Free vs. Local Tissue Transfer and Reconstruction in Pediatric Head and Neck Cancer Patients: A Comparable Complication Outcome Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
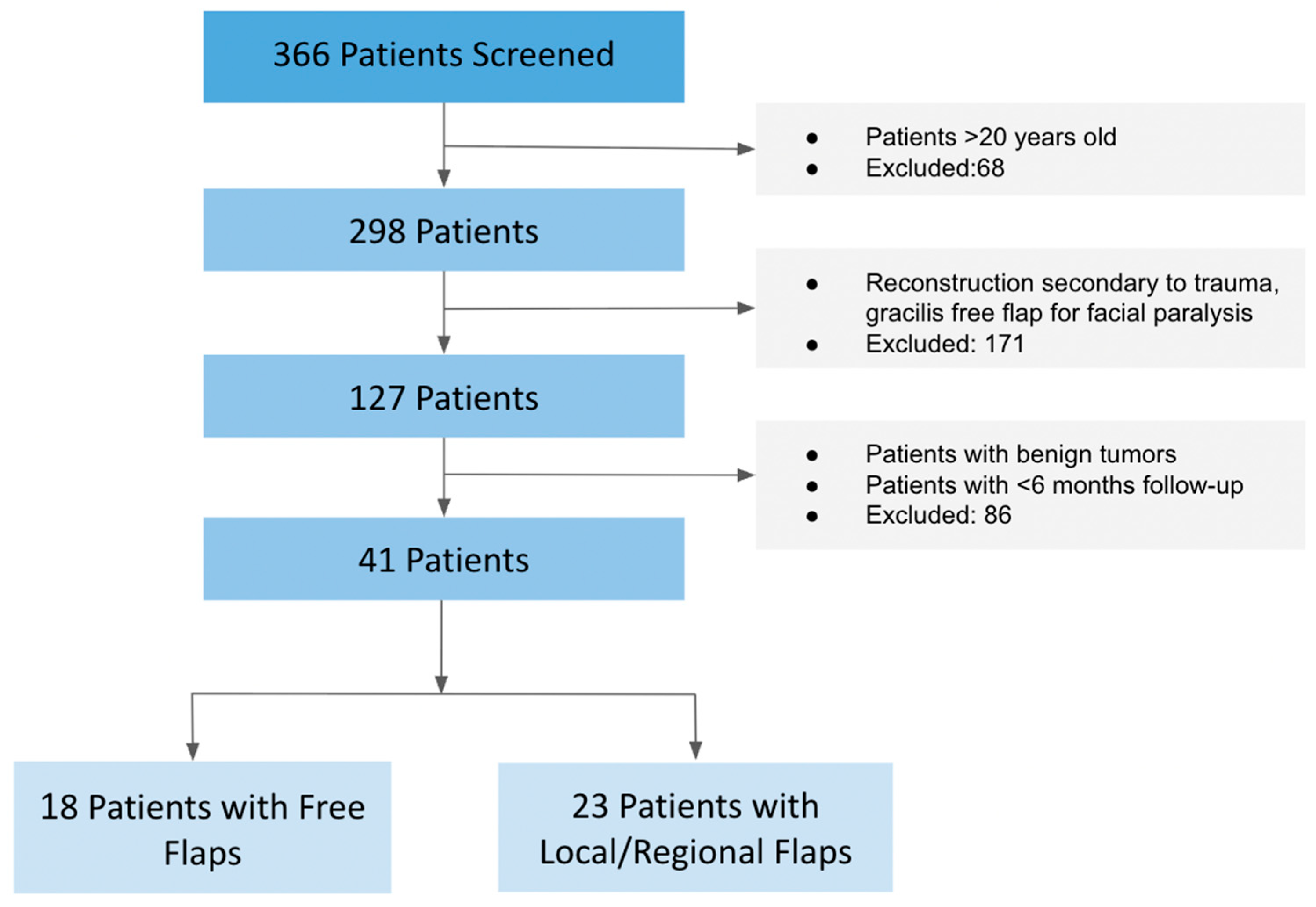
3.1. Patient Selection and Cohort Summary
3.2. Demographics and Tumor Characteristics
3.3. Flap Types and Reconstruction Methods
3.4. Flap Survival and Surgical Complications
3.5. Infectious Complications
3.6. Operative and Hospitalization Metrics
3.7. Donor Site Morbidity and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRS | Plastic and Reconstructive Surgery |
| CSF | Cerebrospinal Fluid |
| CHLA | Children’s Hospital Los Angeles |
| H&N | Head and Neck |
| ALT | Anterolateral Thigh |
References
- Albright, J.T.; Topham, A.K.; Reilly, J.S. Pediatric Head and Neck Malignancies: US Incidence and Trends Over 2 Decades. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.; Hamdi, M.; Blondeel, P.; Tonnard, P.; Verpaele, A.; Monstrey, S. Free perforator flaps in children. Plast. Reconstr. Surg. 2005, 116, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Chim, H.; Salgado, C.J.; Seselgyte, R.; Wei, F.C.; Mardini, S. Principles of head and neck reconstruction: An algorithm to guide flap selection. Semin. Plast. Surg. 2010, 24, 148–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanasono, M.M.; Matros, E.; Disa, J.J. Important aspects of head and neck reconstruction. Plast. Reconstr. Surg. 2014, 134, 968e–980e. [Google Scholar] [CrossRef] [PubMed]
- Ragbir, M.; Brown, J.S.; Mehanna, H. Reconstructive considerations in head and neck surgical oncology: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S191–S197. [Google Scholar] [CrossRef]
- Kim, H.S.; Chung, C.H.; Chang, Y.J. Free-flap reconstruction in recurrent head and neck cancer: A retrospective review of 124 cases. Arch. Craniofac. Surg. 2020, 21, 27–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckardt, A.; Fokas, K. Microsurgical reconstruction in the head and neck region: An 18-year experience with 500 consecutive cases. J. Craniomaxillofac. Surg. 2003, 31, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Amarante, J.; Reis, J.; Costa-Ferreira, A.; Malheiro, E.; Silva, A. Head and neck reconstruction: A review of 117 cases. Eur. J. Plast. Surg. 2000, 23, 404–412. [Google Scholar] [CrossRef]
- Cordeiro, P.G.; Disa, J.J.; Hidalgo, D.A.; Hu, Q.Y. Reconstruction of the mandible with osseous free flaps: A 10-year experience with 150 consecutive patients. Plast. Reconstr. Surg. 1999, 104, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Dorji, K. Local or regional flaps in developing country: Experience from Eastern Bhutan. Int. Wound J. 2024, 21, e14905. [Google Scholar] [CrossRef]
