Real-World Data of First 12-Months of Ofatumumab Treatment in Multiple Sclerosis Patients—A Multicenter Experience from Tertiary Referral Centers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Patient Eligibility for Ofatumumab Therapy
2.3. Evaluation of the Efficacy and Safety of Ofatumumab Treatment
2.4. Statistical Analysis
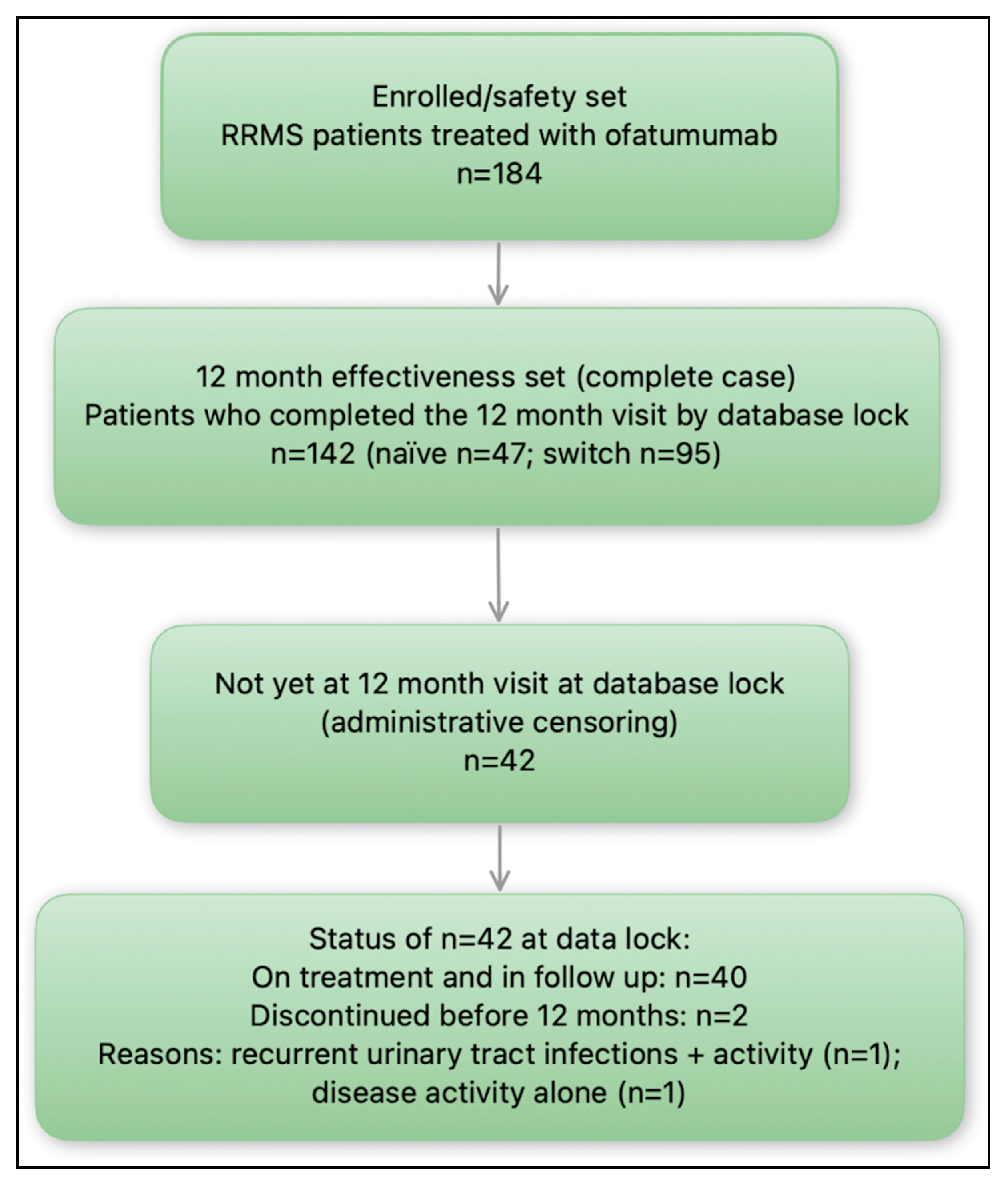
3. Results
3.1. Study Group Characteristics
3.2. Clinical and Radiological Efficacy of Ofatumumab Treatment After First 12-Months
3.3. Adverse Events
3.4. Ofatumumab Discontinuation
3.5. Factors Determining Clinical and Radiological Activity of the Disease
3.6. Reasons in Switching Therapy into Ofatumumab
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| RRMS | Relapsing-Remitting Multiple Sclerosis |
| CDP | Confirmed Disability Progression |
| CNS | Central Nervous System |
| EDSS | Expanded Disability Status Scale |
| DMT | Disease-Modifying Therapy |
| MRI | Magnetic Resonance Imaging |
| T2-w/T1-w | T2-weighted/T1-weighted |
| GELs | Gadolinium-Enhancing Lesions |
| NEDA-3 | No Evidence of Disease Activity-3 |
| IRRs | Infusion-Related Reactions |
| AE/AEs | Adverse Event/s |
| SC | Subcutaneous |
| RCT | Randomized Controlled Trial |
| ARR | Annual Relapse Rate |
| RWE | Real-World Evidence |
| HETA | High-Efficacy Treatment Agents |
| mAb | Monoclonal Antibody |
| HSV | Herpes Simplex Virus |
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Kapica-Topczewska, K.; Kulakowska, A.; Kochanowicz, J.; Brola, W. Epidemiology of multiple sclerosis: Global trends, regional differences, and clinical implications. Neurol. Neurochir. Pol. 2025, 59, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Zainab, R.S.; Khan, J.Z.; Tipu, M.K.; Jahan, F.; Irshad, N. A review on multiple sclerosis: Unravelling the complexities of pathogenesis, progression, mechanisms and therapeutic innovations. Neuroscience 2025, 567, 133–149. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of multiple sclerosis: A review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Hegen, H.; Bsteh, G.; Berger, T. ‘No evidence of disease activity’—is it an appropriate surrogate in multiple sclerosis? Eur. J. Neurol. 2018, 25, 1107-e101. [Google Scholar] [CrossRef]
