Assessing Fibrosis Progression and Endothelial Dysfunction in SSc-ILD and COPD: An Integrated Biomarker and CT Densitometry Approach
Abstract
1. Introduction
2. Materials and Methods
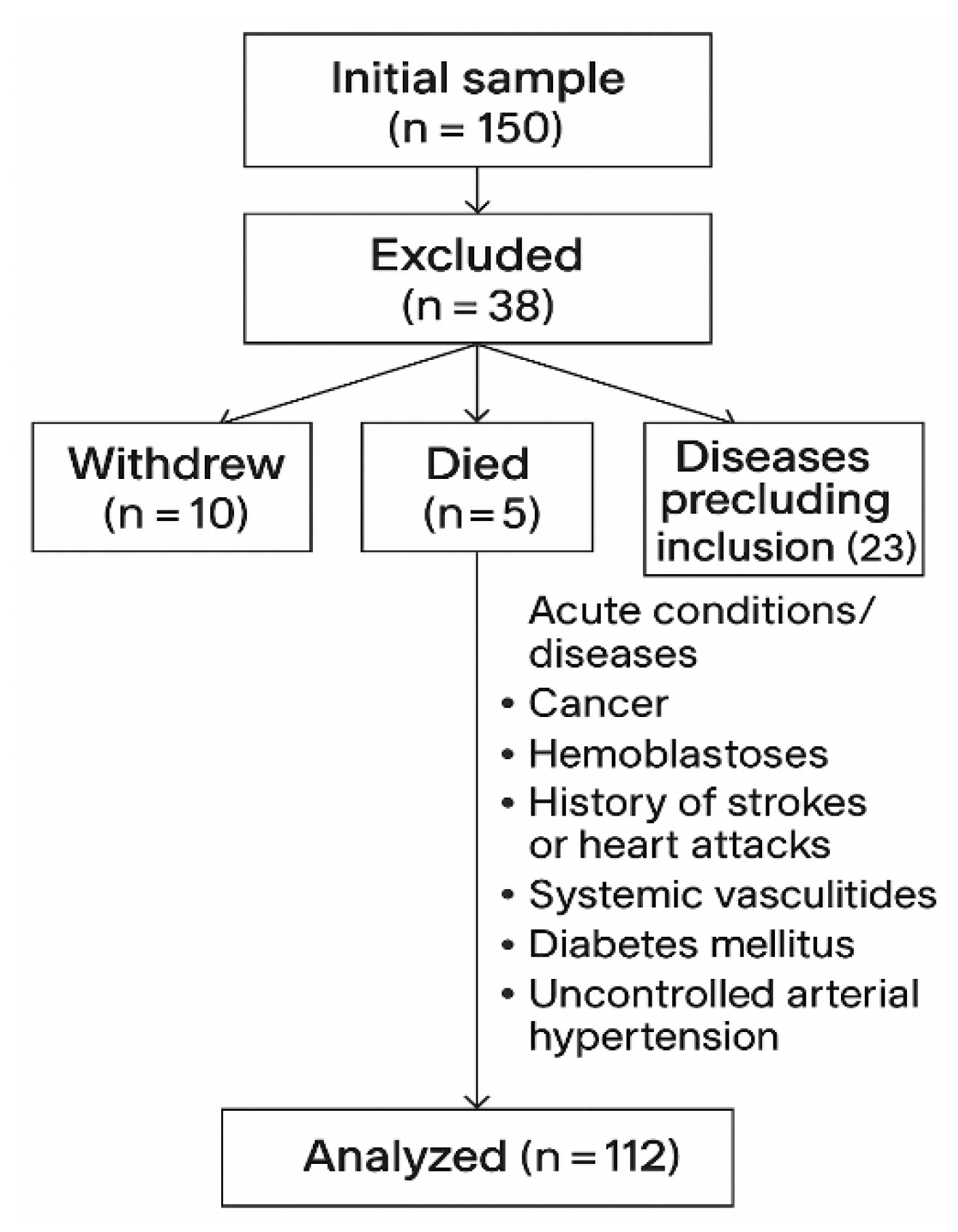
2.1. Study Population
2.2. Exclusion Criteria
2.3. Imaging Protocol
2.4. Biomarker Analysis
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Baseline Characteristics of the Groups
3.2. One-Year Dynamics of Renal Function, Biomarkers, Pulmonary Function, and CT Density in COPD Versus SSc-ILD
3.2.1. Renal Function
3.2.2. Endothelin-1
3.2.3. Galectin-3
3.2.4. Pulmonary Function
3.2.5. CT Densitometry
3.2.6. Functional Outcomes over One Year
3.3. Between-Group Comparisons (COPD vs. SSc-ILD)
3.4. Correlation Analyses
3.4.1. COPD
3.4.2. SSc-ILD
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | Albumin-to-creatinine ratio |
| CKD | Chronic kidney disease |
| CLD | Chronic lung disease |
| COPD | Chronic obstructive pulmonary disease |
| CT | Computed tomography |
| eGFR | Estimated glomerular filtration rate |
| ELISA | Enzyme-linked immunosorbent assay |
| ET-1 | Endothelin-1 |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| Gal-3 | Galectin-3 |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| HRCT | High-resolution computed tomography |
| HU | Hounsfield units |
| ILD | Interstitial lung disease |
| SSc-ILD | Systemic sclerosis-associated interstitial lung disease |
References
- Husain-Syed, F.; McCullough, P.A.; Birk, H.W.; Renker, M.; Brocca, A.; Seeger, W.; Ronco, C. Cardio-pulmonary-renal interactions. J. Am. Coll. Cardiol. 2015, 65, 2433–2448. [Google Scholar] [CrossRef]
- Bollenbecker, S.; Czaya, B.; Gutiérrez, O.M.; Krick, S. Lung–kidney interactions and their role in chronic kidney disease-associated pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L625–L640. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Calimeri, S.; Tranchida, V.; Silipigni, S.; Vella, D.; Ferrara, D.; Spinella, C.; Santoro, D.; Visconti, L. Lung Dysfunction and Chronic Kidney Disease: A Complex Network of Multiple Interactions. J. Pers. Med. 2023, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, Z.; Ding, C. Association between COPD and CKD: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1494291. