Optimizing Cognitive and Physical Gains in Older Adults: Benefits of a Psychomotor Intervention Program Based on Functional Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcome Measures
Complementary Outcomes Measures
2.4. Interactive Cognitive-Motor Program
Psychomotor Intervention Program
2.5. Statistical Analysis
3. Results
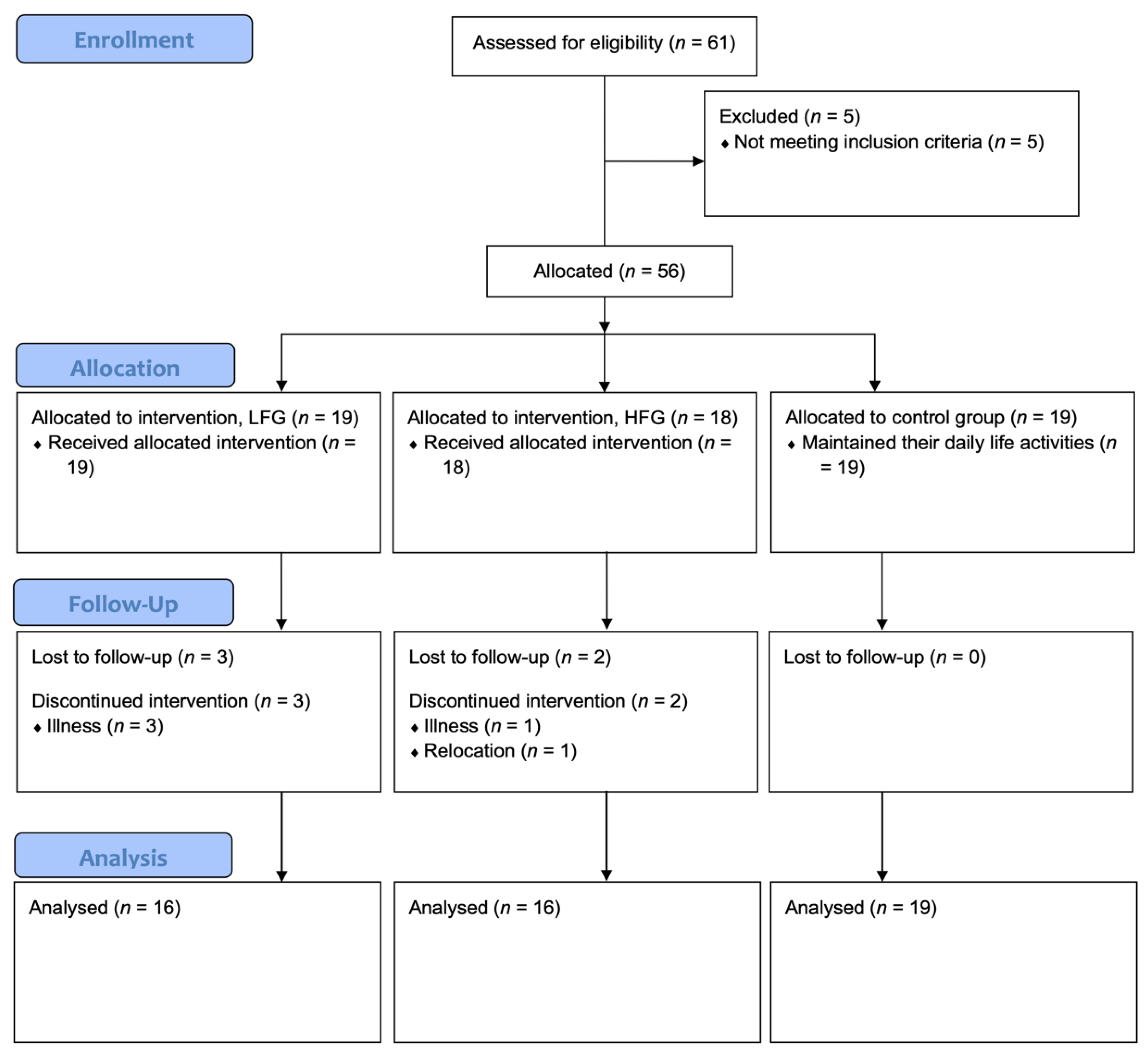
3.1. Sociodemographic Characteristics and Response Rates
3.2. Descriptive Results
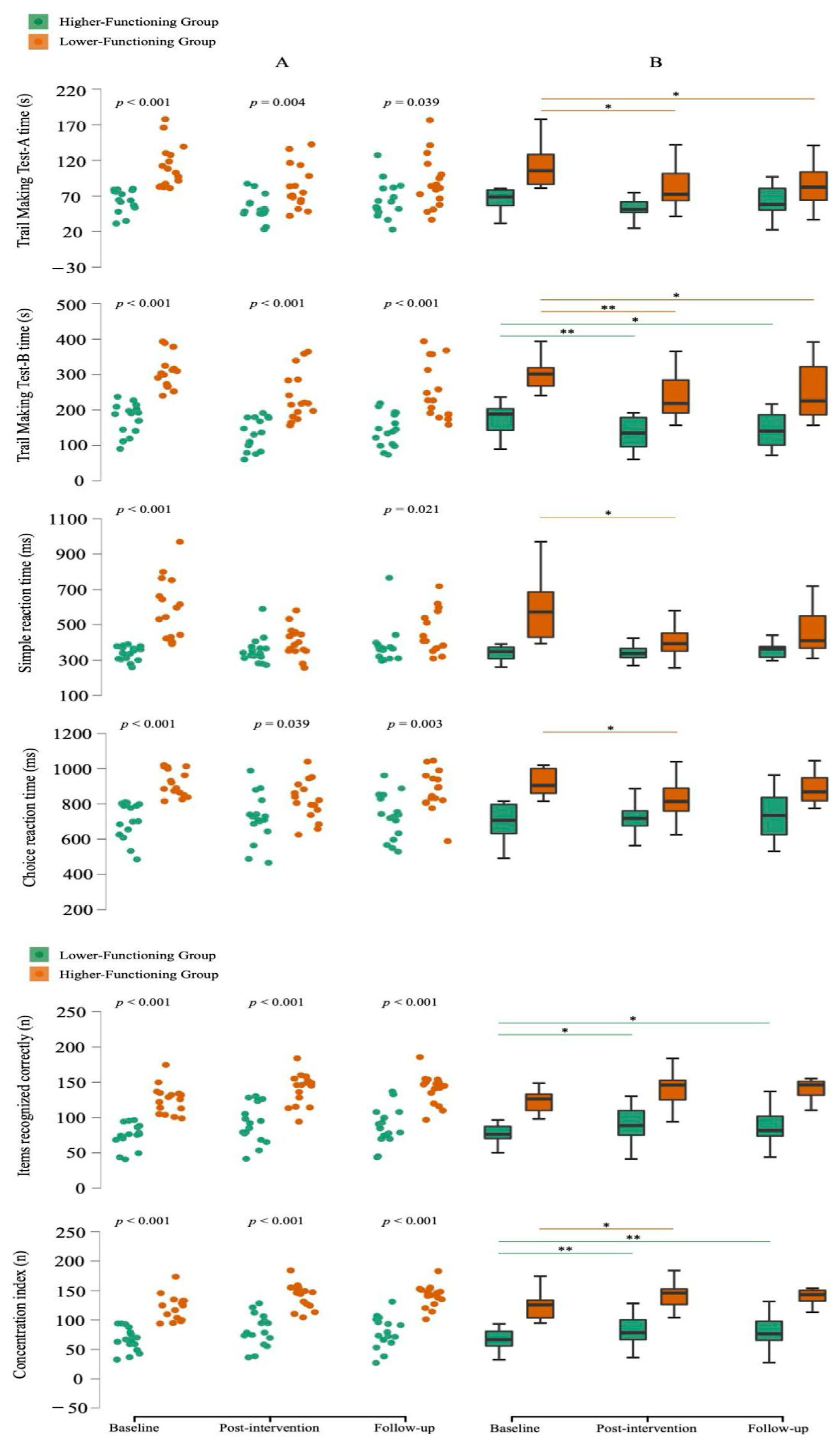
