Comparative Diagnostic and Prognostic Performance of SWI and T2-Weighted MRI in Cerebral Microbleed Detection Following Acute Ischemic Stroke: A Meta-Analysis and SPOT-CMB Study
Abstract
1. Background
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
- (1)
- Study characteristics: author, country, publication year, study name or registry, study design, cohort size;
- (2)
- Participant characteristics: age, sex, comorbidities, number of patients with CMBs at baseline, stroke subtype, CMB location, and specific characteristics of patients with AIS;
- (3)
- Imaging parameters: MRI sequence type for CMB detection, field strength, slice thickness.The ‘SWI and T2*’ subgroup is defined as studies that visualized CMBs in their patients using either SWI or T2* sequences. Slice thickness was extracted as reported and categorized using study-defined thresholds: Thin (≤2 mm), Medium (2.1–4.9 mm), and Thick (≥5 mm), based on radiological conventions commonly applied in neuroimaging studies [15,16];
- (4)
- Definition and criteria of various parameters: CMBs, sICH, poor functional outcome;
- (5)
- Clinical outcomes: occurrence of sICH, HT, and mRS score for functional outcome at 90 days, assessed in relation to the presence or absence of CMBs.
2.4. Methodological Quality Assessment of Included Studies
2.5. Certainty of Evidence Assessment
2.6. Statistical Analyses
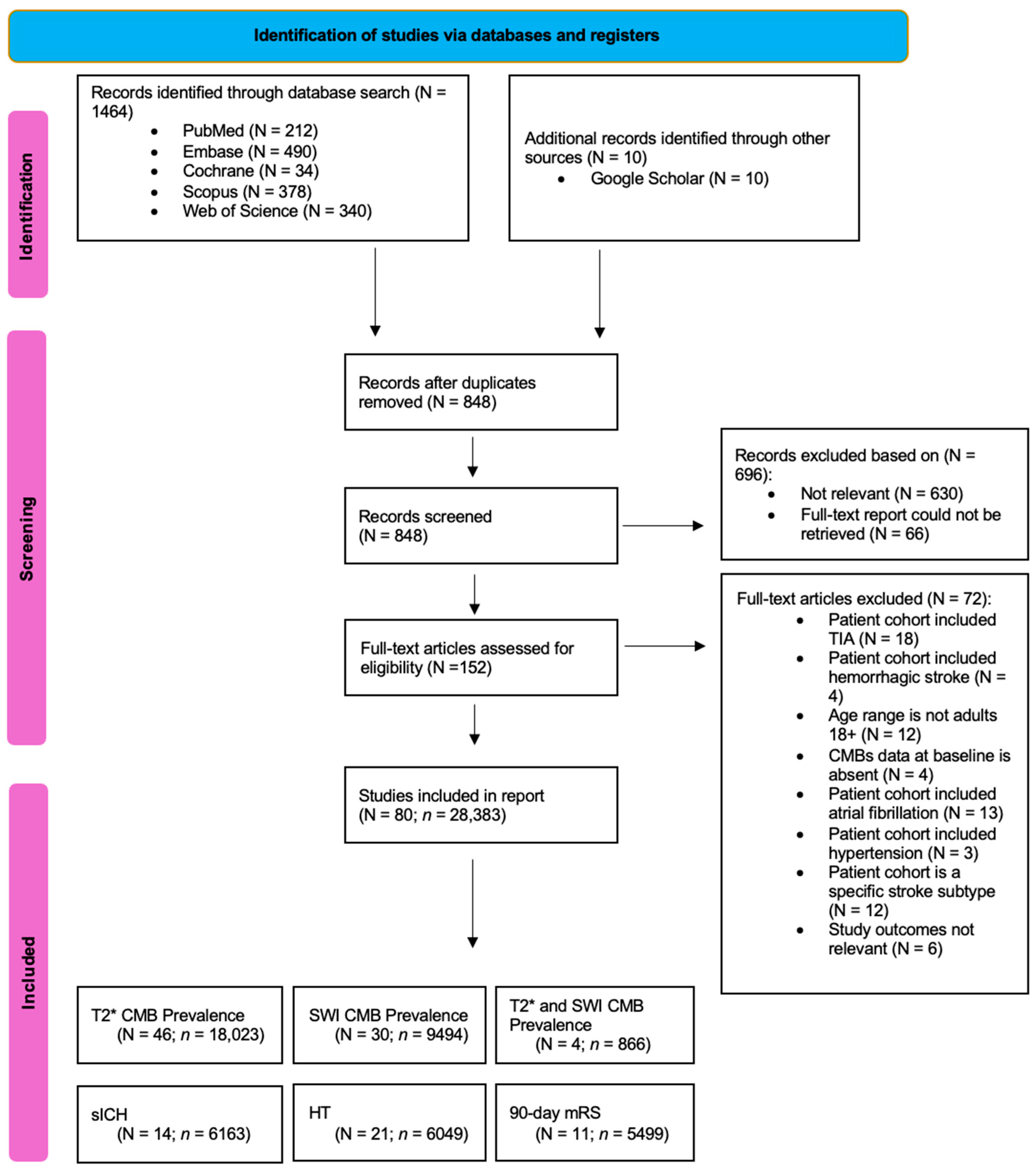
3. Results
3.1. Description of Included Studies
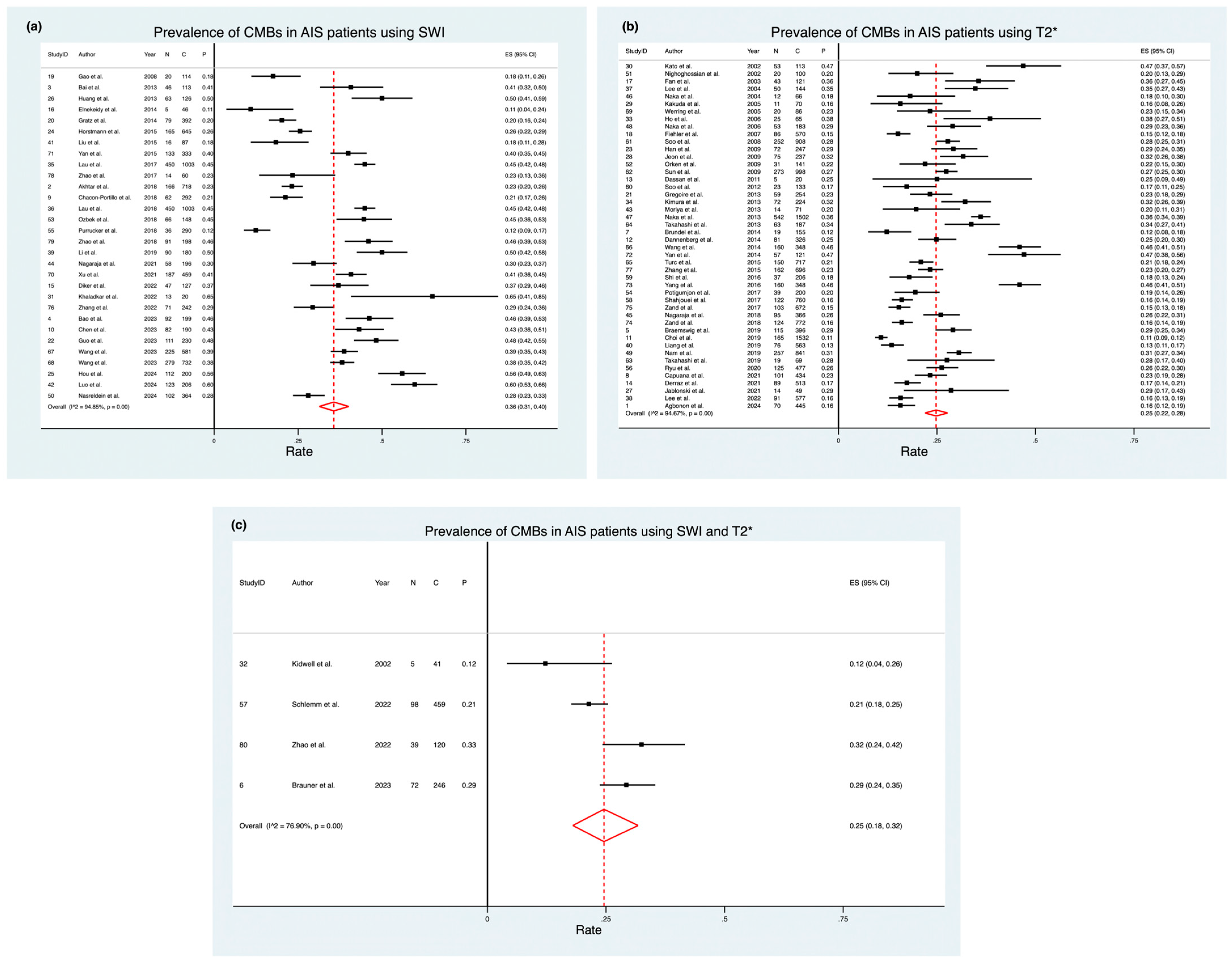
3.2. Prevalence of CMBs Using Different Imaging Modalities
3.2.1. Stratified by Age
3.2.2. Stratified by Hypertension Rates
3.2.3. Stratified by Regional Variation
3.2.4. Stratified by Use of FLAIR
3.2.5. Stratified by Use of NCCT
3.2.6. Stratified by Use of Slice Thickness
3.2.7. Stratified by Field Strength
3.2.8. Stratified by Stroke Subtype
3.2.9. Stratified by CMB Location
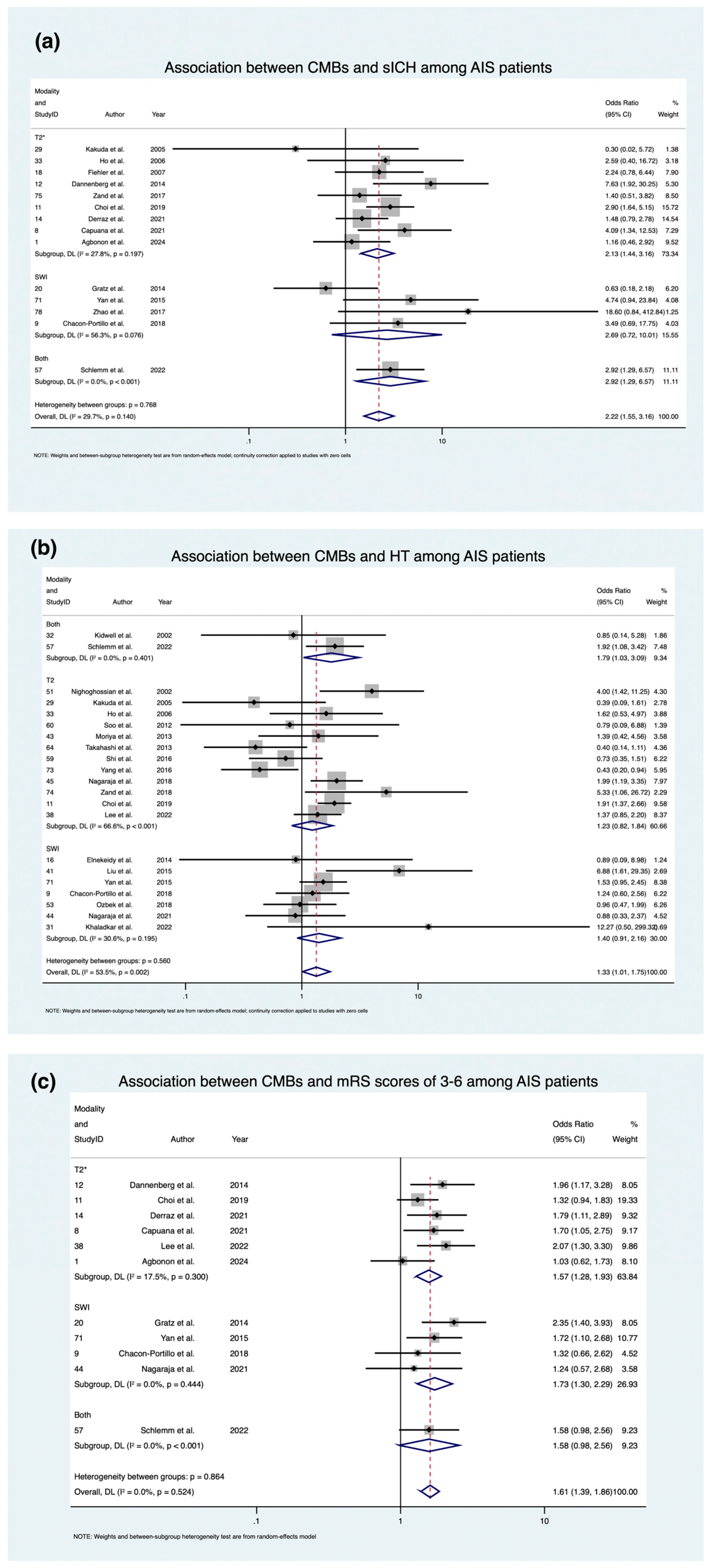
3.3. Association of CMBs with Prognostic Outcomes
3.3.1. Symptomatic Intracranial Hemorrhage (sICH)
3.3.2. Hemorrhagic Transformation (HT)
3.3.3. mRS 3-6 at 90 Days
3.4. Methodological Quality
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Ajmera, P.; Sharma, P.; Kanekar, S. Cerebral microbleeds: Causes, clinical relevance, and imaging approach—A narrative review. J. Neurosci. Rural Pract. 2024, 15, 169–181. [Google Scholar] [CrossRef]
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke 2023, 54, 1330–1339. [Google Scholar] [CrossRef]
- Kilinc, D.; Vernooij, M.W.; Bron, E.E.; Biessels, G.J.; Vinke, E.J. Normative Population–Derived Data for MRI Manifestations of Cerebral Small Vessel Disease. Stroke 2024, 55, 2863–2871. [Google Scholar] [CrossRef]
- Cho, A.H.; Kwon, H.S.; Lee, M.H.; Park, J.H.; Heo, S.H.; Yu, S.; Kwon, S.U. Asia Pacific Stroke Conference 2020. Abstracts of the Annual Conference of the Asia Pacific Stroke Organization (APSO), Virtual Conference, Korea, December 4–6, 2020. Cerebrovasc. Dis. 2020, 49, 1–149. [Google Scholar] [CrossRef]
- Hori, S.; Okamoto, S.; Kubo, M.; Horie, Y.; Kuroda, S. Cerebral microbleeds is a predictor of recurrent small vessel cerebrovascular disease: Evaluation based on the recurrent stroke pattern. J. Stroke Cerebrovasc. Dis. 2024, 33, 107812. [Google Scholar] [CrossRef]
- Ferro, D.A.; van den Brink, H.; Exalto, L.G.; Boomsma, J.M.F.; Barkhof, F.; Prins, N.D.; van der Flier, W.M.; Biessels, G.J.; Scheltens, P.; Teunissen, C.E.; et al. Clinical relevance of acute cerebral microinfarcts in vascular cognitive impairment. Neurology 2019, 92, e1558–e1566. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Saver, J.L.; Villablanca, J.P.; Duckwiler, G.; Fredieu, A.; Gough, K.; Leary, M.C.; Starkman, S.; Gobin, Y.P.; Jahan, R.; et al. Magnetic Resonance Imaging Detection of Microbleeds Before Thrombolysis. Stroke 2002, 33, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Bhaskar, S. Cerebral Microbleeds as Living Lesions: Rethinking Their Role in Stroke Pathophysiology and Prognosis. 2025; Under review. [Google Scholar]
- Haller, S.; Vernooij, M.W.; Kuijer, J.P.A.; Larsson, E.-M.; Jäger, H.R.; Barkhof, F. Cerebral Microbleeds: Imaging and Clinical Significance. Radiology 2018, 287, 11–28. [Google Scholar] [CrossRef]
- Shams, S.; Martola, J.; Cavallin, L.; Granberg, T.; Shams, M.; Aspelin, P.; Wahlund, L.O.; Kristoffersen-Wiberg, M. SWI or T2*: Which MRI Sequence to Use in the Detection of Cerebral Microbleeds? The Karolinska Imaging Dementia Study. Am. J. Neuroradiol. 2015, 36, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, M.; Ge, X.H.; Huang, H.D.; Gao, L.; Qin, J.C. The use of susceptibility-weighted imaging to detect cerebral microbleeds after lacunar infarction. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3105–3112. [Google Scholar]
- Kim, Y.; Luby, M.; Burkett, N.-S.; Norato, G.; Leigh, R.; Wright, C.B.; Kern, K.C.; Hsia, A.W.; Lynch, J.K.; Adil, M.M.; et al. Fluid-Attenuated Inversion Recovery Hyperintense Ischemic Stroke Predicts Less Favorable 90-Day Outcome after Intravenous Thrombolysis. Cerebrovasc. Dis. 2021, 50, 738–745. [Google Scholar] [CrossRef] [PubMed]
- El-Serougy, L.G.; El-Rakhawy, M.M.; Ashamallah, G.A.; Mustafa, W.F. Reliability of magnetic susceptibility weighted imaging in detection of cerebral microbleeds in stroke patients. Egypt. J. Radiol. Nucl. Med. 2017, 48, 225–229. [Google Scholar] [CrossRef][Green Version]
- Puy, L.; Pasi, M.; Rodrigues, M.; van Veluw, S.J.; Tsivgoulis, G.; Shoamanesh, A.; Cordonnier, C. Cerebral microbleeds: From depiction to interpretation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 598–607. [Google Scholar] [CrossRef]
- Nandigam, R.N.K.; Viswanathan, A.; Delgado, P.; Skehan, M.E.; Smith, E.E.; Rosand, J.; Greenberg, S.M.; Dickerson, B.C. MR Imaging Detection of Cerebral Microbleeds: Effect of Susceptibility-Weighted Imaging, Section Thickness, and Field Strength. Am. J. Neuroradiol. 2009, 30, 338–343. [Google Scholar] [CrossRef]
- Charidimou, A.; Krishnan, A.; Werring, D.J.; Rolf Jäger, H. Cerebral microbleeds: A guide to detection and clinical relevance in different disease settings. Neuroradiology 2013, 55, 655–674. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Izumiyama, M.; Izumiyama, K.; Takahashi, A.; Itoyama, Y. Silent Cerebral Microbleeds on T2*-Weighted MRI. Stroke 2002, 33, 1536–1540. [Google Scholar] [CrossRef]
- Nighoghossian, N.; Hermier, M.; Adeleine, P.; Blanc-Lasserre, K.; Derex, L.; Honnorat, J.; Philippeau, F.; Dugor, J.F.; Froment, J.C.; Trouillas, P. Old Microbleeds Are a Potential Risk Factor for Cerebral Bleeding After Ischemic Stroke. Stroke 2002, 33, 735–742. [Google Scholar] [CrossRef]
- Fan, Y.H.; Zhang, L.; Lam, W.W.M.; Mok, V.C.T.; Wong, K.S. Cerebral Microbleeds as a Risk Factor for Subsequent Intracerebral Hemorrhages Among Patients With Acute Ischemic Stroke. Stroke 2003, 34, 2459–2462. [Google Scholar] [CrossRef]
- Lee, S.H.; Bae, H.J.; Kwon, S.J.; Kim, H.; Kim, Y.H.; Yoon, B.W.; Roh, J.K. Cerebral microbleeds are regionally associated with intracerebral hemorrhage. Neurology 2004, 62, 72–76. [Google Scholar] [CrossRef]
- Naka, H.; Nomura, E.; Wakabayashi, S.; Kajikawa, H.; Kohriyama, T.; Mimori, Y.; Nakamura, S.; Matsumoto, M. Frequency of asymptomatic microbleeds on T2*-weighted MR images of patients with recurrent stroke: Association with combination of stroke subtypes and leukoaraiosis. Am. J. Neuroradiol. 2004, 25, 714–719. [Google Scholar] [PubMed]
- Kakuda, W.; Thijs, V.N.; Lansberg, M.G.; Bammer, R.; Wechsler, L.; Kemp, S.; Moseley, M.E.; Marks, M.P.; Albers, G.W. Clinical importance of microbleeds in patients receiving IV thrombolysis. Neurology 2005, 65, 1175–1178. [Google Scholar] [CrossRef]
- Werring, D.J.; Coward, L.J.; Losseff, N.A.; Jager, H.R.; Brown, M.M. Cerebral microbleeds are common in ischemic stroke but rare in TIA. Neurology 2005, 65, 1914–1918. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.H.; Ryu, C.W.; Lee, J.H.; Choi, C.G.; Kim, S.J.; Suh, D.C. Multiple Cerebral Microbleeds in Hyperacute Ischemic Stroke: Impact on Prevalence and Severity of Early Hemorrhagic Transformation After Thrombolytic Treatment. Am. J. Roentgenol. 2006, 186, 1443–1449. [Google Scholar] [CrossRef]
