Accuracy and Early Outcomes of Patient-Specific TKA Using Inertial-Based Cutting Guides: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patients
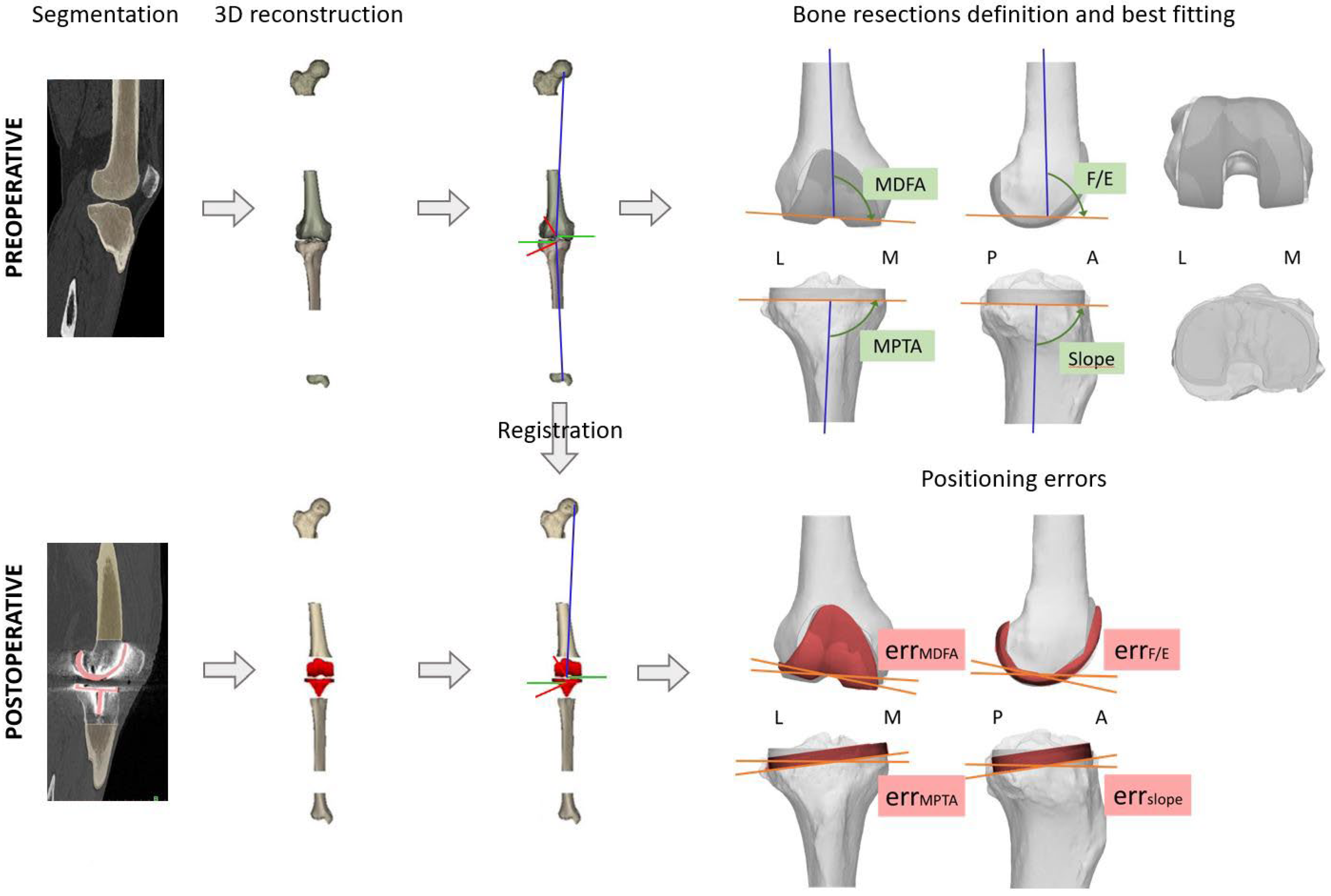
2.3. CT Evaluation: Preoperative Planning and Postoperative Measurements
2.4. Preoperative Planning
2.5. Postoperative Measurements
2.6. Clinical, Functional, and Radiographic Evaluation
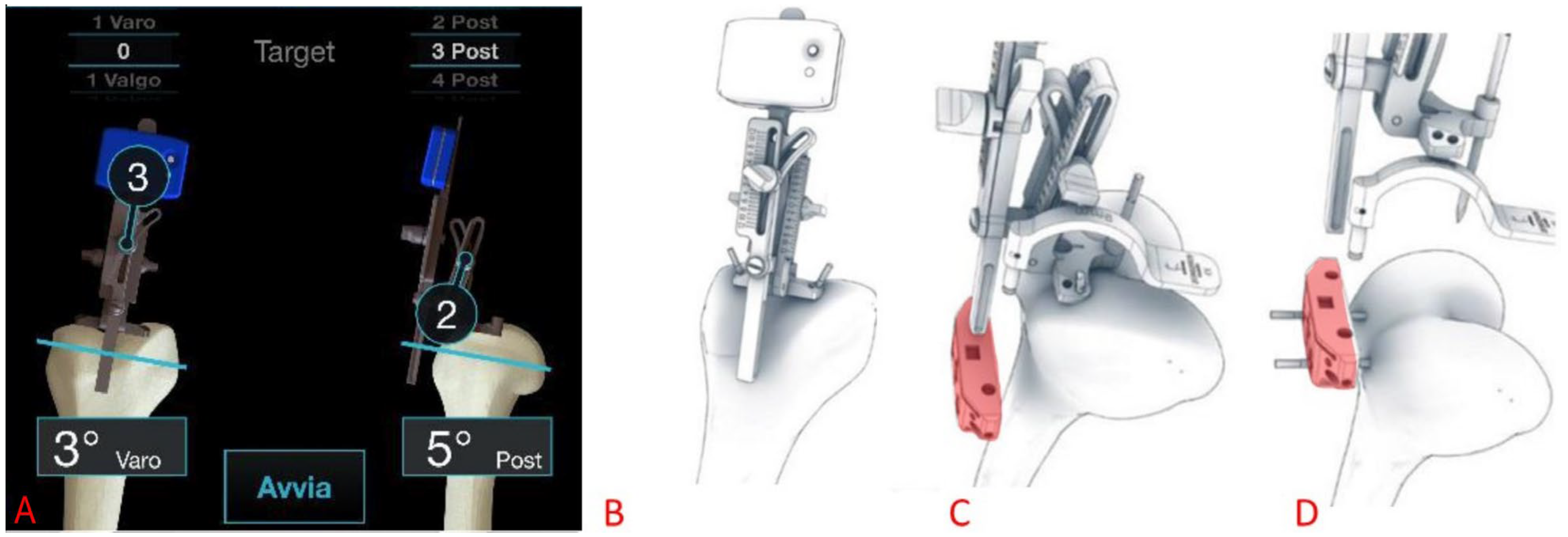
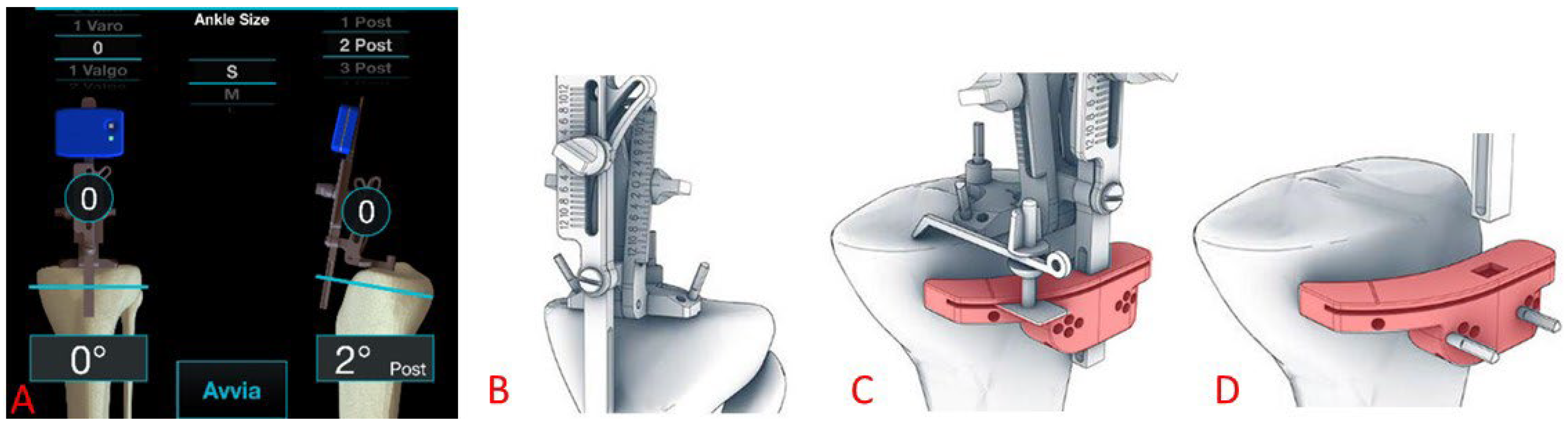
2.7. Surgical Procedure
2.8. Statistical Analysis
3. Results
3.1. Population Demographics
3.2. Positioning Accuracy Achieved with Inertial-Based Extramedullary Cutting Guides
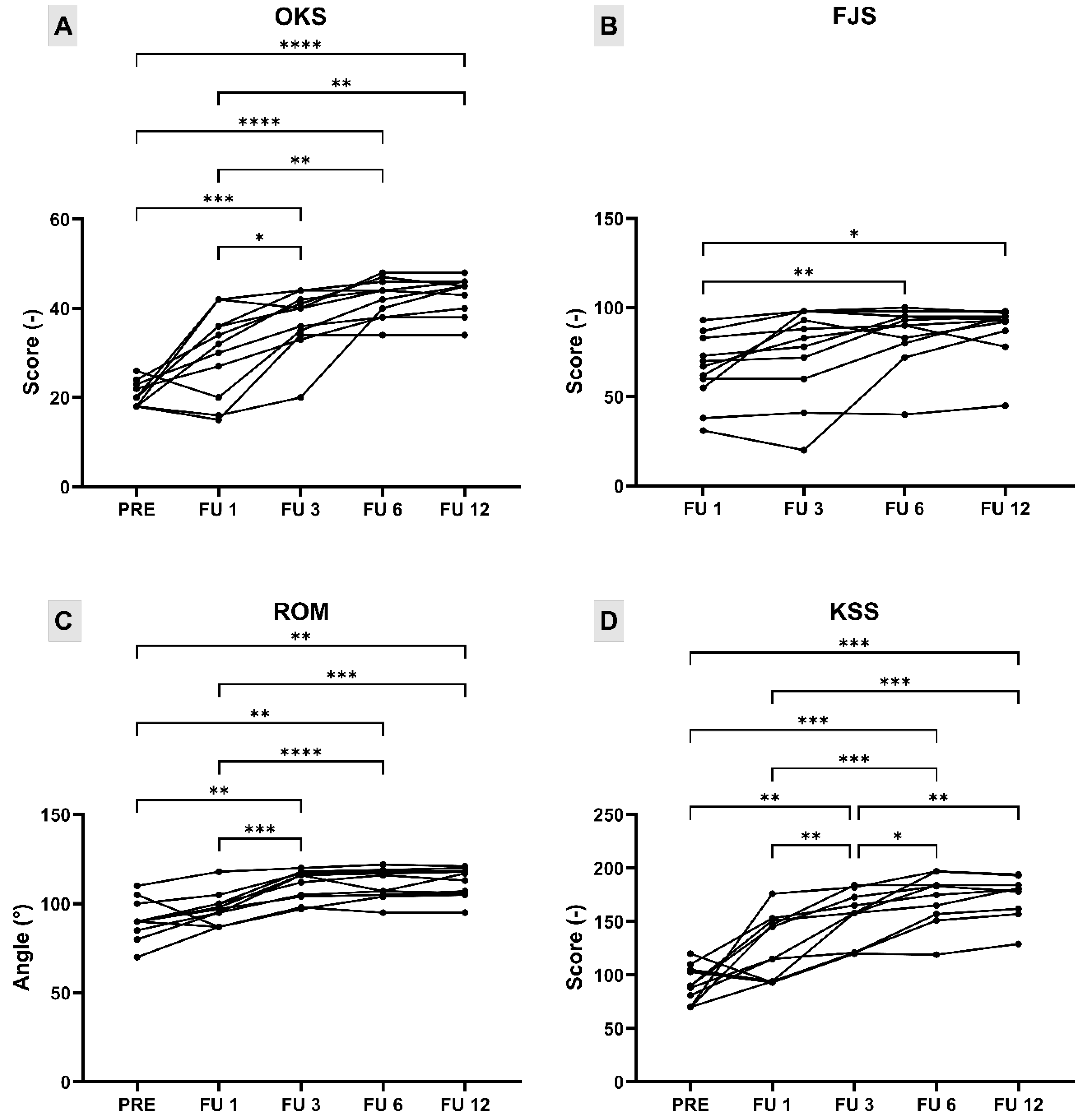
3.3. Postoperative Clinical and Radiographic Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Antero-Posterior |
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| CR | Cruciate Retaining |
| CT | Computed Tomography |
| EQ-5D-3L/EQ-VAS | EuroQol scores |
| FJS | Forgotten Joint Score |
| FLRs | Full Leg Radiographs |
| FU | Follow-Up |
| HAAS | High-Activity Arthroplasty Score |
| CT-derived Hip–Knee–Ankle | |
| JR | Joint Replacement |
| KL | Kellgren–Lawrence |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| KSS | Knee Society Score |
| MA | Mechanical Alignment |
| MDFA | Medial Distal Femoral Angle |
| ML | Medio-Lateral |
| MPTA | Medial Proximal Tibial Angles |
| OA | Osteoarthritis |
| OKS | Oxford Knee Score |
| OTS | Off-the-shelf |
| PRE | Pre-operative |
| PROMs | Patient Reported Outcomes Measures |
| PSC | Patient Specific Components |
| PSI | Patient Specific Instrument |
| ROM | Range of Motion |
| SD | Standard Deviation |
| TKA | Total Knee Arthroplasty |
| VAS | Visual Analogue Scale |
References