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, H.W.; Shin, H.J.; Lee, M.; Leem, A.Y.; Jung, J.Y.; Kim, Y.S.; Park, Y. Longitudinal association between kidney and lung function in the Korean general population. Kidney Res. Clin. Pract. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Han, S.; Xu, Y.; Wang, Y. Association between pulmonary function and rapid kidney function decline: A longitudinal cohort study from CHARLS. BMJ Open Respir. Res. 2024, 11, e002107. [Google Scholar] [CrossRef]
- Van Gestel, Y.R.; Chonchol, M.; Hoeks, S.E.; Welten, G.M.; Stam, H.; Mertens, F.W.; van Domburg, R.T.; Poldermans, D. Association between chronic obstructive pulmonary disease and chronic kidney disease in vascular surgery patients. Nephrol. Dial. Transplant. 2009, 24, 2763–2767. [Google Scholar] [CrossRef]
- Perelas, A.; Silver, R.M.; Arrossi, A.V.; Highland, K.B. Systemic sclerosis-associated interstitial lung disease: Pathophysiology and clinical management. Lancet Respir. Med. 2020, 8, 304–320. [Google Scholar] [CrossRef]
- Roland, M.; Bédard, M.E.; Richer, C.; Maltais, F.; Poirier, P. Plasma endothelin-1 in acute exacerbations of chronic obstructive pulmonary disease. Thorax 2001, 56, 30–35. [Google Scholar] [CrossRef]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045894018758528. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef]
- Gerlach, K.; Köhler-Bachmann, S.; Jungck, D.; Körber, S.; Yanik, S.; Knoop, H.; Wehde, D.; Rheinländer, S.; Walther, J.W.; Kronsbein, J.; et al. Endothelin receptor-antagonists suppress lipopolysaccharide-induced cytokine release from alveolar macrophages of non-smokers, smokers and COPD subjects. Eur. J. Pharmacol. 2015, 768, 123–130. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Genre, F.; López-Mejías, R.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Portilla, V.; Sebastián Mora-Gil, M.; Ocejo-Vinyals, J.G.; Gualillo, O.; Blanco, R.; et al. Endothelin-1 and galectin-3 in interstitial lung disease: Current evidence and future perspectives. Int. J. Mol. Sci. 2023, 24, 1275. [Google Scholar] [CrossRef]
- Zhao, X.; Han, B.; Tang, W.; Ji, S.; Wang, L.; Huang, J.; Hu, Y.; Li, J. Association between serum galectin-3 and chronic obstructive pulmonary disease: A meta-analysis. Biomol. Biomed. 2024, 24, 1491–1500. [Google Scholar] [CrossRef]
