Repurposing Metformin in Precision Oncology: Mechanistic Insights, Biomarker-Guided Strategies, and Translational Imperatives
Abstract
1. Introduction
1.1. Precision Oncology: A Paradigm Shift in Cancer Therapy
1.2. Metformin in Oncology: A Repurposed Candidate
1.3. The Role of Biomarker-Guided Strategies
1.4. Rationale and Scope of This Review
2. Methodology
3. Mechanistic Insights: Metformin’s Anticancer Action Beyond Glycemic Control
3.1. Targeting Cellular Metabolism
3.2. Modulation of Oncogenic Signaling Pathways
3.3. Influence on Cancer Stem Cell Plasticity and Mitochondrial Biogenesis
3.4. Reprogramming the Tumor Microenvironment
4. Preclinical Evidence Supporting Metformin’s Anticancer Effects
5. Clinical Evidence and Observational Studies
5.1. Epidemiological Studies
5.2. Retrospective Analyses
5.3. Prospective Clinical Trials
6. Biomarkers and Personalized Strategies in Metformin Oncology
6.1. Critical Evaluation of Biomarker Use
6.2. Ongoing and Emerging Biomarker-Driven Trials
6.3. Future Directions
7. Long-Term Effects and Safety of Metformin in Cancer Therapy
8. Ethical and Regulatory Challenges
8.1. Regulatory Pathways for Repurposing
8.2. Ethical Implications of Off-Label Use
8.3. Trial Design Complexity and Global Economic Considerations
8.4. Promoting Ethical Global Access
9. Challenges in Integrating Metformin into Oncology
9.1. Challenges and Future Directions in Metformin Integration
9.2. Multidisciplinary Imperatives
9.3. Call to Action: Stakeholder-Specific Priorities
10. Future Perspectives and Clinical Implications
- a
- The absence of biomarkers that guide patient enrollment;
- b
- Heterogeneity among tumor types and clinical endpoints;
- c
- Suboptimal dosing and pharmacokinetic adjustment in cancer care.
- a
- Target non-diabetic subjects as well as participants grouped by metabolic phenotypes;
- b
- Incorporate predictive biomarkers such as OCT1 genotypes and insulin metrics;
- c
- Define tumor-specific endpoints, including progression-free survival, recurrence rates, and quality-of-life measures;
- d
- Embed mechanistic correlative studies that confirm on-target biological effects.
10.1. Personalized and Precision-Based Strategies
- a
- Molecular tumor profiling;
- b
- Metabolic phenotyping;
- c
- Genetic biomarkers (such as OCT1 variants and PI3K/AMPK mutations);
- d
- Omics-guided patient stratification.
10.2. Novel Combinatorial Approaches
- a
- Chemotherapy: By imposing extra metabolic strain, it could render tumor cells more susceptible;
- b
- Radiotherapy: The drug inhibits mitochondrial function, reduces hypoxia, and thus enhances radiosensitivity;
- c
- Immunotherapy: Metformin appears to boost immune cell entry and shrink immunosuppressive populations;
- d
- Targeted therapies: It may work synergistically with drugs that block mTOR, IGF-1R, or angiogenesis.
10.3. Drug Repurposing as a Broader Paradigm
10.4. Clinical Trial Priorities
- a
- Non-diabetic study groups, in which mechanistic actions can be seen apart from blood sugar control;
- b
- Biomarker-driven cohorts, using molecular or metabolic signatures to predict who is likely to respond;
- c
- Tumor-specific endpoints, such as progression-free survival, rates of recurrence, and treatment-related harms;
- d
- Patient-reported metrics, including fatigue, appetite, and overall quality of life, to gauge wider clinical value;
- e
- Cross-disciplinary teamwork among oncologists, endocrinologists, molecular biologists, and trial designers will be vital to creating effective studies.
10.5. Implications for Clinical Practice
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherani, A.M.K.; Khan, M.; Qayyum, M.U.; Hussain, H.K. Synergizing AI and Healthcare: Pioneering advances in cancer medicine for personalized treatment. Int. J. Multidiscip. Sci. Arts 2024, 3, 270–277. [Google Scholar] [CrossRef]
- Dias-Santagata, D.; Heist, R.S.; Bard, A.Z.; da Silva, A.F.L.; Dagogo-Jack, I.; Nardi, V.; Ritterhouse, L.L.; Spring, L.M.; Jessop, N.; Farahani, A.A.; et al. Implementation and clinical adoption of precision oncology workflows across a healthcare network. Oncologist 2022, 27, 930–939. [Google Scholar] [CrossRef]