- Naka, H.; Nomura, E.; Takahashi, T.; Wakabayashi, S.; Mimori, Y.; Kajikawa, H.; Kohriyama, T.; Matsumoto, M. Combinations of the presence or absence of cerebral microbleeds and advanced white matter hyperintensity as predictors of subsequent stroke types. Am. J. Neuroradiol. 2006, 27, 830–835. [Google Scholar]
- Fiehler, J.; Albers, G.W.; Boulanger, J.-M.; Derex, L.; Gass, A.; Hjort, N.; Kim, J.S.; Liebeskind, D.S.; Neumann-Haefelin, T.; Pedraza, S.; et al. Bleeding Risk Analysis in Stroke Imaging Before ThromboLysis (BRASIL). Stroke 2007, 38, 2738–2744. [Google Scholar] [CrossRef] [PubMed]
- Soo, Y.O.; Yang, S.R.; Lam, W.W.; Wong, A.; Fan, Y.H.; Leung, H.H.; Chan, A.Y.; Leung, C.; Leung, T.W.; Wong, L.K. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J. Neurol. 2008, 255, 1679–1686. [Google Scholar] [CrossRef]
- Han, J.; Gao, P.; Lin, Y.; Zhang, J.; Xu, L.; Xue, J. Three-tesla magnetic resonance imaging study of cerebral microbleeds in patients with ischemic stroke. Neurol. Res. 2013, 31, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.B.; Kwon, S.U.; Cho, A.H.; Yun, S.C.; Kim, J.S.; Kang, D.W. Rapid appearance of new cerebral microbleeds after acute ischemic stroke. Neurology 2009, 73, 1638–1644. [Google Scholar] [CrossRef]
- Orken, D.N.; Kenangil, G.; Uysal, E.; Forta, H. Cerebral Microbleeds in Ischemic Stroke Patients on Warfarin Treatment. Stroke 2009, 40, 3638–3640. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Soo, Y.O.; Lam, W.W.; Wong, K.S.; Zeng, J.S.; Fan, Y.H. Different distribution patterns of cerebral microbleeds in acute ischemic stroke patients with and without hypertension. Eur. Neurol. 2009, 62, 298–303. [Google Scholar] [CrossRef]
- Dassan, P.; Brown, M.M.; Gregoire, S.; Keir, G.; Werring, D.J. UK Stroke Forum 2011 Abstracts, Glasgow, 29 November–1 December 2011. Int. J. Stroke 2011, 6, 1–65. [Google Scholar] [CrossRef]
- Soo, Y.O.; Siu, D.Y.; Abrigo, J.; Yu, S.; Ng, N.; Ahuja, A.T.; Wong, L.K.; Leung, T.W. Risk of intracerebral hemorrhage in patients with cerebral microbleeds undergoing endovascular intervention. Stroke 2012, 43, 1532–1536. [Google Scholar] [CrossRef]
- Gregoire, S.M.; Scheffler, G.; Jäger, H.R.; Yousry, T.A.; Brown, M.M.; Kallis, C.; Cipolotti, L.; Werring, D.J. Strictly Lobar Microbleeds Are Associated with Executive Impairment in Patients With Ischemic Stroke or Transient Ischemic Attack. Stroke 2013, 44, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Aoki, J.; Shibazaki, K.; Saji, N.; Uemura, J.; Sakamoto, Y. New Appearance of Extraischemic Microbleeds on T2*-Weighted Magnetic Resonance Imaging 24 Hours After Tissue-type Plasminogen Activator Administration. Stroke 2013, 44, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Moriya, Y.; Takahashi, W.; Kijima, C.; Yutani, S.; Iijima, E.; Mizuma, A.; Honma, K.; Uesugi, T.; Ohnuki, Y.; Nagata, E.; et al. Predictors for hemorrhagic transformation with intravenous tissue plasminogen activator in acute ischemic stroke. Tokai J. Exp. Clin. Med. 2013, 38, 24–27. [Google Scholar]
- Naka, H.; Nomura, E.; Kitamura, J.; Imamura, E.; Wakabayashi, S.; Matsumoto, M. Antiplatelet Therapy as a Risk Factor for Microbleeds in Intracerebral Hemorrhage Patients: Analysis Using Specific Antiplatelet Agents. J. Stroke Cerebrovasc. Dis. 2013, 22, 834–840. [Google Scholar] [CrossRef]
- Takahashi, W.; Moriya, Y.; Mizuma, A.; Uesugi, T.; Ohnuki, Y.; Takizawa, S. Cerebral microbleeds on T2*-weighted images and hemorrhagic transformation after antithrombotic therapies for ischemic stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, e528–e532. [Google Scholar] [CrossRef] [PubMed]
- Brundel, M.; Kwa, V.I.H.; Bouvy, W.H.; Algra, A.; Kappelle, L.J.; Biessels, G.J. Cerebral Microbleeds Are Not Associated with Long-Term Cognitive Outcome in Patients with Transient Ischemic Attack or Minor Stroke. Cerebrovasc. Dis. 2014, 37, 195–202. [Google Scholar] [CrossRef]
- Dannenberg, S.; Scheitz, J.F.; Rozanski, M.; Erdur, H.; Brunecker, P.; Werring, D.J.; Fiebach, J.B.; Nolte, C.H. Number of Cerebral Microbleeds and Risk of Intracerebral Hemorrhage After Intravenous Thrombolysis. Stroke 2014, 45, 2900–2905. [Google Scholar] [CrossRef]
- Wang, B.G.; Yang, N.; Lin, M.; Lu, B. Analysis of risk factors of hemorrhagic transformation after acute ischemic stroke: Cerebral microbleeds do not correlate with hemorrhagic transformation. Cell Biochem. Biophys. 2014, 70, 135–142. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Zhang, X.; Liebeskind, D.S.; Lou, M. New microbleeds after thrombolysis: Contiguous thin-slice 3T MRI. Medicine 2014, 93, e99. [Google Scholar] [CrossRef]
- Turc, G.; Sallem, A.; Moulin, S.; Tisserand, M.; Machet, A.; Edjlali, M.; Baron, J.C.; Leclerc, X.; Leys, D.; Mas, J.L.; et al. Microbleed Status and 3-Month Outcome After Intravenous Thrombolysis in 717 Patients With Acute Ischemic Stroke. Stroke 2015, 46, 2458–2463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; Li, Z.; Wang, Y.; Zhao, X.; Wang, C.; Liu, L.; Pu, Y.; Zou, X.; Pan, Y.; Du, W.; et al. Risk factors of cerebral microbleeds in strictly deep or lobar brain regions differed. J. Stroke Cerebrovasc. Dis. 2015, 24, 24–30. [Google Scholar] [CrossRef]
- Shi, Z.S.; Duckwiler, G.R.; Jahan, R.; Tateshima, S.; Gonzalez, N.R.; Szeder, V.; Saver, J.L.; Kim, D.; Ali, L.K.; Starkman, S.; et al. Mechanical thrombectomy for acute ischemic stroke with cerebral microbleeds. J. Neurointerv. Surg. 2016, 8, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Lin, M.; Wang, B.G.; Zeng, W.Y.; He, Y.F.; Peng, H.Y.; Zeng, J.; Wu, Z.Y.; Zhong, Y. Low level of low-density lipoprotein cholesterol is related with increased hemorrhagic transformation after acute ischemic cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 673–678. [Google Scholar]
- Potigumjon, A.; Watcharakorn, A.; Dharmasaroja, P.A. Prevalence of Cerebral Microbleeds in Thai Patients with Ischemic Stroke. J. Neurosci. Rural Pract. 2019, 08, 216–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahjouei, S.; Tsivgoulis, G.; Singh, M.; McCormack, M.; Noorbakhsh-Sabet, N.; Goyal, N.; Alexandrov, A.W.; Alexandrov, A.V.; Zand, R. Racial Difference in Cerebral Microbleed Burden among Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 2680–2685. [Google Scholar] [CrossRef]
- Zand, R.; Tsivgoulis, G.; Singh, M.; McCormack, M.; Goyal, N.; Ishfaq, M.F.; Shahripour, R.B.; Nearing, K.; Elijovich, L.; Alexandrov, A.W.; et al. Cerebral Microbleeds and Risk of Intracerebral Hemorrhage Post Intravenous Thrombolysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 538–544. [Google Scholar] [CrossRef]
- Nagaraja, N.; Tasneem, N.; Shaban, A.; Dandapat, S.; Ahmed, U.; Policeni, B.; Olalde, H.; Shim, H.; Samaniego, E.A.; Pieper, C.; et al. Cerebral Microbleeds are an Independent Predictor of Hemorrhagic Transformation Following Intravenous Alteplase Administration in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 1403–1411. [Google Scholar] [CrossRef]
- Braemswig, T.B.; Villringer, K.; Turc, G.; Erdur, H.; Fiebach, J.B.; Audebert, H.J.; Endres, M.; Nolte, C.H.; Scheitz, J.F. Predictors of new remote cerebral microbleeds after IV thrombolysis for ischemic stroke. Neurology 2019, 92, e630–e638. [Google Scholar] [CrossRef]
