The Impact of Basal Inflammatory Status on Post-CABG Atrial and Ventricular Ectopy and Remodeling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluated Characteristics [17]
2.3. Statistical Analysis
3. Results
3.1. Cardiovascular Risk Factors and Post-CABG Arrhythmic Burden
3.2. Pre-Procedural Inflammatory Biomarker Levels and Post-CABG PACs and PVCs Burden
3.3. The Impact of Preoperative Inflammation on Post-CABG Outcomes
4. Discussion
4.1. Cardiovascular Risk Factors and Post-CABG PACs and PVCs Burden
4.2. Pre-Procedural Inflammatory Profile and Post-CABG PACs and PVCs
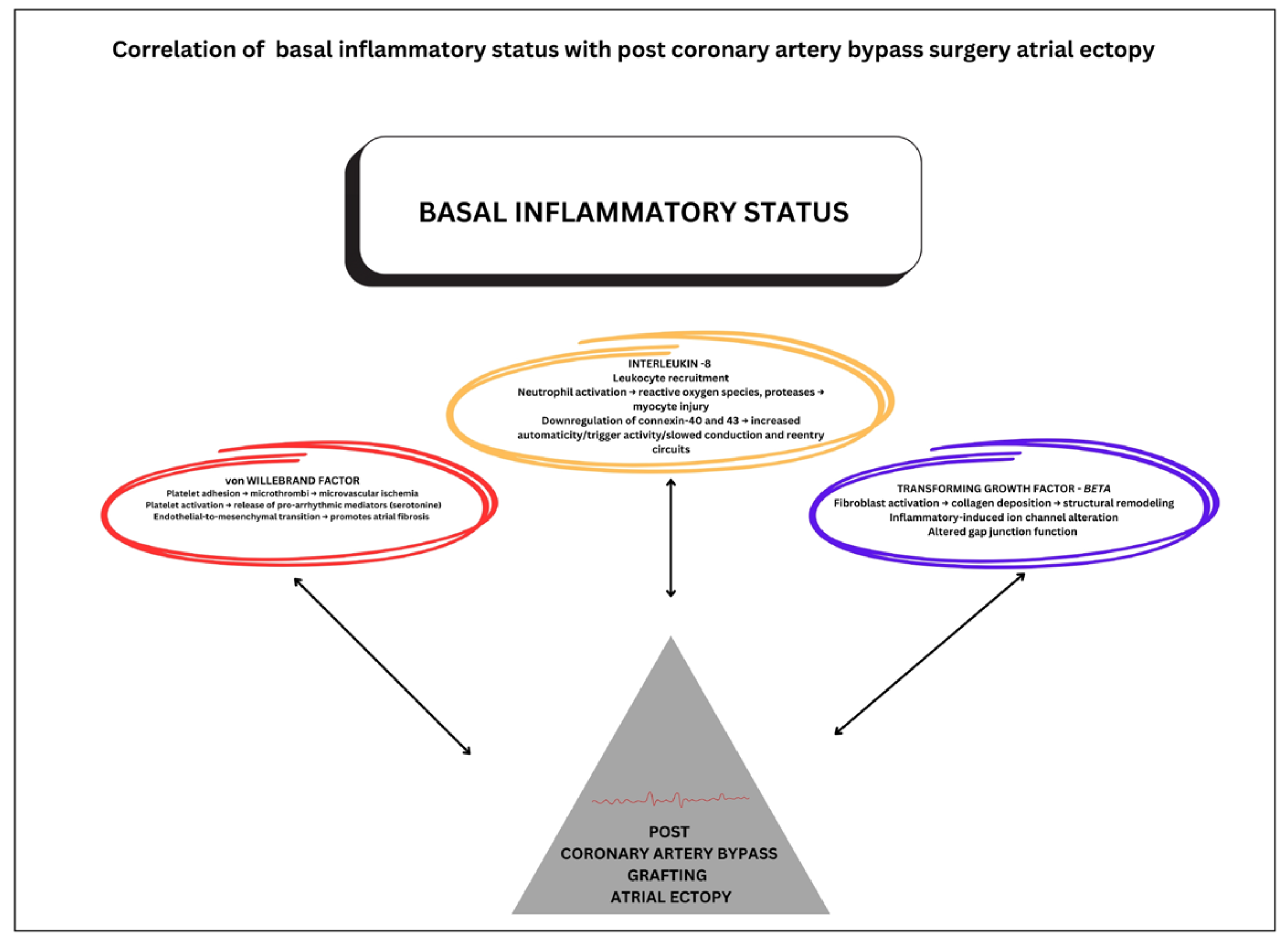
4.3. Atrial Cardiomyopathy and the Relationship with Premature Atrial Contractions in the Context of Coronary Artery Bypass Grafting Surgery
4.4. Clinical Implications of Preoperative Signature Inflammation
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Luengo-Fernandez, R.; Walli-Attaei, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Bairami, F.; Aboyans, V.; Timmis, A.D.; Vardas, P.; et al. Economic burden of cardiovascular diseases in the European Union: A population-based cost study. Eur. Heart J. 2023, 44, 4752–4767. [Google Scholar] [CrossRef] [PubMed]
- Koletsis, E.N.; Prokakis, C.; Crockett, J.R.; Dedeilias, P.; Panagiotou, M.; Panagopoulos, N.; Anastasiou, N.; Dougenis, D.; Apostolakis, E. Prognostic factors of atrial fibrillation following elective coronary artery bypass grafting: The impact of quantified intraoperative myocardial ischemia. J. Cardiothorac. Surg. 2011, 6, 127. [Google Scholar] [CrossRef][Green Version]
- Peretto, G.; Durante, A.; Limite, L.R.; Cianflone, D. Postoperative Arrhythmias after Cardiac Surgery: Incidence, Risk Factors, and Therapeutic Management. Cardiol. Res. Pract. 2014, 2014, 615987. [Google Scholar] [CrossRef]
- Farinha, J.M.; Gupta, D.; Lip, G.Y.H. Frequent premature atrial contractions as a signalling marker of atrial cardiomyopathy, incident atrial fibrillation, and stroke. Cardiovasc. Res. 2023, 119, 429–439. [Google Scholar] [CrossRef]
