Autologous Platelet Concentrates in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Searching and Screening
2.2. Eligibility Criteria
2.3. Data Selection
2.4. Data Extraction
2.5. Quality and Risk of Bias Assessment
3. Results
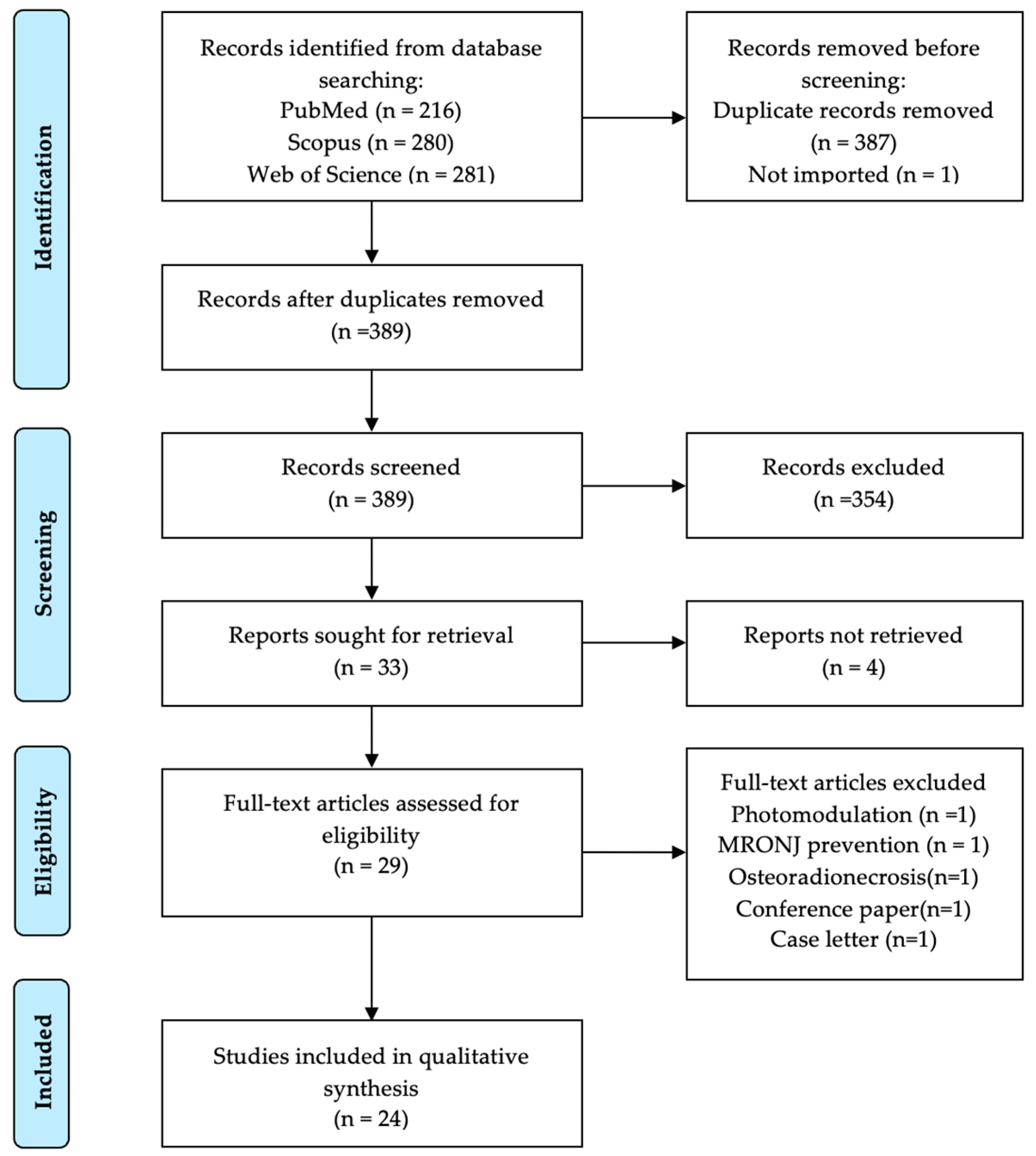
3.1. Study Selection and Screening
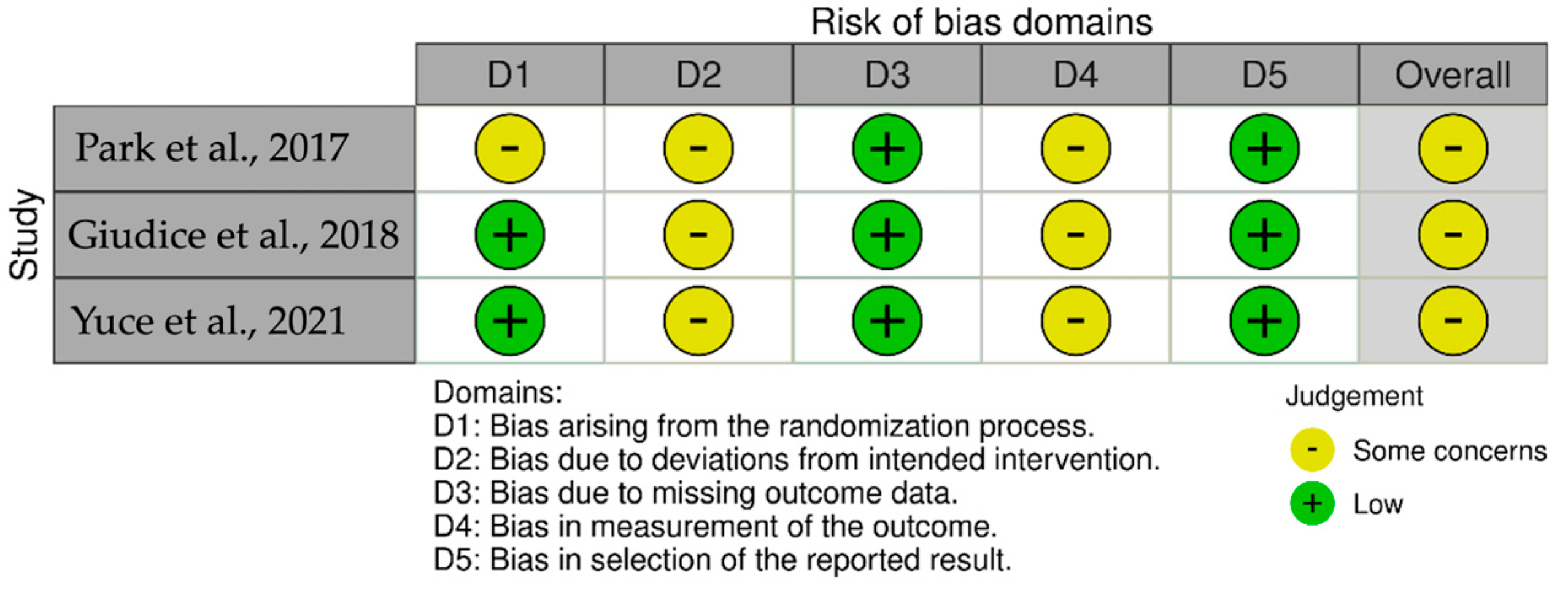
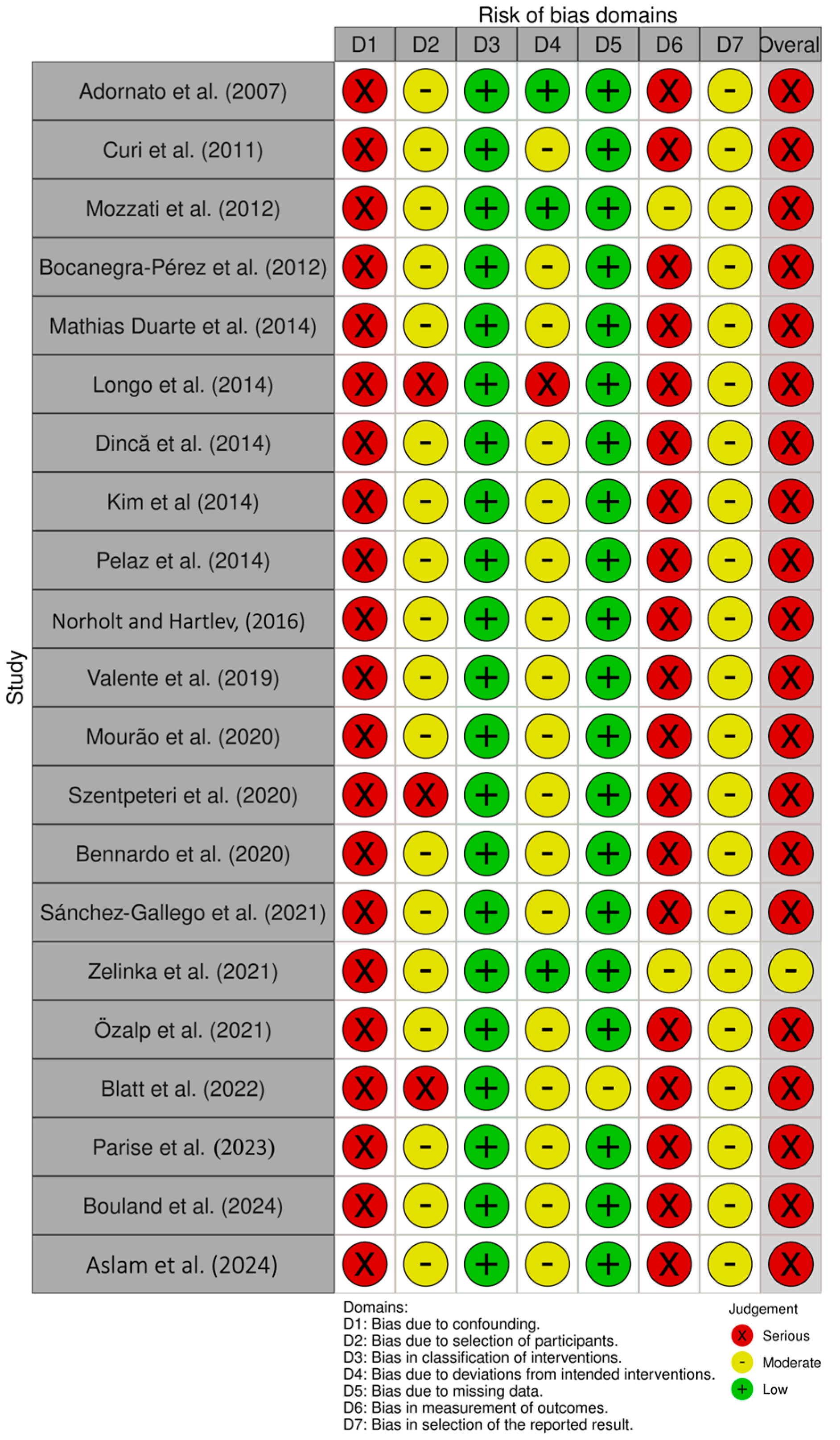
3.2. Risk of Bias Assessment
3.3. Baseline Characteristics of PRP Studies
3.4. Baseline Characteristics of PRF Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Autoimmune disease |
| AF | Atrial fibrillation |
| APC | Autologous platelets concentrates |
| A-PRF | Advanced platelet-rich fibrin |
| AT-SVF | Adipose-tissue stromal vascular fraction |
| BMP2 | Bone morphogenetic protein 2 |
| BPs | Bisphosphonates |
| BRONJ | Bisphosphonate-related osteonecrosis of the jaw |
| CGF | Concentrated growth factor |
| CHX | Chlorhexidine |
| CS | Case Series |
| DM | Diabetes |
| HCL | Hypercholesterolemia |
| HF | Heart Failure |
| HTA | Hypertension |
| i-PRF | Injectable platelet-rich fibrin |
| IV | Intravenous |
| L-PRF | Leukocyte-platelet rich fibrin |
| L-PRP | Leukocyte- and platelet-rich plasma |
| MI | Myocardial infarction |
| Min | Minutes |
| MIU | Million international units |
| MRONJ | Medication-related osteonecrosis of the jaw |
| MTX | Methotrexate |
| N.R. | Not reported |
| ONJ | Osteonecrosis of jaw |
| PDGF | Platelet-derived growth factor |
| PO | Per os |
| PPP | Platelet-poor plasma |
| PRF | Platelet-rich fibrin |
| PRGF | Plasma rich in growth factors |
| PRP | Platelet-rich plasma |
| RCT | Randomized control trial |
| RD | Rheumatoid disease |
| RF | Renal Failure |
| rpm | Rotations per minute |
| SC | Subcutaneous |
| SVF | Stromal vascular fraction |
References
- Pollock, R.A.; Brown, T.W.; Rubin, D.M., Jr. “Phossy Jaw” and “Bis-phossy Jaw” of the 19th and the 21st Centuries: The Diuturnity of John Walker and the Friction Match. Craniomaxillofac. Trauma Reconstr. 2015, 8, 262–270. [Google Scholar] [CrossRef]
- Lončar Brzak, B.; Horvat Aleksijević, L.; Vindiš, E.; Kordić, I.; Granić, M.; Vidović Juras, D.; Andabak Rogulj, A. Osteonecrosis of the Jaw. Dent. J. 2023, 11, 23. [Google Scholar] [CrossRef]
- Wang, J.; Goodger, N.M.; Pogrel, M.A. Osteonecrosis of the jaws associated with cancer chemotherapy. J. Oral Maxillofac. Surg. 2003, 61, 1104–1107. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons' Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Bensi, C.; Giovacchini, F.; Lomurno, G.; Eramo, S.; Barraco, G.; Tullio, A. Quality of life in patients affected by medication-related osteonecrosis of the jaws: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Antonelli, A.; Barone, S.; Adamo, D.; Salviati, M.; Cerra, M.G.; Bennardo, F.; Giudice, A. Oral Health-Related Quality of Life and Mental Health Impairment in Patients Affected by Medication-Related Osteonecrosis of the Jaws: A Case-Control Pilot Study. Dent. J. 2023, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; De Francesco, P.; Giovannacci, I.; Leão, J.C.; Barone, A. Piezoelectric Surgery, Er: YAG Laser Surgery and Nd:YAG Laser Photobiomodulation: A Combined Approach to Treat Medication-Related Osteonecrosis of the Jaws (MRONJ). Dent. J. 2024, 12, 261. [Google Scholar] [CrossRef]
- Hanna, R.; Miron, I.C.; Dalvi, S.; Arany, P.; Bensadoun, R.J.; Benedicenti, S. A Systematic Review of Laser Photobiomodulation Dosimetry and Treatment Protocols in the Management of Medications-Related Osteonecrosis of the Jaws: A Rationalised Consensus for Future Randomised Controlled Clinical Trials. Pharmaceuticals 2024, 17, 1011. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiodt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Kendler, D.L.; Rizzoli, R.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O'Ryan, F.; Reid, I.R.; Ruggiero, S.L.; et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management from the International Task Force on ONJ. J. Clin. Densitom. 2017, 20, 8–24. [Google Scholar] [CrossRef]