- Pelaia, C.; Pastori, D.; Armentaro, G.; Miceli, S.; Cassano, V.; Barbara, K.; Pelaia, G.; Perticone, M.; Maio, R.; Pignatelli, P.; et al. Predictors of Renal Function Worsening in Patients with Chronic Obstructive Pulmonary Disease (COPD): A Multicenter Observational Study. Nutrients. 2021, 13, 2811. [Google Scholar] [CrossRef] [PubMed]
- Romundstad, S.; Naustdal, T.; Romundstad, P.R.; Sorger, H.; Langhammer, A. COPD and microalbuminuria: A 12-year follow-up study. Eur. Respir. J. 2014, 43, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.Y.; San José Estépar, R.; Fain, S.B.; Tal-Singer, R.; Stockley, R.A.; Nordenmark, L.H.; Rennard, S.; Han, M.K.; Merrill, D.; Humphries, S.M.; et al. Relationship between emphysema progression at CT and mortality in ever-smokers: Results from the COPDGene and ECLIPSE cohorts. Radiology 2021, 299, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Baratella, E.; Confalonieri, P.; Wade, B.; Marrocchio, C.; Geri, P.; Busca, A.; Pozzan, R.; Andrisano, A.G.; Cova, M.A.; et al. High-Resolution Computed Tomography: Lights and Shadows in Improving Care for SSc-ILD Patients. Diagnostics 2021, 11, 1960. [Google Scholar] [CrossRef]
- Carnevale, A.; Silva, M.; Maietti, E.; Milanese, G.; Saracco, M.; Parisi, S.; Bravi, E.; De Gennaro, F.; Arrigoni, E.; Bodini, F.C.; et al. Longitudinal change during follow-up of systemic sclerosis: Correlation between high-resolution computed tomography and pulmonary function tests. Clin. Rheumatol. 2021, 40, 213–219. [Google Scholar] [CrossRef]
- Scheja, A.; Bartosik, I.; Wuttge, D.M.; Hesselstrand, R. Renal function is mostly preserved in patients with systemic sclerosis. Scand. J. Rheumatol. 2009, 38, 295–298. [Google Scholar] [CrossRef]
- Bonhomme, O.; André, B.; Gester, F.; de Seny, D.; Moermans, C.; Struman, I.; Louis, R.; Malaise, M.; Guiot, J. Biomarkers in systemic sclerosis-associated interstitial lung disease: Review of the literature. Rheumatology 2019, 58, 1534–1546. [Google Scholar] [CrossRef]
- Koca, S.S.; Akbas, F.; Ozgen, M.; Yolbas, S.; Ilhan, N.; Gundogdu, B.; Isik, A. Serum galectin-3 level in systemic sclerosis. Clin. Rheumatol. 2014, 33, 215–220. [Google Scholar] [CrossRef] [PubMed]
- ISO 15189:2015; Medical laboratories—Requirements for quality and competence. International Organization for Standardization: Geneva, Switzerland, 2015.
