An Update on Cutaneous Metastases of Internal Malignancies
Abstract
1. Introduction
2. Materials and Methods
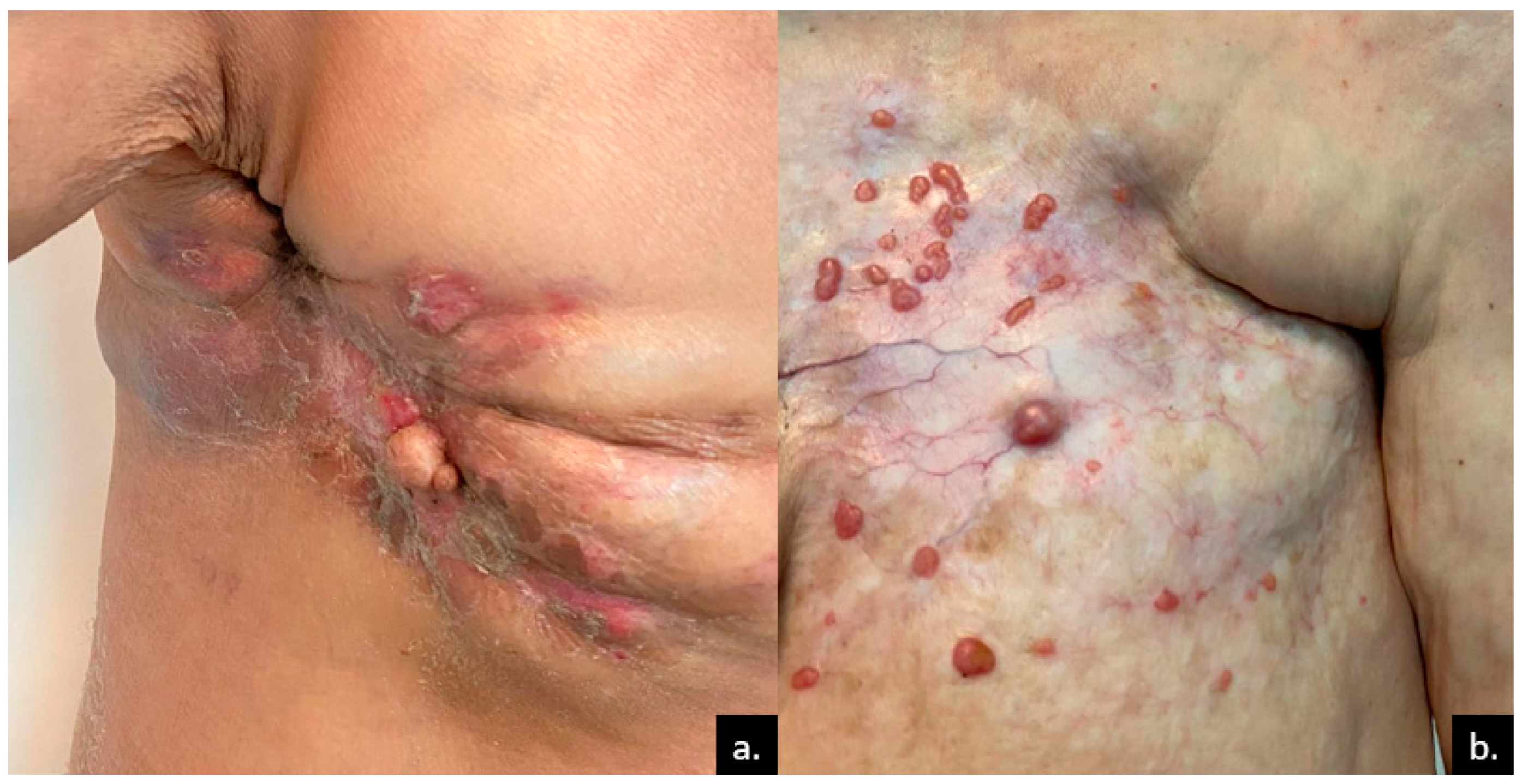
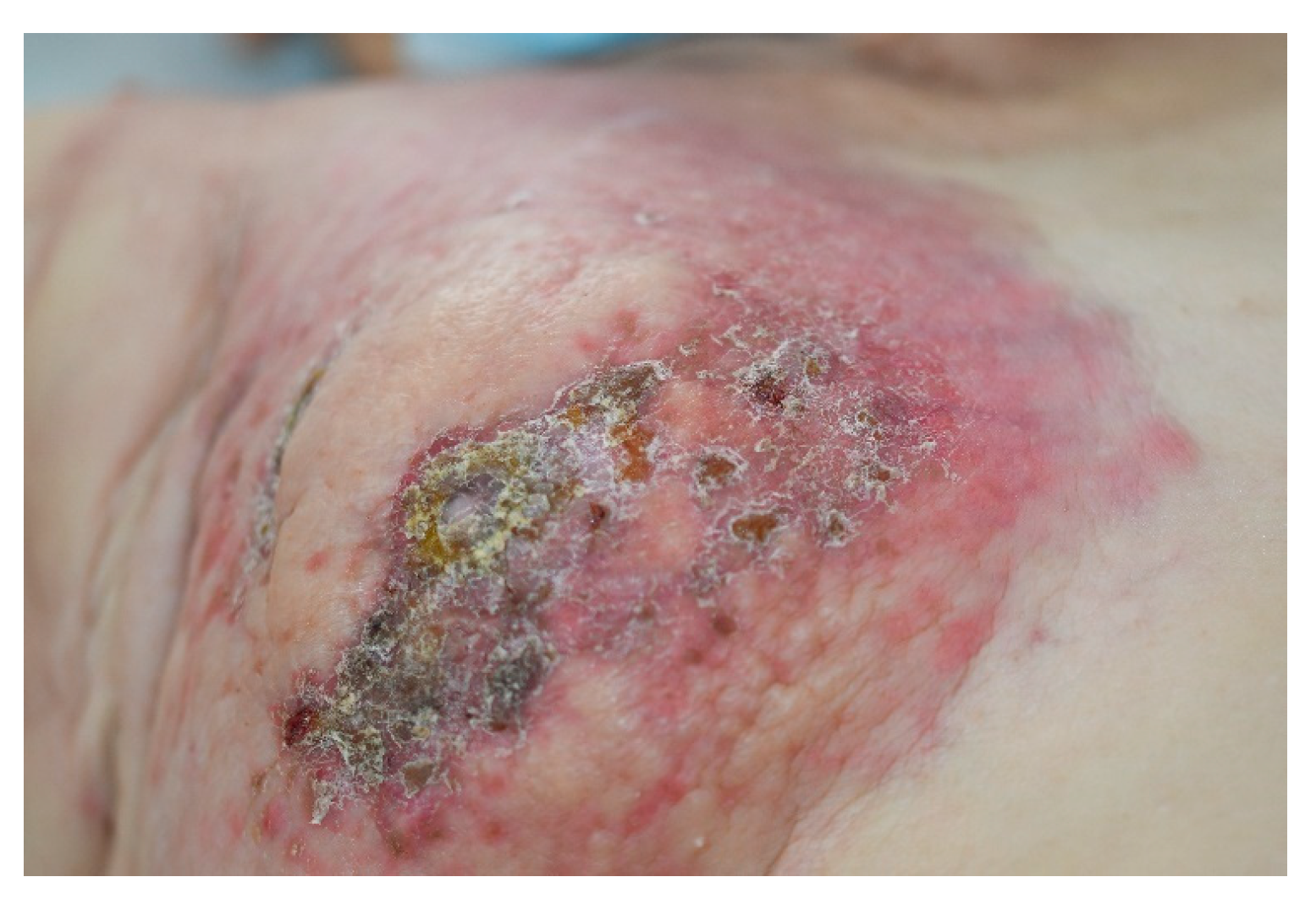
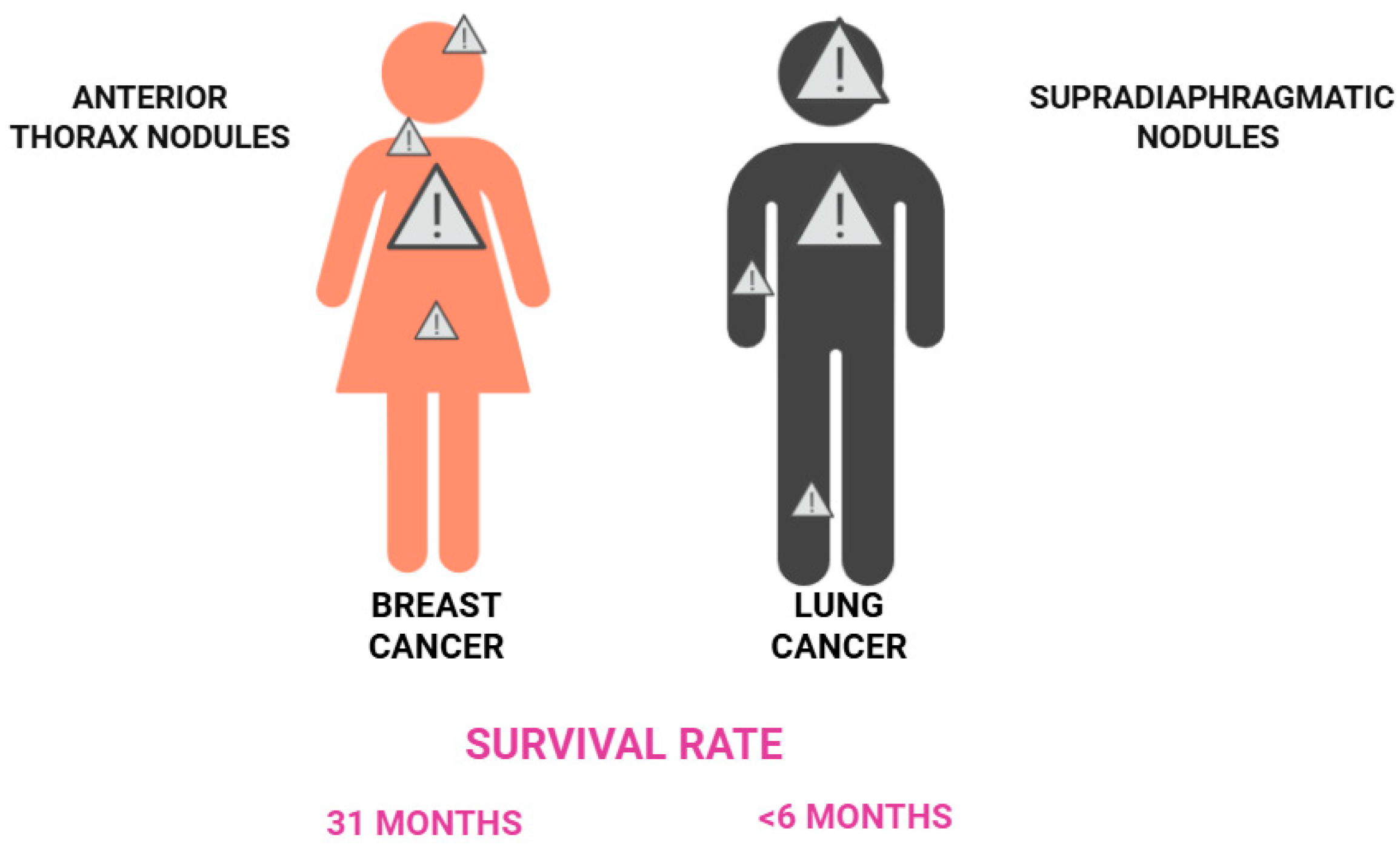
3. Cutaneous Metastases of Breast Cancer
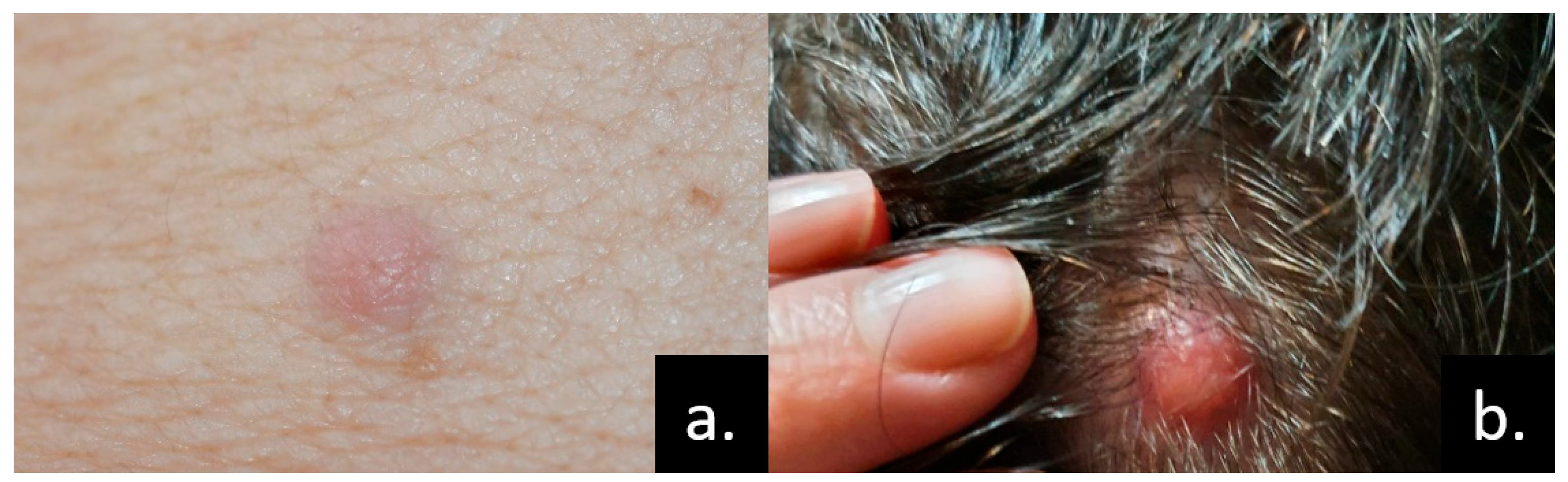
4. Skin Metastases of Lung and Pleural Cancers
4.1. Lung Cancer
4.2. Pleural Cancer (Mesothelioma)
5. Skin Metastases of Gastrointestinal Cancers
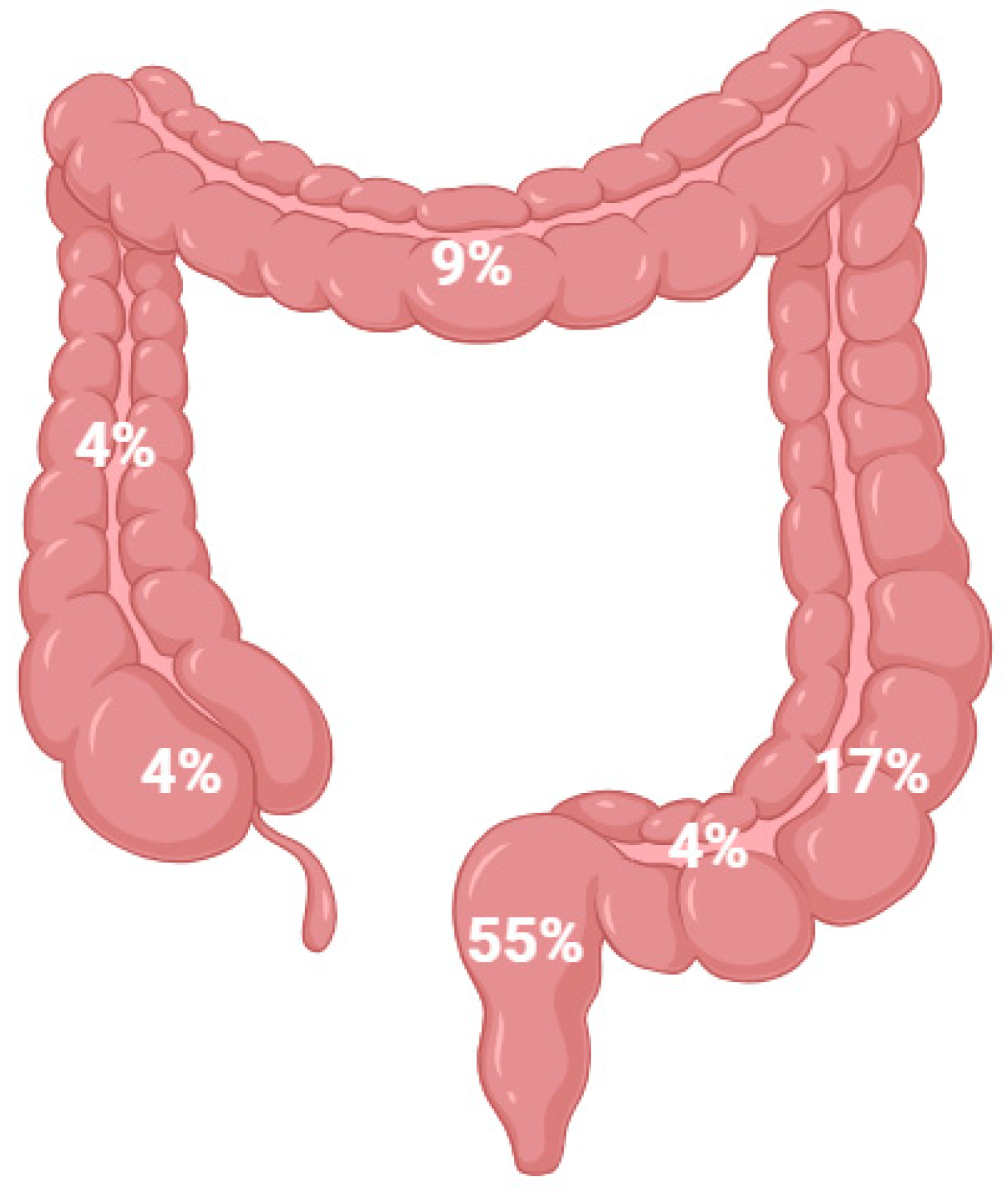
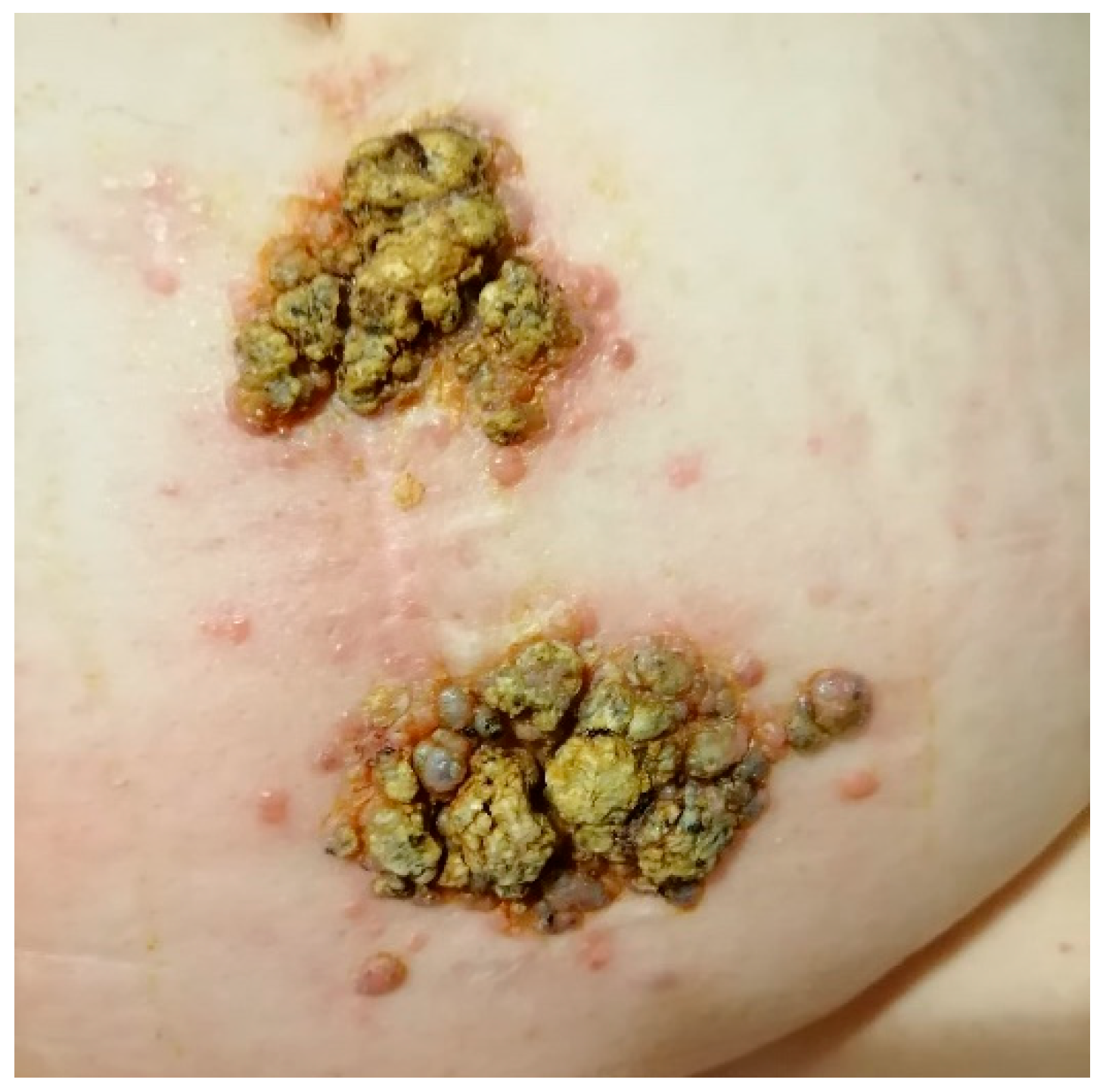
5.1. Colorectal Cancer
5.2. Stomach Cancer
5.3. Pancreatic Cancer
5.4. Liver Cancer
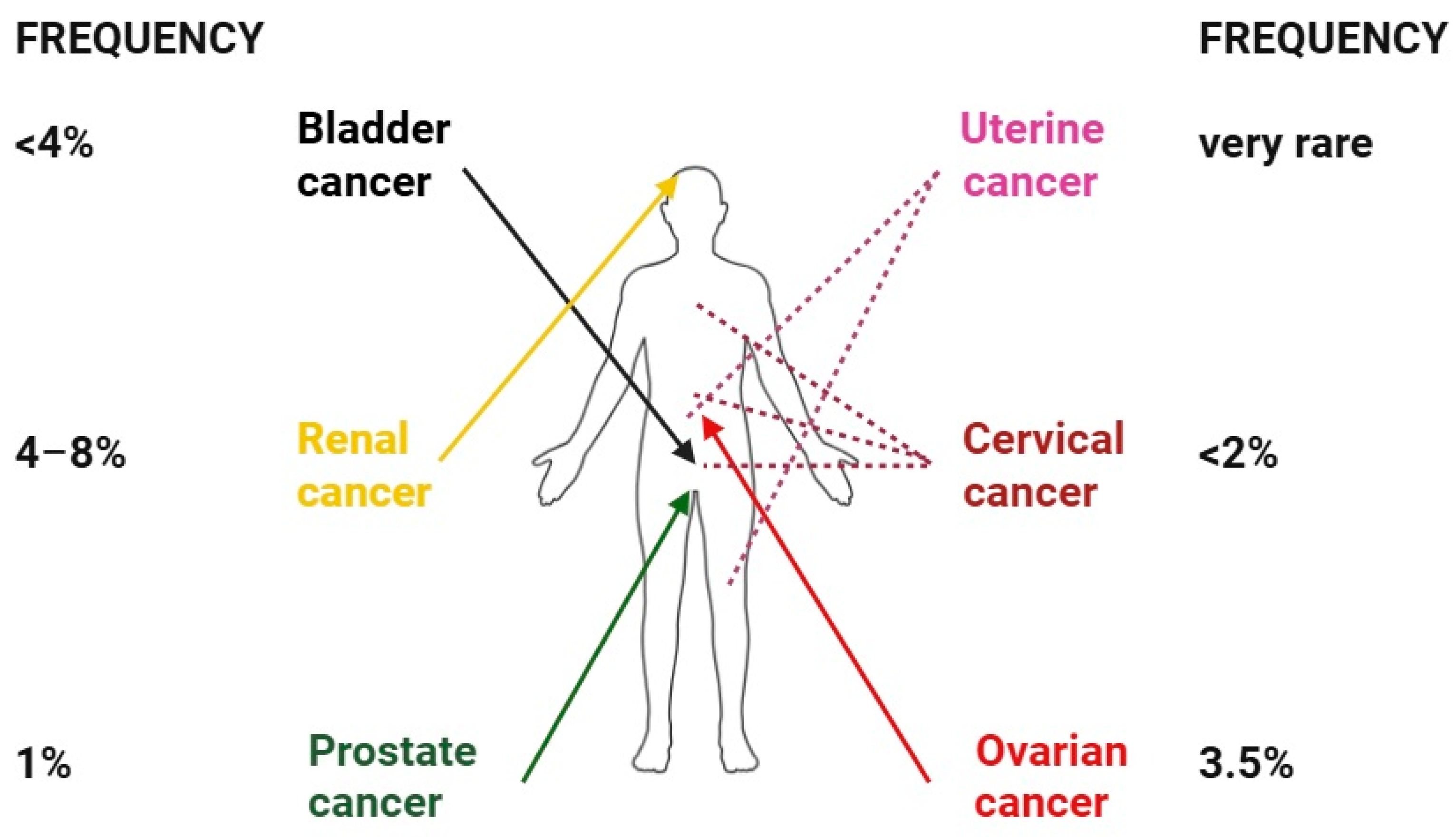
6. Skin Metastases of Renal and Urogenital Cancers
6.1. Bladder Cancer
6.2. Prostate Cancer
6.3. Ovarian Cancer
6.4. Uterine Cancer
6.5. Cervical Cancer
6.6. Renal Cancer
7. Skin Metastases of Head and Neck Cancers
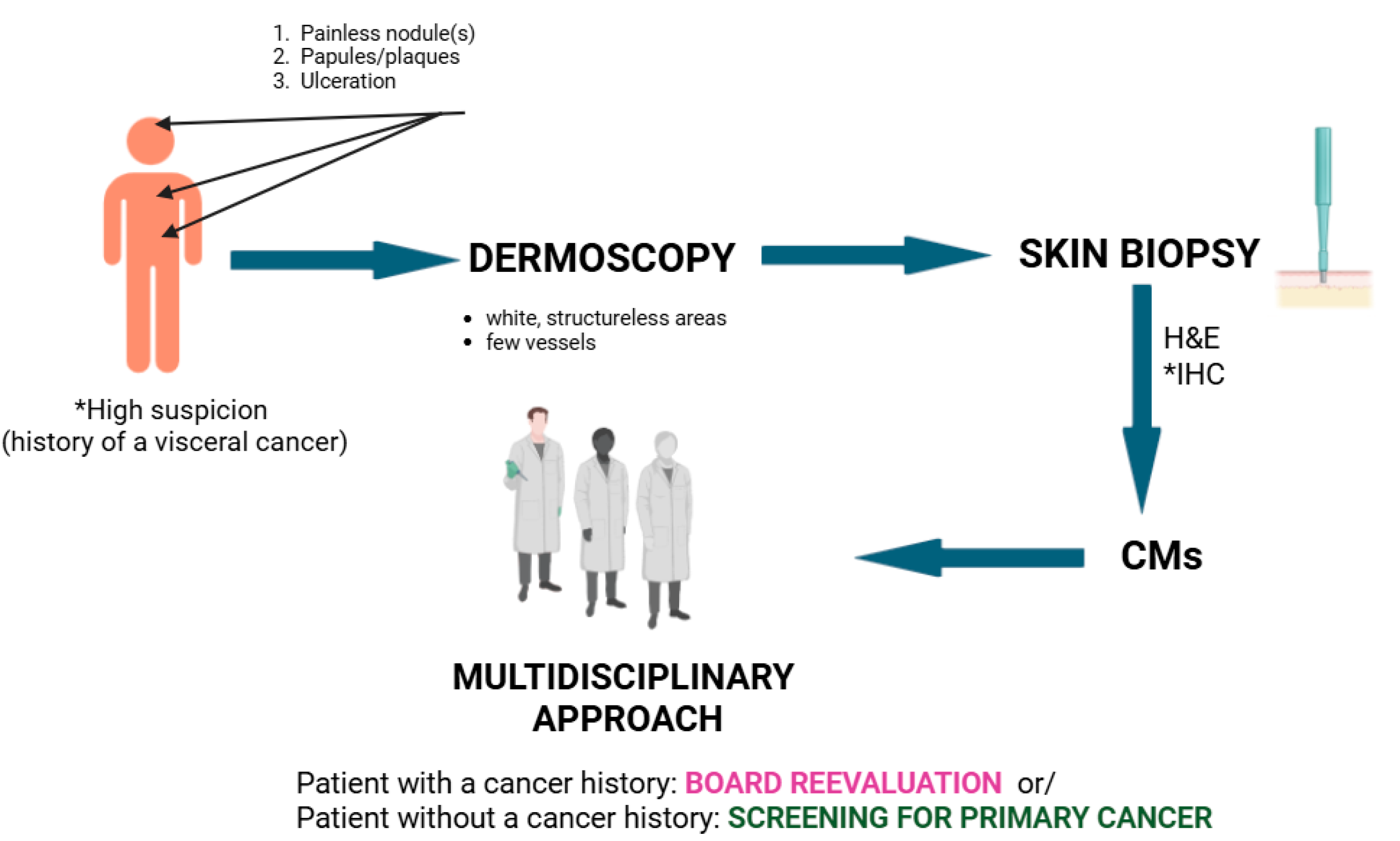
8. Diagnostic Challenges and Differentials
9. Current Treatment Advances
10. Novel Systemic Therapies
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Antón Martínez, M.C.; Parra-Blanco, V.; Avilés Izquierdo, J.A.; Suárez Fernández, R.M. Cutaneous metastases of internal tumors. Actas Dermosifiliogr. 2013, 104, 841–853, (In English and Spanish). [Google Scholar] [CrossRef]
- Sleeman, J.P.; Nazarenko, I.; Thiele, W. Do all roads lead to Rome? Routes to metastasis development. Int. J. Cancer 2011, 128, 2511–2526. [Google Scholar] [CrossRef]
- Hu, S.C.; Chen, G.S.; Lu, Y.W.; Wu, C.S.; Lan, C.C. Cutaneous metastases from different internal malignancies: A clinical and prognostic appraisal. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 735–740. [Google Scholar] [CrossRef]
- Simionescu, O.; Petrică, M.; Avram, A.M.; Costache, M.; Scurtu, L.G.; Tudorache, S.I.; Iorga, P.G.; Grigore, M. Dermoscopy of skin metastases in advanced cancer-systemic (visceral, hematologic) and cutaneous. Front. Med. 2024, 11, 1445811. [Google Scholar] [CrossRef]
- Strickley, J.D.; Jenson, A.B.; Jung, J.Y. Cutaneous metastasis. Hematol. Oncol. Clin. N. Am. 2019, 33, 173–197. [Google Scholar] [CrossRef]
- Gan, E.Y.; Chio, M.T.; Tan, W.P. A retrospective review of cutaneous metastases at the National Skin Centre Singapore. Australas. J. Dermatol. 2015, 56, 1–6. [Google Scholar] [CrossRef]
- Lookingbill, D.P.; Spangler, N.; Helm, K.F. Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J. Am. Acad. Dermatol. 1993, 29 Pt 1, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Helm, M.A.; Kalb, R.E.; Helm, T.N.; Zeitouni, N.C. The presentation, pathology, and current management strategies of cutaneous metastasis. N. Am. J. Med. Sci. 2013, 5, 499–504. [Google Scholar] [CrossRef]
- Sleeman, J.P. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent. Results Cancer Res. 2000, 157, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Khandpur, S.; Khanna, N.; Jain, D. Angioma like carcinoma telangiectoides: An unusual presentation of breast carcinoma metastasis. Indian. J. Dermatol. Venereol. Leprol. 2018, 84, 83. [Google Scholar] [PubMed]
- Schoenlaub, P.; Sarraux, A.; Grosshans, E.; Heid, E.; Cribier, B. Survival after cutaneous metastasis: A study of 200 cases. Ann. Dermatol. Venereol. 2001, 128, 1310–1315. [Google Scholar]
- Bittencourt Mde, J.; Carvalho, A.H.; Nascimento, B.A.; Freitas, L.K.; Parijós, A.M. Cutaneous metastasis of a breast cancer diagnosed 13 years before. An. Bras. Dermatol. 2015, 90 (Suppl. S1), 134–137. [Google Scholar] [CrossRef] [PubMed]
- Araújo, E.; Barbosa, M.; Costa, R.; Sousa, B.; Costa, V. A first sign not to be missed: Cutaneous metastasis from breast cancer. Eur. J. Case Rep. Intern. Med. 2020, 7, 001356. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Alfaioli, B.; Savarese, I.; Corciova, S.A.; Guerriero, G.; Lotti, T. Cutaneous manifestations of breast carcinoma. Dermatol. Ther. 2010, 23, 581–589. [Google Scholar] [CrossRef]
- Bastard, D.P.; Bollea-Garlatti, M.L.; Belatti, A.; Puga, M.C.; Hernández, M.N.; Mazzuoccolo, L.D. Metástasis cutáneas de cáncer de mama: 8 años de revisión en un centro de tercera complejidad. Actas Dermosifiliogr. 2019, 110, 206–211. [Google Scholar] [CrossRef]
- Ferlito, A.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Mondin, V. Incidence and Sites of Distant Metastases from Head and Neck Cancer. ORL 2001, 63, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Nagi, C.; Bleiweiss, I.; Jaffer, S. Epithelial displacement in breast lesions: A papillary phenomenon. Arch. Pathol. Lab. Med. 2005, 129, 1465–1469. [Google Scholar] [CrossRef]
- Kovács, K.A.; Hegedus, B.; Kenessey, I.; Tímár, J. Tumor type-specific and skin region-selective metastasis of human cancers: Another example of the “seed and soil” hypothesis. Cancer Metastasis Rev. 2013, 3, 493–499. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, W.S.; Kim, H.K.; Bae, T.H. Scalp metastasis from an adenocarcinoma of the lung that mimicked a cystic mass. Arch. Craniofac Surg. 2022, 23, 237–240. [Google Scholar] [CrossRef]
- Coslett, L.M.; Katlic, M.R. Lung cancer with skin metastasis. Chest 1990, 97, 757–759. [Google Scholar] [CrossRef]