- Gonzálvez-García, M.; Rodríguez-Lozano, F.J.; Villanueva, V.; Segarra-Fenoll, D.; Rodríguez-González, M.A.; Oñate-Sánchez, R.; Blanquer, M.; Moraleda, J.M. Cell therapy in bisphosphonate-related osteonecrosis of the jaw. J. Craniofac. Surg. 2013, 24, e226–e228. [Google Scholar] [CrossRef]
- Barzegar Amin, A.; Dorpmans, D.; Mufty, H.; Fourneau, I. Treatment of vascular leg ulcers with leukocyte- and platelet-rich fibrin (L-PRF): A systematic review. Phlebology 2024, 39, 512–520. [Google Scholar] [CrossRef]
- Mohale, S.A.; Thakare, P.V.; Gaurkar, S.S.; Bharadia, G.; Acharya, S. Effectiveness of Injectable Platelet-Rich Fibrin Therapy in Alopecia and Facial Rejuvenation: A Systematic Review. Cureus 2024, 16, e62198. [Google Scholar] [CrossRef]
- O'Sullivan, L.; Ni Riordain, R. Autologous platelet concentrates in oral surgery: Protocols, properties, and clinical applications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.D.; de Santana, I.H.G.; Viana, M.R.M.; Freire, J.C.P.; Ferreira-Junior, O.; Sant'Ana, E. Use of platelet- and leukocyte-rich fibrin (L-PRF) as a healing agent in the postoperative period of third molar removal surgeries: A systematic review. Clin. Oral Investig. 2024, 28, 241. [Google Scholar] [CrossRef] [PubMed]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep. 2019, 9, 8310. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Calciolari, E.; Dourou, M.; Akcali, A.; Donos, N. Differences between first- and second-generation autologous platelet concentrates. Periodontology 2000 2025, 97, 52–73. [Google Scholar] [CrossRef]
- Sam, G.; Vadakkekuttical, R.J.; Amol, N.V. In vitro evaluation of mechanical properties of platelet-rich fibrin membrane and scanning electron microscopic examination of its surface characteristics. J. Indian Soc. Periodontol. 2015, 19, 32–36. [Google Scholar] [CrossRef]
- Pereira, V.B.S.; Lago, C.A.P.; Almeida, R.A.C.; Barbirato, D.D.S.; Vasconcelos, B. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 25, 482. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin with the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, E.S.; Mourão, C.F.A.B.; Leite, P.E.C.; Granjeiro, J.M.; Calasans-Maia, M.D.; Alves, G.G. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. J. Biomed. Mater. Res. Part A 2018, 106, 1373–1380. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Adornato, M.C.; Morcos, I.; Rozanski, J. The treatment of bisphosphonate-associated osteonecrosis of the jaws with bone resection and autologous platelet-derived growth factors. J. Am. Dent. Assoc. 2007, 138, 971–977. [Google Scholar] [CrossRef]
- Curi, M.M.; Cossolin, G.S.; Koga, D.H.; Zardetto, C.; Christianini, S.; Feher, O.; Cardoso, C.L.; dos Santos, M.O. Bisphosphonate-related osteonecrosis of the jaws—An initial case series report of treatment combining partial bone resection and autologous platelet-rich plasma. J. Oral Maxillofac. Surg. 2011, 69, 2465–2472. [Google Scholar] [CrossRef]
- Mozzati, M.; Gallesio, G.; Arata, V.; Pol, R.; Scoletta, M. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: A report of 32 cases. Oral Oncol. 2012, 48, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Perez, S.; Vicente-Barrero, M.; Knezevic, M.; Castellano-Navarro, J.M.; Rodriguez-Bocanegra, E.; Rodriguez-Millares, J.; Perez-Plasencia, D.; Ramos-Macias, A. Use of platelet-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Int. J. Oral Maxillofac. Surg. 2012, 41, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Mathias Duarte, L.F.; dos Reis, H.B.; Tucci, R.; Dib, L.L. Bisphosphonate-related osteonecrosis of the jaws: Analysis of a case series at a dental school. Spec. Care Dent. 2014, 34, 77–83. [Google Scholar] [CrossRef]
