocorticoid treatment for tendinopathy: A systematic review of the effects of local glucocorticoid on tendon. Semin. Arthritis Rheum. 2014, 43, 570–576. [Google Scholar] [CrossRef]
- Maxwell, N.J.; Ryan, M.B.; Taunton, J.E.; Gillies, J.H.; Wong, A.D. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: A pilot study. AJR Am. J. Roentgenol. 2007, 189, W215–W220. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.D.; Hassanein, K.M. Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern. Ther. Health Med. 2003, 9, 58–62. [Google Scholar] [PubMed]
- Piraccini, E.; Biondi, G. Prolotherapy: Regenerative Medicine for Lateral Epicondylitis. Turk. J. Anaesthesiol. Reanim. 2020, 48, 509–510. [Google Scholar] [CrossRef]
- Morath, O.; Kubosch, E.J.; Taeymans, J.; Zwingmann, J.; Konstantinidis, L.; Südkamp, N.P.; Hirschmüller, A. The effect of sclerotherapy and prolotherapy on chronic painful Achilles tendinopathy-a systematic review including meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 4–15. [Google Scholar] [CrossRef]
- Lai, W.F.; Yoon, C.H.; Chiang, M.T.; Hong, Y.H.; Chen, H.C.; Song, W.; Chin, Y.P.H. The effectiveness of dextrose prolotherapy in plantar fasciitis: A systemic review and meta-analysis. Medicine 2021, 100, e28216. [Google Scholar] [CrossRef]
- Sit, R.W.S.; Reeves, K.D.; Zhong, C.C.; Wong, C.H.L.; Wang, B.; Chung, V.C.H.; Wong, S.Y.-S.; Rabago, D. Efficacy of hypertonic dextrose injection (prolotherapy) in temporomandibular joint dysfunction: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 14638. [Google Scholar] [CrossRef]
- Refai, H.; Altahhan, O.; Elsharkawy, R. The efficacy of dextrose prolotherapy for temporomandibular joint hypermobility: A preliminary prospective, randomized, double-blind, placebo-controlled clinical trial. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2011, 69, 2962–2970. [Google Scholar] [CrossRef]
- Hauser, R.A.; Matias, D.; Woznica, D.; Rawlings, B.; Woldin, B.A. Lumbar instability as an etiology of low back pain and its treatment by prolotherapy: A review. J. Back Musculoskelet. Rehabil. 2022, 35, 701–712. [Google Scholar] [CrossRef]
- Dagenais, S.; Yelland, M.J.; Del Mar, C.; Schoene, M.L. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst. Rev. 2007, 2007, CD004059. [Google Scholar] [CrossRef] [PubMed]
- Mafhoumi, A.; Nabian, M.H.; Mehrpour, S.R. Prolotherapy in Musculoskeletal Disorders, Guideline for Orthopedic Application. Open J. Regen. Med. 2023, 12, 64–84. [Google Scholar] [CrossRef]
- Bae, G.; Kim, S.; Lee, S.; Lee, W.Y.; Lim, Y. Prolotherapy for the patients with chronic musculoskeletal pain: Systematic review and meta-analysis. Anesth. Pain Med. 2021, 16, 81–95. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vosm, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Low Back Pain [Internet]. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/low-back-pain (accessed on 6 July 2025).
