Interventional Procedures in Deep Venous Thrombosis Treatment: A Review of Techniques, Outcomes, and Patient Selection
Abstract
1. Introduction
2. Methods
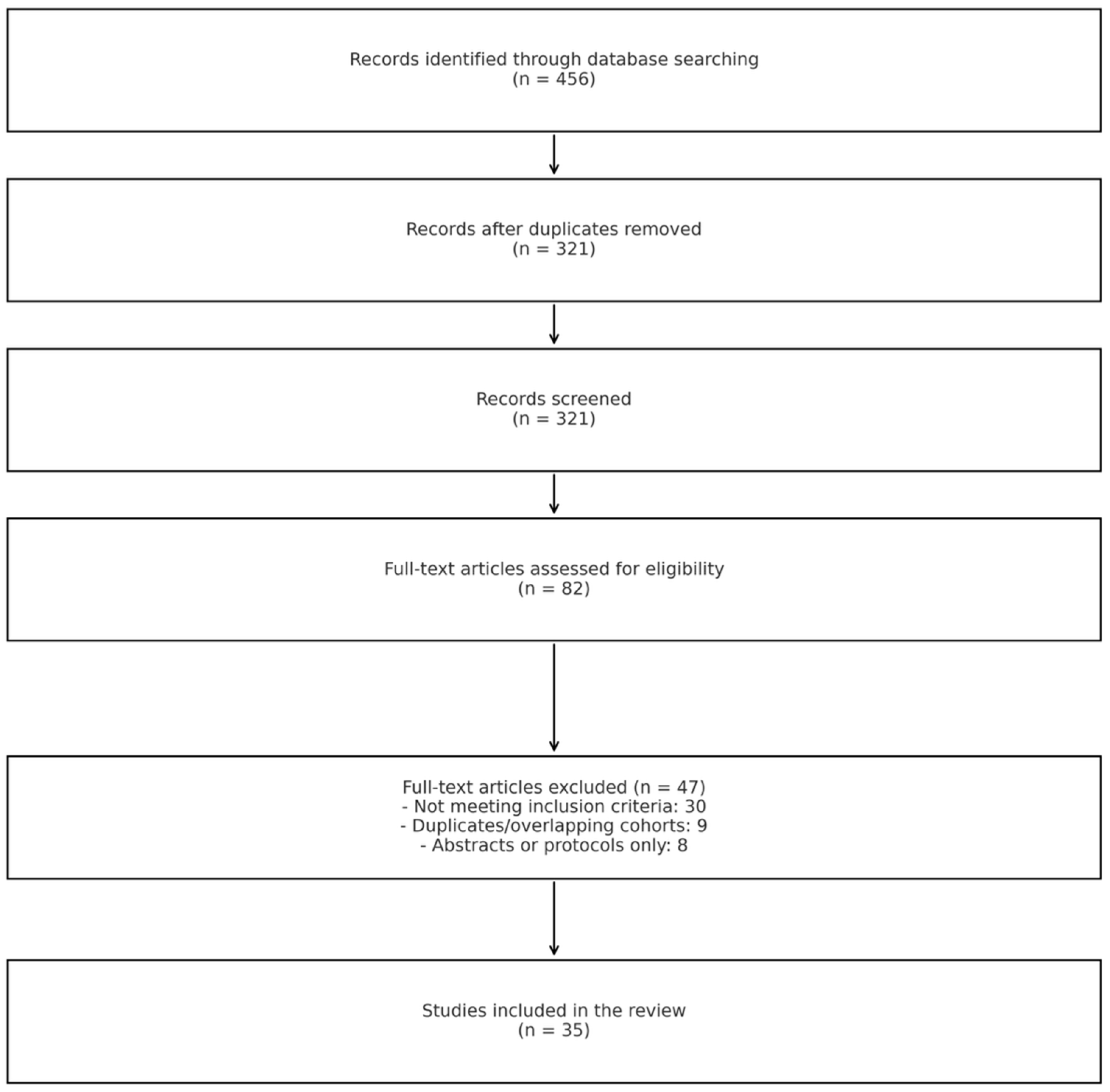
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Outcome Measures
2.4. Data Synthesis and Analysis
3. Conventional Treatment Approaches
3.1. Anticoagulation Therapy
3.1.1. Unfractionated Heparin
3.1.2. Low Molecular Weight Heparin
3.1.3. Direct Oral Anticoagulants
3.1.4. Vitamin K Antagonists
3.2. Systemic Thrombolysis
3.3. Compression Therapy
3.4. Limitations of Conventional Approaches
4. Interventional Procedures for DVT
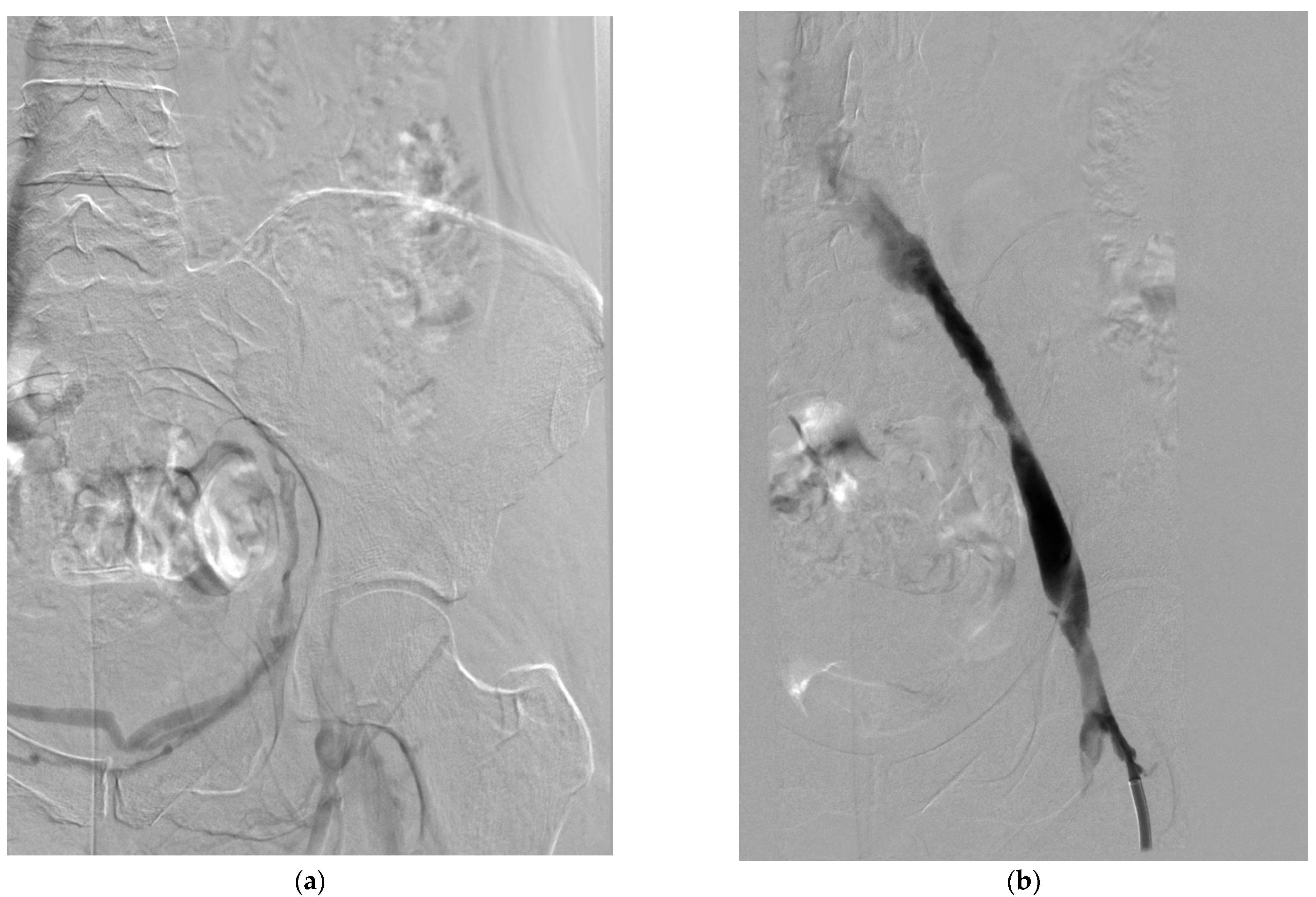
4.1. Catheter-Directed Thrombolysis
4.1.1. Technical Aspects
4.1.2. Adjunctive Techniques
4.2. Mechanical Thrombectomy
4.2.1. Device Categories and Mechanisms
- Rheolytic thrombectomy: These systems use high-velocity saline jets to create a Venturi effect, fragmenting and aspirating thrombus. The AngioJet system (Boston Scientific, Marlborough, MA, USA) is the most widely used device in this category [75]. It employs a dual-lumen catheter with high-pressure jets that create a localized low-pressure zone, drawing thrombus into the catheter for fragmentation and removal [76]. The system can be used in “thrombectomy mode” for pure mechanical removal or in “power pulse mode” for combined pharmacomechanical therapy [70].