- Longo, F.; Guida, A.; Aversa, C.; Pavone, E.; Di Costanzo, G.; Ramaglia, L.; Ionna, F. Platelet rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw: Personal experience and review of the literature. Int. J. Dent. 2014, 2014, 298945. [Google Scholar] [CrossRef]
- Sanchez-Gallego Albertos, C.; Del Castillo Pardo de Vera, J.L.; Viejo Llorente, A.; Cebrian Carretero, J.L. Medication related osteonecrosis of the jaws (MRONJ): Factors related to recurrence after treatment with surgery and platelet rich plasma (PRP) placement. Med. Oral 2021, 26, e684–e690. [Google Scholar] [CrossRef]
- Dinca, O.; Zurac, S.; Staniceanu, F.; Bucur, M.B.; Bodnar, D.C.; Vladan, C.; Bucur, A. Clinical and histopathological studies using fibrin-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Rom. J. Morphol. Embryol. 2014, 55, 961–964. [Google Scholar]
- Kim, J.W.; Kim, S.J.; Kim, M.R. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: A prospective feasibility study. Br. J. Oral Maxillofac. Surg. 2014, 52, 854–859. [Google Scholar] [CrossRef]
- Pelaz, A.; Junquera, L.; Gallego, L.; Garcia-Consuegra, L.; Junquera, S.; Gomez, C. Alternative treatments for oral bisphosphonate-related osteonecrosis of the jaws: A pilot study comparing fibrin rich in growth factors and teriparatide. Med. Oral 2014, 19, e320–e326. [Google Scholar] [CrossRef] [PubMed]
- Norholt, S.E.; Hartlev, J. Surgical treatment of osteonecrosis of the jaw with the use of platelet-rich fibrin: A prospective study of 15 patients. Int. J. Oral Maxillofac. Surg. 2016, 45, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Valente, N.A.; Chatelain, S.; Alfonsi, F.; Mortellaro, C.; Barone, A. Medication-Related Osteonecrosis of the Jaw: The Use of Leukocyte-Platelet-Rich Fibrin as an Adjunct in the Treatment. J. Craniofac. Surg. 2019, 30, 1095–1101. [Google Scholar] [CrossRef]
- Fernando de Almeida Barros Mourao, C.; Calasans-Maia, M.D.; Del Fabbro, M.; Le Drapper Vieira, F.; Coutinho de Mello Machado, R.; Capella, R.; Miron, R.J.; Gomes Alves, G. The use of Platelet-rich Fibrin in the management of medication-related osteonecrosis of the jaw: A case series. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 84–89. [Google Scholar] [CrossRef]
- Szentpeteri, S.; Schmidt, L.; Restar, L.; Csaki, G.; Szabo, G.; Vaszilko, M. The Effect of Platelet-Rich Fibrin Membrane in Surgical Therapy of Medication-Related Osteonecrosis of the Jaw. J. Oral Maxillofac. Surg. 2020, 78, 738–748. [Google Scholar] [CrossRef]
- Bennardo, F.; Bennardo, L.; Del Duca, E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nistico, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef]
- Zelinka, J.; Blahak, J.; Perina, V.; Pacasova, R.; Treglerova, J.; Bulik, O. The use of platelet-rich fibrin in the surgical treatment of medication-related osteonecrosis of the jaw: 40 patients prospective study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2021, 165, 322–327. [Google Scholar] [CrossRef]
- Ozalp, O.; Yildirimyan, N.; Ozturk, C.; Kocabalkan, B.; Simsek Kaya, G.; Sindel, A.; Altay, M.A. Promising results of surgical management of advanced medication related osteonecrosis of the jaws using adjunctive leukocyte and platelet rich fibrin. BMC Oral Health 2021, 21, 613. [Google Scholar] [CrossRef]
- Blatt, S.; Kruger, M.; Kammerer, P.W.; Thiem, D.G.E.; Matheis, P.; Eisenbeiss, A.K.; Wiltfang, J.; Al-Nawas, B.; Naujokat, H. Non-Interventional Prospective Observational Study of Platelet Rich Fibrin as a Therapy Adjunctive in Patients with Medication-Related Osteonecrosis of the Jaw. J. Clin. Med. 2022, 11, 682. [Google Scholar] [CrossRef]
- Parise, G.K.; Costa, B.N.; Nogueira, M.L.; Sassi, L.M.; Schussel, J.L. Efficacy of fibrin-rich platelets and leukocytes (L-PRF) in tissue repair in surgical oral procedures in patients using zoledronic acid-case-control study. Oral Maxillofac. Surg. 2023, 27, 507–512. [Google Scholar] [CrossRef]