- Gunaratne, R.; Pratt, D.N.; Banda, J.; Fick, D.P.; Khan, R.J.K.; Robertson, B.W. Patient Dissatisfaction Following Total Knee Arthroplasty: A Systematic Review of the Literature. J. Arthroplast. 2017, 32, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Bonanzinga, T.; Gambaro, F.M.; Iacono, F.; Marcacci, M. Sub-Optimal Femoral Fit in Total Knee Arthroplasty, a Systematic Review of Human Femoral Data vs off-the-Shelf Contemporary Femoral Components. J. Exp. Orthop. 2023, 10, 41. [Google Scholar] [CrossRef]
- Clementi, D.; Boselli, P.M.; Marzorati, S. Aggiornamento: Sviluppo Della Taglia Nelle Componenti Protesiche Di Ginocchio. G. Ital. Di Ortop. E Traumatol. 2008, 34, 211–215. [Google Scholar]
- Budhiparama, N.C.; Lumban-Gaol, I.; Ifran, N.N.; de Groot, P.C.J.; Nelissen, R.G.H.H. Anthropometric Measurement of Caucasian and Asian Knees, Mismatch with Knee Systems? Orthop. J. Sports Med. 2020, 8, 2325967120S00104. [Google Scholar] [CrossRef]
- Gattu, N.; Sutton, M.; Doherty, D.B.; Lanfermeijer, N.D.; Rodriguez-Quintana, D.; Ismaily, S.K.; Pletka, C.A.; Noble, P.C.; Han, S. Variations in Trochlear Morphology of Contemporary and Legacy Total Knee Arthroplasty Prostheses: A Review of 22 Designs. J. Arthroplast. 2024, 39, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Additive manufacturing applications in orthopaedics: A review. J. Clin. Orthop. Trauma 2018, 9, 202–206. [Google Scholar] [CrossRef]
- Moret, C.S.; Hirschmann, M.T.; Vogel, N.; Arnold, M.P. Customised, Individually Made Total Knee Arthroplasty Shows Promising 1-Year Clinical and Patient Reported Outcomes. Arch. Orthop. Trauma. Surg. 2021, 141, 2217–2225. [Google Scholar] [CrossRef]
- Saffarini, M.; Hirschmann, M.T.; Bonnin, M. Personalisation and Customisation in Total Knee Arthroplasty: The Paradox of Custom Knee Implants. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1193–1195. [Google Scholar] [CrossRef]