- Choi, K.-H.; Kim, J.-H.; Kang, K.-W.; Kim, J.-T.; Choi, S.-M.; Lee, S.-H.; Park, M.-S.; Kim, B.-C.; Kim, M.-K.; Cho, K.-H. Impact of Microbleeds on Outcome Following Recanalization in Patients With Acute Ischemic Stroke. Stroke 2019, 50, 127–134. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.-K.; Liu, Y.-L.; Mok, V.C.T.; Ungvari, G.S.; Chu, W.C.W.; Tang, W.-K.; Kim, J.S.; Kim, J.-M. Exploring causal pathways linking cerebral small vessel diseases burden to poststroke depressive symptoms with structural equation model analysis. J. Affect. Disord. 2019, 253, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kwon, H.M.; Kim, H.L.; Lee, Y.S. Left ventricular ejection fraction is associated with small vessel disease in ischaemic stroke patients. Eur. J. Neurol. 2019, 26, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Saito, S.; Yamamoto, Y.; Uehara, T.; Yokota, C.; Sakai, G.; Nishida, N.; Takahashi, R.; Kalaria, R.N.; Toyoda, K.; et al. Visually-Rated Medial Temporal Lobe Atrophy with Lower Educational History as a Quick Indicator of Amnestic Cognitive Impairment after Stroke. J. Alzheimers Dis. 2019, 67, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Ryu, W.-S.; Jeong, S.-W.; Kim, D.-E. Total small vessel disease burden and functional outcome in patients with ischemic stroke. PLoS ONE 2020, 15, e0242319. [Google Scholar] [CrossRef]
- Capuana, M.L.; Lorenzano, S.; Caselli, M.C.; Paciaroni, M.; Toni, D. Hemorrhagic risk after intravenous thrombolysis for ischemic stroke in patients with cerebral microbleeds and white matter disease. Neurol. Sci. 2020, 42, 1969–1976. [Google Scholar] [CrossRef]
- Derraz, I.; Cagnazzo, F.; Gaillard, N.; Morganti, R.; Dargazanli, C.; Ahmed, R.; Lefevre, P.-H.; Riquelme, C.; Mourand, I.; Gascou, G.; et al. Microbleeds, Cerebral Hemorrhage, and Functional Outcome After Endovascular Thrombectomy. Neurology 2021, 96, E1724–E1731. [Google Scholar] [CrossRef]
- Jabłoński, B.; Gójska-Grymajło, A.; Ossowska, D.; Szurowska, E.; Wyszomirski, A.; Rojek, B.; Karaszewski, B. New Remote Cerebral Microbleeds on T2*-Weighted Echo Planar MRI After Intravenous Thrombolysis for Acute Ischemic Stroke. Front. Neurol. 2022, 12, 744701. [Google Scholar] [CrossRef]
- Lee, S.-J.; Hwang, Y.-H.; Hong, J.M.; Choi, J.W.; Park, J.H.; Park, B.; Kang, D.-H.; Kim, Y.-W.; Kim, Y.-S.; Hong, J.-H.; et al. Influence of cerebral microbleeds on mechanical thrombectomy outcomes. Sci. Rep. 2022, 12, 3637. [Google Scholar] [CrossRef]
- Agbonon, R.; Forestier, G.; Bricout, N.; Benhassen, W.; Turc, G.; Bretzner, M.; Pasi, M.; Benzakoun, J.; Seners, P.; Derraz, I.; et al. Cerebral microbleeds and risk of symptomatic hemorrhagic transformation following mechanical thrombectomy for large vessel ischemic stroke. J. Neurol. 2024, 271, 2631–2638. [Google Scholar] [CrossRef]
- Zand, R.; Shahjouei, S.; Tsivgoulis, G.; Singh, M.; McCormack, M.; Noorbakhsh-Sabet, N.; Goyal, N.; Alexandrov, A.V. Cerebral Microbleeds are Associated with Higher Mortality Among Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2018, 27, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.K.; Wong, Y.K.; Teo, K.C.; Chang, R.S.K.; Tse, M.Y.; Hoi, C.P.; Chan, C.Y.; Chan, O.L.; Cheung, R.H.K.; Wong, E.K.M.; et al. Long-Term Prognostic Implications of Cerebral Microbleeds in Chinese Patients With Ischemic Stroke. J. Am. Heart Assoc. 2017, 6, e007360. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Y.; Zhang, Z. Silent cerebral microbleeds on susceptibility-weighted imaging of patients with ischemic stroke and leukoaraiosis. Neurol. Res. 2013, 30, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Zhao, Z.; Sui, H.; Xie, X.; Chen, J.; Yang, J.; Zhang, L. Susceptibility-weighted imaging for cerebral microbleed detection in super-acute ischemic stroke patients treated with intravenous thrombolysis. Neurol. Res. 2013, 35, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yin, Q.; Sun, W.; Zhu, W.; Li, Y.; Liu, W.; Xiao, L.; Duan, Z.; Cai, Q.; Liu, D.; et al. Microbleeds in ischemic stroke are associated with lower serum adiponectin and higher soluble E-selectin levels. J. Neurol. Sci. 2013, 334, 83–87. [Google Scholar] [CrossRef]
- Elnekeidy, A.E.; Yehia, A.; Elfatatry, A. Importance of susceptibility weighted imaging (SWI) in management of cerebro-vascular strokes (CVS). Alex. J. Med. 2019, 50, 83–91. [Google Scholar] [CrossRef][Green Version]
- Gratz, P.P.; El-Koussy, M.; Hsieh, K.; von Arx, S.; Mono, M.-L.; Heldner, M.R.; Fischer, U.; Mattle, H.P.; Zubler, C.; Schroth, G.; et al. Preexisting Cerebral Microbleeds on Susceptibility-Weighted Magnetic Resonance Imaging and Post-Thrombolysis Bleeding Risk in 392 Patients. Stroke 2014, 45, 1684–1688. [Google Scholar] [CrossRef]
- Horstmann, S.; Möhlenbruch, M.; Wegele, C.; Rizos, T.; Laible, M.; Rauch, G.; Veltkamp, R. Prevalence of atrial fibrillation and association of previous antithrombotic treatment in patients with cerebral microbleeds. Eur. J. Neurol. 2014, 22, 1355–1362. [Google Scholar] [CrossRef]
- Liu, C.; Dong, Z.; Xu, L.; Khursheed, A.; Dong, L.; Liu, Z.; Yang, J.; Liu, J. MR image features predicting hemorrhagic transformation in acute cerebral infarction: A multimodal study. Neuroradiology 2015, 57, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Jin, X.; Zhang, X.; Zhang, S.; Liebeskind, D.S.; Lou, M. Extensive cerebral microbleeds predict parenchymal haemorrhage and poor outcome after intravenous thrombolysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, L.; Wang, Z.; Wang, L.; Cheng, Z.; Lei, H.; Yang, D.; Cui, Y.; Zhang, S. Evaluation of the role of susceptibility-weighted imaging in thrombolytic therapy for acute ischemic stroke. J. Clin. Neurosci. 2017, 40, 175–179. [Google Scholar] [CrossRef]
- Akhtar, N.; Salam, A.; Kamran, S.; D’Souza, A.; Imam, Y.; Own, A.; ElSotouhy, A.; Vattoth, S.; Bourke, P.; Bhutta, Z.; et al. Pre-existing Small Vessel Disease in Patients with Acute Stroke from the Middle East, Southeast Asia, and Philippines. Transl. Stroke Res. 2017, 9, 274–282. [Google Scholar] [CrossRef]
- Chacon-Portillo, M.A.; Llinas, R.H.; Marsh, E.B. Cerebral microbleeds shouldn’t dictate treatment of acute stroke: A retrospective cohort study evaluating risk of intracerebral hemorrhage. BMC Neurol. 2018, 18, 33. [Google Scholar] [CrossRef]
- Lau, K.K.; Lovelock, C.E.; Li, L.; Simoni, M.; Gutnikov, S.; Küker, W.; Mak, H.K.F.; Rothwell, P.M. Antiplatelet Treatment After Transient Ischemic Attack and Ischemic Stroke in Patients With Cerebral Microbleeds in 2 Large Cohorts and an Updated Systematic Review. Stroke 2018, 49, 1434–1442. [Google Scholar] [CrossRef]
- Ozbek, D.; Ozturk Tan, O.; Ekinci, G.; Midi, I. Risk of hemorrhage in ischemic stroke and its relationship with cerebral microbleeds. Clin. Neurol. Neurosurg. 2018, 168, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Purrucker, J.C.; Wolf, M.; Haas, K.; Siedler, T.; Rizos, T.; Khan, S.; Heuschmann, P.U.; Veltkamp, R. Microbleeds in ischemic vs hemorrhagic strokes on novel oral anticoagulants. Acta Neurol. Scand. 2018, 138, 163–169. [Google Scholar] [CrossRef]
- Zhao, F.F.; Gao, H.Y.; Gao, Y.; Zhao, Z.; Li, J.; Ning, F.B.; Zhang, X.N.; Wang, Z.G.; Yu, A.L.; Guo, Y.Y.; et al. A Correlational Study on Cerebral Microbleeds and Carotid Atherosclerosis in Patients with Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 2228–2234. [Google Scholar] [CrossRef]
- Li, G.-F.; Wu, Y.-L.; Wang, S.; Shi, Y.-H.; Zhao, R.; Liu, F.-D.; Liu, Y.-S.; Zhuang, M.-T.; Zhao, Y.; Sun, Q.; et al. Previous chronic symptomatic and asymptomatic cerebral hemorrhage in patients with acute ischemic stroke. Neuroradiology 2018, 61, 103–107. [Google Scholar] [CrossRef]
- Xu, C.X.; Xu, H.; Yi, T.; Yi, X.Y.; Ma, J.P. Cerebral Microbleed Burden in Ischemic Stroke Patients on Aspirin: Prospective Cohort of Intracranial Hemorrhage. Front. Neurol. 2021, 12, 742899. [Google Scholar] [CrossRef] [PubMed]
- Diker, S.; Gelener, P.; Eker, A.; Kaymakamzade, B.; Mut, S.; Erem, A.; Balyemez, U. Association between cerebral microbleeds and inflammatory biomarkers in patients with ischemic stroke. Egypt. J. Neurol. Psychiatr. Neurosurg. 2022, 58, 43. [Google Scholar] [CrossRef]
- Khaladkar, S.M.; Chanabasanavar, V.; Dhirawani, S.; Thakker, V.; Dilip, D.; Parripati, V.K. Susceptibility Weighted Imaging: An Effective Auxiliary Sequence That Enhances Insight Into the Imaging of Stroke. Cureus 2022, 14, e24918. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, N.; Farooqui, A.; Bin Zahid, A.; Kaur, S. Factors associated with the presence of cerebral microbleeds and its influence on outcomes of stroke not treated with alteplase. Clin. Neurol. Neurosurg. 2021, 207, 106798. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Jiang, N.; Liu, Y.; Wang, Q.; Tang, X.; Zhai, Q.; Zhao, L. Correlation of lipoprotein-associated phospholipase A2 and cerebral microbleeds in patients with acute ischaemic stroke. BMC Neurol. 2022, 22, 482. [Google Scholar] [CrossRef]