- Bouland, C.L.; Javadian, R.; Gilis, S.; Yanni, A.; Le Clercq, M.; Mestrallet, P.; Kampouridis, S.; Bron, D.; Lalmand, M.; Vanden Eynden, X.; et al. Treatment of medication-related osteonecrosis of the jaw with cell therapy. Front. Cell Dev. Biol. 2024, 12, 1338376. [Google Scholar] [CrossRef]
- Aslam, R.D.; Pitros, P.; Liew, J.; Besi, E. The adjunctive use of Leukocyte-Platelet Rich Fibrin (L-PRF) in the management of Medication Related Osteonecrosis of the Jaw (MRONJ): A retrospective observational study. Oral Maxillofac. Surg. 2024, 28, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, J.W.; Kim, S.J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2017, 75, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Barone, S.; Giudice, C.; Bennardo, F.; Fortunato, L. Can platelet-rich fibrin improve healing after surgical treatment of medication-related osteonecrosis of the jaw? A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Yuce, M.O.; Adali, E.; Isik, G. The effect of concentrated growth factor (CGF) in the surgical treatment of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: A randomized controlled study. Clin. Oral Investig. 2021, 25, 4529–4541. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef]
- Kuehn, S.; Scariot, R.; Elsalanty, M. Medication-Related Osteonecrosis: Why the Jawbone? Dent. J. 2023, 11, 109. [Google Scholar] [CrossRef]
- Bennett, B.; Tahir, H.; Solanki, K.; Ali, N. An Update on Medication-Related Osteonecrosis of the Jaw in Patients with Osteoporosis. EMJ Rheumatol. 2023, 10. [Google Scholar] [CrossRef]
- Cho, J.; Feldman, G.; Tomlinson, R.; Taub, D.; Diecidue, R. Medication-related osteonecrosis of the jaw (MRONJ) systemic review: Mevalonate pathway mechanisms explored. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 138, 475–483. [Google Scholar] [CrossRef]
- Wei, L.Y.; Chiu, C.M.; Kok, S.H.; Lin, H.Y.; Chiu, W.Y.; Yang, C.W.; Lee, J.J. Prognostic indicators in medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. Osteoporos. Int. 2025, 36, 969–979. [Google Scholar] [CrossRef]
- De Santis, D.; Gelpi, F.; Luciano, U.; Zarantonello, M.; Poscolere, A.; Modena, N.; Faccioni, P.; Causarano, G.; Finotti, M.; Zotti, F.; et al. New trends in adjunctive treatment and diagnosis in medication-related osteonecrosis of the jaw: A 10-year review. J. Biol. Regul. Homeost. Agents 2020, 34, 37–48. [Google Scholar]
- Hwang, S.; Jee, H.K.; Kim, Y.; Yoon, H.-C.; Yun, P.-Y.; Ku, J.-K. Clinical outcome and volumetric 3D analysis of biofluorescence imaging system guided surgery for Medication-Related Osteonecrosis of the Jaw (MRONJ). BMC Oral Health 2025, 25, 123. [Google Scholar] [CrossRef]
- Garzino Demo, P.; Bojino, A.; Roccia, F.; Malandrino, M.C.; Cocis, S.; Ramieri, G. Different Presentation and Outcomes in the Surgical Treatment of Advanced MRONJ in Oncological and Nononcological Patients Taking or Not Corticosteroid Therapy. Biomed. Res. Int. 2021, 2021, 7855497. [Google Scholar] [CrossRef]
- Kammerhofer, G.; Vegh, D.; Bányai, D.; Végh, Á.; Joob-Fancsaly, A.; Hermann, P.; Geczi, Z.; Hegedus, T.; Somogyi, K.S.; Bencze, B.; et al. Association between Hyperglycemia and Medication-Related Osteonecrosis of the Jaw (MRONJ). J. Clin. Med. 2023, 12, 2976. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef] [PubMed]
| Criteria | Determinants |
|---|---|
| Population | Patients with osteonecrosis of the jaw |
| Intervention | Platelet-rich plasma (PRP) protocols |
| Comparator | Platelet-rich fibrin (PRF) protocols |
| Outcome | Effectiveness in treating osteonecrosis of the jaw (e.g., healing rates, symptom relief, bone regeneration) |
| Study Design | Randomized controlled trials, case-controlled trials, cohort studies (prospective and or retrospective), case series (≥5 cases), case reports (≥5 cases) |
| Study ID/Country /Design | Population | APC Type/APC Preparation | Preoperative/Surgery/Postoperative | Results | Complications |
|---|---|---|---|---|---|