- Sheridan, G.A.; Abdelmalek, M.; Howard, L.C.; Neufeld, M.E.; Masri, B.A.; Garbuz, D.S. Navigated Versus Conventional Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Prospective Randomized Controlled Trials. J. Orthop. 2024, 50, 99–110. [Google Scholar] [CrossRef]
- Ekhtiari, S.; Sun, B.; Sidhu, R.; Ade-Conde, A.M.; Chaudhry, H.; Tomescu, S.; Ravi, B.; Mundi, R. Evidence Versus Frenzy in Robotic Total Knee Arthroplasty. J. Bone Jt. Surg. 2024, 106, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Batailler, C.; Swan, J.; Sappey Marinier, E.; Servien, E.; Lustig, S. New Technologies in Knee Arthroplasty: Current Concepts. J. Clin. Med. 2020, 10, 47. [Google Scholar] [CrossRef]
- Bonanzinga, T.; Giuffrida, A.; Di Matteo, B.; Raspugli, G.F.; Iacono, F.; Marcacci, M. In Vitro Validation of a Novel Inertial-Based Cutting Guide for Tibial Resection in Total Knee Arthroplasty. Knee 2020, 27, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Marcheggiani Muccioli, G.M.; Alesi, D.; Russo, A.; Lo Presti, M.; Sassoli, I.; La Verde, M.; Zaffagnini, S. Intra- and Inter-Operator Reliability Assessment of a Novel Extramedullary Accelerometer-Based Smart Cutting Guide for Total Knee Arthroplasty: An in Vivo Study. Int. Orthop. 2023, 47, 83–87. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Gao, X.; Dou, T.; Li, X. Accelerometer-Based Navigation vs. Conventional Techniques for Total Knee Arthroplasty (TKA): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arthroplasty 2022, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Budhiparama, N.C.; Lumban-Gaol, I.; Ifran, N.N.; Parratte, S.; Nelissen, R. Does Accelerometer-Based Navigation Have Any Clinical Benefit Compared with Conventional TKA? A Systematic Review. Clin. Orthop. Relat. Res. 2019, 477, 2017–2029. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A Joint Coordinate System for the Clinical Description of Three-Dimensional Motions: Application to the Knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef]