- Healy, B.C.; Glanz, B.I.; Swallow, E.; Signorovitch, J.; Hagan, K.; Silva, D.; Pelletier, C.; Chitnis, T.; Weiner, H. Confirmed disability progression provides limited predictive information regarding future disease progression in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 2055217321999070. [Google Scholar] [CrossRef]
- Chylińska, M.; Komendziński, J.; Wyszomirski, A.; Karaszewski, B. Brain atrophy as an outcome of disease-modifying therapy for remitting-relapsing multiple sclerosis. Mult. Scler. Int. 2023, 2023, 4130557. [Google Scholar] [CrossRef]
- Sabatino, J.J., Jr.; Zamvil, S.S.; Hauser, S.L. B-cell therapies in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a032037. [Google Scholar] [CrossRef]
- Hauser, S.L.; Waubant, E.; Arnold, D.L.; Vollmer, T.; Antel, J.; Fox, R.J.; Bar-Or, A.; Panzara, M.; Sarkar, N.; Agarwal, S.; et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008, 358, 676–688. [Google Scholar] [CrossRef]
- Mancinelli, C.R.; Rossi, N.; Capra, R. Ocrelizumab for the treatment of multiple sclerosis: Safety, efficacy, and pharmacology. Ther. Clin. Risk Manag. 2021, 17, 765–776. [Google Scholar] [CrossRef]
- Steinman, L.; Fox, E.; Hartung, H.P.; Alvarez, E.; Qian, P.; Wray, S.; Robertson, D.; Huang, D.; Selmaj, K.; Wynn, D.; et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N. Engl. J. Med. 2022, 387, 704–714. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Wiendl, H.; Montalban, X.; Alvarez, E.; Davydovskaya, M.; Delgado, S.R.; Evdoshenko, E.P.; Giedraitiene, N.; Gross-Paju, K.; Haldre, S.; et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase 2 study. Mult. Scler. 2022, 28, 910–924. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.S.; Lisby, S.; Grove, R.; Derosier, F.; Shackelford, S.; Havrdova, E.; Drulovic, J.; Filippi, M. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: A phase 2 study. Neurology 2014, 82, 573–581. [Google Scholar] [CrossRef]
- Bar-Or, A.; Grove, R.A.; Austin, D.J.; Tolson, J.M.; Van-Meter, S.A.; Lewis, E.W.; Derosier, F.J.; Lopez, M.C.; Kavanagh, S.T.; Miller, A.E.; et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018, 90, e1805–e1814. [Google Scholar] [CrossRef]
- Novartis Pharma, A.G. Kesimpta—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf (accessed on 15 May 2025).