- Upton, J.; Guo, L. Pediatric free tissue transfer: A 29-year experience with 433 transfers. Plast. Reconstr. Surg. 2008, 121, 1725–1737. [Google Scholar] [CrossRef]
- Konttila, E.; Koljonen, V.; Kauhanen, S.; Kallio, P.; Tukiainen, E. Microvascular reconstruction in children: A report of 46 cases. J. Trauma 2010, 68, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, M.D.; McNamara, M.G.; Clouston, H.W.; Sutton, P.A.; Hubner, R.A.; Valle, J.W. Patients Undergoing Systemic Anti-Cancer Therapy Who Require Surgical Intervention: What Surgeons Need to Know. Cancers 2023, 15, 3781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arnold, D.J.; Wax, M.K. Microvascular Committee of the American Academy of Otolaryngology-Head and Neck Surgery. Pediatric microvascular reconstruction: A report from the Microvascular Committee. Otolaryngol. Head Neck Surg. 2007, 136, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.M.; Borud, L.J.; Brecht, L.E.; Longaker, M.T.; Siebert, J.W. Microvascular reconstruction of the pediatric mandible. Plast. Reconstr. Surg. 2007, 119, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S.; Wei, F.C.; Cheng, M.H.; Huang, W.C.; Chwei-Chin Chuang, D.; Lin, C.H. Safety and reliability of microsurgical free tissue transfers in paediatric head and neck reconstruction—A report of 72 cases. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 767–771. [Google Scholar] [CrossRef]
- Jacob, L.M.; Dong, W.; Chang, D.W. Outcomes of reconstructive surgery in pediatric oncology patients: Review of 10-year experience. Ann. Surg. Oncol. 2010, 17, 2563–2569. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, J.; Zhu, H.; Xu, L.; Ji, T.; He, Y.; Yang, W.; Hu, Y.; Yang, X.; Zhang, Z. Microsurgical free flap reconstructions of the head and neck region: Shanghai experience of 34 years and 4640 flaps. Int. J. Oral Maxillofac. Surg. 2015, 44, 675–684. [Google Scholar] [CrossRef]
- Weizman, N.; Gil, Z.; Wasserzug, O.; Amir, A.; Gur, E.; Margalit, N.; Fliss, D.M. Surgical ablation and free flap reconstruction in children with malignant head and neck tumors. Skull Base 2011, 21, 165–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakatsuka, T.; Harii, K.; Yamada, A.; Yonehara, Y.; Takato, T.; Kawahara, N.; Sasaki, T.; Yamasoba, T.; Nibu, K.; Ebihara, S. Immediate free flap reconstruction for head and neck pediatric malignancies. Ann. Plast. Surg. 1998, 40, 594–599. [Google Scholar] [CrossRef]