- Pezoulas, V.C.; Hazapis, O.; Lagopati, N.; Exarchos, T.P.; Goules, A.V.; Tzioufas, A.G.; Fotiadis, D.I.; Stratis, I.G.; Yannacopoulos, A.N.; Gorgoulis, V.G. Machine learning approaches on high throughput NGS data to unveil mechanisms of function in biology and disease. Cancer Genom. Proteom. 2021, 18, 605–626. [Google Scholar] [CrossRef]
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an anticancer agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, K.; Ren, Z.; Yin, D.; Zhou, Y. Metformin as anticancer agent and adjuvant in cancer combination therapy: Current progress and future prospect. Transl. Oncol. 2024, 44, 101945. [Google Scholar] [CrossRef]
- Dutta, S.; Shah, R.B.; Singhal, S.; Dutta, S.B.; Bansal, S.; Sinha, S.; Haque, M. Metformin: A review of potential mechanism and therapeutic utility beyond diabetes. Drug Des. Dev. Ther. 2023, 17, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K. Metformin: Activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front. Genet. 2022, 13, 1022739. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.B.; Rangan, A.S.; Govindaraj, V.K.A.; Aamir, S.; Thirugnanam, G.; Kalappan, M.K. Metformin and anti-cancer effects: Systematic insights into its therapeutic potential. Onkol. I Radioter. 2024, 18, 1–7. [Google Scholar]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is it a drug for all reasons and diseases? Metabolism 2022, 133, 155223. [Google Scholar] [CrossRef]
- Mascaraque-Checa, M.; Gallego-Rentero, M.; Nicolás-Morala, J.; Portillo-Esnaola, M.; Cuezva, J.M.; González, S.; Gilaberte, Y.; Juarranz, Á. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol. Metab. 2022, 60, 101496. [Google Scholar] [CrossRef]
- Amin, S.; Lux, A.; O’Callaghan, F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br. J. Clin. Pharmacol. 2019, 85, 37–46. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- Yi, Y.; Chen, D.; Ao, J.; Sun, S.; Wu, M.; Li, X.; Bergholz, J.; Zhang, Y.; Xiao, Z.-X. Metformin promotes AMP-activated protein kinase-independent suppression of ΔNp63α protein expression and inhibits cancer cell viability. J. Biol. Chem. 2017, 292, 5253–5261. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Sotgia, F.; Lisanti, M.P. Cancer stem cells (CSCs): Metabolic strategies for their identification and eradication. Biochem. J. 2018, 475, 1611–1634. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Griss, T.; Vincent, E.E.; Egnatchik, R.; Chen, J.; Ma, E.H.; Faubert, B.; Viollet, B.; DeBerardinis, R.J.; Jones, R.G. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 2015, 13, e1002309. [Google Scholar] [CrossRef]
- Dasgupta, A.; Trucco, M.; Rainusso, N.; Bernardi, R.J.; Shuck, R.; Kurenbekova, L.; Loeb, D.M.; Yustein, J.T. Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties. Oncotarget 2017, 8, 77292. [Google Scholar] [CrossRef]
- You, R.; Wang, B.; Chen, P.; Zheng, X.; Hou, D.; Wang, X.; Zhang, B.; Chen, L.; Li, D.; Lin, X. Metformin sensitizes AML cells to chemotherapy through blocking mitochondrial transfer from stromal cells to AML cells. Cancer Lett. 2022, 532, 215582. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Abdelmoneim, M.; Aboalela, M.A.; Naoe, Y.; Matsumura, S.; Eissa, I.R.; Bustos-Villalobos, I.; Sibal, P.A.; Takido, Y.; Kodera, Y.; Kasuya, H. The impact of metformin on tumor-infiltrated immune cells: Preclinical and clinical studies. Int. J. Mol. Sci. 2023, 24, 13353. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Berstein, L.M.; Popovich, I.G.; Zabezhinski, M.A.; Egormin, P.A.; Piskunova, T.S.; Semenchenko, A.V.; Tyndyk, M.L.; Yurova, M.N.; Kovalenko, I.G. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging 2011, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Chen, X.; Qiu, F.; Zhang, X.; Wu, F.; Zhao, Z.; Xu, M.; Chen, M.; Shen, J.-W.; Shen, Q. Correction: Regulation of cancer-associated fibroblasts for enhanced cancer immunotherapy using advanced functional nanomedicines: An updated review. J. Nanobiotechnol. 2025, 23, 269. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Park, B.-S.; Baek, H.-S.; Kang, H.-M.; Oh, J.-M.; Kim, I.-R. Metformin activates AMPK and mTOR to Inhibit RANKL-stimulated osteoclast formation. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8795–8811. [Google Scholar] [PubMed]
- Hua, Y.; Zheng, Y.; Yao, Y.; Jia, R.; Ge, S.; Zhuang, A. Metformin and cancer hallmarks: Shedding new lights on therapeutic repurposing. J. Transl. Med. 2023, 21, 403. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.H.Y.; Suissa, S. Metformin and cancer: Solutions to a real-world evidence failure. Diabetes Care 2023, 46, 904–912. [Google Scholar] [CrossRef]