- Mollet, T.W.; Garcia, C.A.; Koester, G. Skin metastases from lung cancer. Dermatol. Online J. 2009, 15, 1. [Google Scholar] [CrossRef]
- Alcaraz, I.; Cerroni, L.; Rütten, A.; Kutzner, H.; Requena, L. Cutaneous metastases from internal malignancies: A clinicopathologic and immunohistochemical review. Am. J. Dermatopathol. 2012, 34, 347–393. [Google Scholar] [CrossRef]
- Inamura, K. Update on immunohistochemistry for the diagnosis of lung cancer. Cancers 2018, 10, 72. [Google Scholar] [CrossRef]
- Ward, R.E.; Ali, S.A.; Kuhar, M. Epithelioid malignant mesothelioma metastatic to the skin: A case report and review of the literature. J. Cutan. Pathol. 2017, 44, 1057–1063. [Google Scholar] [CrossRef]
- Mori, T.; Yamamoto, T. Skin metastasis of malignant mesothelioma. An. Bras. Dermatol. 2021, 96, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Elbahaie, A.M.; Kamel, D.E.; Lawrence, J.; Davidson, N.G. Late cutaneous metastases to the face from malignant pleural mesothelioma: A case report and review of the literature. World J. Surg. Oncol. 2009, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.S.L.; Reichrath, J.; Tilgen, W. Disseminated cutaneous metastasis of a biphasic pleural mesothelioma. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 226–227. [Google Scholar] [CrossRef]
- Maiorana, A.; Giusti, F.; Cesinaro, A.M.; Conti, A.; Rossi, G. Cutaneous metastases as the first manifestation of pleural malignant mesothelioma. J. Am. Acad. Dermatol. 2006, 54, 363–365. [Google Scholar] [CrossRef]
- Beer, T.W.; Heenan, P.J. Malignant mesothelioma presenting as a lip tumor: Report of two cases with one unrecognized by 166 pathologists. Am. J. Dermatopathol. 2007, 29, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Boyde, A.M.; Attanoos, R.L. Sister Mary Joseph’s nodule in malignant peritoneal mesothelioma. Histopathology 2003, 43, 303–304. [Google Scholar] [CrossRef]
- Heatley, M.K. Sister Mary Joseph’s nodule in malignant mesothelioma. Histopathology 2004, 45, 299–300. [Google Scholar] [CrossRef]
- Kanbay, A.; Oguzulgen, K.I.; Ozturk, C.; Memis, L.; Demircan, S.; Kurkcuoglu, C.; Akyurek, N.; Kurul, C. Malignant pleural mesothelioma with scalp, cerebellar, and finger metastases: A rare case. South. Med. J. 2007, 100, 63–65. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Xue, W.; Shannon, K.J. Widespread cutaneous and perioral metastases of mesothelioma. J. Cutan. Pathol. 2003, 30, 582–585. [Google Scholar] [CrossRef]
- Dutt, P.L.; Baxter, J.W.; O’Malley, F.P.; Glick, A.D.; Page, D.L. Distant cutaneous metastasis of pleural malignant mesothelioma. J. Cutan. Pathol. 1992, 19, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Shieh, S.; Grassi, M.; Schwarz, J.K.; Cheney, R.T. Pleural mesothelioma with cutaneous extension to chest wall scars. J. Cutan. Pathol. 2004, 31, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Keehn, C.A.; Morgan, M.B. Cutaneous metastasis: A clinical, pathological, and immunohistochemical appraisal. J. Cutan. Pathol. 2004, 31, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Nesseris, I.; Tsamakis, C.; Gregoriou, S.; Ditsos, I.; Christofidou, E.; Rigopoulos, D. Cutaneous metastasis of colon adenocarcinoma: Case report and review of the literature. An. Bras. Dermatol. 2013, 88 (Suppl. S1), 56–58. [Google Scholar] [CrossRef]
- AlSubait, N.A.; BinJadeed, H.F.; AlSaleh, M.R.; AlFaifi, F.S.; AlSaif, F.M.; Arafah, M.A. Dermoscopy of scalp cutaneous metastasis of sigmoid adenocarcinoma. JAAD Case Rep. 2021, 14, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.R.; Donica, W.R.F.; Egger, M.E.; Philips, P.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G., 2nd. Observed changes in the distribution of colon cancer metastasis: A National Cancer Database review institutional experience. Ann. Surg. Oncol. 2025, 32, 418–423. [Google Scholar] [CrossRef]
- Wong, C.Y.; Helm, M.A.; Helm, T.N.; Zeitouni, N. Patterns of skin metastases: A review of 25 years’ experience at a single cancer center. Int. J. Dermatol. 2014, 53, 56–60. [Google Scholar] [CrossRef]
- Şahin, M.; Ekinci, F.; Çelik, C.; Temiz, P.; Erdoğan, A.P.; Göksel, G. A rare case report of skin metastasis in gastric cancer. J. Gastrointest. Cancer 2021, 52, 1156–1158. [Google Scholar] [CrossRef]
- Frey, L.; Vetter-Kauczok, C.; Gesierich, A.; Bröcker, E.B.; Ugurel, S. Cutaneous metastases as the first clinical sign of metastatic gastric carcinoma. J. Dtsch. Dermatol. Ges. 2009, 7, 893–895, (In English and German). [Google Scholar] [CrossRef]
- Takata, T.; Takahashi, A.; Tarutani, M.; Sano, S. A rare case of cellulitis-like cutaneous metastasis of gastric adenocarcinoma. Int. J. Dermatol. 2014, 53, e122–e124. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.H.; Chang, H.M.; Jung, J.Y.; Song, J.H.; Lee, J.L.; Ryu, M.H.; Kim, T.W.; Yook, J.H.; Song, J.S.; Lee, J.S.; et al. Cutaneous metastasis resembling acute dermatitis in patient with advanced gastric cancer. Clin. Exp. Dermatol. 2007, 32, 284–286. [Google Scholar] [CrossRef]