- Aboelatta, Y.A.; Aly, H.M. Free tissue transfer and replantation in pediatric patients: Technical feasibility and outcome in a series of 28 patients. J. Hand Microsurg. 2013, 5, 74–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nepon, H.; Safran, T.; Reece, E.M.; Murphy, A.M.; Vorstenbosch, J.; Davison, P.G. Radiation-Induced Tissue Damage: Clinical Consequences and Current Treatment Options. Semin. Plast. Surg. 2021, 35, 181–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinovic, D.; Tokic, D.; Puizina Mladinic, E.; Usljebrka, M.; Kadic, S.; Lesin, A.; Vilovic, M.; Lupi-Ferandin, S.; Ercegovic, S.; Kumric, M.; et al. Nutritional Management of Patients with Head and Neck Cancer-A Comprehensive Review. Nutrients 2023, 15, 1864. [Google Scholar] [CrossRef]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Jan, W.L.; Chen, H.C.; Chang, C.C.; Chen, H.H.; Shih, P.K.; Huang, T.C. Modified Clavien-Dindo Classification and Outcome Prediction in Free Flap Reconstruction among Patients with Head and Neck Cancer. J. Clin. Med. 2020, 9, 3770. [Google Scholar] [CrossRef]
- Starnes-Roubaud, M.J.; Hanasono, M.M.; Kupferman, M.E.; Liu, J.; Chang, E.I. Microsurgical Reconstruction Following Oncologic Resection in Pediatric Patients: A 15-Year Experience. Ann. Surg. Oncol. 2017, 24, 4009–4016. [Google Scholar] [CrossRef]
- Akçal, A.; Karşıdağ, S.; Sucu, D.Ö.; Turgut, G.; Uğurlu, K. Microsurgical reconstruction in pediatric patients: A series of 30 patients. Ulus. Travma Acil Cerrahi Derg. 2013, 19, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Zavala, A.; Ore, J.F.; Broggi, A.; De Pawlikowski, W. Pediatric Mandibular Reconstruction Using the Vascularized Fibula Free Flap: Functional Outcomes in 34 Consecutive Patients. Ann. Plast. Surg. 2021, 87, 662–668. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.B.; Wang, Y.; Mao, C.; Yu, G.Y.; Peng, X. Long-Term Outcomes After Pediatric Mandibular Reconstruction Using Vascularized Free Fibula Flap. Plast. Reconstr. Surg. 2024, 153, 397E–406E. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.J.; Eley, K.A.; Von Kier, S.; Pearson, O.; Watt-Smith, S.R. Functional fibrinogen to platelet ratio using thromboelastography as a predictive parameter for thrombotic complications following free tissue transfer surgery: A preliminary study. Microsurgery 2012, 32, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.D.; Manna, B. Wound Dehiscence. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK551712/ (accessed on 3 January 2025).