- Burrage, P.S.; Low, Y.H.; Campbell, N.G.; O’Brien, B. New-Onset Atrial Fibrillation in Adult Patients After Cardiac Surgery. Curr. Anesthesiol. Rep. 2019, 9, 174–193. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Sawaya, F.J.; Kilgo, P.; Stein, W.; Halkos, M.; Thourani, V.; Lattouf, O.M.; Delurgio, D.B.; Guyton, R.A.; Puskas, J.D.; et al. Ventricular Arrhythmia After Cardiac Surgery: Incidence, Predictors, and Outcomes. J. Am. Coll. Cardiol. 2012, 60, 2664–2671. [Google Scholar] [CrossRef]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Bader Eddeen, A.; Ruel, M. Long-Term Outcomes in Patients with Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting. JAMA Cardiol. 2020, 5, 631–641. [Google Scholar] [CrossRef]
- Marinheiro, R.; Parreira, L.; Amador, P.; Sá, C.; Duarte, T.; Caria, R. Excessive atrial ectopic activity as an independent risk factor for ischemic stroke. Int. J. Cardiol. 2017, 249, 226–230. [Google Scholar] [CrossRef]
- Helgadottir, S.; Sigurdsson, M.I.; Ingvarsdottir, I.L.; Arnar, D.O.; Gudbjartsson, T. Atrial fibrillation following cardiac surgery: Risk analysis and long-term survival. J. Cardiothorac. Surg. 2012, 7, 87. [Google Scholar] [CrossRef]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; Foundation, E. A Multicenter Risk Index for Atrial Fibrillation After Cardiac Surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef]
- Kant, S.; Banerjee, D.; Sabe, S.A.; Sellke, F.; Feng, J. Microvascular dysfunction following cardiopulmonary bypass plays a central role in postoperative organ dysfunction. Front. Med. 2023, 10, 1110532. [Google Scholar] [CrossRef]
- Alam, S.R.; Stirrat, C.; Spath, N.; Zamvar, V.; Pessotto, R.; Dweck, M.R.; Moore, C.; Semple, S.; El-Medany, A.; Manoharan, D.; et al. Myocardial inflammation, injury and infarction during on-pump coronary artery bypass graft surgery. J. Cardiothorac. Surg. 2017, 12, 115. [Google Scholar] [CrossRef]
- Cameron, A.; Cheng, H.K.; Lee, R.-P.; Doherty, D.; Hall, M.; Khashayar, P.; Lip, G.Y.H.; Quinn, T.; Abdul-Rahim, A.; Dawson, J. Biomarkers for Atrial Fibrillation Detection After Stroke. Neurology 2021, 97, e1775–e1789. [Google Scholar] [CrossRef]
- Li, X.-Y.; Hou, H.-T.; Chen, H.-X.; Liu, X.-C.; Wang, J.; Yang, Q.; He, G.-W. Preoperative plasma biomarkers associated with atrial fibrillation after coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2021, 162, 851–863.e3. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Yamashita, K.; Sharma, V.; Ranjan, R.; Selzman, C.H.; Dosdall, D.J. Perioperative Biomarkers Predicting Postoperative Atrial Fibrillation Risk After Coronary Artery Bypass Grafting: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1933–1941. [Google Scholar] [CrossRef]