- Iacono, F.; Bonanzinga, T.; Di Matteo, B.; Iacomella, A.; Delmedico, M.; Gambaro, F.M.; Favaro, A.; Marcacci, M. The Trochlear Bisector as a New Landmark for Kinematic Alignment in Total Knee Arthroplasty: A Radiographic Study. J. Clin. Med. 2024, 13, 3548. [Google Scholar] [CrossRef]
- Ewald, F.C. The Knee Society Total Knee Arthroplasty Roentgenographic Evaluation and Scoring System. Clin. Orthop. Relat. Res. 1989, 248, 9–12. [Google Scholar] [CrossRef]
- Joseph, L.; Batailler, C.; Lustig, S.; Servien, E. Accuracy of Accelerometer-Based Navigation System Perseus for the Tibial Cut in Total Knee Arthroplasty: No Superiority Compared to Mechanical Instrumentation in Current Practice. Appl. Sci. 2023, 13, 2952. [Google Scholar] [CrossRef]
- Farooq, H.; Deckard, E.R.; Carlson, J.; Ghattas, N.; Meneghini, R.M. Coronal and Sagittal Component Position in Contemporary Total Knee Arthroplasty: Targeting Native Alignment Optimizes Clinical Outcomes. J. Arthroplast. 2023, 38, S245–S251. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, J.-W.; Kim, J.-S.; Park, S.-D. The Relationship between the Survival of Total Knee Arthroplasty and Postoperative Coronal, Sagittal and Rotational Alignment of Knee Prosthesis. Int. Orthop. 2014, 38, 379–385. [Google Scholar] [CrossRef]
- Scott, C.E.H.; Clement, N.D.; Yapp, L.Z.; MacDonald, D.J.; Patton, J.T.; Burnett, R. Association Between Femoral Component Sagittal Positioning and Anterior Knee Pain in Total Knee Arthroplasty. J. Bone Jt. Surg. 2019, 101, 1575–1585. [Google Scholar] [CrossRef]