- Deptuła, M.; Zieliński, J.; Wardowska, A.; Pikuła, M. Wound healing complications in oncological patients: Perspectives for cellular therapy. Postepy Dermatol. Alergol. 2019, 36, 139–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katsnelson, J.Y.; Tyrell, R.; Karadsheh, M.J.; Manstein, E.; Egleston, B.; Deng, M.; Baltodano, P.A.; Shafqat, M.S.; Patel, S.A. Postoperative Complications Associated with the Choice of Reconstruction in Head and Neck Cancer: An Outcome Analysis of 4712 Patients from the ACS-NSQIP Database. J. Reconstr. Microsurg. 2022, 38, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, R.; Colletti, G.; Bonomo, P.; Parrinello, G.; Iavarone, A.; Dolivet, G.; Livi, L.; Deganello, A. Head and neck reconstruction with pedicled flaps in the free flap era. Acta Otorhinolaryngol. Ital. 2016, 36, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Moshal, T.; Lasky, S.; Roohani, I.; Jolibois, M.I.; Manasyan, A.; Munabi, N.C.O.; Fahradyan, A.; Lee, J.A.; Hammoudeh, J.A. The Forgotten Flap: The Pedicled Trapezius Flap’s Utility in Pediatric Head and Neck Reconstruction—A Systematic Review. J. Reconstr. Microsurg. 2025, 41, 113–122. [Google Scholar] [CrossRef]
- Youn, S.; Trotter, C.; O’Brien, D.; Alfeerawi, S.; Choi, D.; Roohani, I.; Shakoori, P.; Fahradyan, A.; Urata, M.M.; Hammoudeh, J.A. A Novel Algorithm for Pediatric Microsurgical Maxillomandibular Reconstruction Using Custom Endoprosthesis. J. Oral Maxillofac. Surg. 2023, 81, S58–S59. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.B.; Yu, Y.; Wang, Y.; Mao, C.; Guo, C.B.; Yu, G.Y.; Peng, X. Free Flap Transfer for Pediatric Head and Neck Reconstruction: What Factors Influence Flap Survival? Laryngoscope 2019, 129, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Liang, T.; Peng, X. Mandibular growth after paediatric mandibular reconstruction with the vascularized free fibula flap: A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 440–447. [Google Scholar] [CrossRef]
- Gil, Z.; Patel, S.G.; Singh, B.; Cantu, G.; Fliss, D.M.; Kowalski, L.P.; Kraus, D.H.; Snyderman, C.; Shah, J.P.; International Collaborative Study Group. Analysis of prognostic factors in 146 patients with anterior skull base sarcoma: An international collaborative study. Cancer 2007, 110, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, P.R.; Graff, J.T.; Cramer, J.D. Association of Surgical Margin Distance with Survival in Patients with Resected Head and Neck Squamous Cell Carcinoma: A Secondary Analysis of a Randomized Clinical Trial. Arch. Otolaryngol.—Head Neck Surg. 2023, 149, 317–326. [Google Scholar] [CrossRef]
- Allam, O.; Shah, R.; Cadwell, J.B.; Dinis, J.; Peck, C.; Junn, A.; Gowda, A.; Alperovich, M. Evaluation of Complication Rates of Free Flap Reconstruction in Pediatric Patients. J. Indian Assoc. Pediatr. Surg. 2022, 27, 428–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| All (n = 41) | Local Flaps (n = 23) | Free Flaps (n = 18) | p-Value | |