3.2.1. Cognitive Function
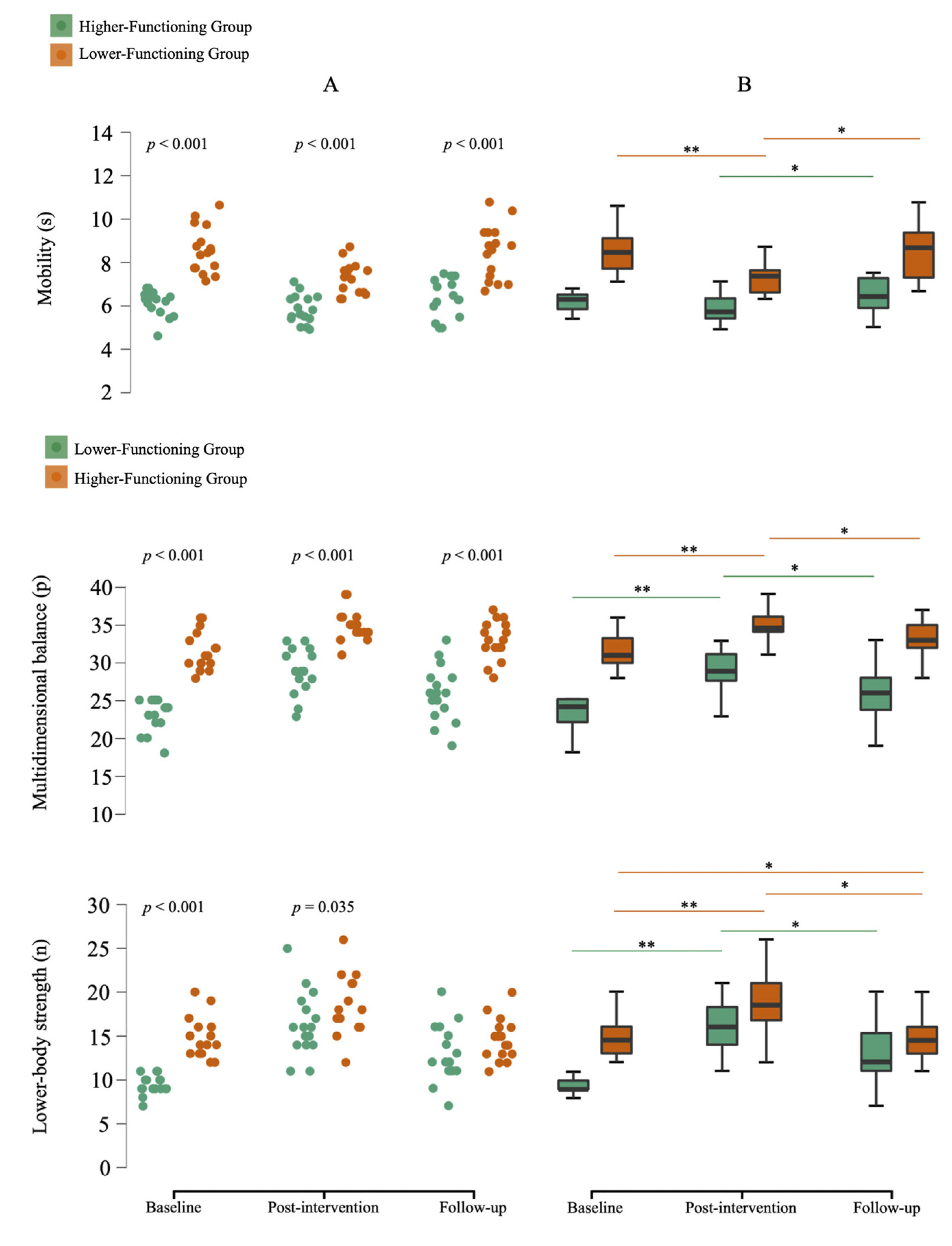
3.2.2. Physical Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Population Structure and Ageing. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing (accessed on 5 May 2025).