- van den Berg, N.W.E.; Neefs, J.; Kawasaki, M.; Nariswari, F.A.; Wesselink, R.; Fabrizi, B.; Jongejan, A.; Klaver, M.N.; Havenaar, H.; Hulsman, E.L.; et al. Extracellular matrix remodeling precedes atrial fibrillation: Results of the PREDICT-AF trial. Heart Rhythm 2021, 18, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.S.; Alonso, S.; Campos, F.O.; Rocha, B.M.; Fernandes, J.F.; Kuehne, T.; dos Santos, R.W. Ectopic beats arise from micro-reentries near infarct regions in simulations of a patient-specific heart model. Sci. Rep. 2018, 8, 16392. [Google Scholar] [CrossRef]
- Zaman, A.G.; Archbold, R.A.; Helft, G.; Paul, E.A.; Curzen, N.P.; Mills, P.G. Atrial Fibrillation After Coronary Artery Bypass Surgery. Circulation 2000, 101, 1403–1408. [Google Scholar] [CrossRef]
- Herrmann, F.E.M.; Taha, A.; Nielsen, S.J.; Martinsson, A.; Hansson, E.C.; Juchem, G.; Jeppsson, A. Recurrence of Atrial Fibrillation in Patients with New-Onset Postoperative Atrial Fibrillation After Coronary Artery Bypass Grafting. JAMA Netw. Open 2024, 7, e241537. [Google Scholar] [CrossRef]
- O’Neal, J.B.; Billings, I.V.F.T.; Liu, X.; Shotwell, M.S.; Liang, Y.; Shah, A.S.; Ehrenfeld, J.M.; Wanderer, J.P.; Shaw, A.D. Effect of Preoperative Beta-Blocker Use on Outcomes Following Cardiac Surgery. Am. J. Cardiol. 2017, 120, 1293–1297. [Google Scholar] [CrossRef]
- Masarone, D.; Martucci, M.L.; Errigo, V.; Pacileo, G. The use of β-blockers in heart failure with reduced ejection fraction. J. Cardiovasc. Dev. Dis. 2021, 8, 101. [Google Scholar] [CrossRef]
- Hoda, M.R.; El-Achkar, H.; Schmitz, E.; Scheffold, T.; Vetter, H.O.; De Simone, R. Systemic Stress Hormone Response in Patients Undergoing Open Heart Surgery With or Without Cardiopulmonary Bypass. Ann. Thorac. Surg. 2006, 82, 2179–2186. [Google Scholar] [CrossRef]
- Hernández-Romero, D.; Lahoz, Á.; Roldan, V.; Jover, E.; Romero-Aniorte, A.I.; Martinez, C.M.; Jara-Rubio, R.; Arribas, J.M.; Garcia-Alberola, A.; Cánovas, S.; et al. Von Willebrand factor is associated with atrial fibrillation development in ischaemic patients after cardiac surgery. EP Eur. 2016, 18, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Ucar, H.I.; Tok, M.; Atalar, E.; Dogan, O.F.; Oc, M.; Farsak, B.; Guvener, M.; Yilmaz, M.; Dogan, R.; Demircin, M.; et al. Predictive significance of plasma levels of interleukin-6 and high-sensitivity C-reactive protein in atrial fibrillation after coronary artery bypass surgery. Heart Surg. Forum 2007, 10, E131–E135. [Google Scholar] [CrossRef] [PubMed]
- Kaireviciute, D.; Lip, G.Y.H.; Balakrishnan, B.; Uzdavinys, G.; Norkunas, G.; Kalinauskas, G.; Sirvydis, V.; Aidietis, A.; Zanetto, U.; Sihota, H.; et al. Intracardiac expression of markers of endothelial damage/dysfunction, inflammation, thrombosis, and tissue remodeling, and the development of postoperative atrial fibrillation. J. Thromb. Haemost. 2011, 9, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Kaireviciute, D.; Blann, A.D.; Balakrishnan, B.; Lane, D.A.; Patel, J.V.; Uzdavinys, G.; Norkunas, G.; Kalinauskas, G.; Sirvydis, V.; Aidietis, A.; et al. Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb. Haemost. 2010, 104, 122–127. [Google Scholar] [CrossRef]
- Biernacka, A.; Marcin, D.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Tarighati Rasekhi, R.; Gill, D.; Alzubi, J.; Mainigi, S.K. How transforming growth factor contributes to atrial fibrillation? Life Sci. 2021, 266, 118823. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Khan, A.A.; Lip, G.Y.H. The prothrombotic state in atrial fibrillation: Pathophysiological and management implications. Cardiovasc. Res. 2019, 115, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Sekiguchi, A.; Sagara, K.; Tanabe, H.; Takamura, M.; Kaneko, S.; Aizawa, T.; Fu, L.-T.; Yamashita, T. Endothelial–mesenchymal transition in human atrial fibrillation. J. Cardiol. 2017, 69, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory signalling in atrial cardiomyocytes: A novel unifying principle in atrial fibrillation pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Fukuda, K.; Pan, J.; Makino, S.; Baba, A.; Hori, S.; Ogawa, S. Leukemia Inhibitory Factor, a Potent Cardiac Hypertrophic Cytokine, Activates the JAK/STAT Pathway in Rat Cardiomyocytes. Circ. Res. 1997, 81, 656–663. [Google Scholar] [CrossRef]