- Rotational thrombectomy: These devices employ rotating components to macerate thrombus. The Aspirex device (Straub Medical, Wangs, Switzerland) uses a rotating helix within a catheter to fragment and aspirate thrombus [77]. Cleaner Rotational Thrombectomy System (Argon Medical Devices, Plano, TX, USA) features a sinusoidal wire that rotates at high speed (3500 rpm) to macerate thrombus without aspiration [65].
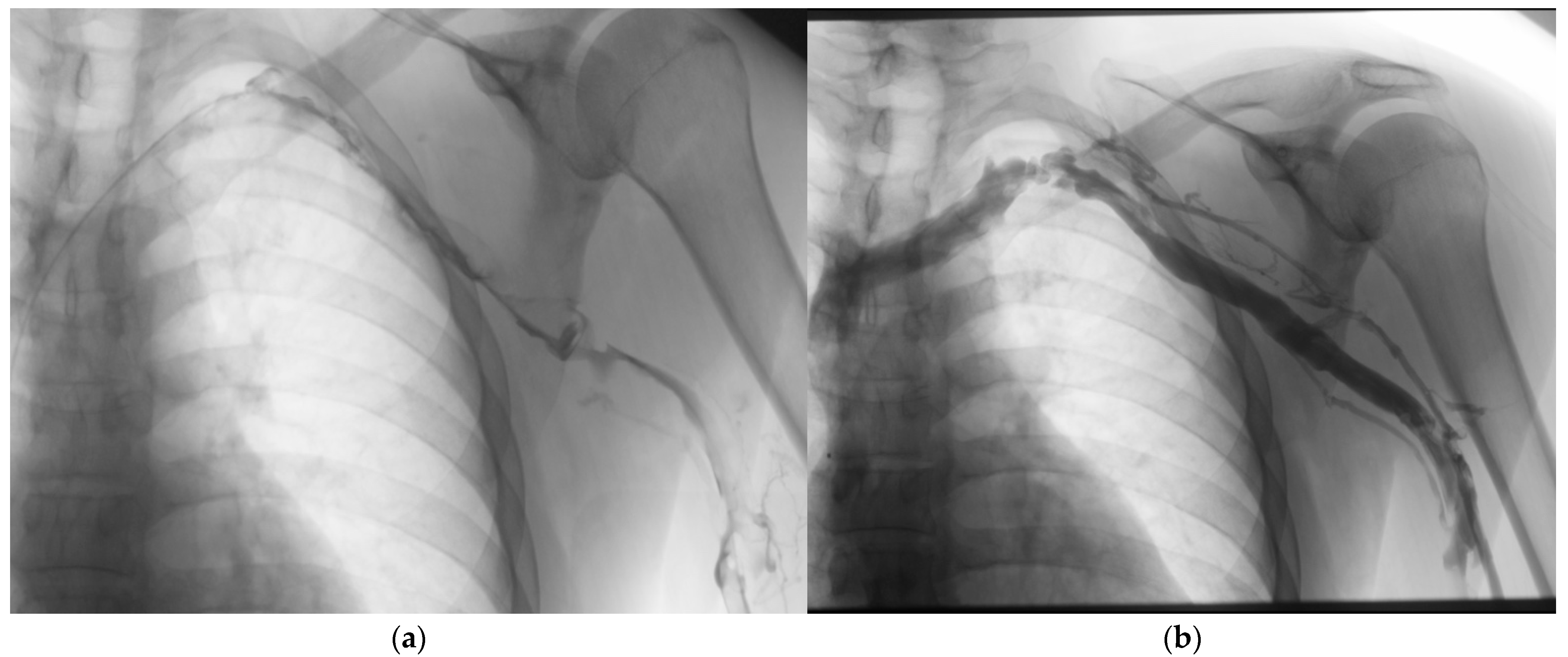
- Aspiration thrombectomy: These systems rely on negative pressure to remove thrombus. The Indigo/Lightening System (Penumbra, Alameda, CA, USA) uses a vacuum pump to generate continuous suction through specially designed catheters [67]. The ClotTriever system (Inari Medical, Irvine, CA, USA) employs a mechanical retriever with a nitinol coring element and attached collection bag to engage and remove thrombus en bloc [78] (Figure 3).