- Hu, S.C.; Chen, G.S.; Wu, C.S.; Chai, C.Y.; Chen, W.T.; Lan, C.C. Rates of cutaneous metastases from different internal malignancies: Experience from a Taiwanese medical center. J. Am. Acad. Dermatol. 2009, 60, 379–387. [Google Scholar] [CrossRef]
- Pliakou, E.; Lampropoulou, D.I.; Nasi, D.; Aravantinos, G. Skin metastases from gastric cancer, a rare entity masquerading as erysipelas: A case report. Mol. Clin. Oncol. 2022, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Wang, X.B.; Gao, F.; Bu, B.; Zhang, S.; Wang, Z. Cutaneous metastasis from pancreatic cancer: A case report and systematic review of the literature. Oncol. Lett. 2014, 8, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Kaoutzanis, C.; Chang, M.C.; Abdul Khalek, F.J.; Kreske, E. Non-umbilical cutaneous metastasis of a pancreatic adenocarcinoma. BMJ Case Rep. 2013, 2013, bcr2012007931. [Google Scholar] [CrossRef]
- Aghighi, M.; Bagher Shokravi, M.; Rahvar, M. Metastatic pancreatic adenocarcinoma to umbilical skin. Cureus 2022, 14, e24568. [Google Scholar] [CrossRef]
- Vekariya, P.; Daneti, D.B.; Senthamizh Selvan, K.; Verma, S.K.; Hamide, A.; Mohan, P. Sister Mary Joseph nodule as an initial presentation of pancreatic adenocarcinoma. ACG Case Rep. J. 2020, 7, e00453. [Google Scholar] [CrossRef]
- Hafez, H.Z. Cutaneous pancreatic metastasis: A case report and review of literature. Indian J. Dermatol. 2008, 53, 206–209. [Google Scholar] [CrossRef]
- Miyahara, M.; Hamanaka, Y.; Kawabata, A.; Sato, Y.; Tanaka, A.; Yamamoto, A.; Ueno, T.; Nishihara, K.; Suzuki, T. Cutaneous metastases from pancreatic cancer. Int. J. Pancreatol. 1996, 20, 127–130. [Google Scholar] [CrossRef]
- Horino, K.; Takamori, H.; Ikuta, Y.; Nakahara, O.; Chikamoto, A.; Ishiko, T.; Beppu, T.; Baba, H. Cutaneous metastases secondary to pancreatic cancer. World J. Gastrointest. Oncol. 2012, 4, 176–180. [Google Scholar] [CrossRef]
- Gu, L.; Mehta, P.P.; Rao, D.; Rotemberg, V.; Capanu, M.; Chou, J.; Lin, S.; Sigel, C.S.; Busam, K.J.; Boyce, L.; et al. Pancreatic cancer: Cutaneous metastases, clinical descriptors and outcomes. Cancer Med. 2023, 12, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Reuben, S.; Owen, D.; Lee, P.; Weiss, A. Hepatocellular carcinoma with cutaneous metastases. Can. J. Gastroenterol. 2009, 23, 23–25. [Google Scholar] [CrossRef]
- Peters, R.L. Metastatic patterns of HCC. In Hepatocellular Carcinoma; Okuda, K., Peters, R.L., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 1976; pp. 156–164. [Google Scholar]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; De Marco, A.; Romita, P.; Foti, C.; Resta, L.; Ingravallo, G. Cutaneous metastases from primary liver cancers: The need for knowledge and differential diagnosis. Life 2021, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Tani, J.; Oura, K.; Tadokoro, T.; Fujita, K.; Masaki, T. Giant cutaneous metastasis from hepatocellular carcinoma. JGH Open 2022, 6, 361–362. [Google Scholar] [CrossRef]
- Ackerman, D.; Barr, R.J.; Elias, A.N. Cutaneous metastases from hepatocellular carcinoma. Int. J. Dermatol. 2001, 40, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Tümen, D.; Heumann, P.; Gülow, K.; Demirci, C.N.; Cosma, L.S.; Müller, M.; Kandulski, A. Pathogenesis and Current Treatment Strategies of Hepatocellular Carcinoma. Biomedicines 2022, 10, 3202. [Google Scholar] [CrossRef]
- Hennedige, T.; Venkatesh, S.K. Imaging of hepatocellular carcinoma: Diagnosis, staging and treatment monitoring. Cancer Imaging 2013, 12, 530–547. [Google Scholar] [CrossRef]
- Amador, A.; Monforte, N.G.; Bejarano, N.; Martí, J.; Artigau, E.; Navarro, S.; Fuster, J. Cutaneous metastasis from hepatocellular carcinoma as the first clinical sign. J. Hepatobiliary Pancreat. Surg. 2007, 14, 328–330. [Google Scholar] [CrossRef]
- Hanazawa, T.; Fukami, Y.; Osawa, T.; Kurahashi, S.; Matsumura, T.; Saito, T.; Komatsu, S.; Kaneko, K.; Tsuzuki, T.; Sano, T. A case of resected hepatocellular carcinoma with gallbladder metastasis. Surg. Case Rep. 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- de Agustín, P.; Conde, E.; Alberti, N.; Pérez-Barrios, A.; López-Ríos, F. Cutaneous metastasis of occult hepatocellular carcinoma: A case report. Acta Cytol. 2007, 51, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Wee, A. Fine needle aspiration biopsy of hepatocellular carcinoma and hepatocellular nodular lesions: Role, controversies and approach to diagnosis. Cytopathology 2011, 22, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Sheefa, H.; Lata, J.; Basharat, M.; Rumana, M.; Veena, M. Utility of FNAC in conjunction with cell block for diagnosing space-occupying lesion (SOL) of liver with emphasis on differentiating hepatocellular carcinoma from metastatic SOL: Analysis of 61 Cases. Oman Med. J. 2016, 31, 135–141. [Google Scholar] [CrossRef]
- Nadal, R.; Bellmunt, J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019, 76, 10–21. [Google Scholar] [CrossRef]
- Hasan, O.; Houlihan, M.; Wymer, K.; Hollowell, C.M.P.; Kohler, T.S. Cutaneous metastasis of bladder urothelial carcinoma. Urol. Case Rep. 2019, 28, 101066. [Google Scholar] [CrossRef]
- Block, C.A.; Dahmoush, L.; Konety, B.R. Cutaneous metastases from transitional cell carcinoma of the bladder. Urology 2006, 67, e15–e17. [Google Scholar] [CrossRef]