- Bar-Or, A.; Schubert-Tennigkeit, A.A.; Mairon, N.; Kerloeguen, C.; Gufran, M.; Shaikh, S.; Das Gupta, A.; Gunisetti, E.; Weckbecker, G.; Su, W.; et al. Dose-dependent tolerability of intravenous and subcutaneous ofatumumab in clinical studies. Mult. Scler. 2020, 26 (Suppl. S3), 118–659. [Google Scholar]
- Ministerstwo Zdrowia. Programy Lekowe. Available online: https://www.gov.pl/web/zdrowie/programy-lekowe (accessed on 15 May 2025).
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Sąsiadek, M.; Hartel, M.; Siger, M.; Katulska, K.; Majos, A.; Kluczewska, E.; Bartosik-Psujek, H.; Kułakowska, A.; Słowik, A.; Steinborn, B.; et al. Recommendations of the Polish Medical Society of Radiology and the Polish Society of Neurology for a protocol concerning routinely used magnetic resonance imaging in patients with multiple sclerosis. Neurol. I Neurochir. Pol. 2020, 54, 410–415. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 22 August 2025).
- Hauser, S.L.; Cohen, J.A.; de Sèze, J.; Meuth, S.G.; Giacomini, P.S.; Nakahara, J.; Oreja-Guevara, C.; Robertson, D.; Wray, S.; Bhatt, A.; et al. Five-year efficacy safety and efficacy outcomes with ofatumumab in patients with relapsing multiple sclerosis. Neurol. Ther. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Amin, M.; Harvey, T.; Pineda, D.M.; Tai, M.H.; Shao, Q.; Brown, B.; Gadkari, A.; Moss, B.; Conway, D.S.; Hersh, C.M. Real-world effectiveness, persistence, tolerability, and safety of ofatumumab in clinical practice. Neurodegener. Dis. Manag. 2025, 15, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.S.; Klimas, R.; Katsimpoura, M.; Sgodzai, M.; Theile-Ochel, S.; Poser, P.L.; Gisevius, B.; Faissner, S.; Salmen, A.; Nastos, I.; et al. Quality of life and tolerability of B-cell directed therapy of multiple sclerosis with ofatumumab in a patient-centered real-world observational study. J. Neurol. 2024, 271, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.; Pogoda-Wesołowska, A.; Staszewski, J.; Brola, W.; Kania, K.; Krzystanek, E.; Rusek, S.; Zajdel, R.; Hałas, M.; Karaszewski, B. Early Treatment with Ofatumumab Increases the Likelihood of Stabilizing Disease in Patients with Relapsing-Remitting Multiple Sclerosis. Arch. Med. Sci. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Tai, M.-H.; Brown, B.; Taiji, R.; Faucher, A.; Vekeman, F.; Ionescu-Ittu, R.; Gadkari, A. Real-World Change in Annualized Relapse Rate and Healthcare Resource Utilization Following Initiation of Ofatumumab in People with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2025, 102, 106617. [Google Scholar] [CrossRef] [PubMed]
- Zanghì, A.; Borriello, G.; Bonavita, S.; Fantozzi, R.; Signoriello, E.; Barone, S.; Abbadessa, G.; Cellerino, M.; Ziccone, V.; Miele, G.; et al. Ocrelizumab and Ofatumumab Comparison: An Italian Real-World Propensity Score Matched Study. J. Neurol. 2024, 271, 4495–4502. [Google Scholar] [CrossRef]