- Balloon-based systems: The FlowTriever system (Inari Medical, Irvine, CA, USA) uses large self-expanding nitinol disks to engage and extract thrombus, with an option for aspiration through the guide catheter [79]. This system is designed for large-vessel thrombectomy without the need for thrombolytics [79].
4.2.2. Procedural Considerations
4.3. Pharmacomechanical Thrombectomy
4.3.1. Techniques and Devices
- Isolated thrombolysis: The Trellis system (Medtronic, now discontinued) isolated the treatment segment between two balloons while a rotating wire fragmented the thrombus and distributed the thrombolytic agent [81]. After a short dwell time (typically 15–20 min), the liquefied thrombus was aspirated before balloon deflation [82].
- Power pulse delivery: The AngioJet system can be used in “power pulse mode,” where thrombolytic agent is forcefully injected into the thrombus, allowed to dwell for 20–30 min, and then removed using the standard thrombectomy mode [70]. This technique has been shown to reduce procedure time and thrombolytic dose compared to standard CDT [76].
- Percutaneous mechanical thrombectomy with thrombolysis: This approach involves initial mechanical thrombectomy followed by a short-duration thrombolytic infusion (typically 4–6 h) to address residual thrombus [72]. This sequential approach may be particularly useful for extensive or partially organized thrombi [73].
4.3.2. Procedural Outcomes
4.4. Venous Stenting
4.4.1. Indications and Technical Considerations
- Residual venous stenosis after thrombolysis/thrombectomy: Significant stenoses (>50% diameter reduction or pressure gradient >2 mmHg) may be treated with stenting to maintain patency and prevent rethrombosis [74].
- Extrinsic venous compression: May–Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) is present in up to 50% of patients with left-sided iliofemoral DVT and typically requires stenting for long-term patency [84].
- Chronic post-thrombotic venous occlusion: In patients with established PTS and venous claudication, recanalization and stenting of chronic occlusions may improve symptoms and quality of life [85].
4.4.2. Outcomes and Complications
4.5. Patient Selection for Interventional Procedures
- Thrombus location and extent: Interventional approaches are generally reserved for proximal DVT, particularly iliofemoral involvement, where the risk of PTS is highest, and the potential benefit of thrombus removal is greatest [19].
- Bleeding risk: Absolute contraindications to thrombolysis include active internal bleeding, recent cerebrovascular event, intracranial neoplasm, or recent major surgery/trauma. Relative contraindications include recent minor surgery, pregnancy, and uncontrolled hypertension [19]. Pure mechanical approaches may be preferred in patients with elevated bleeding risk [67].
- Thrombus characteristics: Fresh, loosely organized thrombus responds better to both thrombolysis and mechanical removal compared to chronic, organized, and wall-adherent thrombus [73].
- Anatomic considerations: Underlying venous anomalies, stenoses, or compression syndromes may influence the choice of intervention and need for adjunctive stenting [84].