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD guideline: Behind the scenes and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Li, R.; Piao, T.H.; Wang, C.L.; Wu, X.L.; Cai, H.Y. Pathological mechanisms and targeted drugs of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1565–1575. [Google Scholar] [CrossRef]
- Gao, J.; Wang, A.; Li, X.; Li, J.; Zhao, H.; Zhang, J.; Liang, J.; Chen, S.; Wu, S. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of chronic kidney diseases. Kidney Blood Press. Res. 2020, 45, 84–94. [Google Scholar] [CrossRef]
- Antus, B.; Kardos, Z. Oxidative stress in COPD: Molecular background and clinical monitoring. Curr. Med. Chem. 2015, 22, 627–650. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Hesse, L.; Faiz, A.; Lubbers, J.; Bodha, P.K.; Ten Hacken, N.H.; van Oosterhout, A.J.; Nawijn, M.C.; Heijink, I.H. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L881–L892. [Google Scholar] [CrossRef]
- Fan, W.; Gui, B.; Zhou, X.; Li, L.; Chen, H. Lung injury: Mechanisms, biomarkers, and monitoring. Crit. Care 2024, 28, 352. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.; Di Stefano, A.; Freato, N.; Bertocchi, L.; Brun, P. Inhibition of pro-fibrotic molecule expression in idiopathic pulmonary fibrosis-derived lung fibroblasts by lactose-modified hyaluronic acid compounds. Polymers 2024, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Faludi, R.; Nagy, G.; Tőkés-Füzesi, M.; Kovács, K.; Czirják, L.; Komócsi, A. Galectin-3 is an independent predictor of survival in systemic sclerosis. Int. J. Cardiol. 2017, 233, 118–124. [Google Scholar] [CrossRef]
- Emre, S.; Fazlıoğlu, N.; Emre, E.; Mutlu, L.C.; Yılmaz, A. Galectin-3 level in idiopathic pulmonary fibrosis and its relationship with response to antifibrotic treatment. Respir. Med. 2025, 240, 108028. [Google Scholar] [CrossRef]