- Cohen, J.A.; Hauser, S.L.; Cross, A.H.; Winthrop, K.; Wiendl, H.; Nicholas, J.; Meuth, S.; Giacomini, P.; Sacca, F.; Zielman, R.; et al. Five-Year Safety of Ofatumumab in People Living with Relapsing Multiple Sclerosis (P8-3.004). Neurology 2023, 100 (Suppl. S2), 2942. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Zhao, X.; Xu, L.; Sun, M.; Meng, Z. The real-world safety of ofatumumab: A pharmacovigilance analysis based on the FDA adverse event reporting system. Front. Immunol. 2025, 16, 1515730. [Google Scholar] [CrossRef]
- Chen, H. Real-World Pharmacovigilance of Ofatumumab in Multiple Sclerosis: A Comprehensive FAERS Data Analysis. Front. Pharmacol. 2025, 15, 1521726. [Google Scholar] [CrossRef]
- Hersh, C.M.; Gorritz, M.; Chen, C.C.; Tuly, R.; Gu, Y.; Gadkari, A.; Brown, B.; Shao, Q. Real-world persistence and adherence of ofatumumab versus oral and injectable disease-modifying therapies in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 91, 105888. [Google Scholar] [CrossRef]
| Characteristics | Patients After Switch form Another DMT (N = 95) | Naïve Patients (N = 47) | p * |
|---|---|---|---|
| Male/female [n, %] | 22/73 (23.2/76.8) | 14/33 (29.8/70.2) | 0.39 |
| Age [years ± SD] | 39.47 ± 11.72 | 33.91 ± 8.71 | 0.29 |
| Duration of disease [years ± SD] | 11.11 ± 7.02 | 2.85 ± 1.48 | <0.001 |
| EDSS, [median, min–max) | 2 (0–5.5) | 2 (0–4.5) | 0.07 |
| Efficacy of Treatment Parameters | Patients After Switch form Another DMT (N = 95) | Naïve Patients (N = 47) | p * |
|---|---|---|---|
| Relapses (n, %) | 12 (12.6) | 5 (10.6) | 0.72 |
| T2-w lesions (n, %) | 12 (12.6) | 6 (12.8) | 0.35 |
| GELs (n, %) | 3 (3.2) | 2 (4.3) | 0.75 |
| EDSS progression (n, %) | 13 (13.7) | 5 (10.6) | 0.59 |
| EDSS improvement (n, %) | 12 (12.4) | 8 (17.1) | 0.49 |
| NEDA-3 (n, %) | 72 (75.8) | 36 (76.6) | 0.92 |
| Adverse Events (AEs) | Patients After Switch form Another DMT (N = 95) | Naïve Patients (N = 47) | p * |
|---|---|---|---|
| Total (n, %) | 62 (65.3) | 34 (72.3) | 0.40 |
| Infusion related reactions (n, %) | 57 (60.0) | 31 (66.0) | 0.49 |
| Infections (n, %) | 18 (18.9) | 7 (14.9) | 0.55 |
| Age | Gender (Female) | Disease Duration | De Novo Treatment | EDSS at Treatment Initiation | |
|---|---|---|---|---|---|
| Relapse Activity | |||||
| OR | 1.02 | 0.69 | 1.01 | 1.09 | 1.28 |
| 95% CI | 0.96–1.09 | 0.21–2.37 | 0.92–1.11 | 0.26–4.71 | 1.08–1.52 |
| p | 0.49 | 0.56 | 0.82 | 0.9 | 0.005 |
| New or enlarged T2-w lesions | |||||
| OR | 1.02 | 1.35 | 0.92 | 0.76 | 1.11 |
| 95% CI | 0.96–1.07 | 0.39–4.66 | 0.82–1.03 | 0.22–2.58 | 0.93–1.31 |
| p | 0.55 | 0.63 | 0.14 | 0.67 | 0.24 |
| GELs | |||||
| OR | 1.07 | 1.03 | 0.99 | 1.15 | 0.88 |
| 95% CI | 1.007–1.67 | 0.92–1.15 | 0.84–1.16 | 0.14–9.19 | 0.65–1.2 |
| p | 0.02 | 0.56 | 0.86 | 0.89 | 0.43 |
| EDSS progression | |||||
| OR | 1.02 | 0.7 | 0.99 | 0.89 | 1.11 |
| 95% CI | 0.96–1.07 | 0.23–2.12 | 0.91–1.09 | 0.25–3.16 | 0.94–1.29 |
| p | 0.56 | 0.53 | 0.95 | 0.86 | 0.22 |