- Baczyński, A.; Howard, N.; Tomaszewski, J.J. Cutaneous metastasis of urothelial carcinoma. Dermatol. Pract. Concept. 2023, 13, e2023225. [Google Scholar] [CrossRef]
- Vasilevska, D.; Rudaitis, V.; Lewkowicz, D.; Širvienė, D.; Mickys, U.; Semczuk, M.; Obrzut, B.; Semczuk, A. Expression patterns of cytokeratins (CK7, CK20, CK19, CK AE1/AE3) in atypical endometrial hyperplasia coexisting with endometrial cancer. Int. J. Mol. Sci. 2024, 25, 9084. [Google Scholar] [CrossRef]
- Krathen, R.A.; Orengo, I.F.; Rosen, T. Cutaneous metastasis: A meta-analysis of data. South. Med. J. 2003, 96, 164–167. [Google Scholar] [CrossRef]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66, 4–34. [Google Scholar] [CrossRef]
- Manzelli, A.; Quaresima, S.; Rossi, P.; Petrou, A.; Ricciardi, E.; Brennan, N.; Kontos, M.; Petrella, G. Solitary skin metastasis from sarcomatoid carcinoma of the bladder: A case report. J. Med. Case Rep. 2011, 5, 484. [Google Scholar] [CrossRef]
- Mitsui, Y.; Arichi, N.; Inoue, K.; Hiraki, M.; Nakamura, S.; Hiraoka, T.; Ishikawa, N.; Maruyama, R.; Yasumoto, H.; Shiina, H. Choroidal and cutaneous metastasis from urothelial carcinoma of the bladder after radical cystectomy: A case report and literature review. Case Rep. Urol. 2014, 2014, 491541. [Google Scholar] [CrossRef]
- Tonni, E.; Oltrecolli, M.; Pirola, M.; Tchawa, C.; Roccabruna, S.; D’Agostino, E.; Matranga, R.; Piombino, C.; Pipitone, S.; Baldessari, C.; et al. New Advances in Metastatic Urothelial Cancer: A Narrative Review on Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 9696. [Google Scholar] [CrossRef]
- Brown, G.T.; Patel, V.; Lee, C.C. Cutaneous metastasis of prostate cancer: A case report and review of the literature with bioinformatics analysis of multiple healthcare delivery networks. J. Cutan. Pathol. 2014, 41, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Mecca, P.S.; Myskowski, P.L.; Slovin, S.F. Scrotal and penile papules and plaques as the initial manifestation of a cutaneous metastasis of adenocarcinoma of the prostate: Case report and review of the literature. J. Cutan. Pathol. 2008, 35, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Rattanasirivilai, A.; Kurban, A.; Lenzy, Y.M.; Yaar, R. Cutaneous metastasis of prostatic adenocarcinoma: A cautionary tale. J. Cutan. Pathol. 2011, 38, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Lookingbill, D.P.; Spangler, N.; Sexton, F.M. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 1990, 22, 19. [Google Scholar] [CrossRef]
- Oh, T.H.; Kim, H.S.; Park, S.C. Scalp nodules as the first presentation of prostate cancer: A CARE-compliant article. Medicine 2023, 102, e36570. [Google Scholar] [CrossRef]
- Stanko, C.; Grandinetti, L.; Baldassano, M.; Mahmoodi, M.; Kantor, G.R. Epidermotropic metastatic prostate carcinoma presenting as an umbilical nodule-Sister Mary Joseph nodule. Am. J. Dermatopathol. 2007, 29, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Ali, T.Z.; Montgomery, E.A.; Begum, S.; Hicks, J.; Goggins, M.; Eberhart, C.G.; Clark, D.P.; Bieberich, C.J.; Epstein, J.I.; et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am. J. Surg. Pathol. 2010, 34, 1097–1105. [Google Scholar] [CrossRef]
- De Bruyne, P.; Schatteman, P.; De Naeyer, G.; Carpentier, P.; Mottrie, A. Port site metastasis in prostate cancer. Can. Urol. Assoc. J. 2015, 9, E387–E389. [Google Scholar] [CrossRef]
- Larrousse, C.; Brasseur, P.; Sukkarieh, F. Métastase orificielle après prostatectomie radicale coelioscopique pour un adénocarcinome mucineux de la prostate [Port-site metastasis following laparoscopic radical prostatectomy for mucinous adenocarcinoma of the prostate]. J. Radiol. 2005, 86, 337–339. (In French) [Google Scholar] [CrossRef] [PubMed]
- Sarangi, S.S.; Singh, V.; Bhirud, D.P. Cutaneous metastasis of castration-resistant prostate cancer: A rare case report and review of literature. Urol. Ann. 2023, 15, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Petcu, E.B.; Gonzalez-Serva, A.; Wright, R.G.; Slevin, M.; Brinzaniuc, K. Prostate carcinoma metastatic to the skin as an extramammary Paget’s disease. Diagn. Pathol. 2012, 7, 106. [Google Scholar] [CrossRef]
- Bailey, C.; Broadbent, A. Cutaneous metastases of prostate cancer. J. Palliat. Med. 2007, 10, 980–982. [Google Scholar] [CrossRef]
- Villalba Bachur Roberto, F.; Florencia, C.; Emilio, L. Prostate cancer cutaneous metastasis: A case report. Urol. Androl. Open J. 2020, 4, 27–29. [Google Scholar] [CrossRef]
- Mak, G.; Chin, M.; Nahar, N.; De Souza, P. Cutaneous metastasis of prostate carcinoma treated with radiotherapy: A case presentation. BMC Res. Notes 2014, 7, 505. [Google Scholar] [CrossRef][Green Version]
- Reingold, I.M. Cutaneous metastases from internal carcinoma. Cancer 1966, 19, 162–168. [Google Scholar] [CrossRef]
- Cormio, G.; Capotorto, M.; Di Vagno, G.; Cazzolla, A.; Carriero, C.; Selvaggi, L. Skin metastases in ovarian carcinoma: A report of nine cases and a review of the literature. Gynecol. Oncol. 2003, 90, 682–685. [Google Scholar] [CrossRef]