- Mu, W.; Jiang, Y.; Liang, G.; Feng, Y.; Qu, F. Metformin: A promising antidiabetic medication for cancer treatment. Curr. Drug Targets 2023, 24, 41–54. [Google Scholar] [CrossRef]
- Sanati, M.; Aminyavari, S.; Mollazadeh, H.; Motamed-Sanaye, A.; Bibak, B.; Mohtashami, E.; Teng, Y.; Afshari, A.R.; Sahebkar, A. The potential therapeutic impact of metformin in glioblastoma multiforme. Curr. Med. Chem. 2023, 30, 857–877. [Google Scholar] [CrossRef]
- Puła, A.; Krzysiek, U.; Podgórska, K.; Gorczyca, K.; Artykiewicz, K.; Słupczyńska, A.; Urbaś, W.; Czarkowski, M.; Kozieł, P.; Grodkiewicz, M. Potential anti-cancer features of metformin. J. Educ. Health Sport 2023, 13, 136–145. [Google Scholar] [CrossRef]
- Mostafavi, S.; Zalpoor, H.; Hassan, Z.M. The promising therapeutic effects of metformin on metabolic reprogramming of cancer-associated fibroblasts in solid tumors. Cell. Mol. Biol. Lett. 2022, 27, 58. [Google Scholar] [CrossRef]
- Cirillo, F.; Scordamaglia, D.; Talia, M.; Santolla, M.F.; Muglia, L.; Zicarelli, A.; De Rosis, S.; Spinelli, A.; Giordano, F.; Miglietta, A.M. Mechanistic Insights on the Anticancer Effects of Metformin in Primary Breast Cancer Cells. Biol. Life Sci. Forum 2023, 21, 18. [Google Scholar] [CrossRef]
- Zhang, F.; Han, S.; Song, W. Anticancer effects of metformin in experimental animal models of different types of cancer: A systematic review and meta-analysis. Lab. Anim. Res. 2022, 38, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Li, H.; Wang, J. Mechanisms of metformin inhibiting cancer invasion and migration. Am. J. Transl. Res. 2020, 12, 4885. [Google Scholar]
- Hajimohammadebrahim-Ketabforoush, M.; Zali, A.; Shahmohammadi, M.; Hamidieh, A.A. Metformin and its potential influence on cell fate decision between apoptosis and senescence in cancer, with a special emphasis on glioblastoma. Front. Oncol. 2024, 14, 1455492. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yan, G.; Yang, L.; Kong, L.; Guan, Y.; Sun, H.; Liu, C.; Liu, L.; Han, Y.; Wang, X. Cancer chemoprevention: Signaling pathways and strategic approaches. Signal Transduct. Target. Ther. 2025, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.C.; Ferrara, A.; Achacoso, N.; Ehrlich, S.F.; Quesenberry, C.P., Jr.; Habel, L.A. A cohort study of metformin and colorectal cancer risk among patients with diabetes mellitus. Cancer Epidemiol. Biomark. Prev. 2018, 27, 525–530. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Sarker, M.M.R.; Zhou, J.-R.; Parhar, I. Metformin in colorectal cancer: Molecular mechanism, preclinical and clinical aspects. J. Exp. Clin. Cancer Res. 2019, 38, 491. [Google Scholar] [CrossRef]
- Anwar, M.A.; Abou Kheir, W.; Eid, S.; Fares, J.; Liu, X.; Eid, A.H.; Eid, A.A. Colorectal and prostate cancer risk in diabetes: Metformin, an actor behind the scene. J. Cancer 2014, 5, 736. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Guo, X.-L. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother. Pharmacol. 2016, 78, 13–26. [Google Scholar] [CrossRef]
- Kheirandish, M.; Mahboobi, H.; Yazdanparast, M.; Kamal, W.; Kamal, M.A. Anti-cancer effects of metformin: Recent evidences for its role in prevention and treatment of cancer. Curr. Drug Metab. 2018, 19, 793–797. [Google Scholar] [CrossRef]
- Chen, Y. Diabetes Medication Use and Risk of Breast, Colorectal, and Pancreatic Cancer: A Population-Based Cohort Study; University of British Columbia: Vancouver, BC, Canada, 2023. [Google Scholar]
- Najafi, F.; Rajati, F.; Sarokhani, D.; Bavandpour, M.; Moradinazar, M. The relationship between metformin consumption and cancer risk: An updated umbrella review of systematic reviews and meta-analyses. Int. J. Prev. Med. 2023, 14, 90. [Google Scholar] [CrossRef]
- Freedman, L.S.; Agay, N.; Farmer, R.; Murad, H.; Olmer, L.; Dankner, R. Metformin treatment among men with diabetes and the risk of prostate cancer: A population-based historical cohort study. Am. J. Epidemiol. 2022, 191, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Mayadunne, T.; Saadati, S.; Asmelash, D.; Mason, T.; Vanky, E.; Teede, H.; Mousa, A. Long-term effects of metformin on offspring health: A review of current evidence and future directions. Diabetes Obes. Metab. 2025, 27, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ao, H.; Guo, G.; Liu, M. The role and mechanism of metformin in inflammatory diseases. J. Inflamm. Res. 2023, 16, 5545–5564. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Castiglione, S.; Pavanello, C. Metformin: From diabetes to cancer to prolongation of life. Pharmacol. Res. 2024, 208, 107367. [Google Scholar] [CrossRef]