- Nishitani, K.; Kuriyama, S.; Nakamura, S.; Song, Y.D.; Morita, Y.; Ito, H.; Matsuda, S. Excessive Flexed Position of the Femoral Component Causes Abnormal Kinematics and Joint Contact/ Ligament Forces in Total Knee Arthroplasty. Sci. Rep. 2023, 13, 6356. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Buehler, K.C.; Markel, D.C. Computer Assisted Navigation in Total Knee Arthroplasty. J. Arthroplast. 2005, 20, 132–138. [Google Scholar] [CrossRef]
- Blakeney, W.G.; Khan, R.J.K.; Wall, S.J. Computer-Assisted Techniques Versus Conventional Guides for Component Alignment in Total Knee Arthroplasty. J. Bone Jt. Surg. 2011, 93, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Maderbacher, G.; Schaumburger, J.; Baier, C.; Zeman, F.; Springorum, H.-R.; Birkenbach, A.-M.; Grifka, J.; Keshmiri, A. Appropriate Sagittal Femoral Component Alignment Cannot Be Ensured by Intramedullary Alignment Rods. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Maderbacher, G.; Matussek, J.; Keshmiri, A.; Greimel, F.; Baier, C.; Grifka, J.; Maderbacher, H. Rotation of Intramedullary Alignment Rods Affects Distal Femoral Cutting Plane in Total Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3311–3316. [Google Scholar] [CrossRef]
- Huang, T.-W.; Peng, K.-T.; Huang, K.-C.; Lee, M.S.; Hsu, R.W.-W. Differences in Component and Limb Alignment between Computer-Assisted and Conventional Surgery Total Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2954–2961. [Google Scholar] [CrossRef]
- Bonanzinga, T.; Tanzi, P.; Pia Neri, M.; Iacono, F.; Mazzola, C.; Belluati, A.; Colombelli, A.; Zaffagnini, S.; Marcacci, M. Evaluation of Blood Loss and Implant Alignment after Total Knee Arthroplasty with Inertial Based Extramedullary Femoral Cutting Guide. Joints 2018, 6, 161–166. [Google Scholar] [CrossRef][Green Version]