- Tavares, V.; Marques, I.S.; Melo, I.G.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. 2024, 25, 1845. [Google Scholar] [CrossRef]
- Zhang, J.; He, W.; Zhang, Z.; Dong, H.; Deng, X.; Wen, Q.; Li, D. Skin metastasis from ovarian cancer with somatic BRCA1 mutation: A case report and literature review. Oncol. Lett. 2024, 28, 348. [Google Scholar] [CrossRef]
- Bayraktar, E.; Chen, S.; Corvigno, S.; Liu, J.; Sood, A.K. Ovarian cancer metastasis: Looking beyond the surface. Cancer Cell. 2024, 42, 1631–1636. [Google Scholar] [CrossRef]
- Singh, R.P.; Tullis, S.; Hatton, M.; Rubin, P.A. Orbital metastasis from ovarian carcinoma in a patient with BRCA-2 mutation. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 298–299. [Google Scholar] [CrossRef]
- Patsner, B.; Mann, W.J.; Chumas, J.; Loesch, M. Herpetiform cutaneous metastases following negative second look laparatomy for ovarian adenocarcinoma. Arch. Gynecol. Obstet. 1988, 244, 63–67. [Google Scholar] [CrossRef]
- McDonald, H.H.; Moore, M.R.; Meffert, J.J. Cutaneous metastases from adenocarcinoma of the ovary. JAAD Case Rep. 2016, 2, 406–407. [Google Scholar] [CrossRef]
- Scheinfeld, N. A review of the cutaneous paraneoplastic associations and metastatic presentations of ovarian carcinoma. Clin. Exp. Dermatol. 2008, 33, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Lu, W.; Wan, X.; Chen, Y.; Xie, X. Extra-abdominal metastases from epithelial ovarian carcinoma: An analysis of 20 cases. Int. J. Gynecol. Cancer 2009, 19, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Matsuura, T. Skin metastases in epithelial ovarian and fallopian tube carcinoma. Medicine 2017, 96, e7798. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I. Cutaneous metastases in ovarian cancer. Cancers 2019, 11, 1292. [Google Scholar] [CrossRef]
- Cheng, H.; Gao, C.; Zhang, R.; Yang, Z.; Zhang, G. Two independent incidences of skin metastases in the umbilicus and abdominal wall in ovarian serous adenocarcinoma: A case report and review of the literature. Medicine 2017, 96, e9118. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Taube, J.; Sharma, M.; McCalmont, T.H.; Kim, J. PAX8 discriminates ovarian metastases from adnexal tumors and other cutaneous metastases. J. Cutan. Pathol. 2010, 37, 938–943. [Google Scholar] [CrossRef]
- Lalich, D.; Tawfik, O.; Chapman, J.; Fraga, G. Cutaneous metastasis of ovarian carcinoma with shadow cells mimicking a primary pilomatrical neoplasm. Am. J. Dermatopathol. 2010, 32, 500–504. [Google Scholar] [CrossRef]
- Charalampidis, C.; Lampaki, S.; Zarogoulidis, P.; Lazaridis, G.; Mpaka, S.; Kosmidis, C.; Tsakiridis, K.; Kioumis, I.; Pavlidis, P.; Karapantzos, I.; et al. Fine-needle aspiration of skin metastasis in ovarian cancer-report of two cases and review of the literature. Ann. Transl. Med. 2016, 4, 447. [Google Scholar] [CrossRef]
- Markowska, A.; Baranowski, W.; Pityński, K.; Chudecka-Głaz, A.; Markowska, J.; Sawicki, W. Metastases and recurrence risk factors in endometrial cancer-the role of selected molecular changes, hormonal factors, diagnostic methods and surgery procedures. Cancers 2023, 16, 179. [Google Scholar] [CrossRef]
- Hussein, M.R. Skin metastasis: A pathologist’s perspective. J. Cutan. Pathol. 2010, 37, e1–e20. [Google Scholar] [CrossRef]
- Debois, J.M. Endometrial adenocarcinoma metastatic to the scalp. Report of two cases. Arch. Dermatol. 1982, 118, 42–43. [Google Scholar] [CrossRef]
- Bashyam, A.; Stewart, A.; Potter, K.; Bagwan, I.; Sunkaraneni, V.S. Metastatic endometrial cancer of the paranasal sinuses. Ann. R. Coll. Surg. Engl. 2018, 100, e161–e164. [Google Scholar] [CrossRef] [PubMed]
- Giardina, V.N.; Morton, B.F.; Potter, G.K.; Mesa-Tejada, R.; Waterfield, W.C. Metastatic endometrial adenocarcinoma to the skin of a toe. Am. J. Dermatopathol. 1996, 18, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Espinós, J.J.; Garcia-Patos, V.; Guiu, X.M.; Delgado, E. Early skin metastasis of endometrial adenocarcinoma: Case report and review of the literature. Cutis 1993, 52, 109–111. [Google Scholar]
- Patel, K.S.; Watkins, R.M. Recurrent endometrial adenocarcinoma presenting as an umbilical metastasis (Sister Mary Joseph’s nodule). Br. J. Clin. Pract. 1992, 46, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.C.; Jobson, V.W.; Homesley, H.D. Umbilical metastasis from gynecologic malignancies: A primary carcinoma of the fallopian tube. Obstet. Gynecol. 1981, 57, 531–533. [Google Scholar]
- Augustin, G.; Kekez, T.; Bogdanic, B. Abdominal papular zosteriform cutaneous metastases from endometrial adenocarcinoma. Int. J. Gynaecol. Obstet. 2010, 110, 74. [Google Scholar] [CrossRef]
- Meedimale, P.; Meedimale, R.S.; Narayen, V.; Chella, V.R.; Patil, P.; Bhende, A.; Panda, J. An exceptional case of carcinoma cervix with distant metastasis to skin. J. Intern. Med. India 2023, 17, 47. [Google Scholar] [CrossRef]
- Agrawal, A.; Yau, A.; Magliocco, A.; Chu, P. Cutaneous metastatic disease in cervical cancer: A case report. J. Obstet. Gynaecol. Can. 2010, 32, 467–472. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, R.; Zhou, Q. Skin metastasis in squamous cell cancer of cervix: A case report and literature review. Precis. Radiat. Oncol. 2023, 7, 142–146. [Google Scholar] [CrossRef]