| [28]/USA/ Case Report | 12 oncologic BON on chemotherapy and corticoids, smoking history, 8 females, (43–83 years) IV BPs | PDGFs/CS6C, Vulcun technologies (automated centrifuge) for 11 min. Thrombin and CaCl2 mixed and added to PRP to form gel. | Minor bone debridement + CHX oral rinse + antibiotics/bone resection + PRP topical gel + resorbable collagen membrane with PRP/clindamycin 300 mg + CHX rinse | 10/12 (83%) complete mucosal and bone healing after 6 months | 2 partial healing |
| [29]/Brazil/ Case Series | 25 oncologic, BRONJ, most on chemotherapy and corticoids, 20 females, 60.7 years (42–85) IV BPs | PRP/SmartPReP system | Minor bone debridement + CHX oral irrigation + antibiotics/IV clindamycin 600 mg + marginal resection (teeth within 1 cm were extracted) + PRP topical/clindamycin 600 mg | 20/25 (80%) complete mucosal healing and no exposed bone after 12 months. | 2 infections during conservative treatment. 1 recurrence |
| [30]/Italy /Case Series | 32 oncologic, BRONJ (IIB) chemotherapy, steroids, smokers, 22 females, 69.7 years (44–83), IV BPs | PRGF/ PRGF System at 1800 rpm for 8 min. PRGF activated with 10% calcium chloride to form a gel | Hygiene session + amoxicillin/marginal resection (teeth within 3 mm removed) + osteoplasty + bone oxygenation + PRGF membrane/amoxicillin | 32/32 (100%) healed No recurrence (48–50 months) | 1 paresthesia (resolved), 4 with pain for 10 days |
| [31]/Spain/Case Series | 6 oncologic and 2 osteoporotic, BRONJ (stage II and < 3 cm), DM, corticosteroids, chemotherapy, former smokers, 6 females, 66 years (55–77), oral and IV BPs | L-PRP/ APC-20 Procedure Pack. PRP activated by mixing with thrombin | N.R./surgical debridement + L-PRP/amoxicillin-clavulanic acid 875 mg + CHX mouthwash + oral hygiene | Lesion healed in 2–4 weeks; asymptomatic, no exposed bone for 12–26 months | N.R. |
| [32]/Brazil/Retrospective case series | 10 oncologic and 3 osteoporotic, BRONJ (stage II), DM, HTA,12 females, 67.3 years (48–84), oral and IV BPs | PRP/N.R. | N.R./ Conservative (n = 3) clindamycin 300 mg + CHX irrigation Surgical resection(n = 10) - with PRP (n = 6) - without PRP (n = 4)/ N.R. | 2/6 (33.3%) healed with PRP 0/4 healed without PRP 2/3 healed with conservative. | 3 patients remained in stage II (1 from each group) 5 regressed to stage I; 2 remained |
| [33]/Italy/Retrospective case series | 72 oncologic, BRONJ, 60 females, 59 years (37–81), BPs | PRP/180 rpm for 10′ min, then at 1800 rpm for 10′ min. Calcium gluconate added to PPP for thrombin formation. 1800 g for 10–15 min. Thrombin ionized Ca added to PRP to form gel. | Oral ciprofloxacin 500 mg + CHX rinse/curettage with or without necrotic bone excision with PRP(n = 34) or without PRP (n = 15)/ Oral ciprofloxacin 500 mg + CHX rinse | 23/72 (32%) healed with conservative treatment 32/34(94%) healed with surgery + PRP 8/15 (53%) healed with surgery alone | 2/34 (6%) partial healing with surgery and PRP 7/15 (47%) partial healing with surgery alone |
| [34]/Spain/Retrospective case series | 34 osteoporotic and 29 oncologic, 7 others, MRONJ, steroids, DM, HTA, smokers, 58 females, most 50–70 years, oral BPs and denosumab | PRP gel/N.R. | N.R./bone resection (teeth extracted if near) + PRP gel/N.R. | 57 (81.4%) had not recurred for 2–52 months. | 13/70 (18.6%) experienced recurrence Smoking independent risk factor for recurrence. |
| Study ID/Country/ Design | Population | APC Type/APC Preparation | Preoperative/ Procedure/Postoperative | Results | Complication |
|---|---|---|---|---|---|
| [35]/Romania/Retrospective Case Series | 10 oncologic, BRONJ (stage II), 6 females, 59 ± 15 years (30–79), IV BPs | A-PRF/1300 rpm, 14 min, no anticoagulant. | Blood analyses/superficial sequestrectomy + PRF clots/clindamycin 0.9 g | 10/10 (100%) complete mucosal healing and no exposed bone at 30 days | N.R. |