- Maniquis-Smigel, L.; Dean Reeves, K.; Jeffrey Rosen, H.; Lyftogt, J.; Graham-Coleman, C.; Cheng, A.L.; Rabago, D. Short Term Analgesic Effects of 5% Dextrose Epidural Injections for Chronic Low Back Pain: A Randomized Controlled Trial. Anesthesiol. Pain Med. 2017, 7, e42550. [Google Scholar] [CrossRef]
- Köroğlu, Ö.; Örsçelik, A.; Karasimav, Ö.; Demir, Y.; Solmaz, İ. Is 5% dextrose prolotherapy effective for radicular low back pain? Gulhane Med. J. 2019, 61, 123. [Google Scholar] [CrossRef]
- Yildirim, T. Comparison of the Effectiveness of Prolotherapy and Facet Joint Injection in the Treatment of Chronic Low Back Pain: A Retrospective Study. Turk. Klin. J. Med. Sci. 2021, 41, 1–6. [Google Scholar]
- Raissi, G.; Ahadi, T.; Forogh, B.; Moradi, K.; Madani, S.P.; Yousefi, N.; Foroumadi, R.; Rahimi-Dehgolan, S. Corticosteroid Versus Dextrose Ultrasound-Guided Injection in the Treatment of Sacroiliac Joint Dysfunction: A Randomized Double-Blinded Clinical Trial. J. Iran. Med. Counc. 2022, 5, 668–676. [Google Scholar] [CrossRef]
- Chen, C.P.C.; Suputtitada, A. Prolotherapy at Multifidus Muscle versus Mechanical Needling and Sterile Water Injection in Lumbar Spinal Stenosis. J. Pain Res. 2023, 16, 2477–2486. [Google Scholar] [CrossRef]
- Pereira Pires, J.A.; Rey Moura, E.C.; Oliveira, C.M.B.; de Vieira Dibai-Filho, A.; Soares Brandão Nascimento Mdo, D.; da Cunha Leal, P. Hypertonic glucose in the treatment of low back pain: A randomized clinical trial. Medicine 2023, 102, e35163. [Google Scholar] [CrossRef]
- Mohamed, Y.R.E.; El-Attar, A.M.I.; Anwar, D.M.F.; Shehab, A.S.A. The efficacy of ultrasound and fluoroscopy-guided caudal epidural prolotherapy versus steroids for chronic pain management in failed back surgery syndrome. Alex. J. Med. 2024, 60, 238–243. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Giordano, L.; Murrell, W.D.; Maffulli, N. Prolotherapy for chronic low back pain: A review of literature. Br. Med. Bull. 2021, 138, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Yelland, M.J.; Glasziou, P.P.; Bogduk, N.; Schluter, P.J.; McKernon, M. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: A randomized trial. Spine 2004, 29, 9–16; Discussion 16. [Google Scholar] [CrossRef]
- Bukina, O.V.; Sinitsyn, A.A.; Pelevin, A.V. Sclerotherapy of telangiectasias: A prospective, randomized, comparative clinical trial of hypertonic glucose versus sodium tetradecyl sulfate. Vasc. Med. 2021, 26, 297–301. [Google Scholar] [CrossRef]
- Bayat, M.; Raeissadat, S.A.; Mortazavian Babaki, M.; Rahimi-Dehgolan, S. Is Dextrose Prolotherapy Superior to Corticosteroid Injection in Patients with Chronic Lateral Epicondylitis?: A Randomized Clinical Trial. Orthop. Res. Rev. 2019, 11, 167–175. [Google Scholar] [CrossRef]