|---|---|---|---|---|
| Age | 10.6 ± 5.6 | 9.6 ± 5.5 | 11.9 ± 4.8 | 0.090 |
| Sex | ||||
| Female | 20 (48.8%) | 11 (47.8%) | 9 (50.0%) | 0.749 |
| Male | 21 (51.2%) | 12 (52.2%) | 9 (50.0%) | |
| Pathology | ||||
| Osteosarcoma | 9 (21.9%) | 5 (21.7%) | 4 (22.2%) | 0.690 |
| Spindle Cell Sarcoma | 6 (14.6%) | 4 (17.4%) | 2 (11.1%) | |
| Rhabdomyosarcoma | 5 (12.2%) | 3 (13%) | 2 (11.1%) | |
| Ewing Sarcoma | 3 (7.3%) | 1 (4.3%) | 2 (11.1%) | |
| Clear Cell Sarcoma | 1 (2.4%) | 1 (4.3%) | - | |
| Epithelioid Sarcoma | 1 (2.4%) | 1 (4.3%) | - | |
| Synovial Sarcoma | 1 (2.4%) | - | 1 (5.6%) | |
| Fibrosarcoma | 1 (2.4%) | 1 (4.3%) | - | |
| Squamous Cell Carcinoma | 2 (4.8%) | 2 (8.7%) | - | |
| Malignant Giant Cell Tumor | 1 (2.4%) | - | 1 (5.6%) | |
| NUT Carcinoma | 4 (9.8%) | 2 (8.7%) | 2 (11.1%) | |
| Medulloblastoma | 5 (12.2%) | 5 (21.7%) | - | |
| Neuroblastoma | 1 (2.4%) | - | 1 (5.6%) | |
| Preoperative Radiation | ||||
| Yes | 27 (65%) | 13 (56.5%) | 14 (76.5%) | 0.154 |
| No | 14 (34.1%) | 10 (43.5%) | 4 (22.2%) |
| Flap Type | Free Flaps (n = 18) |
|---|---|
| Anterolateral Thigh (ALT) | 8 (44.4%) |
| Fibula | 6 (33.3%) |
| Gracilis | 3 (16%) |
| Rectus | 1 (5.6%) |
| Local/Regional Flaps (n = 23) | |
| Facial | 5 (21.7%) |
| Temporalis | 5 (21.7%) |
| Trapezius | 4 (17.4%) |
| Pectoralis | 3 (13.0%) |
| Palatal | 2 (8.7%) |
| Calvarial | 1 (4.4%) |
| Cervicofacial | 1 (4.4%) |
| Pericranial | 1 (4.4%) |
| Platysma | 1 (4.4%) |
| All (n = 41) | Local Flaps (n = 23) | Free Flaps (n = 18) | p-Value | |
|---|---|---|---|---|
| Flap Failure | 1 (2.4%) | - | 1 (5.5%) | 0.252 |
| Major Revisions | 21 (51.2%) | 13 (56.5%) | 8 (44.4%) | 0.443 |
| Partial Flap Necrosis | 6 (12.5%) | 3 (13.0%) | 3 (16.7%) | 0.745 |
| Hardware Exposure | 9 (22.5%) | 8 (34.7%) | 1 (5.5%) | 0.025 * |
| Wound Dehiscence | 11 (25%) | 9 (39.1%) | 1 (5.5%) | 0.013 * |
| Infection | 7 (17.1%) | 4 (17.4%) | 3 (16.7%) | 0.832 |
| Hematoma | 1 (2.4%) | 1 (4.3%) | 1 (5.5%) | 0.859 |
| All (n = 41) | Local Flaps (n = 23) | Free Flaps (n = 18) | p-Value | |
|---|---|---|---|---|
| Operative Time (Hours) | 8.2 ± 5.1 | 6.5 ± 1.2 | 10.3 ± 0.8 | 0.011 * |
| Anesthesia Duration (Hours) | 9.5 ± 5.7 | 7.9 ± 5.9 | 12.5 ± 4.1 | 0.015 * |
| Length of Hospitalization (days) | 20.9 ± 25.6 | 13.9 ± 3.5 | 29.1 ± 7.9 | 0.036 * |
| Readmission within 30 days | 13 (32.5%) | 10 (43.5%) | 3 (17.6%) | 0.085 |
| Follow-up (Years) | 2.3 ± 2.9 | 2.7 ± 3.8 | 1.8 ± 1.8 | 0.788 |
| All (n = 41) | Local Flaps (n = 23) | Free Flaps (n = 18) | p-Value | |
|---|---|---|---|---|
| Donor Site Morbidity | 1 (2.4%) | - | 1 (5.6%) | 0.252 |
| Total Flap Reoperations | 1.4 ± 1.9 | 1.7 ± 2.2 | 1.1 ± 1.7 | 0.822 |
| Mortality | 14 (26.8%) | 6 (26.1%) | 5 (27.8%) | 0.903 |
| Age at Mortality | 13.5 ± 7.1 | 9.8 ± 9.9 | 15.6 ± 4.9 | 0.148 |
| Time from Tissue Transfer to Mortality | 1.9 ± 1.8 | 1.2 ± 0.6 | 2.3 ± 1.0 | 0.235 |