- Shi, P.; Liu, W.; Wang, H.; Li, F.; Zhang, H.; Wu, Y.; Kong, Y.; Zhou, Z.; Wang, C.; Chen, W. Metformin suppresses triple-negative breast cancer stem cells by targeting KLF5 for degradation. Cell Discov. 2017, 3, 17010. [Google Scholar] [CrossRef]
- Chen, B.; Cha, J.-H.; Yan, M.; Cao, N.; Ye, P.; Yan, X.; Yang, W.-H. ATXN7L3B promotes hepatocellular carcinoma stemness and is downregulated by metformin. Biochem. Biophys. Res. Commun. 2021, 573, 1–8. [Google Scholar] [CrossRef]
- Hatoum, D.; McGowan, E.M. Recent advances in the use of metformin: Can treating diabetes prevent breast cancer? BioMed Res. Int. 2015, 2015, 548436. [Google Scholar] [CrossRef]
- Martin-Castillo, B.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; Amillano, K.; Domínguez, S. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: The METTEN study. Oncotarget 2018, 9, 35687. [Google Scholar] [CrossRef]
- Gennari, A.; Foca, F.; Zamarchi, R.; Rocca, A.; Amadori, D.; De Censi, A.; Bologna, A.; Cavanna, L.; Gianni, L.; Scaltriti, L. Insulin-like growth factor-1 receptor (IGF-1R) expression on circulating tumor cells (CTCs) and metastatic breast cancer outcome: Results from the TransMYME trial. Breast Cancer Res. Treat. 2020, 181, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Wu, L.; Zhu, L.; Sun, Z.; Wang, G.; Pan, J.; Zheng, S.; Xu, K.; Du, J.; Jiang, H. Prediction of overall survival for metastatic pancreatic cancer: Development and validation of a prognostic nomogram with data from open clinical trial and real-world study. Cancer Med. 2018, 7, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-X.; Wu, W.-W.; Zhao, Y.-Y.; Xu, H.-L.; Chen, G.-C.; Jin, S.-Y.; Chen, J.; Xian, S.-X.; Liang, J.-H. Evidence from a meta-analysis and systematic review reveals the global prevalence of mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1227112. [Google Scholar] [CrossRef]
- Dickerman, B.A.; García-Albéniz, X.; Logan, R.W.; Denaxas, S.; Hernán, M.A. Evaluating metformin strategies for cancer prevention: A target trial emulation using electronic health records. Epidemiology 2023, 34, 690–699. [Google Scholar] [CrossRef]
- BM, M.B.; Kumar, A.; Batra, A.; Sharma, V.; Kataria, B.; Gogia, A.; Mathur, S.; Sharma, A. Metformin with Neoadjuvant Chemotherapy in Localized Triple Negative and HER2neu-Positive Breast Cancer: A Prospective Phase 2 Open Label Randomised Controlled Trial (McBETH); American Society of Clinical Oncology: Alexandria, VA, USA, 2023. [Google Scholar]
- Papadakos, S.P.; Ferraro, D.; Carbone, G.; Frampton, A.E.; Vennarecci, G.; Kykalos, S.; Schizas, D.; Theocharis, S.; Machairas, N. The emerging role of metformin in the treatment of hepatocellular carcinoma: Is there any value in repurposing metformin for HCC immunotherapy? Cancers 2023, 15, 3161. [Google Scholar] [CrossRef]
- Serageldin, M.A.; Kassem, A.B.; El-Kerm, Y.; Helmy, M.W.; El-Mas, M.M.; El-Bassiouny, N.A. The effect of metformin on chemotherapy-induced toxicities in non-diabetic breast cancer patients: A randomised controlled study. Drug Saf. 2023, 46, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Kicinski, M.; Valpione, S.; Gandini, S.; Suciu, S.; Blank, C.U.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M. Prognostic and predictive value of metformin in the European Organisation for Research and Treatment of Cancer 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur. J. Cancer 2023, 189, 112900. [Google Scholar] [CrossRef]
- Wen, J.; Yi, Z.; Chen, Y.; Huang, J.; Mao, X.; Zhang, L.; Zeng, Y.; Cheng, Q.; Ye, W.; Liu, Z. Efficacy of metformin therapy in patients with cancer: A meta-analysis of 22 randomised controlled trials. BMC Med. 2022, 20, 402. [Google Scholar] [CrossRef]
- Bilusic, M.; Toney, N.J.; Donahue, R.N.; Karzai, F.; Madan, R.A.; Schlom, J.; Gulley, J.L.; Rai, P.; Due, C.; Gregory, P. (Eds.) Metformin for Biochemical Recurrence of Prostate Cancer: Immune Data from a Phase 2 Study; American Society of Clinical Oncology: Alexandria, VA, USA, 2023. [Google Scholar]