- The Society of Interventional Radiology and the American Heart Association have published consensus guidelines for patient selection, generally recommending consideration of interventional treatment for patients with acute (<14 days) iliofemoral DVT, severe symptoms, low bleeding risk, good functional status, and life expectancy > 1 year [17,19]. However, these recommendations continue to evolve as new evidence emerges from clinical trials and registries.
5. Clinical Outcomes of Interventional DVT Treatment
5.1. Randomized Controlled Trials
5.1.1. The CaVenT Trial
5.1.2. The ATTRACT Trial
5.2. Other Randomized Trials
5.3. Observational Studies and Registries
5.4. Comparative Effectiveness of Interventional Approaches
6. Safety Outcomes
7. Cost-Effectiveness
8. Synthesis of Evidence
9. Conclusions
- Prospective studies focusing specifically on patients with iliofemoral DVT, where the benefit of intervention appears greatest;
- Head-to-head comparisons of different interventional techniques, particularly newer mechanical thrombectomy devices versus traditional pharmacomechanical approaches;
- Development and validation of risk prediction models to identify patients most likely to benefit from intervention;
- Longer-term follow-up studies to assess the durability of treatment effects and late complications;
- Cost-effectiveness analyses incorporating real-world practice patterns and long-term outcomes.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Heit, J.A.; Spencer, F.A.; White, R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 3–14. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Kahn, S.R.; Comerota, A.J.; Cushman, M.; Evans, N.S.; Ginsberg, J.S.; Goldenberg, N.A.; Gupta, D.K.; Prandoni, P.; Vedantham, S.; Walsh, M.E.; et al. The postthrombotic syndrome: Evidence-based prevention, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 2014, 130, 1636–1661. [Google Scholar] [CrossRef]
- Esmon, C.T. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009, 23, 225–229. [Google Scholar] [CrossRef]
- Anderson, F.A.; Spencer, F.A. Risk factors for venous thromboembolism. Circulation 2003, 107, I9–I16. [Google Scholar] [CrossRef]
- Douketis, J.D.; Crowther, M.A.; Foster, G.A.; Ginsberg, J.S. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am. J. Med. 2001, 110, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Shrier, I.; Julian, J.A.; Ducruet, T.; Arsenault, L.; Miron, M.-J.; Roussin, A.; Desmarais, S.; Joyal, F.; Kassis, J.; et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann. Intern. Med. 2008, 149, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The long-term clinical course of acute deep venous thrombosis. Ann. Intern. Med. 1996, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Shbaklo, H.; Lamping, D.L.; Holcroft, C.A.; Shrier, I.; Miron, M.J.; Roussin, A.; Desmarais, S.; Joyal, F.; Kassis, J.; et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J. Thromb. Haemost. 2008, 6, 1105–1112. [Google Scholar] [CrossRef]
- Kahn, S.R.; Ducruet, T.; Lamping, D.L.; Arsenault, L.; Miron, M.J.; Roussin, A.; Desmarais, S.; Joyal, F.; Kassis, J.; Solymoss, S.; et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch. Intern. Med. 2005, 165, 1173–1178. [Google Scholar] [CrossRef]
- Grosse, S.D.; Nelson, R.E.; Nyarko, K.A.; Richardson, L.C.; Raskob, G.E. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb. Res. 2016, 137, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.H.; Manzo, R.A.; Bergelin, R.O.; Markel, A.; Strandness, D.E. Deep venous insufficiency: The relationship between lysis and subsequent reflux. J. Vasc. Surg. 1993, 18, 596–605; discussion 606. [Google Scholar] [CrossRef]
- Comerota, A.J.; Aldridge, S.C. Thrombolytic therapy for deep venous thrombosis: A clinical review. Can. J. Surg. 1993, 36, 359–364. [Google Scholar]
- Plate, G.; Einarsson, E.; Ohlin, P.; Jensen, R.; Qvarfordt, P.; Eklöf, B. Thrombectomy with temporary arteriovenous fistula: The treatment of choice in acute iliofemoral venous thrombosis. J. Vasc. Surg. 1984, 1, 867–876. [Google Scholar] [CrossRef]