- Steffens, D.; Karunaratne, S.; McBride, K.; Gupta, S.; Horsley, M.; Fritsch, B. Implementation of robotic-assisted total knee arthroplasty in the public health system: A comparative cost analysis. Int. Orthop. 2022, 46, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Reimann, P.; Brucker, M.; Arbab, D.; Lüring, C. Patient Satisfaction—A Comparison between Patient-Specific Implants and Conventional Total Knee Arthroplasty. J. Orthop. 2019, 16, 273–277. [Google Scholar] [CrossRef]
- Wendelspiess, S.; Kaelin, R.; Vogel, N.; Rychen, T.; Arnold, M.P. No Difference in Patient-Reported Satisfaction after 12 Months between Customised Individually Made and off-the-Shelf Total Knee Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2948–2957. [Google Scholar] [CrossRef]
- Schroeder, L.; Dunaway, A.; Dunaway, D. A Comparison of Clinical Outcomes and Implant Preference of Patients with Bilateral TKA. JBJS Rev. 2022, 10, e20. [Google Scholar] [CrossRef]
- Vogel, N.; Kaelin, R.; Rychen, T.; Wendelspiess, S.; Müller-Gerbl, M.; Arnold, M.P. Satisfaction after Total Knee Arthroplasty: A Prospective Matched-pair Analysis of Patients with Customised Individually Made and Off-the-shelf Implants. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5873–5884. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Kaelin, R.; Arnold, M.P. Custom Total Knee Arthroplasty with Personalised Alignment Showed Better 2-year Functional Outcome Compared to Off-the-shelf Arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 3220–3229. [Google Scholar] [CrossRef]
- Ratano, S.; Müller, J.H.; Daxhelet, J.; Beckers, L.; Bondoux, L.; Tibesku, C.O.; Aït-Si-Selmi, T.; Bonnin, M.P. Custom TKA Combined with Personalised Coronal Alignment Yield Improvements That Exceed KSS Substantial Clinical Benefits. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, L.; Dobbelaere, A.; Ratano, S.; Bondoux, L.; Müller, J.H.; Dubreuil, S.; Saffarini, M.; Tibesku, C.O.; Aït-Si-Selmi, T.; Bonnin, M.P. Custom Total Knee Arthroplasty Combined with Personalised Alignment Grants 94% Patient Satisfaction at Minimum Follow-up of 2 Years. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1276–1283. [Google Scholar] [CrossRef]
- Fang, D.M.; Ritter, M.A.; Davis, K.E. Coronal Alignment in Total Knee Arthroplasty: Just How Important Is It? J. Arthroplast. 2009, 24, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, K.J.; Yang, D.S.; Jeung, S.W.; Choi, H.G.; Choy, W.S. Screw-Home Movement of the Tibiofemoral Joint during Normal Gait: Three-Dimensional Analysis. Clin. Orthop. Surg. 2015, 7, 303. [Google Scholar] [CrossRef]
- Ritter, M.A.; Davis, K.E.; Meding, J.B.; Pierson, J.L.; Berend, M.E.; Malinzak, R.A. The Effect of Alignment and BMI on Failure of Total Knee Replacement. J. Bone Jt. Surg. Am. 2011, 93, 1588–1596. [Google Scholar] [CrossRef]

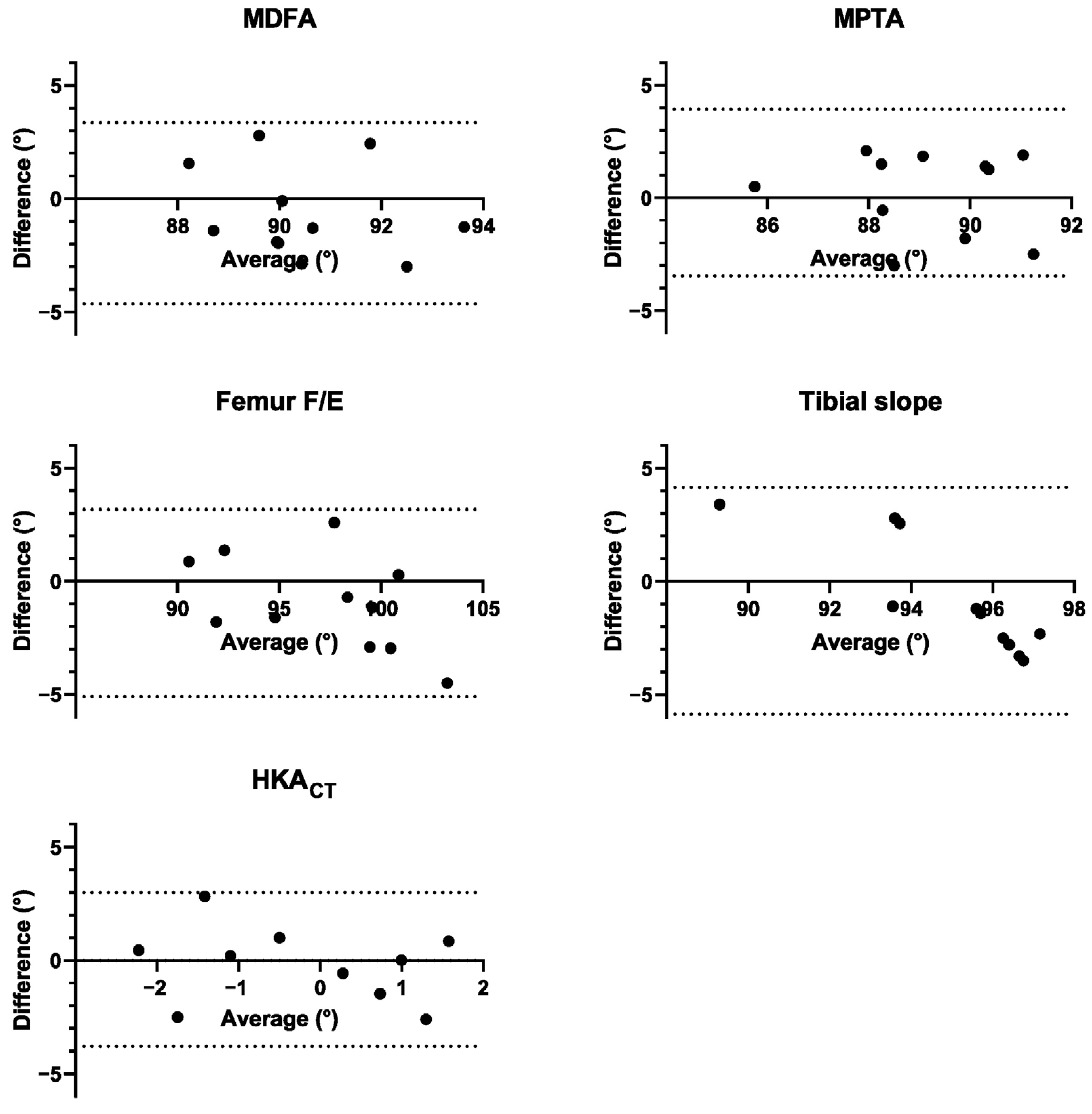
| Planned Mean (SD) | Post-Op Mean (SD) | Abs Error Mean (SD) | % <3° | B-A Bias (SD) | B-A 95% LoA | |
|---|---|---|---|---|---|---|
| MDFA (°) | 90.18 (1.66) | 90.82 (2.10) | 1.87 (0.87) | 100% | −0.63 (2.04) | [−4.64,3.37] |
| MPTA (°) | 89.27 (1.79) | 89.03 (1.96) | 1.67 (0.75) | 100% | 0.24 (1.89) | [−3.46, 3.95] |
| Femur F/E (°) | 96.73 (3.77) | 97.68 (4.79) | 1.89 (1.24) | 91% | −0.95 (2.11) | [−5.09, 3.19] |
| Tibial slope (°) | 94.55 (1.27) | 95.4 (3.46) | 2.45 (0.87) | 73% | −0.85 (2.56) | [−5.86, 4.16] |
| HKACT (°) | −0.55 (1.57) | −0.16 (−0.27) | 1.36 (1.06) | 100% | −0.39 (1.73) | [−3.79, 3.01] |
| PRE Mean (SD) | FU 1 Mean (SD) | FU 3 Mean (SD) | FU 6 Mean (SD) | FU 12 Mean (SD) | p-Value | |
|---|---|---|---|---|---|---|
| OKS (score) | 20.64 (2.77) | 30 (9.54) | 37.18 (6.90) | 42.27 (4.34) | 43 (4.12) | p < 0.0001 |