- Essa, N.M.; Salem, H.F.; Elgendy, M.O.; Gabr, A.; Omran, M.M.; Hassan, N.A.; Tashkandi, H.M.; Harakeh, S.; Boshra, M.S. Efficacy of metformin as adjuvant therapy in metastatic breast cancer treatment. J. Clin. Med. 2022, 11, 5505. [Google Scholar] [CrossRef]
- Barrios-Bernal, P.; Zatarain-Barrón, Z.L.; Hernández-Pedro, N.; Orozco-Morales, M.; Olivera-Ramírez, A.; Ávila-Moreno, F.; Colín-González, A.L.; Cardona, A.F.; Rosell, R.; Arrieta, O. Will we unlock the benefit of metformin for patients with lung cancer? Lessons from current evidence and new hypotheses. Pharmaceuticals 2022, 15, 786. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Acosta, R.; Vintea, I.; Koeken, I.; Hassannia, B.; Vanden Berghe, T. Harnessing ferroptosis for precision oncology: Challenges and prospects. BMC Biol. 2025, 23, 57. [Google Scholar] [CrossRef]
- Andrzejewski, S.; Siegel, P.M.; St-Pierre, J. Metabolic profiles associated with metformin efficacy in cancer. Front. Endocrinol. 2018, 9, 372. [Google Scholar] [CrossRef]
- Udumula, M.P.; Poisson, L.M.; Dutta, I.; Tiwari, N.; Kim, S.; Chinna-Shankar, J.; Allo, G.; Sakr, S.; Hijaz, M.; Munkarah, A.R. Divergent metabolic effects of metformin merge to enhance eicosapentaenoic acid metabolism and inhibit ovarian cancer in vivo. Cancers 2022, 14, 1504. [Google Scholar] [CrossRef]
- Ayoub, R.; Ruddy, R.M.; Cox, E.; Oyefiade, A.; Derkach, D.; Laughlin, S.; Ades-Aron, B.; Shirzadi, Z.; Fieremans, E.; MacIntosh, B.J. Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin. Nat. Med. 2020, 26, 1285–1294. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, S.-Y.; Sun, T. Pharmacogenomic studies of current antidiabetic agents and potential new drug targets for precision medicine of diabetes. Diabetes Ther. 2020, 11, 2521–2538. [Google Scholar] [CrossRef]
- Xu, H.; Chen, K.; Jia, X.; Tian, Y.; Dai, Y.; Li, D.; Xie, J.; Tao, M.; Mao, Y. Metformin use is associated with better survival of breast cancer patients with diabetes: A meta-analysis. Oncologist 2015, 20, 1236–1244. [Google Scholar] [CrossRef]
- Tulipano, G. Integrated or independent actions of metformin in target tissues underlying its current use and new possible applications in the endocrine and metabolic disorder area. Int. J. Mol. Sci. 2021, 22, 13068. [Google Scholar] [CrossRef]
- Diniz, M.d.F.H.S.; Beleigoli, A.M.R.; Schmidt, M.I.; Duncan, B.B.; Ribeiro, A.L.P.; Vidigal, P.G.; Benseñor, I.M.; Lotufo, P.A.; Santos, I.S.; Griep, R.H. Homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic syndrome at baseline of a multicentric Brazilian cohort: ELSA-Brasil study. Cad. Saude Publica 2020, 36, e00072120. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Sourlas, A.; Oikonomakis, K.; Zoumi, E.-A.; Papadimitriou, A.; Kostara, C.E. Biomarkers of insulin sensitivity/resistance. J. Int. Med. Res. 2024, 52, 03000605241285550. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients with Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lupini, L.; Scutiero, G.; Iannone, P.; Martinello, R.; Bassi, C.; Ravaioli, N.; Soave, I.; Bonaccorsi, G.; Lanza, G.; Gafà, R. Molecular biomarkers predicting early development of endometrial carcinoma: A pilot study. Eur. J. Cancer Care 2019, 28, e13137. [Google Scholar] [CrossRef]
- Mehta, R.; Frakes, J.; Kim, J.; Nixon, A.; Liu, Y.; Howard, L.; Martinez Jimenez, M.E.; Carballido, E.; Imanirad, I.; Sanchez, J. Phase I study of lenvatinib and capecitabine with external radiation therapy in locally advanced rectal adenocarcinoma. Oncologist 2022, 27, 621–e617. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Cao, L.; Yin, Y.; Shen, Y.; Zhu, W. Prognostic value of metformin in cancers: An updated meta-analysis based on 80 cohort studies. Medicine 2022, 101, e31799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Khin, N.A.; Grandinetti, C.; Dixey, H.; Yu, B.; Skeete, R.; Ayalew, K.; Budwal-Jagait, M.; Cho, S.J.; Dasgupta, A.; Fisher, A. Tackling challenging data integrity topics in 2020: Update on good clinical practice perspectives from the US FDA and MHRA UK. Clin. Pharmacol. Ther. 2022, 112, 31–43. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef]
- Saengboonmee, C.; Sanlung, T.; Wongkham, S. Repurposing metformin for cancer treatment: A great challenge of a promising drug. Anticancer. Res. 2021, 41, 5913–5918. [Google Scholar] [CrossRef]