| [36]/South Korea/Prospective feasibility study | 32 osteoporotic and 2 oncologic, BRONJ, chemotherapy, steroids, DM, obesity, RF, 34 females, 71 ± 13 years IV and oral BPs | L-PRF/3000 rpm, 10 min, no anticoagulant | IV cephalosporin 1 g + analgesics + CHX irrigation + antibacterial rinse + professional hygiene session/necrotic bone removal, sequestrectomy, ostectomy+ antibiotics irrigation + L-PRF/antibacterial rinse + systemic antibiotics | 26/34 (77%) no exposed or necrotic bone at the site, full coverage by mucosa, and no pain at 1 month Association between the response to treatment and the stage of BRONJ (p = 0.002) No association between the response to treatment and sCTX concentrations, actinomycosis or site of lesion. | 6/34 (18%) expose or necrotic bone at 1 month but resolved at 4 months (delayed). 2/34 (6%) no healing |
| [37]/Spain/Non-randomized comparative pilot study | PRF 3 osteoporosis and 2 osteoporosis corticoids related, 72.8 years (60–87) Teriparatide 2 osteoporosis and 2 osteoporosis corticoids related, 73.5 years (64–84) BRONJ (stage III), RD, corticoids, MTX, 9 females Oral BPs | PRGF/Vivostat PRF® System (automated) | N.R./ PRF sequestrectomy + Curettage + PRF Teriparatide teriparatide 20 µg/day, 4–10 months/ PRF amoxicillin-clavulanic acid 4 g/day | PRF 5/5 (100%) complete healing Teriparatide 1/4 (25%) complete healing | PRF Lip anesthesia (1), oro-antral communication (1)—resolved Teriparatide Bone exposure (3) (1 symptomatic) |
| [38]/Denmark/Prospective case series | 8 oncologic and 7 osteoporotic, BRONJ (stage II and III), 68.5 years (54–83), 11 females; BPs and denosumab | PRF membrane/1300 rpm, 14 min (L-PRF centrifuge). Fibrin clots pressed to form a membrane | PO 2 MIU penicillin (if allergy: clindamycin) + metronidazole 1 g/IV antibiotic + bone resection + PRF membranes/metronidazole 0.5 g + 1 MIU penicillin (if allergy: clindamycin) + prothesis avoidance + CHX rinse + soft diet | 14/15 (93%) complete mucosal healing and no symptoms (follow-up: 7–20 months) | 1/15 (7%) recurrence with bone exposure (high dose, bilateral mandibular involvement, stage III, died of cancer after 14 months) |
| [39]/Switzerland/Retrospective clinical study | 8 osteoporotic and 7 oncologic, MRONJ, HTA, HCL, smoking, DM, AF, previous MI, corticoids, 9 females,69 years (56–71); IV, oral BPs Denosumab SC | L-PRF/N.R. | Antibiotic Therapy (15): amoxicillin 2–3 g clindamycin 900–1200 mg ciprofloxacin 500 mg /sequestrectomy + PRF + antibiotics (1) bone debridement + PRF + antibiotics (13) Number of surgeries: 1 (3), 2 (8), 3 (3) /antibiotic cycle repetition | 11/15 (73.3%) treated with L-PRF achieved complete healing (mean period: 42.2 months) | 8/11 reintervened (>1) for healing 4/15(26.7%) relapsed |
| [40]/Brazil/Case Series | 11 osteoporotic, MRONJ (stage II), HTA, DM, 9 females, 67.7 ± 14.6 years (38–84), oral BPs | PRF/2700 rpm, 12 min without anticoagulants (Intra-Spin EBA 200). PRF clot pressed with PRF-Box1 (Intra-Lock System, Miami-FL, USA) | Amoxicillin-clavulanic acid 875/125 mg /necrotic bone removal + debridement + PRF membranes/oral amoxicillin-clavulanic acid 875/125 mg + soft diet + topical CHX | 11/11 (100%) complete healing at 2 weeks | N.R. |
| [41]/Hungary/Retrospective Cohort Study | PRF 25 oncology and 3 osteoporosis, 68.42 years Control 61 oncology and 12 osteoporosis, 63.97 years. MRONJ (stage II and III), chemotherapy, antihormonal therapy, 74 females oral and IV BPs | A-PRF membrane/ 3000 rpm, 8 min. without anticoagulants (PRF Duo Centrifuge System, Process for PRF), A-PRF layer processed into a membrane | Oral amoxicillin-clavulanic acid 875 mg/125 mg (if allergy: clindamycin 300 mg)/necrotic bone debridement ± PRF membranes/ same regimen | Control: 38/73 (58.46%) wound healing at 4 weeks 54/70 (77.14%) stage improvement PRF: 23/28 (82.14%) wound healing at 4 weeks 28/28 (100%) stage improvement Wound healing (p = 0.022), stage improvement (p = 0.005), and reduced relapse rate (p < 0.001) significantly superior in PRF group | Control: 25/38 (65.78%) relapsed PRF: 5/23 (21.74%) relapsed |