- Aromolaran, A.S.; Srivastava, U.; Alí, A.; Chahine, M.; Lazaro, D.; El-Sherif, N.; Capecchi, P.L.; Laghi-Pasini, F.; Lazzerini, P.E.; Boutjdir, M.; et al. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS ONE 2018, 13, e0208321. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Accioli, R.; Acampa, M.; Zhang, W.-H.; Verrengia, D.; Cartocci, A.; Bacarelli, M.R.; Xin, X.; Salvini, V.; Chen, K.-S.; et al. Interleukin-6 Elevation Is a Key Pathogenic Factor Underlying COVID-19-Associated Heart Rate-Corrected QT Interval Prolongation. Front. Cardiovasc. Med. 2022, 9, 893681. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Cupelli, M.; Cartocci, A.; Bertolozzi, I.; Salvini, V.; Accioli, R.; Salvadori, F.; Marzotti, T.; Verrengia, D.; Cevenini, G.; et al. Elevated Interleukin-6 Levels Are Associated with an Increased Risk of QTc Interval Prolongation in a Large Cohort of US Veterans. J. Am. Heart Assoc. 2024, 13, e032071. [Google Scholar] [CrossRef]
- Tong, D.C.; Whitbourn, R.; MacIsaac, A.; Wilson, A.; Burns, A.; Palmer, S.; Layland, J. High-Sensitivity C-Reactive Protein Is a Predictor of Coronary Microvascular Dysfunction in Patients with Ischemic Heart Disease. Front. Cardiovasc. Med. 2018, 4, 81. [Google Scholar] [CrossRef]
- Salvarani, N.; Maguy, A.; De Simone, S.A.; Miragoli, M.; Jousset, F.; Rohr, S. TGF-β1 (Transforming Growth Factor-β1) Plays a Pivotal Role in Cardiac Myofibroblast Arrhythmogenicity. Circ. Arrhythmia Electrophysiol. 2017, 10, e004567. [Google Scholar] [CrossRef]
- Zhao, T.X.; Kostapanos, M.; Griffiths, C.; Arbon, E.L.; Hubsch, A.; Kaloyirou, F.; Helmy, J.; Hoole, S.P.; Rudd, J.H.F.; Wood, G.; et al. Low-dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndromes (LILACS): Protocol and study rationale for a randomised, double-blind, placebo-controlled, phase I/II clinical trial. BMJ Open 2018, 8, e022452. [Google Scholar] [CrossRef]
- Pierucci, N.; Mariani, M.V.; Iannetti, G.; Maffei, L.; Coluccio, A.; Laviola, D.; Palombi, M.; Trivigno, S.; Spadafora, L.; Chourda, E.; et al. Atrial cardiomyopathy: New pathophysiological and clinical aspects. Minerva Cardiol. Angiol. 2025, 73. [Google Scholar] [CrossRef]
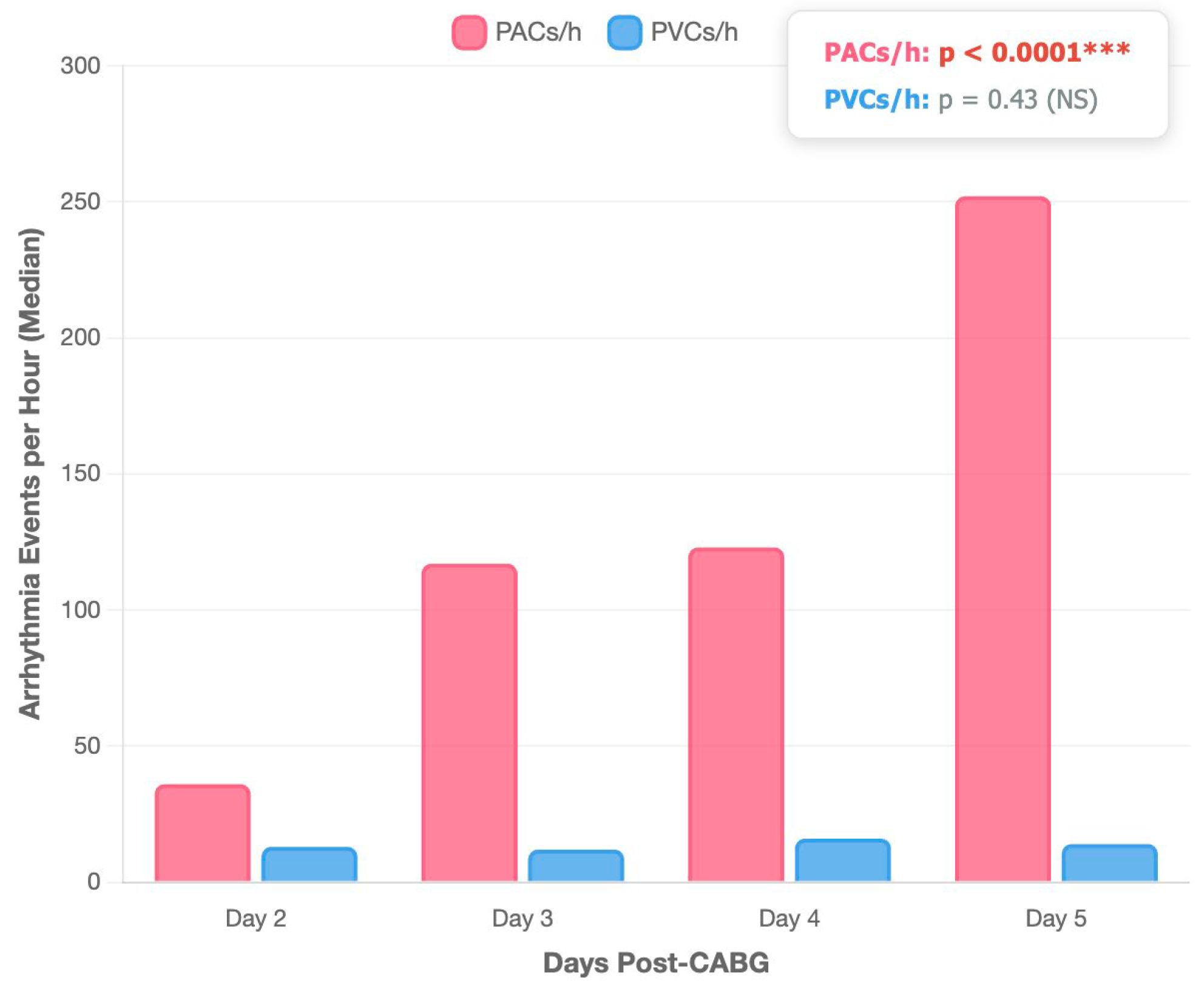
| Post-CABG Arrhythmic Burden | Parameters | Spearman r | 95% CI | p-Value |
|---|---|---|---|---|
| PACs | Age | 0.12 | −0.07 and 0.31 | 0.20 |
| Body mass index | 0.25 | 0.05 and 0.43 | <0.001 | |
| Abdominal circumference | 0.24 | 0.04 and 0.42 | 0.01 | |