- Nicotera, G.; Sferrazza, G.; Serafino, A.; Pierimarchi, P. The iterative development of medicines through the European medicine agency’s adaptive pathway approach. Front. Med. 2019, 6, 148. [Google Scholar] [CrossRef]
- Wu, H.; Huang, D.; Zhou, H.; Sima, X.; Wu, Z.; Sun, Y.; Wang, L.; Ruan, Y.; Wu, Q.; Wu, F. Metformin: A promising drug for human cancers. Oncol. Lett. 2022, 24, 204. [Google Scholar] [CrossRef]
- Hanchard, M.S. Debates over orphan drug pricing: A meta-narrative literature review. Orphanet J. Rare Dis. 2025, 20, 107. [Google Scholar] [CrossRef]
- Cramer, A.; Sørup, F.K.H.; Christensen, H.R.; Petersen, T.S.; Karstoft, K. Cancer drug applications to the EMA and the FDA: A comparison of new drugs and extension of indication in terms of approval decisions and time in review. Br. J. Clin. Pharmacol. 2025, 91, 1431–1438. [Google Scholar] [CrossRef]
- Grieselhuber, N.R.; Kodner, I.J.; Brown, D.; Yu, J. Confronting the therapeutic misconception. Surgery 2017, 162, 183–187. [Google Scholar] [CrossRef]
- Christopher, P.P.; Appelbaum, P.S.; Truong, D.; Albert, K.; Maranda, L.; Lidz, C. Reducing therapeutic misconception: A randomized intervention trial in hypothetical clinical trials. PLoS ONE 2017, 12, e0184224. [Google Scholar] [CrossRef]
- Rich, B.A. Off-label prescribing: In search of a reasonable patient-centered approach. J. Pain Palliat. Care Pharmacother. 2012, 26, 131–133. [Google Scholar] [CrossRef]
- Shore, C.; Hinners, J.; Khandekar, E.; Wizemann, T. Reflections on Sharing Clinical Trial Data: Challenges and a Way Forward: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Grumbling, E. Privacy Research and Best Practices: Summary of a Workshop for the Intelligence Community; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Getz, K.A.; Campo, R.A. Trial watch: Trends in clinical trial design complexity. Nat. Rev. Drug Discov. 2017, 16, 307–308. [Google Scholar] [CrossRef]
- Gumber, L.; Agbeleye, O.; Inskip, A.; Fairbairn, R.; Still, M.; Ouma, L.; Lozano-Kuehne, J.; Bardgett, M.; Isaacs, J.D.; Wason, J.M. Operational complexities in international clinical trials: A systematic review of challenges and proposed solutions. BMJ Open 2024, 14, e077132. [Google Scholar] [CrossRef] [PubMed]
- Daniels, N.; del Pilar Guzmán Urrea, M.; Rentmeester, C.A.; Kotchian, S.A.; Fontaine, S.; Hernández-Aguado, I.; Lumbreras, B.; Blacksher, E.; Goold, S.D.; Gómez, M.I. Resource allocation and priority setting. In Public Health Ethics: Cases Spanning the Globe; Springer: Berlin/Heidelberg, Germany, 2016; pp. 61–94. [Google Scholar]
- Herman, W.H.; Ratner, R.E. Metformin should be used to treat prediabetes in selected individuals. Diabetes Care 2020, 43, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Karamzad, N.; Kaufman, J.S.; Bell, A.W.; Nejadghaderi, S.A.; Sullman, M.J.; Moradi-Lakeh, M.; Collins, G.; Kolahi, A.-A. Prevalence, deaths and disability-adjusted-life-years (DALYs) due to type 2 diabetes and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the global burden of disease study 2019. Front. Endocrinol. 2022, 13, 838027. [Google Scholar] [CrossRef] [PubMed]
- Gharaee, H.; Tabrizi, J.S.; Azami-Aghdash, S.; Farahbakhsh, M.; Karamouz, M.; Nosratnejad, S. Analysis of public-private partnership in providing primary health care policy: An experience from Iran. J. Prim. Care Community Health 2019, 10, 2150132719881507. [Google Scholar] [CrossRef]
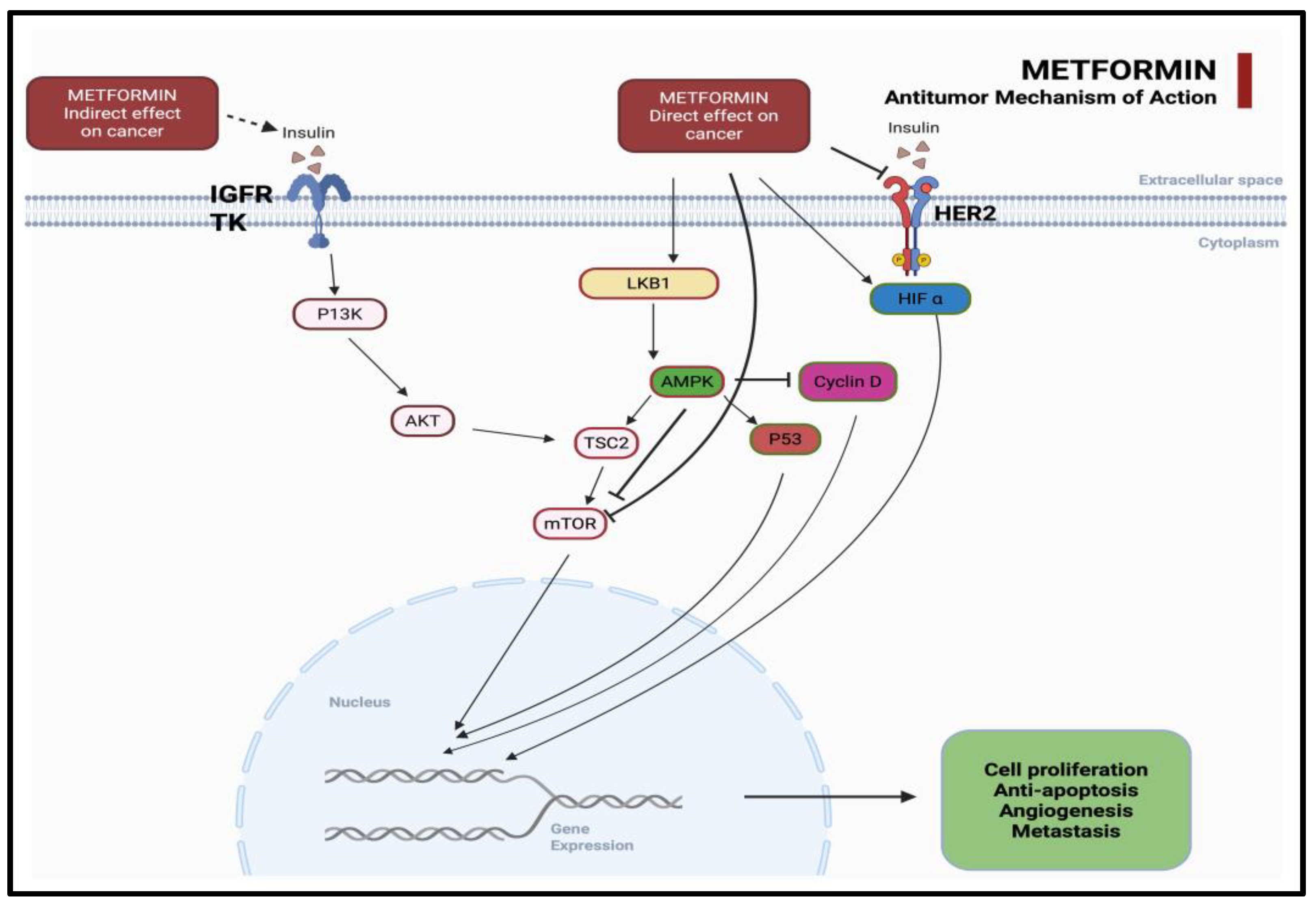
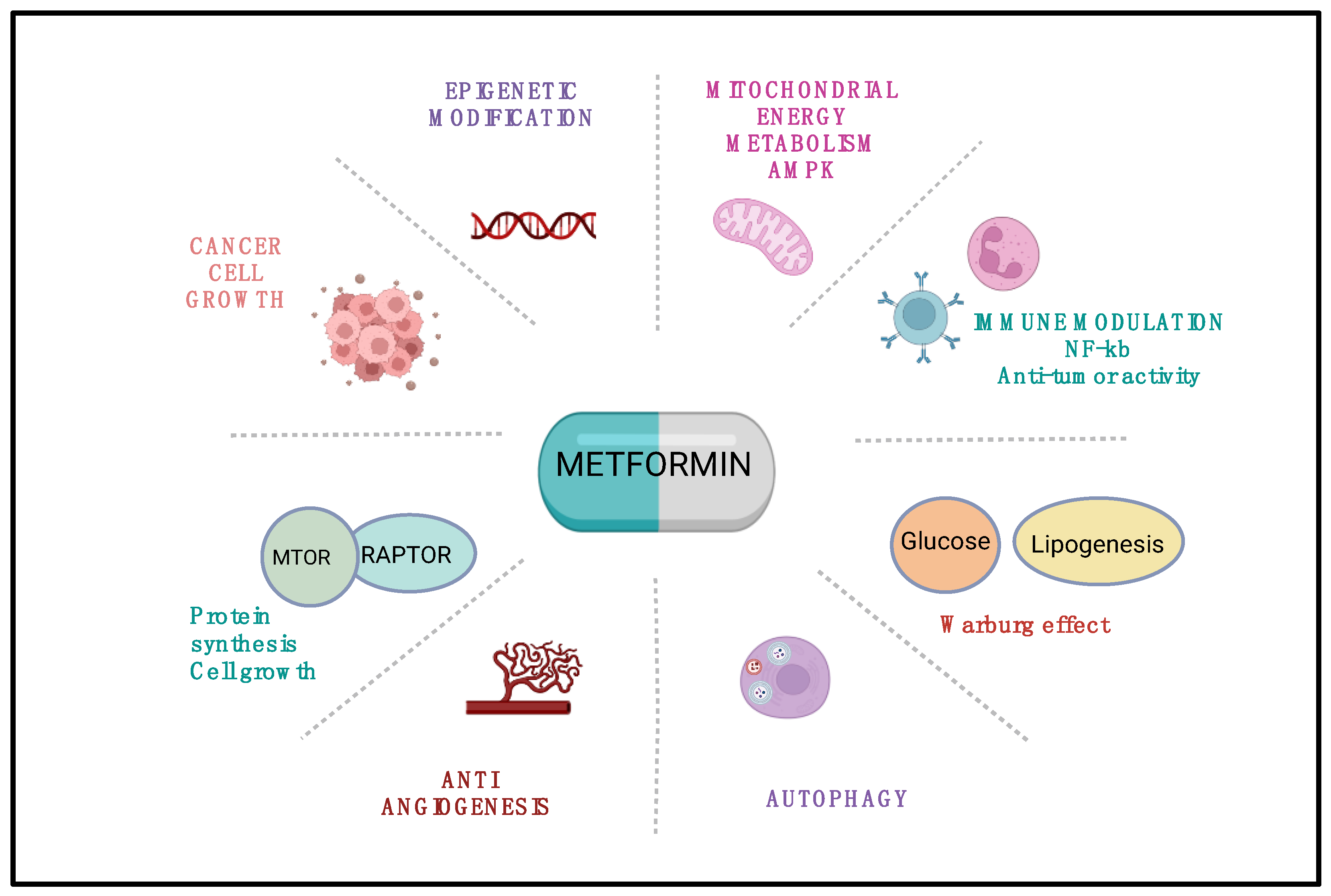
| Canonical Pathways (AMPK-Dependent) | Non-Canonical Pathways (AMPK-Independent) |
|---|---|
| Activation of AMPK (energy stress sensor) | Inhibition of mitochondrial complex I |
| Inhibition of mTOR signaling → ↓ protein synthesis, cell growth | Altered NAD+/NADH ratio and redox status |
| Inhibition of hepatic gluconeogenesis → ↓ insulin/IGF-1 signaling | Modulation of microRNA expression |
| Suppression of cancer cell proliferation via cell cycle arrest | Disruption of cancer stem cell dynamics |
| Enhanced immune response via T cell activation and PD-L1 downregulation | Epigenetic remodeling and chromatin accessibility changes |
| Study Reference | Category | Cancer Type | Key Findings |
|---|---|---|---|