| [42]/Italy/Case report | 5 osteoporotic and 3 oncologic, MRONJ with facial sinus tracts (stage III), 6 females (65–82 years), BPs | i-PRF/700 rpm for 3 min without additives (i-PRF, process for PRF) | Oral hygiene session + CHX mouthwash + amoxicillin 1 g (if allergy: clindamycin 600 mg) + metronidazole 250 mg/bone debridement + Sinus tract management (1 mL of PRF near fistula, weekly for 1 month)/same antibiotic regimen | 6/8 (75%) healing of sinus tract and bone lesion at 4 weeks pain relief 8/8 (100%) within 2 days | 1 (12.5%) persistent bone lesion and fistula 1 (12.5%) incomplete mucosal cover at 4 weeks |
| [43]/Czech Republic/Prospective case series | 34 oncologic and 6 osteoporotic, MRONJ, DM, corticosteroids, chemotherapy, smoking, 24 females, 69 years (37–85), BP or/and denosumab | L-PRF/ 3200 rpm, 10 min. without anticoagulants (EBA 20). | Amoxicillin + clavulanic acid 875 mg/125 mg (if allergy clindamycin 300 mg)/sequestromy + debridement necrotic bone (except 4 cases) + PRF clots/antibiotics | 34/40 (85%) complete healing at 12 months Significant association between size of necrotic bone and treatment response (p = 0.014) | 6/40 (15%) recurred 4/6 incomplete necrotic bone removal 1/6 extraoral fistula 1/6 multiple intraoral fistulas |
| [44]/Turkey/Clinical study | 10 oncologic and 3 osteoporotic, MRONJ (stage II and III), 7 females, 72.4 ± 10.61 years (54–84); IV, oral BPs and denosumab | L-PRF/2700 rpm, 12 min. without anticoagulants | Oral Amoxicillin-clavulanate 875/125 mg, (allergy: clindamycin 150 mg)/ Marginal resection + L-PRF (3) sequestrectomy + peripheral ostectomy + L-PRF (9) curettage + L-PRF (1)/ systemic antibiotics + sterile saline irrigation | 9/13 (69.2%) complete healing | 4/13 (30.8%) incomplete healing 1 oro-antral fistula partially resolved 1 chronic fistula and pain resolved after 3 surgeries 1 persistent exposed bone after 4 surgeries 1 resolved after 2nd intervention |
| [45]/Germany/Non-Interventional Prospective Observational Study | 52 oncologic, MRONJ, DM, RD osteoporosis, smoking, immunomodulatory therapy, 27 females, 71.5 ± 8.6 years, IV BPs or denosumab SC | PRF membrane/1200 rpm, 8 min (duo centrifuge, process for PRF) PRF membrane | Ampicillin/sulbactam 3 g IV, (if allergy: clindamycin 600 mg PO) + tube feeding/ Arm A (n = 22): surgical resection Arm B (n = 30): surgical resection + PRF/ same regimen | No significant wound healing, downstaging, pain reduction, or quality of life | 16 (30.76%) wound dehiscence |
| [46]/Brazil/Case-control study | 20 oncologic, MRONJ, 12 females, 61.9 years (41–91), IV BPs | L-PRF/2700 rpm, 12 min (DT4000, Daiki) | CHX rinse + amoxicillin 500 mg + metronidazole 400 mg/ Group 1 tooth extractions Group 2 tooth extractions + L-PRF Group 3 (MRONJ) surgery+ L-PRF/pasty liquid diet + analgesics + oral amoxicillin 500 mg + metronidazole 400 mg | Group 1 4/7 (57%) Group 2 8/8 (100%) Group 3 4/5 (80%) achieved mucosal healing and symptom resolution | 1 in group 3 had recurred 2 in group 1 developed MRONJ Delayed healing (8–12 weeks) in Group 1 |
| [47]/Belgium/Prospective clinical study | 9 oncologic, MRONJ (stage II and III), HTA, DM, HF, RF, 6 females, 68 ± 8 years, BPs or denosumab | L-PRF/AT-SVF/2700 rpm, 12 min, no anticoagulants to obtain L-PRF. Lipoaspiration. Enzymatic treatment and centrifugation. SVF pellet harvested + saline solution. AT-SVF injected into L-PRF (Intra-SpinEBA 200) | N.R./ debridement + AT-SVF/L-PRF/analgesics + amoxicillin-clavulanic acid, 875 mg | 9/10 (90%) lesions of oral mucosa healed within 1 month. 8/9 imaging showed bone healing at 6 months | 2/9 new MRONJ lesions In different locations 1/9 died from cancer after 6 months of follow-up 1/9 imaging showed rejected the bony sequestrum naturally after 12 months |