- Koga, Y.; Motegi, M.; Ono, A.; Hachisu, Y.; Utsugi, M.; Sunaga, N.; Takise, A.; Sato, M.; Kuwako, T.; Osaki, T.; et al. Serum galectin-3 as a biomarker of progression of idiopathic pulmonary fibrosis treated with nintedanib. Respir. Investig. 2025, 63, 394–398. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pasichnyk, K.; Lopez-Guisa, J.M.; Collins, S.; Hsu, D.K.; Liu, F.T.; Eddy, A.A. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am. J. Physiol. Renal Physiol. 2011, 300, F245–F253. [Google Scholar] [CrossRef]
- Fairley, J.L.; Ross, L.; Paratz, E.; McKelvie, P.; MBiostatistics, D.H.; Stevens, W.; La Gerche, A.; Nikpour, M. Pathological contributors to organ damage and mortality in systemic sclerosis: A nationwide matched case-control study. Semin. Arthritis Rheum. 2025, 73, 152739. [Google Scholar] [CrossRef] [PubMed]
- Saldana, D.C.; Hague, C.J.; Murphy, D.; Coxson, H.O.; Tschirren, J.; Peterson, S.; Sieren, J.P.; Kirby, M.; Ryerson, C.J. Association of computed tomography densitometry with disease severity, functional decline, and survival in systemic sclerosis-associated interstitial lung disease. Ann. Am. Thorac. Soc. 2020, 17, 813–820. [Google Scholar] [CrossRef] [PubMed]


| Slice | Anatomical Landmark (Right Lung) | Slice | Anatomical Landmark (Left Lung) |
|---|---|---|---|
| R1 | Aortic arch—right side | L1 | Aortic arch—left side |
| R2 | 1 cm below the carina—right side | L2 | 1 cm below the carina—left side |
| R3 | At the level of pulmonary vein entry—right | L3 | At the level of pulmonary vein entry—left |
| R4 | Midpoint between R3 and R5—right side | L4 | Midpoint between L3 and L5—left side |
| R5 | 2 cm above the hemidiaphragm—right side | L5 | 2 cm above the hemidiaphragm—left side |
| R6 | 1 cm below the hemidiaphragm—right side | L6 | 1 cm below the hemidiaphragm—left side |
| Variable | COPD 58 n (%) | SSc-ILD 54 n (%) |
|---|---|---|
| Gender | ||
| Male | 50 (86.3%) | 12 (22.2%) |
| Female | 8 (13.7%) | 42 (77.8%) |
| Median [QR] | Median [QR] | |
| Age | 56.0 [52.0–60.0] | 53 [49.0–57.0] |
| Smoking status | 58 (100%) | 3 (5.5%) |
| Spirometry Median [QR] | ||
| FEV1% predicted | 69.1 [61.0–76.0] | 72.5 [66.0–80.0] |
| FVC% predicted | 69.7 [64.0–76.5] | 77.5 [71.0–84.0] |
| Marker (Unit) | COPD | SSc-ILD | ||||||
|---|---|---|---|---|---|---|---|---|