| Hua et al. [27] | In Vitro Studies | Various | Metformin modulates cancer hallmarks via AMPK activation and insulin pathway inhibition. |
| Yu et al. [28] | In Vitro Studies | Various | Observational studies showed benefit; RCTs did not confirm reduction in cancer outcomes. |
| Mu et al. [29] | In Vitro Studies | Various | Activated AMPK, inhibited proliferation, and promoted apoptosis in cancer cells. |
| Sanati et al. [30] | In Vitro Studies | Glioblastoma multiforme | Reviewed metformin’s anticancer potential against GBM. |
| Puła et al. [31] | In Vitro Studies | Various | Suggested preventive and therapeutic potential of metformin in neoplasms. |
| Mostafavi et al. [32] | In Vivo Studies | Solid tumors | Metformin reprograms CAFs and impairs tumor-supportive environment. |
| Cirillo et al. [33] | Mechanistic Studies | Breast | Metformin inhibits the PI3K/AKT pathway and metastasis-related CXCR4 expression. |
| Zhang et al. [34] | Mechanistic Studies | Multiple | Meta-analysis confirmed tumor burden reduction across cancer types. |
| Reference | Study Type | Cancer Type | Key Findings | Limitations | Clinical Implications |
|---|---|---|---|---|---|
| Dickerman et al. [56] | Target trial emulation | Multiple cancers | No significant effect on cancer incidence | Observational design constraints | Limited role in prevention |
| Kumar et al. [57] | RCT (neoadjuvant chemo + metformin) | Breast (HER2+, TNBC) | Higher pathological response rates with metformin | Small sample, open-label | Possible synergy with chemotherapy |
| Papadakos et al. [58] | Preclinical + early clinical | Hepatocellular carcinoma | Suggests benefit with immunotherapy | Lack of standardized dosing | Potential role in HCC; needs further trials |
| Kassem et al. [59] | RCT (non-diabetic breast cancer) | Breast | Reduced chemotherapy-related toxicities (neuropathy, mucositis, fatigue) | Open-label, no biomarker validation | Supportive role to reduce toxicity |
| Kennedy et al. [60] | RCT (pembrolizumab ± metformin) | Melanoma | No survival benefit observed | Small metformin subgroup | Limited effect in immunotherapy setting |
| Wen et al. [61] | Meta-analysis (22 RCTs) | Multiple cancers | No OS benefit; modest PFS benefit in reproductive cancers | Heterogeneity across trials | Benefits may be tumor-type-specific |
| Biomarker Type | Details | Examples | Predictive Utility | Limitations |
|---|---|---|---|---|
| Genetic | Influence metformin transport, metabolism, and pathway interaction | OCT1 (SLC22A1) polymorphisms, PI3K/AKT/mTOR mutations, PTEN loss, AMPK SNPs | Predict drug uptake; insulin pathway sensitivity | Inconsistent translation across tumor types; limited specificity; transporter expression varies by tissue and tumor context |
| Metabolic | Reflect systemic/tumor metabolic state and insulin sensitivity | Fasting insulin, glucose, HOMA-IR, metabolomic signatures | May indicate response to metabolic stress or AMPK activation | Confounded by comorbidities (e.g., obesity, T2DM); not tumor-specific |
| Imaging | Monitor real-time metabolic/structural tumor adaptation | 18F-FDG PET, DCE-MRI, diffusion-weighted MRI | Non-invasive, dynamic assessment | Variability in resolution and interpretation; lacks mechanistic depth |
| Multi-Omics | Layered profiling for comprehensive tumor biology | Genomic + transcriptomic + proteomic + metabolomic integration | Improves patient stratification and therapeutic targeting | High cost; complex data interpretation; not yet standardized |
| Domain | Key Findings | Evidence Level | Remarks | Key Reference |
|---|---|---|---|---|
| Overall Survival (OS) | Improved OS in metformin users across cancers (e.g., breast, lung, prostate); reduced cancer-related mortality in diabetics | Moderate (meta-analyses, observational studies) | Needs validation from randomized controlled trials (RCTs) | Yang J et al. [77] |
| Safety Profile | Generally well tolerated; GI side effects most common; rare lactic acidosis in high-risk patients | High (clinical practice data) | Risk stratification essential for non-diabetic populations | UK NICE Guidelines [78] |
| Contraindications | eGFR < 30 mL/min/1.73 m2; severe hepatic or cardiac dysfunction; respiratory failure; hypersensitivity history | High (established clinical guidelines) | Pretreatment screening is mandatory | UK MHRA [79] |