- Pion, C.H.; Barbat-Artigas, S.; St-Jean-Pelletier, F.; Chevalier, S.; Gaudreau, P.; Gouspillou, G.; Morais, J.A.; Belanger, M.; Aubertin-Leheudre, M. Muscle strength and force development in high- and low-functioning elderly men: Influence of muscular and neural factors. Exp. Gerontol. 2017, 96, 19–28. [Google Scholar] [CrossRef]
- Rieker, J.A.; Reales, J.M.; Muinos, M.; Ballesteros, S. The Effects of Combined Cognitive-Physical Interventions on Cognitive Functioning in Healthy Older Adults: A Systematic Review and Multilevel Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 838968. [Google Scholar] [CrossRef]
- Vaughan, S.; Wallis, M.; Polit, D.; Steele, M.; Shum, D.; Morris, N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: A randomised controlled trial. Age Ageing 2014, 43, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Linde, K.; Alfermann, D. Single versus combined cognitive and physical activity effects on fluid cognitive abilities of healthy older adults: A 4-month randomized controlled trial with follow-up. J. Aging Phys. Act. 2014, 22, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Mohamed, O.; White, B.; Singh-Carlson, S.; Krishnan, V. The effects of a multicomponent intervention program on clinical outcomes associated with falls in healthy older adults. Aging Clin. Exp. Res. 2018, 30, 1101–1110. [Google Scholar] [CrossRef]
- Teraz, K.; Slosar, L.; Paravlic, A.H.; de Bruin, E.D.; Marusic, U. Impact of Motor-Cognitive Interventions on Selected Gait and Balance Outcomes in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Psychol. 2022, 13, 837710. [Google Scholar] [CrossRef]
- Blasco-Lafarga, C.; Cordellat, A.; Forte, A.; Roldan, A.; Monteagudo, P. Short and Long-Term Trainability in Older Adults: Training and Detraining Following Two Years of Multicomponent Cognitive-Physical Exercise Training. Int. J. Environ. Res. Public Health 2020, 17, 5984. [Google Scholar] [CrossRef]
- Olyaei, G.; Khanmohammadi, R.; Talebian, S.; Hadian, M.R.; Bagheri, H.; Najafi, M. The effect of exergaming on cognition and brain activity in older adults: A motor- related cortical potential study. Physiol. Behav. 2022, 255, 113941. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Rosado, H.; Cruz-Ferreira, A.; Marmeleira, J. Effects of a 10-week multimodal exercise program on physical and cognitive function of nursing home residents: A psychomotor intervention pilot study. Aging Clin. Exp. Res. 2018, 30, 471–479. [Google Scholar] [CrossRef]
- Kwag, E.; Stuckenschneider, T.; Schneider, S.; Abeln, V. The effect of a psychomotor intervention on electroencephalography and neuropsychological performances in older adults with and without mild cognitive impairment. Psychogeriatrics 2021, 21, 528–539. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, C.; Barreyro, J.P.; Tartaglini, M.F.; Hermida, P.D.; Moya Garcia, L.; Benetti, L.; Somale, M.V.; Allegri, R. Estimation of cognitive reserve and its impact on cognitive performance in older adults. Appl. Neuropsychol. Adult. 2021, 31, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.S.; Hosseini, S.M.H. The Effect of Baseline Performance and Age on Cognitive Training Improvements in Older Adults: A Qualitative Review. J. Prev. Alzheimer’s Dis. 2021, 8, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, F.; Poppe, L.; DeSmet, A.; Swinnen, S.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S.; Fias, W. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef]