| Beta-blocker | −0.01 | −0.21 and 0.18 | 0.80 | |
| Renin-angiotensin-aldosterone inhibitors | 0.10 | −0.09 and 0.29 | 0.29 | |
| Statins | −0.02 | −0.22 and 0.17 | 0.80 | |
| CHA2DS2-VASc score | 0.04 | −0.15 and 0.24 | 0.63 | |
| SYNTAX II score | −0.35 | −0.51 and 0.16 | <0.0001 | |
| Left ventricle ejection fraction | 0.10 | −0.05 and 0.33 | 0.13 | |
| Left atrium area | 0.06 | −0.30 and 0.41 | 0.74 | |
| PVCs | Age | −0.002 | −0.20 and 0.19 | 0.90 |
| Body mass index | −0.04 | −0.23 and 0.16 | 0.68 | |
| Abdominal circumference | −0.14 | −0.33 and 0.06 | 0.15 | |
| Beta-blocker | −0.13 | −0.32 and 0.07 | 0.1 | |
| Renin-angiotensin-aldosterone inhibitors | 0.28 | 0.08 and 0.45 | <0.001 | |
| Statins | −0.28 | −0.45 and 0.08 | <0.001 | |
| CHA2DS2-VASc score | 0.01 | −0.18 and 0.21 | 0.84 | |
| SYNTAX II score | 0.07 | −0.12 and 0.27 | 0.44 | |
| Left ventricle ejection fraction | −0.39 | −0.55 and 0.21 | <0.0001 |
| Inflammatory Biomarkers | Median Pre-CABG Plasma Level (n = 102) |
|---|---|
| Highly sensitive C-reactive protein (mg/L) | 1.67 (0.52–5.52) |
| Von Willebrand factor (IU/dL) | 1.78 (1.45–3.14) |
| Transforming growth factor-β (ng/mL) | 68,680 (52,684–74,835) |
| Interleukin-1b (pg/mL) | 12.86 (5.45–29.66) |
| Interleukin-2 (pg/mL) | 5.73 (2.97–14.09) |
| Interleukin-6 (pg/mL) | 8.38 (2.80–18.48) |
| Interleukin-8 (pg/mL) | 7.61 (3.31–21.29) |
| Vascular endothelial growth factor (pg/mL) | 631.60 (422.3–1025) |
| Post-CABG Arrhythmic Burden | Parameters | Spearman r | 95% CI | p-Value |
|---|---|---|---|---|
| PACs | Highly sensitive C-reactive protein | 0.14 | −0.06–0.33 | 0.10 |
| Von Willebrand factor | 0.3 | 0.12–0.48 | <0.001 | |
| Transforming growth factor β | 0.2 | 0.01–0.39 | 0.03 | |
| Interleukin-1b | −0.1 | −0.29–0.09 | 0.20 | |
| Interleukin-2 | −0.09 | −0.28–0.11 | 0.30 | |
| Interleukin-6 | −0.14 | −0.33–0.06 | 0.10 | |
| Interleukin-8 | 0.25 | 0.05–0.43 | <0.001 | |
| Vascular endothelial growth factor | −0.11 | −0.30–0.08 | 0.20 | |
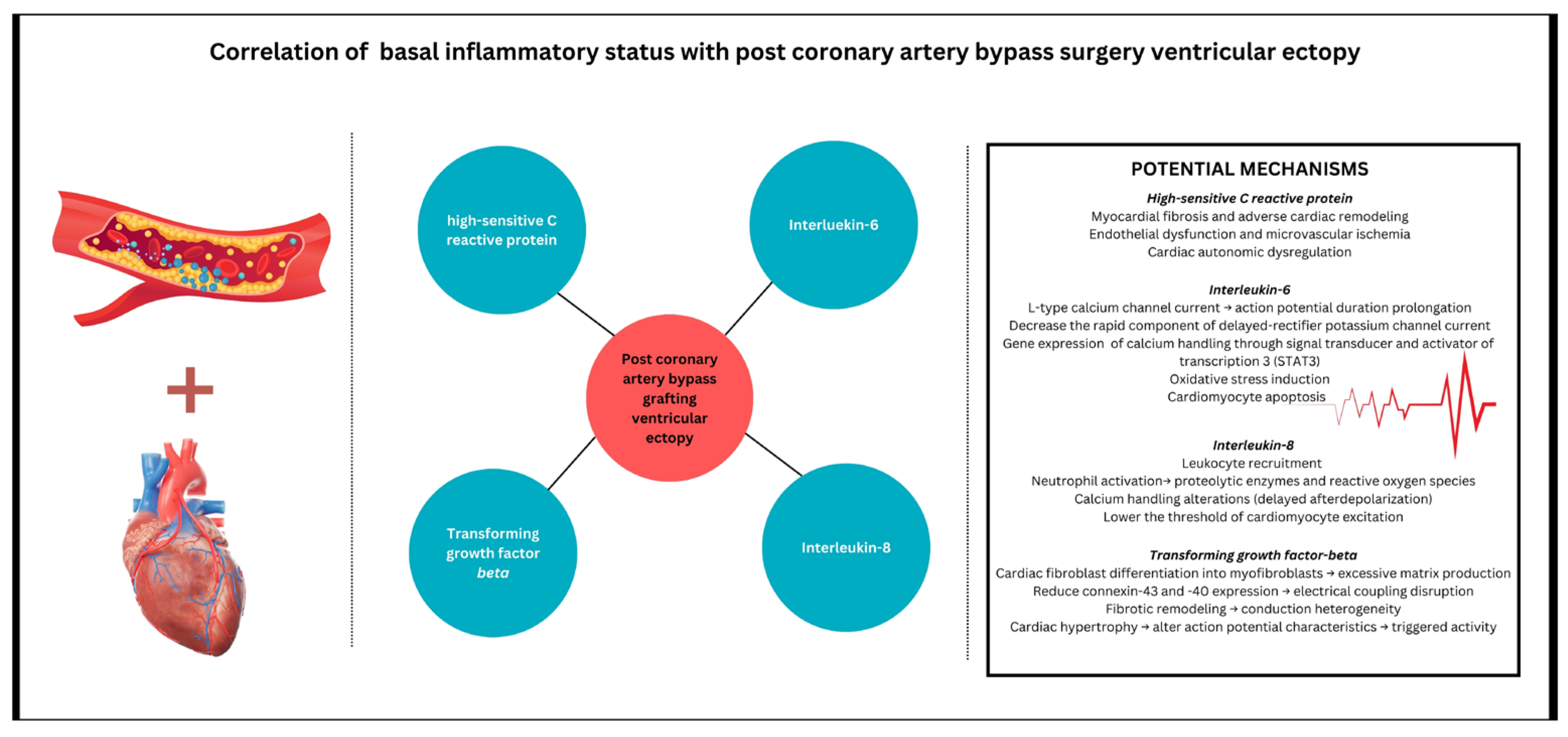
| PVCs | High-sensitive C-reactive protein | 0.67 | 0.55–0.77 | <0.0001 |
| Von Willebrand factor | 0.08 | −0.11–0.28 | 0.30 | |
| Transforming growth factor β | 0.32 | 0.13–0.49 | <0.0001 | |
| Interleukin-1b | 0.16 | −0.03–0.35 | 0.09 | |
| Interleukin-2 | 0.07 | −0.12–0.27 | 0.40 | |