| NEDA-3 | |||||
| OR | 0.99 | 1.21 | 1.01 | 0.98 | 0.91 |
| 95% CI | 0.95–1.04 | 0.49–2.92 | 0.93–1.09 | 0.38–2.54 | 0.8–1.04 |
| p | 0.86 | 0.68 | 0.81 | 0.97 | 0.17 |
| Author, Year | Study Design, Region | N (Population) | Follow-Up | Prior DMT Exposure | Relapse Outcomes | NEDA-3 | Safety/Persistence Highlights |
|---|---|---|---|---|---|---|---|
| Amin, 2025 [25] | Retrospective EMR, 2 US MS centers) | 175 (80% RRMS/CIS; 15.4% SPMS; 4.6% PPMS) | 12 mo | 86% previously treated | 1 relapse over 12 mo (~0.6%); marked drop in new T2 and GELs | NR (NEDA-2 85% in subset) | 90.6% on-treatment at 12 mo; systemic IRR in 36% (mostly early); IgG stable, IgM ↓ but not linked to infections |
| Karl, 2024 [26] | Prospective, Germany | 81 RMS | ~6 mo visit (~10 mo on OFA at FU) | Mixed | 4 relapses (5%); EDSS stable; no discontinuations | NR | Headache and limb pain persisted; handling rated ‘very easy’ by 88% |
| Stępień, 2025 [27] | Multicentre, Poland | 430 RRMS | 12 mo (EDSS to 24 mo) | 73% previously treated; 27% naïve | Relapse-free: 45% → 88% | 72.7% | AEs in 18.6% (mostly flu-like/weakness); no discontinuations; lymphocytes dipped at 2 mo then normalized |
| Tai, 2025 [28] | US claims, pre-post | 779 MS | Mean 1.36 yrs | 63% had DMT pre-index | ARR 0.41 → 0.10 (75% ↓); hospitalizations 90% ↓ | NR | Safety not captured in claims |
| Zanghì, 2024 [29] | Italy; OFA vs. OCR, PSM; OFA arm | 180 RMS | Mean 13.2 mo | Mix of naïve/switch; high-efficacy pre-DMT excluded | ARR 0.038; MRI activity 2.7% | 94.4% | IRR 17.2%, URTI 11.1%, UTI 3.3%; no SAEs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galus, W.; Kaczmarczyk, A.; Walawska-Hrycek, A.; Siuda, J.; Polewka, M.; Lasek-Bal, A.; Puz, P. Real-World Data of First 12-Months of Ofatumumab Treatment in Multiple Sclerosis Patients—A Multicenter Experience from Tertiary Referral Centers. Medicina 2025, 61, 1568. https://doi.org/10.3390/medicina61091568
Galus W, Kaczmarczyk A, Walawska-Hrycek A, Siuda J, Polewka M, Lasek-Bal A, Puz P. Real-World Data of First 12-Months of Ofatumumab Treatment in Multiple Sclerosis Patients—A Multicenter Experience from Tertiary Referral Centers. Medicina. 2025; 61(9):1568. https://doi.org/10.3390/medicina61091568
Chicago/Turabian StyleGalus, Weronika, Aleksandra Kaczmarczyk, Anna Walawska-Hrycek, Joanna Siuda, Milena Polewka, Anetta Lasek-Bal, and Przemysław Puz. 2025. "Real-World Data of First 12-Months of Ofatumumab Treatment in Multiple Sclerosis Patients—A Multicenter Experience from Tertiary Referral Centers" Medicina 61, no. 9: 1568. https://doi.org/10.3390/medicina61091568
APA StyleGalus, W., Kaczmarczyk, A., Walawska-Hrycek, A., Siuda, J., Polewka, M., Lasek-Bal, A., & Puz, P. (2025). Real-World Data of First 12-Months of Ofatumumab Treatment in Multiple Sclerosis Patients—A Multicenter Experience from Tertiary Referral Centers. Medicina, 61(9), 1568. https://doi.org/10.3390/medicina61091568