- de Labra, C.; Guimaraes-Pinheiro, C.; Maseda, A.; Lorenzo, T.; Millan-Calenti, J.C. Effects of physical exercise interventions in frail older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2015, 15, 154. [Google Scholar] [CrossRef]
- Mason, R.C.; Horvat, M.; Nocera, J. The Effects of Exercise on the Physical Fitness of High and Moderate-Low Functioning Older Adult Women. J. Aging Res. 2016, 2016, 8309284. [Google Scholar] [CrossRef]
- Mack, M.; Stojan, R.; Bock, O.; Voelcker-Rehage, C. Cognitive-motor multitasking in older adults: A randomized controlled study on the effects of individual differences on training success. BMC Geriatr. 2022, 22, 581. [Google Scholar] [CrossRef]
- Rosado, H.; Bravo, J.; Raimundo, A.; Carvalho, J.; Almeida, G.; Pereira, C. Can two multimodal psychomotor exercise programs improve attention, affordance perception, and balance in community dwellings at risk of falling? A randomized controlled trial. BMC Public Health 2022, 21, 2336. [Google Scholar] [CrossRef]
- Rosado, H.; Bravo, J.; Raimundo, A.; Carvalho, J.; Marmeleira, J.; Pereira, C. Effects of two 24-week multimodal exercise programs on reaction time, mobility, and dual-task performance in community-dwelling older adults at risk of falling: A randomized controlled trial. BMC Public Health 2021, 21, 408. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontol. 2013, 53, 255–267. [Google Scholar] [CrossRef]
- Cavaco, S.; Goncalves, A.; Pinto, C.; Almeida, E.; Gomes, F.; Moreira, I.; Fernandes, J.; Teixeira-Pinto, A. Trail Making Test: Regression-based norms for the Portuguese population. Arch. Clin. Neuropsychol. 2013, 28, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Deary, I.J.; Liewald, D.; Nissan, J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: The Deary-Liewald reaction time task. Behav. Res. Methods 2011, 43, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Brickenkamp, R. d2 Test of Attention; CEGOC-TEA: Lisbon, Portugal, 2007. [Google Scholar]
- Hernandez, D.; Rose, D.J. Predicting which older adults will or will not fall using the Fullerton Advanced Balance scale. Arch. Phys. Med. Rehabil. 2008, 89, 2309–2315. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Yoshihara, S.; Kanno, N.; Fukuda, H.; Arisaka, O.; Arita, M.; Sekine, K.; Yamaguchi, K.; Tsuchida, A.; Yamada, Y.; Watanabe, T.; et al. Caregiver treatment satisfaction is improved together with children’s asthma control: Prospective study for budesonide monotherapy in school-aged children with uncontrolled asthma symptoms. Allergol. Int. 2015, 64, 371–376. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Liguori, G. Exercise Prescription for Healthy Populations with Special Considerations. In ACSM’S Guidelines For Exercise Testing And Prescription; Kluwer, W., Ed.; LWW: Philadelphia, PA, USA, 2021. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates, Publishers: Hillsdale, NJ, USA, 1998. [Google Scholar]
- Tse, A.C.; Wong, T.W.; Lee, P.H. Effect of Low-intensity Exercise on Physical and Cognitive Health in Older Adults: A Systematic Review. Sports Med. Open 2015, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Ageing and Health. Available online: https://apps.who.int/iris/handle/10665/186463 (accessed on 5 May 2025).