| Median Δ (2024–2023) | Median_log2FC | p | Δ% | Median Δ (2024–2023) | Median_log2FC | p | Δ% | |
| GFR (mL/min/1.73 m2) | 90.37 | −0.101 | 0.001 * | −6.76 | 92.4 | −0.046 | 0.029 * | −3.16 |
| Endothelin-1 (pg/mL) | 38.9 | 0.878 | 0.0002 * | 83.78 ** | 47.1 | 0.308 | 0.0001 * | 23.83 ** |
| Galectin-3 (ng mL) | 16.3 | 0.018 | 0.964 | 1.26 | 21.3 | 0.140 | 0.043 | 10.20 |
| FVC (% predicted) | 68.1 | −0.059 | 0.01 * | −4.01 | 78.25 | 0.041 | 0.326 | 2.90 |
| ACR (mg g) | 3.05 | 0.275 | 0.281 | 21.00 ** | 4.65 | 0.092 | 0.681 | 6.58 |
| FEV1 (% predicted) | 42 | −0.103 | 0.584 | −6.89 | 73.8 | 0.068 | 0.128 | 4.86 |
| Total lung volume (cm3) | 683.3 | 0.042 | 0.146 | 2.95 | 3254.8 | −0.091 | 0.020 * | −6.08 |
| R1 density (HU) | −877 | 0.001 | 0.001 * | 0.07 | −800.8 | −0.007 | 0.109 | −0.50 |
| L1 density (HU) | −870.8 | 0.003 | 0.004 * | 0.21 | −797.5 | 0.002 | 0.92 | 0.13 |
| R2 density (HU) | −874.5 | 0.0001 | 0.002 * | 0.01 | −788.5 | −0.007 | 0.651 | −0.50 |
| L2 density (HU) | −871.8 | 0.001 | 0.414 | 0.07 | −801 | 0.001 | 0.484 | 0.06 |
| R3 density (HU) | −877.5 | −0.003 | 0.13 | −0.21 | −787.3 | 0.003 | 0.565 | 0.19 |
| L3 density (HU) | −876.3 | 0.007 | 0.95 | 0.49 | −797.3 | 0.009 | 0.726 | 0.69 |
| R4 density (HU) | −879.5 | −0.005 | 0.016 * | −0.35 | −785.5 | −0.02 | 0.505 | −1.39 |
| L4 density (HU) | −885.5 | −0.006 | 0.354 | −0.42 | −785 | −0.009 | 0.321 | −0.64 |
| R5 density (HU) | −884 | −0.011 | 0.113 | −0.76 | −784.5 | 0.003 | 0.908 | 0.19 |
| L5 density (HU) | −888.5 | −0.002 | 0.694 | −0.14 | −780.5 | 0.004 | 0.353 | 0.32 |
| R6 density (HU) | −895 | −0.003 | 0.564 | −0.21 | −782.75 | −0.01 | 0.108 | −0.70 |
| L6 density (HU) | −895.3 | −0.003 | 0.117 | −0.21 | −788.5 | −0.002 | 0.004 * | −0.13 |
| SpO2 after 6MWT (%) | 0 | −0.015 | 0.128 | −1.058 | 0 | −0.023 | 0.015 * | −1.604 |
| Borg scale after 6MWT (score) | 0 | 0.193 | 0.671 | 14.286 | 0 | 0.415 | 0.002 * | 33.333 |
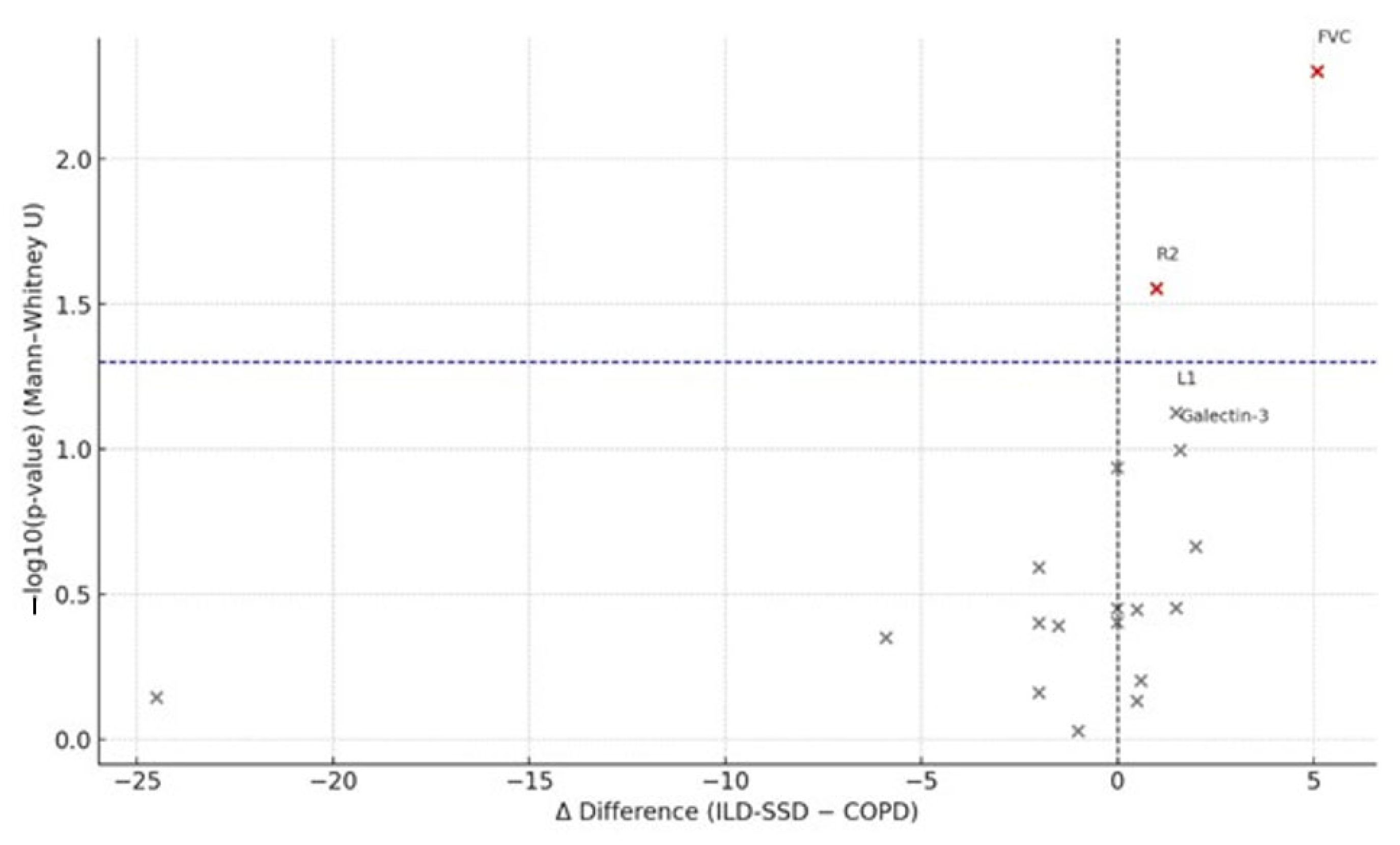
| Marker (unit) | Median Δ (COPD) | Median Δ (SSc-ILD) | Δ Difference (SSc-ILD − COPD) | p-Value (Mann–Whitney U) |
|---|---|---|---|---|