- Bao, Y.; Gu, J.; Lv, T.; Chen, M.; Zhao, K.; Yang, Y.; Gu, D. Correlation between blood pressure variability and deep cerebral microbleeds in patients with acute ischemic stroke. Folia Neuropathol. 2023, 61, 309–316. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Tian, X.; Zhang, T.; Zhang, J.; Ran, F. Impact of cerebral microbleeds on cognitive functions and its risk factors in acute cerebral infarction patients. Neurol. Res. 2023, 45, 564–571. [Google Scholar] [CrossRef]
- Guo, X.; Xing, Y.; Teng, Z.; Shen, Z.; Guo, X.; Lv, P.; Tian, S. Gender heterogeneity in the influencing factors for cerebral microbleeds in acute ischemic stroke patients. Curr. Med. Res. Opin. 2023, 39, 1045–1054. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Wang, Y.; Luan, M.; Xu, L.; Zhong, M.; Zheng, X. Dual antiplatelet therapy in acute ischaemic stroke with or without cerebral microbleeds. Eur. J. Neurosci. 2023, 57, 1197–1207. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Wang, Y.; Luan, M.; Zhong, M.; Xu, L.; Zheng, X. Comparative effectiveness of dual to single antiplatelet therapy after one year versus seven years in patients with acute ischemic stroke combined with cerebral microbleeds. J. Clin. Neurosci. 2023, 112, 73–79. [Google Scholar] [CrossRef]
- Hou, Y.; Xiao, Q.; Chen, Z.; Cheng, J.; Shi, Z.; Zhu, Q.; Liu, C. Risk factors and prognosis of acute ischemic stroke complicated with cerebral microbleeds. Neurol. Asia 2024, 29, 591–596. [Google Scholar] [CrossRef]
- Luo, Y.; Gao, K.; Fawaz, M.; Wu, B.; Zhong, Y.; Zhou, Y.; Haacke, E.M.; Dai, Y.; Liu, S. Automatic detection of cerebral microbleeds using susceptibility weighted imaging and artificial intelligence. Quant. Imaging Med. Surg. 2024, 14, 2640–2654. [Google Scholar] [CrossRef] [PubMed]
- Nasreldein, A.; Shoamnesh, A.; Foli, N.; Makboul, M.; Salah, S.; Faßbender, K.; Walter, S. Prevalence and Risk Factors of Cerebral Microbleeds among Egyptian Patients with Acute Ischemic Stroke. Neuroepidemiology 2024, 59, 1–9. [Google Scholar] [CrossRef]
- Schlemm, L.; Braemswig, T.B.; Boutitie, F.; Vynckier, J.; Jensen, M.; Galinovic, I.; Simonsen, C.Z.; Cheng, B.; Cho, T.-H.; Fiehler, J.; et al. Cerebral Microbleeds and Treatment Effect of Intravenous Thrombolysis in Acute Stroke. Neurology 2022, 98, E302–E314. [Google Scholar] [CrossRef]
- Zhao, D.X.; Gootee, E.; Johansen, M.C. Atrial cardiopathy is associated with cerebral microbleeds in ischemic stroke patients. Front. Neurol. 2022, 13, 982926. [Google Scholar] [CrossRef]
- Brauner, R.; Gory, B.; Lapergue, B.; Sibon, I.; Richard, S.; Kyheng, M.; Labreuche, J.; Desilles, J.P.; Blanc, R.; Piotin, M.; et al. Effect of small vessel disease severity on blood pressure management after endovascular therapy in theBP TARGETtrial. Eur. J. Neurol. 2023, 30, 1676–1685. [Google Scholar] [CrossRef]
- De Simone, M.; Fontanella, M.M.; Choucha, A.; Schaller, K.; Machi, P.; Lanzino, G.; Bijlenga, P.; Kurz, F.T.; Lövblad, K.-O.; De Maria, L. Current and Future Applications of Arterial Spin Labeling MRI in Cerebral Arteriovenous Malformations. Biomedicines 2024, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Fandler-Höfler, S.; Eppinger, S.; Ambler, G.; Nash, P.; Kneihsl, M.; Lee, K.-J.; Lim, J.-S.; Shiozawa, M.; Koga, M.; Li, L.; et al. Sex Differences in Frequency, Severity, and Distribution of Cerebral Microbleeds. JAMA Netw. Open 2024, 7, e2439571. [Google Scholar] [CrossRef]
- Liang, C.; Wang, J.; Feng, M.; Zhang, N.; Guo, L. White matter changes, duration of hypertension, and age are associated with cerebral microbleeds in patients with different stages of hypertension. Quant. Imaging Med. Surg. 2022, 12, 119–130. [Google Scholar] [CrossRef]
- Lyu, L.; Shen, J.; Zeng, C.; Ji, J.; Hu, W.; Wei, T.; Mao, W. Cerebral microbleeds are associated with blood pressure levels in individuals with hypertension. Clin. Exp. Hypertens. 2019, 42, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Alemany, M.; Stenborg, A.; Terent, A.; Sonninen, P.; Raininko, R. Coexistence of Microhemorrhages and Acute Spontaneous Brain Hemorrhage: Correlation with Signs of Microangiopathy and Clinical Data. Radiology 2006, 238, 240–247. [Google Scholar] [CrossRef]
- Stehling, C.; Wersching, H.; Kloska, S.P.; Kirchhof, P.; Ring, J.; Nassenstein, I.; Allkemper, T.; Knecht, S.; Bachmann, R.; Heindel, W. Detection of asymptomatic cerebral microbleeds: A comparative study at 1.5 and 3.0 T. Acad. Radiol. 2008, 15, 895–900. [Google Scholar] [CrossRef] [PubMed]
- De Kort, A.M.; Verbeek, M.M.; Schreuder, F.H.B.M.; Klijn, C.J.M.; Jäkel, L. Prevalence of Cerebral Amyloid Angiopathy Pathology and Strictly Lobar Microbleeds in East-Asian Versus Western Populations: A Systematic Review and Meta-Analysis. J. Stroke 2024, 26, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ramirez, S.; Greenberg, S.M.; Viswanathan, A. Cerebral microbleeds: Overview and implications in cognitive impairment. Alzheimer’s Res. Ther. 2014, 6, 33. [Google Scholar] [CrossRef]
- Yilmaz, P.; Ikram, M.A.; Ikram, M.K.; Niessen, W.J.; Viswanathan, A.; Charidimou, A.; Vernooij, M.W. Application of an Imaging-Based Sum Score for Cerebral Amyloid Angiopathy to the General Population: Risk of Major Neurological Diseases and Mortality. Front. Neurol. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Tipirneni, S.; Stanwell, P.; Weissert, R.; Bhaskar, S.M.M. Prevalence and Impact of Cerebral Microbleeds on Clinical and Safety Outcomes in Acute Ischaemic Stroke Patients Receiving Reperfusion Therapy: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2865. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Zand, R.; Katsanos, A.H.; Turc, G.; Nolte, C.H.; Jung, S.; Cordonnier, C.; Fiebach, J.B.; Scheitz, J.F.; Klinger-Gratz, P.P.; et al. Risk of Symptomatic Intracerebral Hemorrhage After Intravenous Thrombolysis in Patients With Acute Ischemic Stroke and High Cerebral Microbleed Burden: A Meta-analysis. JAMA Neurol. 2016, 73, 675–683. [Google Scholar] [CrossRef]
- Wilson, D.; Charidimou, A.; Ambler, G.; Fox, Z.V.; Gregoire, S.; Rayson, P.; Imaizumi, T.; Fluri, F.; Naka, H.; Horstmann, S.; et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: A meta-analysis. Neurology 2016, 87, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Chojdak-Łukasiewicz, J.; Dziadkowiak, E.; Zimny, A.; Paradowski, B. Cerebral small vessel disease: A review. Adv. Clin. Exp. Med. 2021, 30, 349–356. [Google Scholar] [CrossRef]
- Daugherty, A.M.; Raz, N. Incident risk and progression of cerebral microbleeds in healthy adults: A multi-occasion longitudinal study. Neurobiol. Aging 2017, 59, 22–29. [Google Scholar] [CrossRef]
- Schrag, M.; Greer, D.M. Clinical Associations of Cerebral Microbleeds on Magnetic Resonance Neuroimaging. J. Stroke Cerebrovasc. Dis. 2014, 23, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Yates, P.A.; Villemagne, V.L.; Ellis, K.A.; Desmond, P.M.; Masters, C.L.; Rowe, C.C. Cerebral microbleeds: A review of clinical, genetic, and neuroimaging associations. Front. Neurol. 2014, 4, 205. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.; Auger, F.; Cordonnier, C.; Deramecourt, V.; Durieux, N.; Pasquier, F.; Bordet, R.; Maurage, C.A.; Leys, D. Comparison of 7.0-T T2*-magnetic resonance imaging of cerebral bleeds in post-mortem brain sections of Alzheimer patients with their neuropathological correlates. Cerebrovasc. Dis. 2011, 31, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer-Hanke, D.; Mayer, T.; Muller, H.P.; Neugebauer, H.; Abaei, A.; Scheuerle, A.; Weis, J.; Forsberg, K.M.E.; Althaus, K.; Meier, J.; et al. Histological correlates of postmortem ultra-high-resolution single-section MRI in cortical cerebral microinfarcts. Acta Neuropathol. Commun. 2020, 8, 33. [Google Scholar] [CrossRef]