| Drug Interactions | May interact with chemotherapy and immunotherapy; potential for altered pharmacokinetics and immune-related side effects | Moderate (emerging data from clinical settings) | Monitor closely when combined with novel agents | Heckman-Stoddard BM et al. [80] |
| Challenge | Description | Proposed Solution |
|---|---|---|
| Patient Heterogeneity | Variability in tumor type, disease stage, metabolic status, and genetic background affects response to metformin. | Adopt biomarker-guided, stratified trial designs to identify responsive subgroups. |
| Tumor Type and Biological Complexity | Efficacy varies across cancers due to differing oncogenic drivers and metabolic profiles. | Conduct tumor-specific studies and mechanistic research to define indications. |
| Biological Redundancy and Resistance Mechanisms | Cancer cells may bypass AMPK/mTOR inhibition via alternate metabolic or signaling pathways. | Design combination regimens that target compensatory mechanisms. |
| Pharmacokinetic and Dosing Variability | Standard antidiabetic dosing may not ensure therapeutic levels for antitumor effects. | Perform oncology-specific pharmacokinetic and dose optimization studies. |
| Drug Interactions and Treatment Integration | Metformin may alter efficacy or toxicity profiles of concurrent chemotherapy or immunotherapy. | Design rational combination trials with integrated pharmacovigilance. |
| Lack of Dedicated Oncology Trials | Most data are derived from retrospective or diabetic cohorts, limiting applicability. | Launch well-powered RCTs in non-diabetic patients with tumor-specific endpoints. |
| Stakeholder | Priority Actions |
|---|---|
| Researchers | Establish reliable biological markers that reliably predict patient sensitivity to metformin. Pursue laboratory investigations using cancer stem cell models and platforms that integrate immunometabolic pathways. Systematically test whether metformin enhances the efficacy of paired modalities such as immune checkpoint inhibitors or focused radiotherapy. |
| Clinicians | Clinicians should exercise restraint when prescribing off-label, favoring indications supported by rigorous clinical trials. Biomarker assessment, when accessible, can direct therapy toward the patients most likely to benefit. In non-diabetic cohorts, vigilant monitoring of metabolic indices and known contraindications remains essential to prevent avoidable harm. |
| Regulators and Policymakers | Encourage the use of adaptable regulatory routes such as the FDA 505(b)(2) pathway and the EMA’s Adaptive Pathways. Increase public–private funding for studies that test old drugs in new settings. Weave QALY and DALY metrics into pricing and access rules from the start. |
| Global Health Stakeholders | Strengthen fair access in low- and middle-income countries by using pooled purchasing. Include these nations early on when drafting clinical protocols. Prevent misuse by banning off-label applications that lack proper review. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.S.; Rangraze, I.R.; Wali, A.F.; Jhancy, M.; Attia, R.A.; Elshamly, H.A.H.; Adam, S.; Elbeshbeishy, R.A.M. Repurposing Metformin in Precision Oncology: Mechanistic Insights, Biomarker-Guided Strategies, and Translational Imperatives. Medicina 2025, 61, 1577. https://doi.org/10.3390/medicina61091577
Khan SS, Rangraze IR, Wali AF, Jhancy M, Attia RA, Elshamly HAH, Adam S, Elbeshbeishy RAM. Repurposing Metformin in Precision Oncology: Mechanistic Insights, Biomarker-Guided Strategies, and Translational Imperatives. Medicina. 2025; 61(9):1577. https://doi.org/10.3390/medicina61091577
Chicago/Turabian StyleKhan, Shehla Shafi, Imran Rashid Rangraze, Adil Farooq Wali, Malay Jhancy, Rasha Aziz Attia, Hesham Amin Hamdy Elshamly, Shukri Adam, and Rana Aly Mohamed Elbeshbeishy. 2025. "Repurposing Metformin in Precision Oncology: Mechanistic Insights, Biomarker-Guided Strategies, and Translational Imperatives" Medicina 61, no. 9: 1577. https://doi.org/10.3390/medicina61091577
APA StyleKhan, S. S., Rangraze, I. R., Wali, A. F., Jhancy, M., Attia, R. A., Elshamly, H. A. H., Adam, S., & Elbeshbeishy, R. A. M. (2025). Repurposing Metformin in Precision Oncology: Mechanistic Insights, Biomarker-Guided Strategies, and Translational Imperatives. Medicina, 61(9), 1577. https://doi.org/10.3390/medicina61091577