- Bayat, M.; Hojjati, F.; Boland Nazar, N.S.; Modabberi, M.; Rahimi, M.S. Comparison of Dextrose Prolotherapy and Triamcinolone Intraarticular Injection on Pain and Function in Patients with Knee Osteoarthritis—A Randomized Clinical Trial. Anesthesiol. Pain Med. 2023, 13, e134415. [Google Scholar] [CrossRef]
- Suputtitada, A.; Chen, C.P.C.; Pongpirul, K. Mechanical Needling with Sterile Water Versus Lidocaine Injection for Lumbar Spinal Stenosis. Glob. Spine J. 2024, 14, 82–92. [Google Scholar] [CrossRef]
- Iliescu, D.M.; Micu, S.I.; Ionescu, C.; Bulbuc, I.; Bordei, P.; Obada, B.; Voinea, F.; Gheorghe, E.; Iliescu, M.G. Axial and para axial loading response evaluation on human cadaver harvested lumbar vertebral blocks: In vitro experiment with possible clinical implications for clinical practice. Exp. Ther. Med. 2021, 22, 1192. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.A.; Oprea, D.; Obada, B.; Iliescu, D.M.; Botnarciuc, M.; Ionescu, A.M.; Mihailov, C.I.; Iliescu, M.G.; Caraban, B.M. Variation of serum serotonin values under specific peloido-therapy in patients with degenerative pathology of the lumbar spine. Balneo PRM Res. J. 2023, 14, 588. [Google Scholar] [CrossRef]
- Buonanno, P.; Marra, A.; Iacovazzo, C.; Vargas, M.; Nappi, S.; Squillacioti, F.; de Siena, A.U.; Servillo, G. The Patient Approach: A New Bundle for the Management of Chronic Pain. J. Pers. Med. 2023, 13, 1551. [Google Scholar] [CrossRef]
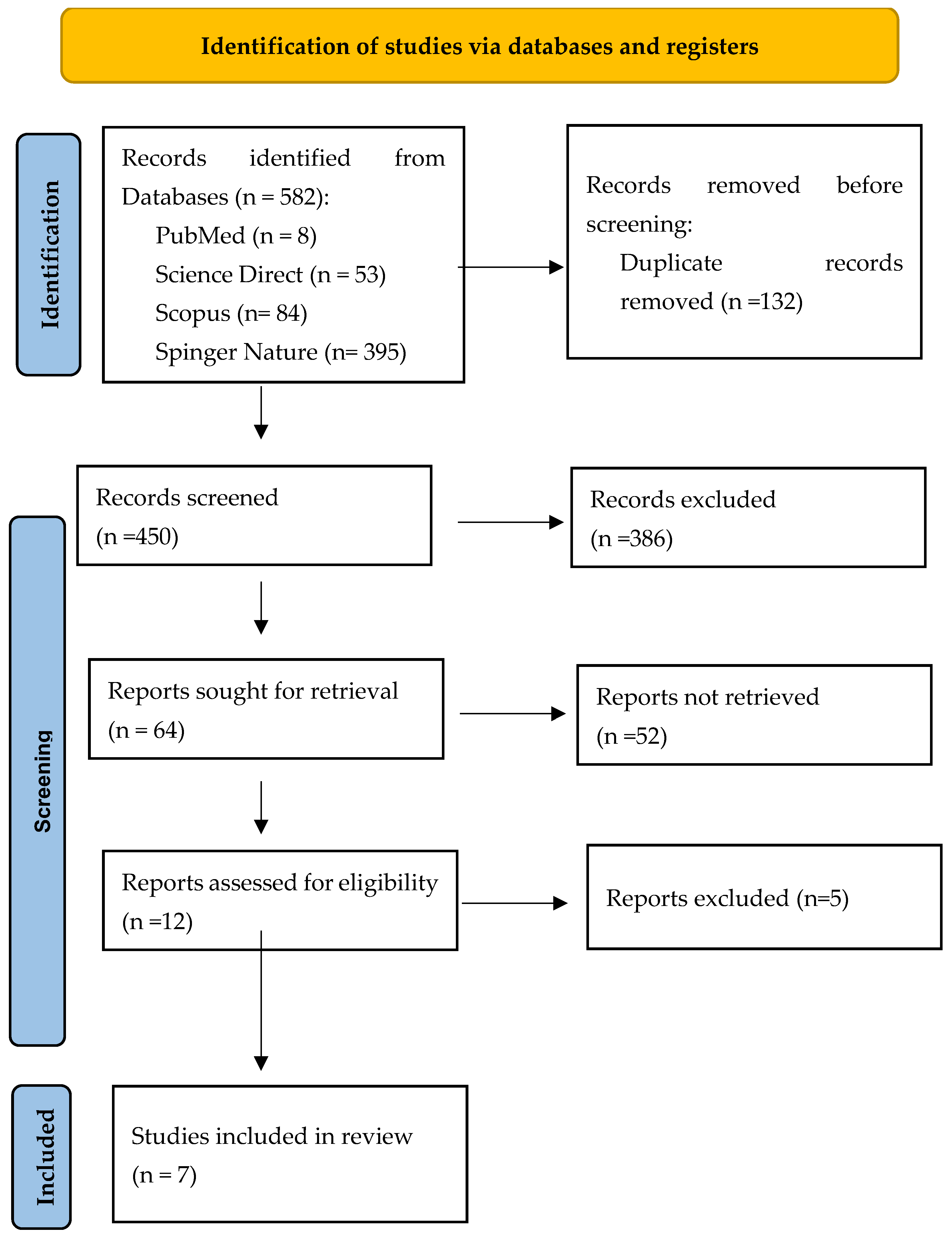
| Keyword Combinations | PubMed | Science Direct | Scopus | Springer Nature | Web of Science | Cochrane | Total |
|---|---|---|---|---|---|---|---|
| Prolotherapy AND low back pain | 2 | 19 | 59 | 89 | 25 | 0 | 194 |
| Hypertonic glucose AND low back pain | 1 | 25 | 5 | 246 | 4 | 0 | 281 |
| Dextrose prolotherapy AND low back pain | 2 | 7 | 20 | 47 | 11 | 0 | 87 |
| D-glucose prolotherapy AND low back pain | 3 | 2 | 0 | 13 | 2 | 0 | 20 |
| Total | 8 | 53 | 84 | 395 | 42 | 0 | 582 |
| Authors and Publication Year | Mean Grading | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total * | |
| Liza Maniquis-Smigel et al., 2017 [59] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 9/10 |
| Özlem Köroğlu et al., 2019 [60] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5/10 |
| Timur Yildirim et al., 2021 [61] | Yes | No | No | No | No | No | No | No | Yes | Yes | Yes | 3/10 |
| Gholamreza Raissi et al., 2022 [62] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 8/10 |
| Carl PC Chen et al., 2023 [63] | Yes | No | No | Yes | No | No | No | Yes | No | Yes | Yes | 4/10 |