| Author | Year | Continent | Study Design | Cohort | Age Mean (±Standard Deviation (SD)) | Male, n (n%) | Number of CMBs | CMB Definition | CMB Imaging | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Cerebral Microbleed (CMB) | No CMB | |||||||||
| Agbonon et al. [63] | 2024 | Europe | Retrospective | 445 | 68.3 (±15.2) | 71.7 (±13) | - | 229 (51) | 70 | - | T2 Gradient Echo Imaging (T2*GRE) |
| Akhtar et al. [75] | 2018 | Asia | Retrospective | 718 | 54.7 (±14) | - | - | 594 (83) | 166 | <5 mm | Susceptibility Weighted Imaging (SWI) |
| Bai et al. [67] | 2013 | Asia | Prospective | 113 | 61.6 (±10.8) | - | - | - | 46 | - | SWI |
| Bao et al. [87] | 2023 | Asia | Retrospective | 199 | - | - | - | - | 92 | 2–10 mm | SWI |
| Braemswig et al. [53] | 2019 | Europe | Prospective | 396 | - | - | - | 103 (26) | 115 | <10 mm | T2*GRE |
| Brauner et al. [97] | 2023 | Europe | Prospective | 246 | 73.6 (±13.3) | - | - | 117 (48) | 72 | - | T2*GRE, SWI |
| Brundel et al. [41] | 2014 | Europe | Prospective | 155 | - | - | - | - | 19 | - | T2*GRE |
| Capuana et al. [59] | 2021 | Europe | Prospective | 434 | 68.3 (±13.3) | 69 (±12.6) | 68.1 (±13.8) | 264 (61) | 101 | <10 mm | T2*GRE |
| Chacon-Portillo et al. [76] | 2018 | North America | Retrospective | 292 | 63 (±15) | - | - | 240 (82) | 62 | 2–10 mm | SWI |
| Chen et al. [88] | 2023 | Asia | Retrospective | 190 | - | - | - | 104 (55) | 82 | <10 mm | SWI |
| Choi et al. [54] | 2019 | Asia | Prospective | 1532 | 69.4 (±11.8) | 72 (±11.2) | 68.9 (±11.9) | 855 (56) | 165 | - | T2*GRE |
| Dannenberg et al. [42] | 2014 | Europe | Prospective | 326 | - | - | - | 159 (49) | 81 | ≤10 mm | T2*GRE |
| Dassan et al. [34] | 2011 | Europe | Retrospective | 20 | - | - | - | - | 5 | - | T2*GRE |
| Derraz et al. [60] | 2021 | Europe | Prospective | 513 | 69.4 (±25.9) | 80.8 (±15.7) | 67.3 (±25.4) | 243 (47) | 89 | ≤10 mm | T2*GRE |
| Diker et al. [83] | 2022 | Europe | Retrospective | 127 | 66.6 (±14.4) | 68.5 (±12.9) | 63.6 (±15.6) | 74 (58) | 47 | <10 mm | SWI |
| Elnekeidy et al. [69] | 2014 | Africa | Prospective | 46 | - | - | - | - | 5 | - | SWI |
| Fan et al. [21] | 2003 | Asia | Prospective | 121 | 68 (±11) | 69.5 (±11) | 67.1 (±10.9) | 82 (68) | 43 | - | T2*GRE |
| Fiehler et al. [28] | 2007 | Multinational | Retrospective | 570 | 68.3 (±13.3) | - | - | 341 (60) | 86 | <5 mm | T2*GRE |
| Gao et al. [66] | 2008 | Asia | Retrospective | 114 | - | - | - | - | 20 | <10 mm | SWI |
| Gratz et al. [70] | 2014 | Europe | Prospective | 392 | 68.1 (±13.7) | - | - | 223 (57) | 79 | <5 mm | SWI |
| Gregoire et al. [36] | 2013 | Europe | Prospective | 254 | - | - | - | - | 59 | - | T2*GRE |
| Guo et al. [89] | 2023 | Asia | Retrospective | 230 | 63.8 (±11) | 66.5 (±10.8) | 61.3 (±11.1) | 160 (70) | 111 | 2–10 mm | SWI |
| Han et al. [30] | 2009 | Asia | Retrospective | 247 | 61.3 (±11.4) | 64.6 (±11) | 60 (±11.6) | 176 (71) | 72 | ≤5 mm | T2*GRE |
| Horstmann et al. [71] | 2015 | Europe | Prospective | 645 | - | - | - | - | 165 | ≤10 mm | SWI |
| Hou et al. [92] | 2024 | Asia | Retrospective | 200 | 68.3 (±9.5) | 70.7 (±8.6) | 65.3 (±10.5) | 144 (72) | 112 | - | SWI |
| Huang et al. [68] | 2013 | Asia | Prospective | 126 | 63.8 (±13) | 64.6 (±12.7) | 63.2 (±13.3) | 83 (66) | 63 | 2–10 mm | SWI |
| Jablonski et al. [61] | 2021 | Europe | Prospective | 49 | - | - | - | 23 (47) | 14 | - | T2*GRE |
| Jeon et al. [31] | 2009 | Area | Retrospective | 237 | 64 (±12.8) | - | - | 142 (60) | 75 | ≤5 mm | T2*GRE |
| Kakuda et al. [24] | 2005 | Multinational | Prospective | 70 | 70.8 (±29.2) | 70 (±32) | 71 (±29) | 31 (44) | 11 | <5 mm | T2*GRE |
| Kato et al. [19] | 2002 | Asia | Retrospective | 113 | - | - | - | 65 (58) | 53 | - | T2*GRE |
| Khaladkar et al. [84] | 2022 | Asia | Prospective | 20 | - | - | - | - | 13 | - | SWI |
| Kidwell et al. [7] | 2002 | North America | Retrospective | 41 | - | - | - | - | 5 | <5 mm | T2*GRE, SWI |
| Ho et al. [26] | 2006 | Asia | Retrospective | 65 | - | - | - | 37 (57) | 25 | <5 mm | T2*GRE |
| Kimura et al. [37] | 2013 | Asia | Prospective | 224 | 76.2 (±10.6) | - | - | 121 (54) | 72 | - | T2*GRE |
| Lau et al. [65] | 2017 | Asia | Prospective | 1003 | 69 (±12) | - | - | 601 (60) | 450 | <10 mm | SWI |
| Lau et al. [77] | 2018 | Asia | Prospective | 1003 | - | - | - | 601 (60) | 450 | - | SWI |
| Lee et al. [22] | 2004 | Asia | Retrospective | 144 | 64.6 (±9.1) | - | - | 75 (52) | 50 | ≤5 mm | T2*GRE |
| Lee et al. [62] | 2022 | Asia | Retrospective | 577 | 67 (±13) | 70.8 (±10.4) | 66.7 (±12.8) | 322 (56) | 91 | <10 mm | T2*GRE |
| Li et al. [81] | 2019 | Asia | Retrospective | 180 | 71.5 (±12.4) | - | - | 100 (56) | 90 | 2–10 mm | SWI |
| Liang et al. [55] | 2019 | Asia | Prospective | 563 | 67 (±10.2) | - | - | 333 (59) | 76 | - | T2*GRE |
| Liu et al. [72] | 2015 | Asia | Prospective | 87 | 67.3 (±12.5) | - | - | 49 (56) | 16 | 2–5 mm | SWI |
| Luo et al. [93] | 2024 | Asia | Retrospective | 206 | - | - | - | - | 123 | ≤10 mm | SWI |
| Moriya et al. [38] | 2013 | Asia | Retrospective | 71 | 73 (±10) | - | - | 50 (70) | 14 | - | T2*GRE |
| Nagaraja et al. [85] | 2021 | North America | Retrospective | 196 | 66.1 (±14) | 72 (±13) | 63.6 (±14.4) | 98 (50) | 58 | 2–10 mm | SWI |
| Nagaraja et al. [52] | 2018 | North America | Retrospective | 366 | 67 (±15) | 74.1 (±12.5) | 64.9 (±15.2) | 198 (54) | 95 | <10 mm | T2*GRE |
| Naka et al. [23] | 2004 | Asia | Prospective | 66 | - | - | - | - | 12 | - | T2*GRE |
| Naka et al. [39] | 2013 | Asia | Prospective | 1502 | 72.6 (±12) | - | - | 881 (59) | 542 | <10 mm | T2*GRE |
| Naka et al. [27] | 2006 | Asia | Prospective | 183 | - | - | - | - | 53 | - | T2*GRE |
| Nam et al. [56] | 2019 | Asia | Prospective | 841 | 68 | - | - | 516 (61) | 257 | <10 mm | T2*GRE |
| Nasreldein et al. [94] | 2024 | Africa | Prospective | 364 | - | - | - | - | 102 | - | SWI |
| Nighoghossian et al. [20] | 2002 | Europe | Prospective | 100 | 60 (±13) | - | - | 58 (58) | 20 | 2–5 mm | T2*GRE |
| Orken et al. [32] | 2009 | Europe | Prospective | 141 | 65.8 (±12.2) | 69.6 (±10.7) | 64.7 (±12.4) | 82 (58) | 31 | <5 mm | T2*GRE |
| Ozbek et al. [78] | 2018 | Europe | Prospective | 148 | 68 (±14.8) | - | - | 84 (57) | 66 | 2–10 mm | SWI |
| Potigumjon et al. [49] | 2017 | Asia | Retrospective | 200 | 61 | 66 | 60 | 126 (63) | 39 | <10 mm | T2*GRE |
| Purrucker et al. [79] | 2018 | Europe | Prospective | 290 | 78.6 | - | - | 150 (52) | 36 | 2–10 mm | SWI |
| Ryu et al. [58] | 2020 | Asia | Prospective | 477 | 66 (±14) | - | - | 294 (62) | 125 | ≤10 mm | T2*GRE |
| Schlemm et al. [95] | 2022 | Europe | Prospective | 459 | 68 | 71.7 | 67 | 289 (63) | 98 | ≤10 mm | T2*GRE, SWI |
| Shahjouei et al. [50] | 2017 | North America | Retrospective | 760 | 62.1 (±13.9) | - | - | 391 (51) | 122 | ≤10 mm | T2*GRE |
| Shi et al. [47] | 2016 | Asia | Prospective | 206 | 66.8 (±17.6) | 77 (±14) | 65 (±18) | 87 (42) | 37 | <10 mm | T2*GRE |
| Soo et al. [35] | 2012 | Asia | Prospective | 133 | 67.3 | 67 | 67.4 | - | 23 | 2–10 mm | T2*GRE |
| Soo et al. [29] | 2008 | Asia | Prospective | 908 | 68.4 (±11.9) | 71.2 (±10) | 67.3 (±11.8) | 524 (58) | 252 | - | T2*GRE |
| Sun et al. [33] | 2009 | Asia | Retrospective | 998 | 68.3 (±11.7) | 71.4 (±10) | 67.2 (±12) | 588 (59) | 273 | 2–10 mm | T2*GRE |
| Takahashi et al. [57] | 2019 | Asia | Prospective | 69 | - | - | - | 45 (65) | 19 | - | T2*GRE |
| Takahashi et al. [40] | 2013 | Asia | Retrospective | 187 | 74 (±11) | - | - | 112 (60) | 63 | - | T2*GRE |
| Turc et al. [45] | 2015 | Europe | Prospective | 717 | - | - | - | 351 (49) | 150 | ≤10 mm | T2*GRE |
| Wang et al. [43] | 2014 | Asia | Prospective | 348 | 65.2 (±13.1) | - | - | 207 (59) | 160 | 2–5 mm | T2*GRE |
| Wang et al. [90] | 2023 | Asia | Retrospective | 581 | 64.3 | 65.6 | 63.5 | 388 (67) | 225 | <10 mm | SWI |
| Wang et al. [91] | 2023 | Asia | Retrospective | 732 | - | - | - | - | 279 | <10 mm | SWI |