| Interleukin-6 | 0.21 | 0.01–0.3 | 0.02 | |
| Interleukin-8 | 0.69 | 0.57–0.78 | <0.0001 | |
| Vascular endothelial growth factor | 0.13 | −0.06–0.32 | 0.10 |
| Parameters | Total (n = 102) | Acute Kidney Failure (n = 25) | Without Acute Kidney Failure (n = 77) | p-Value |
|---|---|---|---|---|
| Age (years) | 60 (58–67) | 65 (58–73) | 59 (57–66) | 0.03 |
| Abdominal circumference (cm) | 102 (92.7–112) | 104 (95.2–113) | 100 (92–113) | 0.50 |
| Male gender (n, %) | 58 (56%) | 17 (68%) | 59 (76%) | 0.50 |
| Highly sensitive C-reactive protein (mg/L) | 1.67 (0.52–5.52) | 3.26 (0.52–7.44) | 1.42 (0.52–5.22) | 0.25 |
| Von Willebrand factor (IU/dL) | 1.78 (1.45–3.14) | 1.59 (1.09–2.79) | 1.85 (1.57–3.23) | 0.08 |
| Transforming growth factor-β (ng/mL) | 68,680 (52,684–74,835) | 68,680 (51,657–72,165) | 70,670 (52,684–85,758) | 0.35 |
| Interleukin-1b (pg/mL) | 12.86 (5.45–29.66) | 13.04 (5.04–28.11) | 12.79 (6.49–31.00) | 0.61 |
| Interleukin-2 (pg/mL) | 5.73 (2.97–14.09) | 6.35 (2.17–14.34) | 5.12 (3.12–13.19) | 0.86 |
| Interleukin-6 (pg/mL) | 8.38 (2.80–18.48) | 10.58 (3.78–17.71) | 7.08 (2.80–18.48) | 0.71 |
| Interleukin-8 (pg/mL) | 7.61 (3.31–21.29) | 4.37 (3.10–17.17) | 7.88 (3.31–23.76) | 0.27 |
| Vascular endothelial growth factor (pg/mL) | 631.60 (422.3–1025) | 674 (380.7–1052) | 621 (422.30–1038) | 0.95 |
| Parameters | Total (n = 102) | Acute hepatic failure (n = 21) | Without acute hepatic failure (n = 81) | p-value |
| Age (years) | 60 (58–67) | 64 (58–70) | 59 (57–67) | 0.10 |
| Abdominal circumference (cm) | 102 (92.7–112) | 99 (92.5–115) | 102 (92.5–111) | 0.70 |
| Male gender (n, %) | 58 (56%) | 19 (90.4%) | 57 (70.3%) | 0.10 |
| Highly sensitive C-reactive protein (mg/L) | 1.67 (0.52–5.52) | 1.67 (0.49–7.44) | 1.67 (0.53–5.22) | 0.71 |
| Von Willebrand factor (IU/dL) | 1.78 (1.45–3.14) | 1.75 (1.45–2.27) | 1.85 (1.45–3.44) | 0.51 |
| Transforming growth factor-β (ng/mL) | 68,680 (52,684–74,835) | 63,019 (52,155–85,758) | 70,670 (52,684–73,697) | 0.90 |
| Interleukin-1b (pg/mL) | 12.86 (5.45–29.66) | 13.04 (10.50–33.86) | 12.79 (5.45–29.21) | 0.17 |
| Interleukin-2 (pg/mL) | 5.73 (2.97–14.09) | 7.01 (3.79–17.35) | 4.57 (2.52–13.19) | 0.10 |
| Interleukin-6 (pg/mL) | 8.38 (2.80–18.48) | 10.81 (4.40–22.18) | 6.76 (2.80–18.07) | 0.12 |