- Alrefaie, S.I.; Alshamrani, H.M.; Abduljabbar, M.H.; Hariri, J.O. Skin metastasis from squamous cell carcinoma of the cervix to the lower extremities: Case report and review of the literature. J. Fam. Med. Prim. Care 2019, 8, 3443–3446. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Ke, X.; Liu, Y.; Zang, C. Cutaneous metastasis from cervical cancer to the scalp and trunk: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 435. [Google Scholar] [CrossRef] [PubMed]
- Tharakaram, S.; Rajendran, S.S.; Premalatha, S.; Yesudian, P.; Zahara, A. Cutaneous metastasis from carcinoma cervix. Int. J. Dermatol. 1985, 24, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed]
- Daw, E.; Riley, S. Umbilical metastasis from squamous carcinoma of the cervix: Case report. Br. J. Obstet. Gynaecol. 1982, 89, 1066. [Google Scholar] [CrossRef]
- Behtash, N.; Mehrdad, N.; Shamshirsaz, A.; Hashemi, R.; Amouzegar Hashemi, F. Umblical metastasis in cervical cancer. Arch. Gynecol Obstet. 2008, 278, 489–491. [Google Scholar] [CrossRef]
- Maheshwari, G.K.; Baboo, H.A.; Ashwathkumar, R.; Dave, K.S.; Wadhwa, M.K. Scalp metastasis from squamous cell carcinoma of the cervix. Int. J. Gynecol Cancer. 2001, 11, 244–246. [Google Scholar] [CrossRef]
- Takagi, H.; Miura, S.; Matsunami, K.; Ikeda, T.; Imai, A. Cervical cancer metastasis to the scalp: Case report and literature review. Eur. J. Gynaecol. Oncol. 2010, 31, 217–218. [Google Scholar]
- Kovács, K.A.; Kenessey, I.; Tímár, J. Skin metastasis of internal cancers: A single institution experience. Pathol. Oncol. Res. 2013, 19, 515–520. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers. 2017, 3, 17009. [Google Scholar] [CrossRef]
- Errami, M.; Margulis, V.; Huerta, S. Renal cell carcinoma metastatic to the scalp. Rare Tumors 2016, 8, 6400. [Google Scholar] [CrossRef] [PubMed]
- Snow, S.; Madjar, D.; Reizner, G.; Mack, E.; Bentz, M. Renal cell carcinoma metastatic to the scalp: Case report and review of the literature. Dermatol. Surg. 2001, 27, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.J.; Wu, H.; Greenberg, R.E.; Hudes, G.; Topham, N.; Lessin, S.R.; Uzzo, R.G. Cutaneous metastases from geniourinary malignancies. Urology 2004, 63, 1021–1026. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, A.; Tan, D.; Du, J.; Wang, Y.; Tang, P.Y.; Sim, A.S.P. Cutaneous metastasis of renal cell carcinoma. Lancet Oncol. 2020, 21, e292. [Google Scholar] [CrossRef]
- Leve, P.P.; Felício, J.; Carneiro, R.; Zagalo, C. Recurrent renal cell carcinoma presenting as a cutaneous metastasis: A case report and review of the literature. Urol. Ann. 2021, 13, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Bujons, A.; Pascual, X.; Martínez, R.; Rodríguez, O.; Palou, J.; Villavicencio, H. Cutaneous metastases in renal cell carcinoma. Urol. Int. 2008, 80, 111–112. [Google Scholar] [CrossRef]
- de Paula, T.A.; da Silva, P.S.; Berriel, L.G. Renal cell carcinoma with cutaneous metastasis: Case report. J. Bras. Nefrol. 2010, 32, 213–215. [Google Scholar] [PubMed]
- Ferhatoglu, M.F.; Senol, K.; Filiz, A.I. Skin metastasis of renal cell carcinoma: A case report. Cureus 2018, 10, e3614. [Google Scholar] [CrossRef]
- Singh, P.; Somani, K. Latent distant metastasis of renal cell carcinoma to skin: A case report. Clin. Case Rep. 2020, 8, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Teramoto, S.; Kitamura, T. Metachronous bilateral renal cell carcinoma with an interval of more than 10 years. Int. Urol. Nephrol. 2009, 41, 843–846. [Google Scholar] [CrossRef]
- Pitman, K.T.; Johnson, J.T. Skin metastases from head and neck squamous cell carcinoma: Incidence and impact. Head Neck 1999, 21, 560–565. [Google Scholar] [CrossRef]
- Kmucha, S.T.; Troxel, J.M. Dermal metastases in epidermoid carcinoma of the head and neck. Arch. Otolaryngol. Head. Neck Surg. 1993, 119, 326–330. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92, Erratum in Nat. Rev. Dis. Primers 2023, 9, 4. https://doi.org/10.1038/s41572-023-00418-5. [Google Scholar] [CrossRef]
- Crosetti, E.; Arrigoni, G.; Cerutti, M.; Succo, G. Atypical carcinoid of the larynx. Ear Nose Throat J. 2020, 99, 369–370. [Google Scholar] [CrossRef]
- Thariat, J.; Badoual, C.; Hans, S.; Meatchi, T.; Housset, M. Skin metastasis of head and neck carcinoma predictive for dismal outcome. Dermatol. Online J. 2008, 14, 8. [Google Scholar] [CrossRef]
- El Khoury, J.; Khalifeh, I.; Kibbi, A.G.; Abbas, O. Cutaneous metastasis: Clinicopathological study of 72 patients from a tertiary care center in Lebanon. Int. J. Dermatol. 2014, 53, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gor, D.; Ghimire, B.; Abbas, O.; Diab, M. Cutaneous metastases without a known primary: A clinical conundrum. Cureus 2025, 17, e83591. [Google Scholar] [CrossRef]
- Virmani, N.C.; Sharma, Y.K.; Panicker, N.K.; Dash, K.N.; Patvekar, M.A.; Deo, K.S. Zosteriform skin metastases: Clue to an undiagnosed breast cancer. Indian. J. Dermatol. 2011, 56, 726–727. [Google Scholar] [CrossRef]