| Werring et al. [25] | 2005 | Europe | Prospective | 86 | 62.1 (±16.1) | - | - | 57 (66) | 20 | <10 mm | T2*GRE |
| Xu et al. [82] | 2021 | Asia | Prospective | 459 | 67.3 (±11.7) | 69 (±11.3) | 66.1 (±12) | 314 (68) | 187 | 2–10 mm | SWI |
| Yan et al. [73] | 2015 | Asia | Retrospective | 333 | 66.2 (±13) | - | - | 223 (67) | 133 | ≤10 mm | SWI |
| Yan et al. [44] | 2014 | Asia | Prospective | 121 | 67.3 (±12.5) | 72.2 (±13) | - | 77 (64) | 57 | ≤10 mm | T2*GRE |
| Yang et al. [48] | 2016 | Asia | Prospective | 348 | 65.2 (±13.1) | - | - | 207 (59) | 160 | 2–5 mm | T2*GRE |
| Zand et al. [64] | 2018 | North America | Retrospective | 772 | 61.9 (±14.2) | 64.9 (±13.2) | 61.3 (±14.3) | 398 (52) | 124 | ≤10 mm | T2*GRE |
| Zand et al. [51] | 2017 | North America | Prospective | 672 | 62 (±14) | 64.8 (±14.1) | 61 (±14) | 350 (52) | 103 | ≤10 mm | T2*GRE |
| Zhang et al. [86] | 2022 | Asia | Prospective | 242 | 67.5 (±9.5) | 69.5 (±9.9) | 66.7 (±9.2) | 158 (65) | 71 | ≤10 mm | SWI |
| Zhang et al. [46] | 2015 | Asia | Retrospective | 696 | 60 | 66 | 59 | 516 (74) | 162 | ≤10 mm | T2*GRE |
| Zhao et al. [74] | 2017 | Asia | Prospective | 60 | 62.3 (±12.5) | - | - | 38 (63) | 14 | 2–5 mm | SWI |
| Zhao et al. [80] | 2018 | Asia | Prospective | 198 | 68.1 (±8.7) | - | - | 109 (55) | 91 | <10 mm | SWI |
| Zhao et al. [96] | 2022 | North America | Prospective | 120 | 59.6 | - | - | 65 (54) | 39 | <10 mm | T2*GRE, SWI |
| Clinical Risk Factors, n (n%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Year | Atrial Fibrillation | Hyper-lipidaemia | Hypertension | Coronary Artery Disease | Prior Stroke/Transient Ischemic Stroke | Smoking | Diabetes Mellitus |
| Agbonon et al. [63] | 2024 | - | 25 (14) | 46 (18) | - | - | - | 6 (10) |
| Akhtar et al. [75] | 2018 | - | - | - | - | - | - | - |
| Bai et al. [67] | 2013 | - | - | - | - | - | - | - |
| Bao et al. [87] | 2023 | - | - | - | - | - | - | - |
| Braemswig et al. [53] | 2019 | - | - | - | - | - | - | - |
| Brauner et al. [97] | 2023 | - | - | - | - | - | - | - |
| Brundel et al. [41] | 2014 | - | - | - | - | - | - | - |
| Capuana et al. [59] | 2021 | 17 (24) | - | 77 (27) | - | - | 18 (22) | 15 (20) |
| Chacon-Portillo et al. [76] | 2018 | - | - | - | - | - | - | - |
| Chen et al. [88] | 2023 | - | - | 35 (61) | 17 (47) | - | 37 (44) | 26 (42) |
| Choi et al. [54] | 2019 | - | - | - | - | - | - | - |
| Dannenberg et al. [42] | 2014 | - | - | - | - | - | - | - |
| Dassan et al. [34] | 2011 | - | - | - | - | - | - | - |
| Derraz et al. [60] | 2021 | 38 (25) | 36 (23) | 65 (21) | 21 (25) | 19 (31) | 28 (14) | 14 (21) |
| Diker et al. [83] | 2022 | 21 (42) | 17 (38) | 34 (41) | 11 (44) | 7 (27) | - | 15 (35) |
| Elnekeidy et al. [69] | 2014 | - | - | - | - | - | - | - |
| Fan et al. [21] | 2003 | 3 (50) | 11 (41) | 32 (38) | - | - | 17 (34) | 11 (28) |
| Fiehler et al. [28] | 2007 | - | - | - | - | - | - | - |
| Gao et al. [66] | 2008 | - | - | - | - | - | - | - |
| Gratz et al. [70] | 2014 | - | - | - | - | - | - | - |
| Gregoire et al. [36] | 2013 | - | - | - | - | - | - | - |
| Guo et al. [89] | 2023 | - | 32 (38) | 88 (53) | - | - | 30 (43) | 34 (45) |
| Han et al. [30] | 2009 | - | - | 63 (40) | - | 26 (40) | 34 (26) | 17 (24) |
| Horstmann et al. [71] | 2015 | - | - | - | - | - | - | - |
| Hou et al. [92] | 2024 | 9 (56) | - | 83 (58) | 11 (61) | - | 50 (55) | 55 (63) |
| Huang et al. [68] | 2013 | - | 14 (38) | 53 (56) | - | - | 18 (49) | 10 (59) |
| Jablonski et al. [61] | 2021 | - | - | - | - | - | - | - |
| Jeon et al. [31] | 2009 | - | - | - | - | - | - | - |
| Kakuda et al. [24] | 2005 | - | 2 (12) | 8 (19) | - | - | 6 (20) | 4 (21) |
| Kato et al. [19] | 2002 | - | - | - | - | - | - | - |
| Khaladkar et al. [84] | 2022 | - | - | - | - | - | - | - |
| Kidwell et al. [7] | 2002 | - | - | - | - | - | - | - |
| Ho et al. [26] | 2006 | - | - | - | - | - | - | - |
| Kimura et al. [37] | 2013 | - | - | - | - | - | - | - |
| Lau et al. [65] | 2017 | - | - | - | - | - | - | - |
| Lau et al. [77] | 2018 | - | - | - | - | - | - | - |
| Lee et al. [22] | 2004 | - | - | - | - | - | - | - |
| Lee et al. [62] | 2022 | 42 (15) | - | 71 (20) | - | 24 (24) | 19 (14) | 27 (17) |
| Li et al. [81] | 2019 | - | - | - | - | - | - | - |
| Liang et al. [55] | 2019 | - | - | - | - | - | - | - |
| Liu et al. [72] | 2015 | - | - | - | - | - | - | - |
| Luo et al. [93] | 2024 | - | - | - | - | - | - | - |
| Moriya et al. [38] | 2013 | - | - | - | - | - | - | - |
| Nagaraja et al. [85] | 2021 | 15 (58) | 27 (38) | 52 (34) | 15 (34) | 33 (48) | - | 20 (29) |
| Nagaraja et al. [52] | 2018 | 14 (24) | 48 (33) | 67 (31) | 22 (39) | 25 (49) | 19 (19) | 23 (28) |
| Naka et al. [23] | 2004 | - | - | - | - | - | - | - |
| Naka et al. [39] | 2013 | - | - | - | - | - | - | - |
| Naka et al. [27] | 2006 | - | - | - | - | - | - | - |
| Nam et al. [56] | 2019 | - | - | - | - | - | - | - |
| Nasreldein et al. [94] | 2024 | - | - | - | - | - | - | - |
| Nighoghossian et al. [20] | 2002 | - | - | - | - | - | - | - |
| Orken et al. [32] | 2009 | - | - | 27 (24) | - | 7 (27) | 5 (13) | 6 (22) |
| Ozbek et al. [78] | 2018 | - | - | - | - | - | - | - |
| Potigumjon et al. [49] | 2017 | 6 (15) | 21 (18) | 33 (27) | 1 (10) | 9 (25) | 10 (20) | 10 (18) |
| Purrucker et al. [79] | 2018 | - | - | - | - | - | - | - |
| Ryu et al. [58] | 2020 | - | - | - | - | - | - | - |
| Schlemm et al. [95] | 2022 | 16 (32) | - | 64 (26) | - | 14 (24) | - | 22 (30) |
| Shahjouei et al. [50] | 2017 | - | - | - | - | - | - | - |
| Shi et al. [47] | 2016 | 16 (20) | 10 (16) | 26 (19) | 11 (26) | 5 (15) | - | 13 (30) |
| Soo et al. [35] | 2012 | - | 20 (18) | 20 (20) | - | 12 (22) | 12 (21) | 8 (20) |
| Soo et al. [29] | 2008 | 19 (28) | 138 (25) | 200 (32) | 19 (25) | 83 (46) | 64 (34) | 76 (26) |
| Sun et al. [33] | 2009 | 19 (28) | 148 (25) | 211 (32) | - | - | - | 81 (25) |
| Takahashi et al. [57] | 2019 | - | - | - | - | - | - | - |
| Takahashi et al. [40] | 2013 | - | - | - | - | - | - | - |
| Turc et al. [45] | 2015 | - | - | - | - | - | - | - |
| Wang et al. [43] | 2014 | - | - | - | - | - | - | - |
| Wang et al. [90] | 2023 | - | 82 (36) | 174 (44) | - | - | - | 81 (42) |
| Wang et al. [91] | 2023 | - | - | - | - | - | - | - |
| Werring et al. [25] | 2005 | - | - | - | - | - | - | - |
| Xu et al. [82] | 2021 | 10 (43) | 4 (31) | 120 (45) | - | - | 99 (44) | 44 (39) |
| Yan et al. [73] | 2015 | - | - | - | - | - | - | - |
| Yan et al. [44] | 2014 | - | - | - | - | - | - | - |
| Yang et al. [48] | 2016 | - | - | - | - | - | - | - |
| Zand et al. [64] | 2018 | 13 (17) | 51 (20) | 110 (18) | - | 43 (22) | 45 (16) | 44 (17) |
| Zand et al. [51] | 2017 | - | - | - | - | - | - | - |
| Zhang et al. [86] | 2022 | 9 (30) | - | 54 (34) | 16 (27) | - | 26 (30) | 27 (39) |
| Zhang et al. [46] | 2015 | - | 124 (22) | 149 (27) | - | - | 68 (21) | 53 (19) |
| Zhao et al. [74] | 2017 | - | - | - | - | - | - | - |
| Zhao et al. [80] | 2018 | - | - | 25 (52) | - | - | 44 (46) | 13 (54) |
| Zhao et al. [96] | 2022 | - | - | - | - | - | - | - |
| Author | Year | Reperfusion Therapy | Symptomatic Intracranial Hemorrhage (sICH) Definition | sICH, n (n%) | Hemorrhagic Transformation (HT), n (n%) | Modified Ranking Scale (mRS) 3–6 at 90 Days, n (n%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Cerebral Microbleed (CMB) | No-CMB | Overall | CMB | No-CMB | Overall | CMB | No-CMB | ||||
| Agbonon et al. [63] | 2024 | Endovascular Thrombolysis (EVT) | ECASS-II | 34 (7.6) | 6 (1.4) | 28 (6.3) | - | - | - | 194 (43.6) | 31 (7.0) | 163 (36.6) |
| Capuana et al. [59] | 2021 | Intravenous Thrombolysis (IVT) | SITS-MOST | 13 (3.0) | 7 (1.6) | 6 (1.4) | - | - | - | 130 (30.0) | 39 (9.0) | 91 (21.0) |
| Chacon-Portillo et al. [76] | 2018 | IVT | NINDS | 6 (2.0) | 3 (1.0) | 3 (1.0) | 46 (15.8) | 12 (4.1) | 34 (11.6) | 63 (21.6) | 16 (6.2) | 42 (14.4) |
| Choi et al. [54] | 2019 | IVT/EVT | ECASS-I | 69 (4.5) | 17 (1.1) | 52 (3.4) | 420 (27.4) | 66 (4.3) | 354 (23.1) | 865 (56.4) | 103 (6.7) | 763 (49.8) |