| ROM (°) | 90.91 (11.14) | 97.18 (9.12) | 109.09 (9.07) | 110.36 (8.44) | 111.45 (8.21) | p < 0.0001 |
| KSS f (score) | 46.09 (11.54) | 59.55 (17.81) | 74.09 (13.75) | 85.00 (12.45) | 86.82 (10.07) | p < 0.0001 |
| KSS k (score) | 44.55 (11.28) | 65.55 (12.72) | 76.91 (12.16) | 84.36 (11.86) | 85.82 (9.73) | p < 0.0001 |
| KSS tot (score) | 90.64 (17.25) | 125.09 (30.19) | 151.00 (25.57) | 169.36 (23.57) | 172.64 (19.46) | p < 0.0001 |
| FJS (score) | 65.34 (19.24) | 75.39 (25.65) | 85.09 (17.14) | 87.97 (15.28) | p = 0.0031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piovan, G.; Amarossi, A.; Bertolino, L.; Bardi, E.; Favaro, A.; Povegliano, L.; Screpis, D.; Iacono, F.; Bonanzinga, T. Accuracy and Early Outcomes of Patient-Specific TKA Using Inertial-Based Cutting Guides: A Pilot Study. Medicina 2025, 61, 1554. https://doi.org/10.3390/medicina61091554
Piovan G, Amarossi A, Bertolino L, Bardi E, Favaro A, Povegliano L, Screpis D, Iacono F, Bonanzinga T. Accuracy and Early Outcomes of Patient-Specific TKA Using Inertial-Based Cutting Guides: A Pilot Study. Medicina. 2025; 61(9):1554. https://doi.org/10.3390/medicina61091554
Chicago/Turabian StylePiovan, Gianluca, Andrea Amarossi, Luca Bertolino, Elena Bardi, Alberto Favaro, Lorenzo Povegliano, Daniele Screpis, Francesco Iacono, and Tommaso Bonanzinga. 2025. "Accuracy and Early Outcomes of Patient-Specific TKA Using Inertial-Based Cutting Guides: A Pilot Study" Medicina 61, no. 9: 1554. https://doi.org/10.3390/medicina61091554
APA StylePiovan, G., Amarossi, A., Bertolino, L., Bardi, E., Favaro, A., Povegliano, L., Screpis, D., Iacono, F., & Bonanzinga, T. (2025). Accuracy and Early Outcomes of Patient-Specific TKA Using Inertial-Based Cutting Guides: A Pilot Study. Medicina, 61(9), 1554. https://doi.org/10.3390/medicina61091554