| Interleukin-8 (pg/mL) | 7.61 (3.31–21.29) | 4.44 (3.10–15.23) | 7.88 (3.37–23.76) | 0.30 |
| Vascular endothelial growth factor (pg/mL) | 631.60 (422.3–1025) | 713.80 (526.6–1092.0) | 621 (401.5–949.2) | 0.25 |
| Post-CABG Outcome | Parameters | Spearman r | 95% CI | p-Value |
|---|---|---|---|---|
| Days of inotropic support | Highly sensitive C-reactive protein | 0.008 | −0.19–0.20 | 0.90 |
| Von Willebrand factor | −0.10 | −0.29–0.09 | 0.29 | |
| Transforming growth factor-β | 0.001 | −0.19–0.20 | 0.90 | |
| Interleukin-1b | −0.02 | −0.22–0.17 | 0.83 | |
| Interleukin-2 | −0.002 | −0.20–0.19 | 0.90 | |
| Interleukin-6 | 0.06 | −0.13–0.26 | 0.49 | |
| Interleukin-8 | 0.06 | −0.13–0.26 | 0.48 | |
| Vascular endothelial growth factor | 0.005 | −0.19–0.20 | 0.90 | |
| Days of hospitalization | High-sensitive C reactive protein | −0.01 | −0.21–0.18 | 0.87 |
| Von Willebrand factor | −0.04 | −0.24–0.15 | 0.63 | |
| Transforming growth factor-β | −0.14 | −0.33–0.05 | 0.14 | |
| Interleukin-1b | 0.06 | −0.14–0.25 | 0.55 | |
| Interleukin-2 | 0.05 | −0.14–0.25 | 0.50 | |
| Interleukin-6 | 0.04 | −0.15–0.24 | 0.63 | |
| Interleukin-8 | 0.07 | −0.12–0.27 | 0.43 | |
| Vascular endothelial growth factor | 0.09 | −0.11–0.28 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozac, D.-A.; Somkereki, C.; Huțanu, A.; Nicoara, T.R.; Scridon, A. The Impact of Basal Inflammatory Status on Post-CABG Atrial and Ventricular Ectopy and Remodeling Pathways. Medicina 2025, 61, 1545. https://doi.org/10.3390/medicina61091545
Cozac D-A, Somkereki C, Huțanu A, Nicoara TR, Scridon A. The Impact of Basal Inflammatory Status on Post-CABG Atrial and Ventricular Ectopy and Remodeling Pathways. Medicina. 2025; 61(9):1545. https://doi.org/10.3390/medicina61091545
Chicago/Turabian StyleCozac, Dan-Alexandru, Cristina Somkereki, Adina Huțanu, Tunde Renata Nicoara, and Alina Scridon. 2025. "The Impact of Basal Inflammatory Status on Post-CABG Atrial and Ventricular Ectopy and Remodeling Pathways" Medicina 61, no. 9: 1545. https://doi.org/10.3390/medicina61091545
APA StyleCozac, D.-A., Somkereki, C., Huțanu, A., Nicoara, T. R., & Scridon, A. (2025). The Impact of Basal Inflammatory Status on Post-CABG Atrial and Ventricular Ectopy and Remodeling Pathways. Medicina, 61(9), 1545. https://doi.org/10.3390/medicina61091545