| Dannenberg et al. [42] | 2014 | IVT | ECASS-III | 10 (3.1) | 7 (2.1) | 3 (0.9) | - | - | - | 158 (48.4) | 50 (15.3) | 108 (33.1) |
| Derraz et al. [60] | 2021 | EVT | ECASS-II | 66 (12.9) | 15 (2.9) | 51 (9.9) | - | - | - | 281 (54.8) | 59 (11.5) | 222 (43.3) |
| Elnekeidy et al. [69] | 2014 | - | - | - | - | - | 10 (21.7) | 1 (2.2) | 9 (19.6) | - | - | - |
| Fiehler et al. [28] | 2007 | IVT | ECASS-I | 18 (3.2) | 5 (0.9) | 13 (2.3) | - | - | - | - | - | - |
| Gratz et al. [70] | 2014 | IVT/EVT | PROACT-II | 21 (5.4) | 3 (0.8) | 18 (4.6) | - | - | - | 193 (49.2) | 52 (13.3) | 141 (36.0) |
| Kakuda et al. [24] | 2005 | IVT | ECASS-II | 7 (10.0) | 0 (0) | 7 (10.0) | 32 (45.7) | 3 (4.3) | 29 (41.4) | - | - | - |
| Khaladkar et al. [84] | 2022 | - | - | - | - | - | 18 (90) | 13 (65) | 5 (25) | - | - | - |
| Kidwell et al. [7] | 2002 | IVT | - | - | - | - | 15 (36.6) | 2 (4.9) | 13 (31.7) | - | - | - |
| Ho et al. [26] | 2006 | IVT | - | 5 (12.2) | 3 (7.3) | 2 (4.9) | 17 (41.5) | 8 (19.5) | 9 (22.0) | - | - | - |
| Lee et al. [62] | 2022 | EVT | - | - | - | - | 170 (29.5) | 32 (55.5) | 138 (21.9) | 288 (49.9) | 59 (10.2) | 229 (39.7) |
| Liu et al. [72] | 2015 | - | - | - | - | - | 17 (19.5) | 5 (5.7) | 12 (13.8) | - | - | - |
| Moriya et al. [38] | 2013 | IVT | - | - | - | - | 26 (36.6) | 6 (8.5) | 20 (28.2) | - | - | - |
| Nagaraja et al. [52] | 2018 | - | - | - | - | - | 87 (23.8) | 32 (8.7) | 55 (15.0) | - | - | - |
| Nagaraja et al. [85] | 2021 | - | - | - | - | - | 22 (11.2) | 6 (3.1) | 16 (8.2) | 36 (18.4) | 12 (6.1) | 24 (12.2) |
| Nighoghossian et al. [20] | 2002 | IVT | - | - | - | - | 26 (26.0) | 10 (10.0) | 16 (16.0) | - | - | - |
| Ozbek et al. [78] | 2018 | - | - | - | - | - | 41 (27.7) | 18 (12.2) | 23 (15.5) | - | - | - |
| Schlemm et al. [95] | 2022 | IVT | SITS-MOST, ECASS-II, ECASS-III, NINDS | 26 (5.7) | 11 (2.4) | 15 (3.3) | 102 (22.2) | 21 (4.6) | 46 (10.0) | 125 (27.2) | 34 (7.4) | 91 (19.8) |
| Shi et al. [47] | 2016 | EVT | - | - | - | - | 91 (44.2) | 14 (6.8) | 77 (37.4) | - | - | - |
| Soo et al. [35] | 2012 | EVT | - | - | - | - | 7 (5.3) | 1 (0.8) | 6 (4.5) | - | - | - |
| Takahashi et al. [40] | 2013 | - | - | - | - | - | 27 (14.4) | 5 (2.7) | 22 (11.8) | - | - | - |
| Yan et al. [73] | 2015 | IVT | ECASS-II | 8 (2.4) | 6 (1.8) | 2 (0.6) | 102 (30.6) | 48 (14.4) | 54 (16.2) | 206 (61.9) | 140 (42.0) | 66 (19.8) |
| Yang et al. [48] | 2016 | - | - | - | - | - | 35 (10.0) | 10 (2.9) | 25 (7.2) | - | - | - |
| Zand et al. [51] | 2017 | IVT | ECASS-II | 25 (3.7) | 5 (0.7) | 20 (3.0) | - | - | - | - | - | - |
| Zand et al. [64] | 2018 | IVT | - | - | - | - | 6 (0.8) | 3 (0.4) | 3 (0.4) | - | - | - |
| Zhao et al. [74] | 2017 | IVT | ECASS-II | 2 (3.3) | 2 (3.3) | 0 (0) | - | - | - | - | - | - |
| Modality | Subgroup | Pooled Prevalence (Effect Size) | 95% Confidence Interval | Weight (%) | Heterogeneity χ2 (Degrees of Freedom) | p-Value | I2 (%) | z-Score | p-Value (z-Test) |
|---|---|---|---|---|---|---|---|---|---|
| T2 Gradient Echo Imaging (T2*) | - | 0.25 | 0.22–0.28 | 57.74 | 844.41 (45) | 0 | 94.67 | 28.82 | 0 |
| Susceptibility Weighted Imaging (SWI) | - | 0.36 | 0.31–0.41 | 37.44 | 563.55 (29) | 0 | 94.85 | 25.61 | 0 |
| Both | - | 0.25 | 0.18–0.32 | 4.82 | 12.99 (3) | 0 | 76.90 | 11.67 | 0 |
| Overall | - | 0.29 | 0.26–0.31 | 100 | 1912.84 (79) | 0 | 95.87 | 35.04 | 0 |
| Age | |||||||||
| T2* | <65 years | 0.22 | 0.18–0.26 | 29.20 | 75.77 (9) | 0 | 88.12 | 18.58 | 0 |
| ≥65 years | 0.25 | 0.21–0.30 | 70.80 | 674.41 (23) | 0 | 96.59 | 19.14 | 0 | |
| Overall | 0.24 | 0.21–0.28 | 100 | 779.72 (33) | 0 | 95.77 | 24.20 | 0 | |
| SWI | <65 years | 0.36 | 0.27–0.47 | 31.66 | 103.61 (5) | 0 | 95.17 | 11.57 | 0 |
| ≥65 years | 0.35 | 0.28–0.43 | 68.34 | 261.71 (12) | 0 | 95.41 | 15.56 | 0 | |
| Overall | 0.36 | 0.30–0.42 | 100 | 377.01 (18) | 0 | 95.23 | 19.92 | 0 | |
| Hypertension | |||||||||
| T2* | <65% HTN | 0.21 | 0.17–0.27 | 44.34 | 161.27 (11) | 0 | 93.18 | 14.73 | 0 |
| ≥65% HTN | 0.26 | 0.23–0.29 | 55.66 | 107.71 (14) | 0 | 87.00 | 27.15 | 0 | |
| Overall | 0.24 | 0.21–0.27 | 100 | 300.82 (26) | 0 | 91.38 | 27.94 | 0 | |
| SWI | <65% HTN | 0.37 | 0.27–0.48 | 26.22 | 65.62 (4) | 0 | 93.90 | 10.96 | 0 |
| ≥65% HTN | 0.36 | 0.29–0.43 | 73.78 | 351.70 (13) | 0 | 96.30 | 16.52 | 0 | |
| Overall | 0.36 | 0.30–0.42 | 100 | 418.37 (18) | 0 | 95.70 | 20.24 | 0 | |
| Fluid Attenuated Inversion Recovery (FLAIR) | |||||||||
| T2* | FLAIR | 0.24 | 0.21–0.27 | 60.69 | 333.22 (27) | 0 | 91.90 | 25.27 | 0 |
| No FLAIR | 0.26 | 0.20–0.31 | 39.31 | 499.28 (17) | 0 | 96.60 | 15.95 | 0 | |
| Overall | 0.25 | 0.22–0.28 | 100 | 844.41 (45) | 0 | 94.67 | 28.82 | 0 | |
| SWI | FLAIR | 0.38 | 0.31–0.44 | 63.04 | 348.57 (16) | 0 | 95.41 | 18.86 | 0 |
| No FLAIR | 0.33 | 0.25–0.42 | 36.96 | 185.34 (9) | 0 | 95.14 | 12.30 | 0 | |
| Overall | 0.36 | 0.31–0.41 | 100 | 555.50 (26) | 0 | 95.32 | 22.70 | 0 | |
| Non-contrast Computed Tomography (NCCT) | |||||||||
| T2* | NCCT | 0.27 | 0.21–0.33 | 35.74 | 573.23 (15) | 0 | 97.38 | 14.89 | 0 |
| No NCCT | 0.24 | 0.21–0.26 | 64.26 | 260.83 (29) | 0 | 88.88 | 28.43 | 0 | |
| Overall | 0.25 | 0.22–0.28 | 100 | 844.41 (45) | 0 | 94.67 | 28.82 | 0 | |
| SWI | NCCT | 0.44 | 0.34–0.54 | 22.39 | 62.59 (5) | 0 | 92.01 | 13.27 | 0 |
| No NCCT | 0.33 | 0.28–0.39 | 77.61 | 467.38 (20) | 0 | 95.72 | 19.05 | 0 | |
| Overall | 0.36 | 0.31–0.41 | 100 | 555.50 (26) | 0 | 95.32 | 22.70 | 0 | |
| Field Strength in Tesla (T) | |||||||||
| T2* | 1.5 | 0.27 | 0.23–0.31 | 68.57 | 252.74 (21) | 0 | 91.76 | 22.77 | 0 |
| 3T | 0.23 | 0.18–0.28 | 31.43 | 112.65 (8) | 0 | 92.90 | 16.68 | 0 | |
| Overall | 0.25 | 0.22–0.29 | 100 | 460.20 (30) | 0 | 93.48 | 26.04 | 0 | |
| SWI | 1.5T | 0.36 | 0.26–0.47 | 35.48 | 106.63 (7) | 0 | 93.44 | 10.85 | 0 |
| 3T | 0.37 | 0.31–0.43 | 64.52 | 261.40 (13) | 0 | 95.03 | 19.39 | 0 | |
| Overall | 0.37 | 0.32–0.42 | 100 | 370.43 (21) | 0 | 94.33 | 23.04 | 0 | |
| Slice Thickness | |||||||||
| Overall | Thin ≤ 2 mm | 0.40 | 0.32–0.49 | 13.36 | 139.05 (10) | 0 | 92.81 | 14.10 | 0 |
| Medium 2.1–4.9 mm | 0.23 | 0.18–0.28 | 5 | 10.84 (3) | 0.01 | 72.33 | 15.56 | 0 | |
| Thick ≥ 5 mm | 0.25 | 0.22–0.29 | 41.78 | 545.62 (32) | 0 | 94.14 | 25.02 | 0 | |
| Overall | 0.28 | 0.25–0.31 | 100 | 809.02 (47) | 0 | 94.19 | 29.72 | 0 | |
| Region | |||||||||
| T2* | Asia | 0.28 | 0.24–0.33 | 59.14 | 645.90 (26) | 0 | 95.97 | 21.40 | 0 |
| Europe | 0.21 | 0.19–0.24 | 27.25 | 41.79 (12) | 0 | 71.29 | 25.63 | 0 | |
| North America | 0.18 | 0.14–0.22 | 9.4 | 19.97 (3) | 0 | 84.97 | 16.17 | 0 | |
| Multinational | 0.15 | 0.12–0.18 | 4.21 | - | - | - | 18.06 | 0 | |
| Overall | 0.25 | 0.22–0.28 | 100 | 844.41 (45) | 0 | 94.67 | 28.82 | 0 | |
| SWI | Africa | 0.26 | 0.22–0.30 | 6.4 | - | - | - | 19.45 | 0 |
| Asia | 0.41 | 0.37–0.46 | 68.91 | 260.13 (19) | 0 | 92.70 | 28.14 | 0 | |
| Europe | 0.27 | 0.18–0.37 | 17.63 | 69.37 (4) | 0 | 94.23 | 9.34 | 0 | |
| North America | 0.24 | 0.21–0.28 | 7.06 | - | - | - | 21.50 | 0 | |
| Overall | 0.36 | 0.32–0.41 | 100 | 559.19 (28) | 0 | 94.99 | 25.44 | 0 | |
| Stroke Subtype | |||||||||
| T2* | Atherothrombotic | 0.25 | 0.12–0.39 | 28.03 | 46.29 (4) | 0 | 91.36 | 5.74 | 0 |
| Lacunar | 0.39 | 0.25–0.53 | 29.73 | 35.05 (4) | 0 | 88.59 | 8.24 | 0 | |
| Cardioembolic | 0.24 | 0.14–0.35 | 6.59 | 11.31 (4) | 0.02 | 64.65 | 7.09 | 0 | |
| Undetermined | 0.27 | 0.20–0.33 | 17.11 | - | - | - | 12.71 | 0 | |
| Overall | 0.29 | 0.23–0.36 | 100 | 119.90 (17) | 0 | 85.82 | 14.31 | 0 | |
| SWI | Atherothrombotic | 0.23 | 0.08–0.42 | 27.31 | 104.67 (4) | 0 | 96,18 | 4.19 | 0 |
| Lacunar | 0.26 | 0.17–0.37 | 26.62 | 19.57 (4) | 0 | 79.56 | 8.45 | 0 | |
| Cardioembolic | 0.25 | 0.11–0.43 | 26.37 | 61.15 (4) | 0 | 93.46 | 4.96 | 0 | |
| Undetermined | 0.20 | 0.10–0.32 | 19.7 | 11.40 (3) | 0.01 | 73.69 | 5.60 | 0 | |
| Overall | 0.24 | 0.18–0.30 | 100 | 229.98 (18) | 0 | 92.17 | 11.94 | 0 | |
| Cerebral Microbleed Location | |||||||||
| T2* | Deep | 0.33 | 0.20–0.47 | 19.76 | 60.02 (6) | 0 | 90.00 | 7.47 | 0 |
| Infratentorial | 0.08 | 0.02–0.19 | 13.69 | 19.12 (4) | 0 | 79.08 | 3.15 | 0 | |