- Park, D.C.; Bischof, G.N. The aging mind: Neuroplasticity in response to cognitive training. Dialogues Clin. Neurosci. 2013, 15, 109–119. [Google Scholar] [CrossRef]
- Desjardins-Crepeau, L.; Berryman, N.; Fraser, S.A.; Vu, T.T.; Kergoat, M.J.; Li, K.Z.; Bosquet, L.; Bherer, L. Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clin. Interv. Aging 2016, 11, 1287–1299. [Google Scholar] [CrossRef]
- van het Reve, E.; de Bruin, E.D. Strength-balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: A multicenter randomized-controlled trial. BMC Geriatr. 2014, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, P.; Schumacher, V.; Angst, M.; Theill, N.; de Bruin, E.D. Does multicomponent physical exercise with simultaneous cognitive training boost cognitive performance in older adults? A 6-month randomized controlled trial with a 1-year follow-up. Clin. Interv. Aging 2015, 10, 1335–1349. [Google Scholar] [CrossRef]
- Lopez-Higes, R.; Martin-Aragoneses, M.T.; Rubio-Valdehita, S.; Delgado-Losada, M.L.; Montejo, P.; Montenegro, M.; Prados, J.M.; de Frutos-Lucas, J.; Lopez-Sanz, D. Efficacy of Cognitive Training in Older Adults with and without Subjective Cognitive Decline Is Associated with Inhibition Efficiency and Working Memory Span, Not with Cognitive Reserve. Front. Aging Neurosci. 2018, 10, 23. [Google Scholar] [CrossRef]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front. Psychol. 2022, 13, 831819. [Google Scholar] [CrossRef]
- Brahms, C.M.; Hortobagyi, T.; Kressig, R.W.; Granacher, U. The Interaction between Mobility Status and Exercise Specificity in Older Adults. Exerc. Sport Sci. Rev. 2021, 49, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Chalavi, S.; Swinnen, S.P. Aging and brain plasticity. Aging 2018, 10, 1789–1790. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Stahelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef]
| Experimental LFG Mean ± SD | Experimental HFG Mean ± SD | p-Value | |
|---|---|---|---|
| Cognitive function | |||
| Trail Making Test-A (time) (s) | |||
| Age (years) | 77.9 ± 4.3 | 71.1 ± 3.9 | <0.001 |
| Educational level (years) | 4.9 ± 2.1 | 7.3 ± 3.3 | 0.051 |
| Trail Making Test-B time (s) | |||
| Age (years) | 75.1 ± 5.1 | 73.9 ± 5.6 | 0.491 |
| Educational level (years) | 4.8 ± 1.8 | 7.3 ± 3.5 | 0.035 |
| Simple reaction time (ms) | |||
| Age (years) | 74.6 ± 6.0 | 74.4 ± 4.7 | 0.985 |
| Educational level (years) | 5.1 ± 2.3 | 7.0 ± 3.4 | 0.160 |
| Choice reaction time (ms) | |||
| Age (years) | 75.6 ± 5.3 | 73.4 ± 5.3 | 0.270 |
| Educational level (years) | 4.5 ± 1.7 | 7.6 ± 3.2 | 0.010 |
| Items recognized correctly (n) | |||
| Age (years) | 76.1 ± 5.3 | 72.9 ± 5.0 | 0.110 |
| Educational level (years) | 5.1 ± 2.4 | 7.0 ± 3.3 | 0.119 |
| Concentration index (n) | |||
| Age (years) | 75.8 ± 4.9 | 73.2 ± 5.6 | 0.160 |
| Educational level (years) | 4.4 ± 1.5 | 7.8 ± 3.2 | 0.002 |
| Physical function | |||
| Lower-body strength (n) | |||
| Age (years) | 73.5 ± 5.6 | 75.5 ± 5.0 | 0.323 |
| Educational level (years) | 6.4 ± 3.4 | 5.7 ± 2.5 | 0.590 |
| Multidimensional balance (points) | |||
| Age (years) | 75.9 ± 6.1 | 73.1 ± 4.1 | 0.138 |
| Educational level (years) | 5.2 ± 3.3 | 6.9 ± 2.4 | 0.026 |
| Mobility (s) | |||
| Age (years) | 75.4 ± 5.7 | 73.6 ± 4.9 | 0.323 |