| GFR (mL/min/1.73 m2) | −3.3 | −2.65 | 0.6 | 0.628 |
| Endothelin-1 (pg/mL) | 10.722 | 4.7231 | −5.9 ** | 0.447 |
| Galectin-3 (ng mL) | −0.25 | 1.3 | 1.6 ** | 0.101 |
| FVC (% predicted) | −4 | 1.05 | 5.1 ** | 0.005 * |
| ACR (mg g) | 0 | 0 | 0 | 0.396 |
| FEV1 (% predicted) | 1.5 | 2 | 0.5 | 0.358 |
| Total lung volume (cm3) | −93 | −117.5 | −24.5 ** | 0.718 |
| R1 density (HU) | −2 | −2 | 0 | 0.355 |
| L1 density (HU) | −2.5 | −1 | 1.5 ** | 0.075 |
| R2 density (HU) | −2 | −1 | 1 | 0.028 * |
| L2 density (HU) | 1 | −1 | −2 ** | 0.256 |
| R3 density (HU) | 2 | 0 | −2 ** | 0.691 |
| L3 density (HU) | 0.5 | −0.5 | −1 | 0.937 |
| R4 density (HU) | 2 | 0.5 | −1.5 ** | 0.407 |
| L4 density (HU) | 1.5 | 2 | 0.5 | 0.739 |
| R5 density (HU) | 2 | 0 | −2 ** | 0.398 |
| L5 density (HU) | −1 | 0.5 | 1.5 ** | 0.353 |
| R6 density (HU) | 1 | 3 | 2 ** | 0.217 |
| L6 density (HU) | 2.5 | 2.5 | 0 | 0.116 |
| SpO2 after 6MWT (%) | 0.0 | 0.0 | 0.0 | 0.575 |
| Borg scale after 6MWT (score) | 0.0 | 0.0 | 0.0 | 0.018 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrayeva, L.; Bacheva, I.; Alina, A.; Klassen, O. Assessing Fibrosis Progression and Endothelial Dysfunction in SSc-ILD and COPD: An Integrated Biomarker and CT Densitometry Approach. Medicina 2025, 61, 1572. https://doi.org/10.3390/medicina61091572
Ibrayeva L, Bacheva I, Alina A, Klassen O. Assessing Fibrosis Progression and Endothelial Dysfunction in SSc-ILD and COPD: An Integrated Biomarker and CT Densitometry Approach. Medicina. 2025; 61(9):1572. https://doi.org/10.3390/medicina61091572
Chicago/Turabian StyleIbrayeva, Lyazat, Irina Bacheva, Assel Alina, and Olga Klassen. 2025. "Assessing Fibrosis Progression and Endothelial Dysfunction in SSc-ILD and COPD: An Integrated Biomarker and CT Densitometry Approach" Medicina 61, no. 9: 1572. https://doi.org/10.3390/medicina61091572
APA StyleIbrayeva, L., Bacheva, I., Alina, A., & Klassen, O. (2025). Assessing Fibrosis Progression and Endothelial Dysfunction in SSc-ILD and COPD: An Integrated Biomarker and CT Densitometry Approach. Medicina, 61(9), 1572. https://doi.org/10.3390/medicina61091572