| Lobar | 0.37 | 0.29–0.46 | 34.78 | 93.38 (11) | 0 | 88.22 | 13.21 | 0 | |
| Mixed | 0.46 | 0.36–0.55 | 31.76 | 84.44 (10) | 0 | 88.16 | 14.15 | 0 | |
| Overall | 0.34 | 0.28–0.41 | 100 | 446 (34) | 0 | 92.38 | 16.16 | 0 | |
| SWI | Deep | 0.18 | 0.14–0.21 | 23.04 | 19.94 (8) | 0.01 | 59.87 | 16.52 | 0 |
| Infratentorial | 0.12 | 0.07–0.19 | 23.04 | 86.75 (8) | 0 | 90.78 | 6.55 | 0 | |
| Lobar | 0.29 | 0.24–0.34 | 28.25 | 45.14 (10) | 0 | 77.85 | 18.79 | 0 | |
| Mixed | 0.49 | 0.39–0.60 | 25.68 | 155.02 (9) | 0 | 94.19 | 13.46 | 0 | |
| Overall | 0.27 | 0.21–0.33 | 100 | 1021.49 (38) | 0 | 96.28 | 14.86 | 0 | |
| Outcome | Modality | Effect Measure | Summary Effects | Heterogeneity ⍺ | Heterogeneity Variance Estimates | ||||
|---|---|---|---|---|---|---|---|---|---|
| DerSimonian and Laird Random-Effects Method (REDL) | Tests of Overall Effect | Cochran’s Q | H | I2 ≤ * | p-Value | τ2 ≤ † | |||
| Odds Ratio (OR) (95% Confidence Interval) | |||||||||
| Symptomatic intracranial hemorrhage (sICH) | T2 Gradient Echo Imaging (T2*) | OR | 2.13 [1.435; 3.160] | p = 0.000, z = 3.754 | 11.08 | 1.18 | 27.8% | 0.197 | 0.0949 |
| Susceptibility Weighted Imaging (SWI) | OR | 2.687 [0.722; 10.007] | p = 0.141, z = 1.474 | 6.86 | 1.51 | 56.3% | 0.076 | 0.972 | |
| Both | OR | 2.916 [1.294; 6.574] | p = 0.010, z = 2.581 | 0.00 | - | - | - | 0 | |
| Overall | OR | 2.216 [1.555; 3.159] | p = 0.000, z = 4.402 | 18.49 | 1.19 | 29.7% | 0.140 | 0.122 | |
| Hemorrhagic transformation (HT) | T2* | OR | 1.229 [0.820; 1.843] | p = 0.319, z = 0.997 | 32.95 | 1.73 | 66.6% | 0.001 | 0.282 |
| SWI | OR | 1.402 [0.910; 2.163] | p = 0.125, z = 1.535 | 8.64 | 1.20 | 30.6% | 0.195 | 0.0956 | |
| Both | OR | 1.788 [1.033; 3.094] | p = 0.038, z = 2.076 | 0.70 | 0.84 | 0.0% | 0.401 | 0 | |
| Overall | OR | 1.332 [1.013; 1.750] | p = 0.040, z = 2.054 | 1.16 | 1.47 | 53.5% | 0.002 | 0.174 | |
| Modified Ranking Scale (mRS) 3–6 at 90 Days | T2* | OR | 1.572 [1.282; 1.927] | p = 0.000, z = 4.346 | 6.06 | 1.10 | 17.5% | 0.300 | 0.0114 |
| SWI | OR | 1.727 [1.303; 2.289] | p = 0.000, z = 3.798 | 2.68 | 0.95 | 0.0% | 0.444 | 0 | |
| Both | OR | 1.579 [0.976; 2.555] | p = 0.063, z = 1.859 | 0.00 | - | - | - | 0 | |
| Overall | OR | 1.606 [1.387; 1.858] | p = 0.000, z = 6.344 | 9.09 | 0.95 | 0.0% | 0.524 | 0 | |
| Outcome | Modality | Parameter | Estimate | 95% Confidence Interval (CI) |
|---|---|---|---|---|
| Symptomatic Intracranial Hemorrhage (sICH) | Susceptibility Weighted Imaging (SWI) | Sensitivity | 0.05 | [0.03; 0.08] |
| Specificity | 0.98 | [0.95; 0.99] | ||
| Positive Likelihood Ratio | 2.8 | [0.7; 11.2 | ||
| Negative Likelihood Ratio | 0.97 | [0.93; 0.1.01] | ||
| Diagnostic Odds Ratio | 3 | [1; 12] | ||
| Pretest Probability of Disease | 0.04 | - | ||
| Area under ROC Curve (AUROC) | 0.11 | [0.08; 0.14] | ||
| Interstudy Variation in Sensitivity (ICC_SEN) | 0.01 | [0.00; 0.07] | ||
| Interstudy Variation in Specificity (ICC_SPE) | 0.17 | [0.00; 0.50] | ||
| Heterogeneity (Chi-square) | 2.333, degrees of freedom (df) = 2, p = 0.156 | |||
| Inconsistency (I2) | 14 | [0; 100] | ||
| T2 Gradient Echo Imaging (T2*) | Sensitivity | 0.09 | [0.07; 0.12] | |
| Specificity | 0.96 | [0.93; 0.97] | ||
| Positive Likelihood Ratio | 2.1 | [1.4; 3.1] | ||
| Negative Likelihood Ratio | 0.95 | [0.93; 0.97] | ||
| Diagnostic Odds Ratio | 2 | [1; 3] | ||
| Pretest Probability of Disease | 0.16 | - | ||
| AUROC | 0.30 | [ 0.26; 0.34] | ||
| ICC_SEN | 0.02 | [0.00; 0.07] | ||
| ICC_SPE | 0.11 | [0.00; 0.22] | ||
| Heterogeneity (Chi-square) | 29.382, df = 2, p < 0.0001 | |||
| I2 | 93 | [87; 99] | ||
| Hemorrhagic Transformation (HT) | SWI | Sensitivity | 0.34 | [0.15; 0.61] |
| Specificity | 0.75 | [0.62; 0.85] | ||
| Positive Likelihood Ratio | 1.4 | [1.0; 2.0] | ||
| Negative Likelihood Ratio | 0.87 | [0.69, 1.11] | ||
| Diagnostic Odds Ratio | 2 | [1, 3] | ||
| Pretest Probability of Disease | 0.23 | - | ||
| AUROC | 0.65 | [0.61; 0.69] | ||
| ICC_SEN | 0.37 | [0.03; 0.72] | ||
| ICC_SPE | 0.16 | [0.00; 0.37] | ||
| Heterogeneity (Chi-square) | 44.168, df = 2, p < 0.001 | - | ||
| I2 | 95 | [92; 99] | ||
| T2* | Sensitivity | 0.21 | [0.12; 0.35] | |
| Specificity | 0.82 | [0.69; 0.90] | ||
| Positive Likelihood Ratio | 1.2 | [0.8; 1.7] | ||
| Negative Likelihood Ratio | 0.96 | [0.88; 1.05] | ||
| Diagnostic Odds Ratio | 1 | [1; 2] | ||
| Pretest Probability of Disease | 0.21 | - | ||
| AUROC | 0.52 | [0.48; 0.56] | ||
| ICC_SEN | 0.30 | [0.10; 0.50] | ||
| ICC_SPE | 0.32 | [0.13; 0.52] | ||
| Heterogeneity (Chi-square) | 334.234, df = 2, p < 0.001 | - | ||
| I2 | 99 | [99; 100] | ||
| Modified Rankin Scale (mRS) 3-6 at 90 days | Overall | Sensitivity | 0.49 | [0.41; 0.58] |
| Specificity | 0.62 | [0.54; 0.69] | ||
| Positive Likelihood Ratio | 1.3 | [1.2; 1.4] | ||
| Negative Likelihood Ratio | 0.82 | [0.75; 0.89] | ||
| Diagnostic Odds Ratio | 2 | [1; 2] | ||
| Pretest Probability of Disease | 0.46 | - | ||
| AUROC | 0.58 | [0.54; 0.62] | ||
| ICC_SEN | 0.09 | [0.05; −0.12] | ||
| ICC_SPE | 0.08 | [0.05; 0.10] | ||
| Heterogeneity (Chi-square) | 170.018, df = 2, p < 0.0001 | - | ||
| I2 | 99 | [98; 99] |
| Outcome | No. of Studies (Participants) | Study Design | Relative Effect (95% CI) | Assumed Risk (control) | Risk with CMBs | Absolute Effect | Certainty of Evidence | Reasons |
|---|---|---|---|---|---|---|---|---|
| Symptomatic intracerebral hemorrhage (sICH) | 14 (~6163) | Observational (meta-analysis, random-effects) | OR 2.22 (1.56–3.16) | 40 per 1000 | 88 per 1000 | 48 more per 1000 | ⊕⊕◯◯ Low to Moderate | −1 risk of bias (variable definitions), −1 imprecision (subgroup variability), +1 consistent association |
| Hemorrhagic transformation (HT) | 21 (~6049) | Observational (meta-analysis, random-effects) | OR 1.33 (1.01–1.75) | 150 per 1000 | 190 per 1000 | 40 more per 1000 | ⊕⊕◯◯ Low | −1 risk of bias, −1 inconsistency (I2 = 53.5%), −1 indirectness (definitions variable) |
| Poor functional outcome (mRS 3–6 at 90 days) | 11 (~5499) | Observational (meta-analysis, random-effects) | OR 1.61 (1.39–1.86) | 350 per 1000 | 470 per 1000 | 120 more per 1000 | ⊕⊕⊕◯ Moderate | −1 risk of bias, +1 consistency (I2 = 0%) |
| CMB prevalence by imaging modality (SWI vs. T2*) | 80 (~28,383) | Observational (meta-analysis) | SWI 36% (95% CI: 31–41); T2* 25% (22–28) | — | — | 11% higher detection with SWI | ⊕⊕◯◯ Low | −1 inconsistency (high heterogeneity), −1 indirectness, +1 strong magnitude of effect |
| Diagnostic accuracy for sICH prediction | 14 (~6163) | Observational (diagnostic meta-analysis) | AUC 0.29; DOR 2–3 | — | — | Poor sensitivity (<10%) but high specificity (>95%) | ⊕◯◯◯ Very low | −1 risk of bias, −1 indirectness, −1 imprecision |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.; Spring, K.J.; Killingsworth, M.; Bhaskar, S. Comparative Diagnostic and Prognostic Performance of SWI and T2-Weighted MRI in Cerebral Microbleed Detection Following Acute Ischemic Stroke: A Meta-Analysis and SPOT-CMB Study. Medicina 2025, 61, 1566. https://doi.org/10.3390/medicina61091566
Tan R, Spring KJ, Killingsworth M, Bhaskar S. Comparative Diagnostic and Prognostic Performance of SWI and T2-Weighted MRI in Cerebral Microbleed Detection Following Acute Ischemic Stroke: A Meta-Analysis and SPOT-CMB Study. Medicina. 2025; 61(9):1566. https://doi.org/10.3390/medicina61091566
Chicago/Turabian StyleTan, Rachel, Kevin J. Spring, Murray Killingsworth, and Sonu Bhaskar. 2025. "Comparative Diagnostic and Prognostic Performance of SWI and T2-Weighted MRI in Cerebral Microbleed Detection Following Acute Ischemic Stroke: A Meta-Analysis and SPOT-CMB Study" Medicina 61, no. 9: 1566. https://doi.org/10.3390/medicina61091566
APA StyleTan, R., Spring, K. J., Killingsworth, M., & Bhaskar, S. (2025). Comparative Diagnostic and Prognostic Performance of SWI and T2-Weighted MRI in Cerebral Microbleed Detection Following Acute Ischemic Stroke: A Meta-Analysis and SPOT-CMB Study. Medicina, 61(9), 1566. https://doi.org/10.3390/medicina61091566