| Educational level (years) | 5.1 ± 3.1 | 7.1 ± 2.6 | 0.026 |
| Baseline (Mean ± SD) | ∆ Post-Intervention (Mean ± SD) | ∆ Follow-Up (Mean ± SD) | |
|---|---|---|---|
| Cognitive function | |||
| Trail Making Test-A (time) (s) | |||
| Experimental LFG | 111.9 ± 29.9 a | −29.3 ± 21.6 # | 6.6 ± 22.5 |
| Experimental HFG | 64.6 ± 16.0 | −10.2 ± 13.9 # | 9.6 ± 17.8 # |
| CG | 84.4 ± 39.7 b | −3.9 ± 25.6 b | −2.9 ± 16.7 |
| Trail Making Test-B time (s) | |||
| Experimental LFG | 304.4 ± 47.4 a | −66.6 ± 42.1 #,a | 11.7 ± 38.0 |
| Experimental HFG | 174.4 ± 43.1 | −43.6 ± 38.4 # | 10.5 ± 26.6 |
| CG | 207.2 ± 80.4 b | −3.3 ± 44.8 b,c | −13.8 ± 31.4 b |
| Simple reaction time (ms) | |||
| Experimental LFG | 588.3 ± 171.5 a | −183.3 ± 172.4 #,a | 51.4 ± 105.8 |
| Experimental HFG | 339.9 ± 40.3 | 17.5 ± 73.8 | 21.5 ± 49.5 |
| CG | 418.7 ± 143.6 b | 41.8 ± 81.5 #,b | 3.1 ± 82.6 |
| Choice reaction time (ms) | |||
| Experimental LFG | 1061.5 ± 89.2 a | −117.4 ± 116.8 #,a | 61.8 ± 111.2 # |
| Experimental HFG | 800.8 ± 125.0 | 20.6 ± 95.6 | 7.1 ± 91.3 |
| CG | 916.4 ± 172.7 b | 99.5 ± 158.6 #,b | −53.1 ± 112.0 #,b |
| Items recognized correctly (n) | |||
| Experimental LFG | 73.8 ± 17.3 a | 16.4 ± 24.2 # | −3.2 ± 11.0 |
| Experimental HFG | 124.5 ± 19.9 | 15.8 ± 11.9 # | 0.4 ± 6.7 |
| CG | 95.5 ± 34.8 c | 0.5 ± 16.2 c | 3.9 ± 10.3 |
| Concentration index (n) | |||
| Experimental LFG | 65.9 ± 19.8 a | 16.1 ± 26.5 # | −3.1 ± 11.7 |
| Experimental HFG | 121.8 ± 21.5 | 18.6 ± 12.6 # | −0.5 ± 10.8 |
| CG | 89.8 ± 38.1 c | −0.6 ± 21.3 c | 6.8 ± 14.3 |
| Physical function | |||
| Lower-body strength (n) | |||
| Experimental LFG | 9.4 ± 1.1 a | 6.9 ± 3.5 # | −3.4 ± 1.7 # |
| Experimental HFG | 14.9 ± 2.3 | 3.8 ± 2.6 # | −4.1 ± 2.4 # |
| CG | 12.7 ± 3.2 b | −0.6 ± 2.1 b,c | −0.4 ± 2.2 b,c |
| Multidimensional balance (points) | |||
| Experimental LFG | 23.1 ± 2.1 a | 5.9 ± 2.1 # | −3.1 ± 1.7 # |
| Experimental HFG | 31.6 ± 2.5 | 3.3 ± 1.6 # | −1.9 ± 1.5 # |
| CG | 29.7 ± 3.2 b | −0.2 ± 1.8 b,c | −0.6 ± 1.4 b |
| Mobility (s) | |||
| Experimental LFG | 8.5 ± 1.1 a | −1.3 ± 1.0 #,a | 1.2 ± 1.0 # |
| Experimental HFG | 6.1 ± 0.6 | −0.3 ± 0.6 # | 0.6 ± 0.7 # |
| CG | 7.0 ± 1.5 b | 0.5 ± 0.9 #,b | 0.4 ± 1.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosado, H.; Bravo, J.; Raimundo, A.; Carvalho, J.; Pereira, C. Optimizing Cognitive and Physical Gains in Older Adults: Benefits of a Psychomotor Intervention Program Based on Functional Level. Medicina 2025, 61, 1584. https://doi.org/10.3390/medicina61091584
Rosado H, Bravo J, Raimundo A, Carvalho J, Pereira C. Optimizing Cognitive and Physical Gains in Older Adults: Benefits of a Psychomotor Intervention Program Based on Functional Level. Medicina. 2025; 61(9):1584. https://doi.org/10.3390/medicina61091584
Chicago/Turabian StyleRosado, Hugo, Jorge Bravo, Armando Raimundo, Joana Carvalho, and Catarina Pereira. 2025. "Optimizing Cognitive and Physical Gains in Older Adults: Benefits of a Psychomotor Intervention Program Based on Functional Level" Medicina 61, no. 9: 1584. https://doi.org/10.3390/medicina61091584
APA StyleRosado, H., Bravo, J., Raimundo, A., Carvalho, J., & Pereira, C. (2025). Optimizing Cognitive and Physical Gains in Older Adults: Benefits of a Psychomotor Intervention Program Based on Functional Level. Medicina, 61(9), 1584. https://doi.org/10.3390/medicina61091584