| Jose Alberto Pereira Pires et al., 2023 [64] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6/10 |
| Yasmine Ragab Elsayed Mohamed et al., 2024 [65] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7/10 |
| Outcomes | Anticipated Absolute Effects (95% CI) | Relative Effect (95% CI) | № of Participants (Studies) | Certainty of the Evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with [Comparison] | Risk with [Intervention] | ||||
| Pain Evaluation assessed with VAS | The mean pain Evaluation was 0 | 0 (0 to 0) | - | 586 (6 studies) | -a,b,c,d |
| Pain Evaluation assessed with NRS | The mean pain Evaluation was 0 | 0 (0 to 0) | - | 35 (1 RCT) | -r |
| Pain characterization assessed with MPQ | The mean pain characterization was 0 | 0 (0 to 0) | - | 83 (1 RCT) | -n |
| Pain-related impact on daily activities assessed with DPQ | The mean pain-related impact on daily activities was 0 | 0 (0 to 0) | - | 36 (1 RCT) | ⨁◯◯◯ * e,f,g |
| Functional disability assessed with ODI | The mean functional disability was 0 | 0 (0 to 0) | - | 301 (3 studies) | -h,i,j,k |
| Functional disability assessed with RMDQ | The mean functional disability was 0 | 0 (0 to 0) | - | 38 (1 RCT) | -s |
| Quality of life assessed with Sf-36 | The mean quality of life was 0 | 0 (0 to 0) | - | 40 (1 study) | -l,m |
| Walking distance assessed with meters before calf pain | The mean walking distance was 0 | 0 (0 to 0) | - | 211 (1 study) | -o,p,q |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mociu, S.-I.; Nedelcu, A.-D.; Lupu, A.-A.; Uzun, A.-B.; Iliescu, D.-M.; Ionescu, E.-V.; Iliescu, M.-G. Prolotherapy as a Regenerative Treatment in the Management of Chronic Low Back Pain: A Systematic Review. Medicina 2025, 61, 1588. https://doi.org/10.3390/medicina61091588
Mociu S-I, Nedelcu A-D, Lupu A-A, Uzun A-B, Iliescu D-M, Ionescu E-V, Iliescu M-G. Prolotherapy as a Regenerative Treatment in the Management of Chronic Low Back Pain: A Systematic Review. Medicina. 2025; 61(9):1588. https://doi.org/10.3390/medicina61091588
Chicago/Turabian StyleMociu, Stelian-Ilie, Andreea-Dalila Nedelcu, Andreea-Alexandra Lupu, Andreea-Bianca Uzun, Dan-Marcel Iliescu, Elena-Valentina Ionescu, and Madalina-Gabriela Iliescu. 2025. "Prolotherapy as a Regenerative Treatment in the Management of Chronic Low Back Pain: A Systematic Review" Medicina 61, no. 9: 1588. https://doi.org/10.3390/medicina61091588
APA StyleMociu, S.-I., Nedelcu, A.-D., Lupu, A.-A., Uzun, A.-B., Iliescu, D.-M., Ionescu, E.-V., & Iliescu, M.-G. (2025). Prolotherapy as a Regenerative Treatment in the Management of Chronic Low Back Pain: A Systematic Review. Medicina, 61(9), 1588. https://doi.org/10.3390/medicina61091588