| [48]/UK/Retrospective Observational study | Control 8 oncologic, 2 osteoporosis and 1 other, PRF 7 osteoporosis, 3 oncologic and 1 other MRONJ, smoking, steroids, 72 ± 8.08 years (58–87), 20 females oral, IV BPs and denosumab | L-PRF/2700 rpm, 12–18 min, no anticoagulants | Oral hygiene improvement + CHX mouthwash + oral antibiotics (if infection)/ Bone debridement/ sequestrectomy ± L-PRF/N.R. | Control 5/11 (45.5%) complete healing PRF 11/11 (100%) complete healing Statistically significant (p = 0.004) | 1 further reintervention 2 deceased with exposed bone 3 required additional treatments. |
| [49]/South Korea/RCT | PRF 22 osteoporosis and 3 bone metastases, 75.24 years (59–97) PRF+BMP2 26 osteoporosis, 4 bone metastasis, 75.2 years (60–85) MRONJ, DM, steroids, 51 females oral, IV BPs | L-PRF/3000 rpm, 10 min. | IV 3rd generation cephalosporin 1 g + Analgesics + CHX irrigation + professional dental prophylaxis/ necrotic bone debridement + antibiotic irrigation + L-PRF ± collagen sponge with rhBMP-2/ antibacterial mouth rinse + antibiotics | PRF 9/25 (36.0%) complete healing at 4 weeks PRF+BMP2 18/30 (60.0%) healing at 4 weeks Statistically significant (p = 0.028) Bacterial colonies significant negative factor affecting healing (p =0.017) | 13/25 (52.0%) delayed healing with PRF 11/30 (36.7%) delayed healing with PRF+BMP2 3/25 (12%) no healing with PRF alone 1/30 no healing with PRF+BMP2 |
| [50]/Italy/RCT | PRF 19 oncologic and 5 osteoporotic, 75.5 ± 5.6 Control 16 oncologic and 7 osteoporotic, 73.9 ± 7.4 years MRONJ (stage II and III), 24 females, Oral, IV BPs and SC denosumab | A-PRF membrane/1300 rpm, 8 min (in a specific centrifuge, process for PRF). PRF Box surgical kit to form membrane | Professional hygiene session + amoxicillin 1 g (allergy: clindamycin 600 mg) + metronidazole 250 mg + CHX mouthwash/ debridement ± A-PRF membrane/ same antibiotic regimen + prothesis avoidance | Mucosal Integrity PRF = 87.5%, Control = 60.9% (p < 0.05) at 1 month No significant differences at 6 months or 1 year Reduced pain and fewer postoperative infections in PRF group at 1 month (p < 0.05) | Reinterventions 1 month PRF = 3 patients Control = 9 patients 6 months PRF =1 patients Control =2 patients (p < 0.05) Signs of fistulas 6 months PRF = 1 patients Control = 1 patients (both received high dose therapy) |
| [51]/Turkey/RCT | CGF 14 osteoporotic, 73.57 ± 5.1 (65–81) Control 14 osteoporotic, 73.64 ± 5.49 (65–81) MRONJ (stage II and III), DM, HTA, AD, 28 females, 65–81 years Oral BPs | CGF/30 s accelerations, 2700 rpm, 2 min, 2400 rpm, 4 min, 2700 rpm, 4 min, 3000 rpm, 3 min, 36 s deceleration. No anticoagulants | Dental examination + PO amoxicillin-clavulanic acid 2 g/ sequestrectomy + curettage + CGF clots/ Soft diet + CHX irrigation+ antibiotic (if infection) | CGF 11/14 (78.6%) healed at 6 months Control 8/14 (57.1%) healed at 6 months No statistically significant differences (p > 0.05) | CGF: 3 bone exposure (1 also infected) Control: 6 bone exposure (3 also infection) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, F.; Faria, C.; Pozza, D.H. Autologous Platelet Concentrates in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Medicina 2025, 61, 1496. https://doi.org/10.3390/medicina61081496
Ferreira F, Faria C, Pozza DH. Autologous Platelet Concentrates in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Medicina. 2025; 61(8):1496. https://doi.org/10.3390/medicina61081496
Chicago/Turabian StyleFerreira, Filipa, Carlos Faria, and Daniel Humberto Pozza. 2025. "Autologous Platelet Concentrates in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review" Medicina 61, no. 8: 1496. https://doi.org/10.3390/medicina61081496
APA StyleFerreira, F., Faria, C., & Pozza, D. H. (2025). Autologous Platelet Concentrates in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Medicina, 61(8), 1496. https://doi.org/10.3390/medicina61081496