- Savoia, P.; Fava, P.; Deboli, T.; Quaglino, P.; Bernengo, M.G. Zosteriform cutaneous metastases: A literature meta-analysis and a clinical report of three melanoma cases. Dermatol. Surg. 2009, 35, 1355–1363. [Google Scholar] [CrossRef]
- Dumlao, J.K.G.; Cubillan, E.L.A.; Villena, J.P.D.S. Clinical and histopathologic profile of patients with cutaneous metastasis in a tertiary hospital in the Philippines. Dermatopathology 2022, 9, 392–407. [Google Scholar] [CrossRef]
- Bolognia, J.L.; Schaffer, J.V.; Cerroni, L. Dermatology, 4th ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2018. [Google Scholar]
- de Carvalho, M.; Valejo Coelho, M.M.; Bajanca, R. Metástases cutâneas de carcinoma de células renais: A propósito de dois casos clínicos. Acta Med. Port. 2025, 38, 125–126. (In Portuguese) [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Gordon Spratt, E.A.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: A meta-analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef]
- Russano, F.; Del Fiore, P.; Di Prata, C.; Pasqual, A.; Marconato, R.; Campana, L.G.; Spina, R.; Gianesini, C.M.; Collodetto, A.; Tropea, S.; et al. The role of electrochemotherapy in the cutaneous and subcutaneous metastases from breast cancer: Analysis of predictive factors to treatment from an Italian cohort of patients. Front. Oncol. 2021, 11, 772144. [Google Scholar] [CrossRef] [PubMed]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, É.; Gehl, J.; et al. Evaluation of calcium electroporation for the treatment of utaneous metastases: A double blinded randomised controlled phase II trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef]
- Morley, J.; Grocott, P.; Purssell, E.; Murrells, T. Electrochemotherapy for the palliative management of cutaneous metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Belkacémi, Y.; Bourgier, C.; Cowen, D.; Cutuli, B.; Fourquet, A.; Hannoun-Lévi, J.M.; Pasquier, D.; Racadot, S.; Rivera, S. Radiotherapy of breast cancer. Cancer Radiother. 2022, 26, 221–230. [Google Scholar] [CrossRef]
- Nakamura, N.; Kawamori, J.; Takahashi, O.; Shikama, N.; Sekiguchi, K.; Takahashi, T.; Kato, S.; Ogita, M.; Motegi, A.; Akimoto, T. Palliative radiotherapy for breast cancer patients with skin invasion: A multi-institutional prospective observational study. Jpn. J. Clin. Oncol. 2018, 48, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Mu, F.; Zhang, C.; Li, Y.; Liu, W.; Jiang, F. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology 2013, 67, 17–22. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, L.; Mu, F.; Wang, X.; Zeng, J.; Yao, F.; Jiang, F.; He, L.; Chen, J.; Li, J.; et al. Therapeutic outcomes of combining cryotherapy, chemotherapy and DC-CIK immunotherapy in the treatment of metastatic non-small cell lung cancer. Cryobiology 2013, 67, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Niu, L.; Mu, F.; Liu, S.; Leng, Y.; Liao, M.; Zeng, J.; Yao, F.; Chen, J.; Li, J.; et al. Percutaneous comprehensive cryoablation for metastatic esophageal cancer after failure of radical surgery. Cryobiology 2013, 67, 363–368. [Google Scholar] [CrossRef]
- Chen, F.Z.; Zhao, X.K. Prostate cancer: Current treatment and prevention strategies. Iran. Red. Crescent Med. J. 2013, 15, 279–284. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Tsai, C.-L.; Huang, H.-P. Optimizing Osimertinib for NSCLC: Targeting Resistance and Exploring Combination Therapeutics. Cancers 2025, 17, 459. [Google Scholar] [CrossRef]
- Ahn, J.; Nagasaka, M. Spotlight on Cemiplimab-rwlc in the Treatment of Non-Small Cell Lung Cancer (NSCLC): Focus on Patient Selection and Considerations. Cancer Manag. Res. 2023, 15, 627–634. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Niciforovic, D.; Papic, D.; Milojevic, K.; Markovic, M. CDK4/6 inhibitors: Basics, pros, and major cons in breast cancer treatment with specific regard to cardiotoxicity—A narrative review. Ther. Adv. Med. Oncol. 2023, 15, 17588359231205848. [Google Scholar] [CrossRef] [PubMed]
- Gion, M.; Blancas, I.; Cortez-Castedo, P.; Cortés-Salgado, A.; Marmé, F.; Blanch, S.; Morales, S.; Díaz, N.; Calvo-Plaza, I.; Recalde, S.; et al. Atezolizumab plus paclitaxel and bevacizumab as first-line treatment of advanced triple-negative breast cancer: The ATRACTIB phase 2 trial. Nat. Med. 2025, 31, 2746–2754. [Google Scholar] [CrossRef]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Doleschal, B.; Petzer, A.; Rumpold, H. Current concepts of anti-EGFR targeting in metastatic colorectal cancer. Front. Oncol. 2022, 12, 1048166. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Funk-Debleds, P.; Cattey-Javouhey, A.; Desseigne, F.; Guibert, P.; Marolleau, P.; Rochefort, P.; de la Fouchardière, C. Targeting HER2 in metastatic gastroesophageal adenocarcinomas: What is new? Bull. Cancer 2023, 110, 552–559. [Google Scholar] [CrossRef]
- Liu, J.; Matulonis, U.A. Update on PARP inhibitors for the treatment of ovarian cancer. Clin. Adv. Hematol. Oncol. 2025, 23, 100–110. [Google Scholar]