| TARGETS | Local Tissue Transfer | Free Tissue Transfer |
|---|---|---|
| Type of tissue | Suitable for soft tissue-only defects. | Preferred for composite tissue requirements (bone, muscle, skin). |
| Ability to heal | Local flaps may be more prone to wound dehiscence, especially in irradiated or thin tissue beds. | Free flaps provide well-vascularized tissue that may reduce the risk of dehiscence and promote more reliable healing. |
| Restoration of function/ radiation | Adequate for simpler functional restorations. Radiation might affect regional tissue quality and vascularity, making local tissue transfer less optimal. | Superior for restoring complex functions like speech or chewing. Offers well-vascularized, non-irradiated tissue for repair. |
| Growth potential | May require additional surgical revisions and adjustments as the child grows. | Greater recruitment of soft and bone tissue may better accommodate the child’s growth over time. |
| Exposure | May offer adequate soft tissue coverage for hardware. May need additional revisions for hardware exposure. | May offer greater recruitment of soft tissue for hardware long-term coverage. |
| Tumor margins | May not adequately address extensive margins. Sufficient for small defects. | Allows for wide reconstruction with extensive margins. Preferred for larger defects. |
| Site of resection | Limited to regional tissue near the defect; may not suffice for extensive or deep defects. | Enables reconstruction across distant or complex anatomical sites where local tissue is insufficient. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejia, V.; Pekcan, A.; Bakovic, M.; Patel, R.K.; Turk, M.; Roohani, I.; Shakoori, P.; Urata, M.; Hammoudeh, J.A. Free vs. Local Tissue Transfer and Reconstruction in Pediatric Head and Neck Cancer Patients: A Comparable Complication Outcome Review. Medicina 2025, 61, 1477. https://doi.org/10.3390/medicina61081477
Mejia V, Pekcan A, Bakovic M, Patel RK, Turk M, Roohani I, Shakoori P, Urata M, Hammoudeh JA. Free vs. Local Tissue Transfer and Reconstruction in Pediatric Head and Neck Cancer Patients: A Comparable Complication Outcome Review. Medicina. 2025; 61(8):1477. https://doi.org/10.3390/medicina61081477
Chicago/Turabian StyleMejia, Valeria, Asli Pekcan, Melanie Bakovic, Raina Kushal Patel, Marvee Turk, Idean Roohani, Pasha Shakoori, Mark Urata, and Jeffrey A. Hammoudeh. 2025. "Free vs. Local Tissue Transfer and Reconstruction in Pediatric Head and Neck Cancer Patients: A Comparable Complication Outcome Review" Medicina 61, no. 8: 1477. https://doi.org/10.3390/medicina61081477
APA StyleMejia, V., Pekcan, A., Bakovic, M., Patel, R. K., Turk, M., Roohani, I., Shakoori, P., Urata, M., & Hammoudeh, J. A. (2025). Free vs. Local Tissue Transfer and Reconstruction in Pediatric Head and Neck Cancer Patients: A Comparable Complication Outcome Review. Medicina, 61(8), 1477. https://doi.org/10.3390/medicina61081477