- Lorusso, D.; Colombo, N.; Dubot, C.; Cáceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Salman, P.; Yañez, E.; Gümüş, M.; Olivera, M.; et al. Pembrolizumab plus chemotherapy for advanced and recurrent cervical cancer: Final analysis according to bevacizumab use in the randomized KEYNOTE-826 study. Ann. Oncol. 2025, 36, 65–75. [Google Scholar] [CrossRef]
- Liolis, E.; Mulita, F.; Koutras, A.; Makatsoris, T.; Sivolapenko, G. Exploring Bevacizumab’s Role in Gynecological Cancers: An Up-to-Date Narrative Review Focusing on Ovarian Cancer. Mater. Sociomed. 2024, 36, 268–279. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Ahmed, I.; Bhise, R.; Mohanti, B.K.; Sharma, A.; Rieckmann, T.; Paterson, C.; Bonomo, P. The dogma of Cetuximab and Radiotherapy in head and neck cancer—A dawn to dusk journey. Clin. Transl. Radiat. Oncol. 2022, 34, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chang, R.; Liu, J.; Wang, Y.; Ren, M.; Xin, K.; Liu, B.; Xie, J.; Yang, Y. Case report: A case of advanced gastric cancer with multiple skin metastases, with significant relief from immunotherapy. Front. Immunol. 2024, 15, 1356350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Xu, P.F.; Nie, Y.L. Partial response to posterior line immunotherapy for more than 15 months in a pMMR patient with cutaneous metastasis of rectal carcinoma: A case report. Ther. Adv. Gastroenterol. 2025, 18, 17562848251338673. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef]
- Pandey, S.; Bradley, L.; Del Fabbro, E. Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions. Cancers 2024, 16, 1696. [Google Scholar] [CrossRef]


| Category | Differentials |
|---|---|
| Inflammatory | - Reactive granulomatous dermatitis - Sarcoidosis - Rheumatoid nodules - Erythema nodosum - Panniculitis - Pseudolymphoma - Lupus erythematosus profundus -Vasculitis - Morphea (nodular subtype) - Lichen nitidus |
| Infectious | - Bacterial abscesses - Tuberculosis cutis (scrofuloderma, lupus vulgaris) - Fungal infections (blastomycosis, histoplasmosis, coccidioidomycosis) - Leishmaniasis - Nocardiosis - Bacterial folliculitis - Bullous impetigo - Actinomycosis - Herpes zoster (for ZMs) |
| Benign tumors | - Lipoma - Dermatofibroma - Sebaceous cysts - Syringoma - Hemangioma - Fibroma - Seborrheic keratosis - Pyogenic granuloma - Glomus tumor |
| Malignant tumors | - Cutaneous lymphomas (mostly B cell type) - Basal cell carcinoma (nodular subtype) - Squamous cell carcinoma - Melanoma - Merkel cell carcinoma - Angiosarcoma |
| Visceral Carcinoma | Localization | Clinical Appearance | Immunohistochemistry Markers | Life Expectancy After Diagnosis (Months) |
|---|---|---|---|---|
| Breast [7,8,9,10,11,12,14] | Anterior chest wall | Nodules | CK7, CK19, S100, CAM 5.2, gamma-globulin, estrogen receptor, progesterone receptor, GCDFP-15 | 31 |
| Lung [19,21,22,23] | Not specific | Not specific | CK7, CAM 5.2, TTF-1, Ber-EP4, naspsin A | <6 |
| Mesothelioma [24,25,26,27,28,34,35,36] | Abdomen | Sister Mary Joseph Nodules | Calretinin, HBME-1, CK5, CK6, orthokeratin, vimentin, EMA | N/A |
| Colorectal [7,12,36,37,38,39,40] | Abdominal surgery scar | Nodules | CK19, CK20, CDX2, CK20, CAM 5.2, CEA, Ber-EP4 | <6 |
| Stomach [7,22,41,42,43,44,45,46] | Not specific | Nodules | CK20, CEA, EMA, CDX2, HIK1083, Ber-EP4, CAM 5.2 | <16 |
| Pancreas [7,47,48,49,50,51,52,53] | Umbilicus | Sister Mary Joseph Nodules | CK7, CK19, CA 19-9, Ber-EP4, CAM 5.2 | 5 |
| Liver [7,56,57,58,59,60,61,62,63,64] | Not specific | Nodules | alfa-fetoprotein, CAM 5.2 | 7 |
| Kidney [7,131,132,133,134,135,136,137,138] | Scalp | Nodules | CK7, EMA, vimentin, keratin, CD10, CEA | <21 |
| Bladder [67,68,69,70,71,75,76] | Not specific | Not specific | CK7, CK20 | <12 |
| Prostate [7,76,77,78,79,80,81,83,86,89] | Inguinal region, abdomen | Nodules | NKX3.1, PSA, PAP, neuroendocrine markers, Ber-EP4, CAM 5.2 | 7 |
| Ovaries [91,92,93,94,100,101,102,103,104,105,106] | Abdomen | Nodules | progesterone receptors, estrogen receptors, CK7, CA125, vimentin, mesothelin, Glut1, PAX8 | <12 |
| Uterus [7,22,109,110,111,112,113,114] | Not specific | Nodules | CK7, PAX8 | 34 |
| Cervix [108,116,117,118,119,120,121] | Not specific | Nodules | CK5, CK6, p63, p16 | <9 |
| Head and neck [16,139,140,141,142,143] | Neck, face | Nodules | CK5, CK6, p16, EMA, EGFR | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorga, P.G.; Dragomirescu, A.; Scurtu, L.G.; Simionescu, O. An Update on Cutaneous Metastases of Internal Malignancies. Medicina 2025, 61, 1570. https://doi.org/10.3390/medicina61091570
Iorga PG, Dragomirescu A, Scurtu LG, Simionescu O. An Update on Cutaneous Metastases of Internal Malignancies. Medicina. 2025; 61(9):1570. https://doi.org/10.3390/medicina61091570
Chicago/Turabian StyleIorga, Polixenia Georgeta, Andreea Dragomirescu, Lucian G. Scurtu, and Olga Simionescu. 2025. "An Update on Cutaneous Metastases of Internal Malignancies" Medicina 61, no. 9: 1570. https://doi.org/10.3390/medicina61091570
APA StyleIorga, P. G., Dragomirescu, A., Scurtu, L. G., & Simionescu, O. (2025). An Update on Cutaneous Metastases of Internal Malignancies. Medicina, 61(9), 1570. https://doi.org/10.3390/medicina61091570