- Semba, C.P.; Dake, M.D. Iliofemoral deep venous thrombosis: Aggressive therapy with catheter-directed thrombolysis. Radiology 1994, 191, 487–494. [Google Scholar] [CrossRef]
- Vedantham, S.; Goldhaber, S.Z.; Julian, J.A.; Kahn, S.R.; Jaff, M.R.; Cohen, D.J.; Magnuson, E.; Razavi, M.K.; Comerota, A.J.; Gornik, H.L.; et al. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N. Engl. J. Med. 2017, 377, 2240–2252. [Google Scholar] [CrossRef]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, S.; Sista, A.K.; Klein, S.J.; Nayak, L.; Razavi, M.K.; Kalva, S.P.; Saad, W.E.; Dariushnia, S.R.; Caplin, D.M.; Chao, C.P.; et al. Quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J. Vasc. Interv. Radiol. 2014, 25, 1317–1325. [Google Scholar] [CrossRef]
- Kahn, S.R.; Julian, J.A.; Kearon, C.; Gu, C.-S.; Cohen, D.J.; Magnuson, E.A.; Comerota, A.J.; Goldhaber, S.Z.; Jaff, M.R.; Razavi, M.K.; et al. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 8–23.e18. [Google Scholar] [CrossRef] [PubMed]
- Enden, T.; Haig, Y.; Kløw, N.-E.; Slagsvold, C.-E.; Sandvik, L.; Ghanima, W.; Hafsahl, G.; Holme, P.A.; Holmen, L.O.; Njaastad, A.M.; et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): A randomised controlled trial. Lancet 2012, 379, 31–38. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e419S–e496S. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z. RE-COVER Study Group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef]
- EINSTEIN–PE Investigators; Büller, H.R.; Prins, M.H.; Lensin, A.W.A.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; Wells, P.; et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar] [CrossRef]
- Ageno, W.; Mantovani, L.G.; Haas, S.; Kreutz, R.; Monje, D.; Schneider, J.; van Eickels, M.; Gebel, M.; Zell, E.; Turpie, A.G.G. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): An international, prospective, non-interventional study. Lancet Haematol. 2016, 3, e12–e21. [Google Scholar] [CrossRef]
- Hirsh, J.; Dalen, J.; Anderson, D.R.; Poller, L.; Bussey, H.; Ansell, J.; Deykin, D. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001, 119, 8S–21S. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.; Hirsh, J.; Hylek, E.; Jacobson, A.; Crowther, M.; Palareti, G.; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 160S–198S. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H.; et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e152S–e184S. [Google Scholar] [CrossRef]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G.; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e44S–e88S. [Google Scholar] [CrossRef]
- Witt, D.M.; Nieuwlaat, R.; Clark, N.P.; Ansell, J.; Holbrook, A.; Skov, J.; Shehab, N.; Mock, J.; Myers, T.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018, 2, 3257–3291. [Google Scholar] [CrossRef]
- Büller, H.R.; Davidson, B.L.; Decousus, H.; Gallus, A.; Gent, M.; Piovella, F.; Prins, M.H.; Raskob, G.; Segers, A.E.M.; Cariou, R.; et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: A randomized trial. Ann. Intern. Med. 2004, 140, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A. Fondaparinux sodium: A selective inhibitor of factor Xa. Am. J. Health Syst. Pharm. 2001, 58 (Suppl. 2), S14–S17. [Google Scholar] [CrossRef]
- Büller, H.R.; Davidson, B.L.; Decousus, H.; Gallus, A.; Gent, M.; Piovella, F.; Prins, M.H.; Raskob, G.; van den Berg-Segers, A.E.M.; Cariou, R.; et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N. Engl. J. Med. 2003, 349, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Donat, F.; Duret, J.P.; Santoni, A.; Cariou, R.; Necciari, J.; Magnani, H.; de Greef, R. The pharmacokinetics of fondaparinux sodium in healthy volunteers. Clin. Pharmacokinet. 2002, 41 (Suppl. 2), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Pai, M.; Linkins, L.-A. Direct oral anticoagulants for treatment of HIT: Update of Hamilton experience and literature review. Blood 2017, 130, 1104–1113. [Google Scholar] [CrossRef]
- Kahn, S.R.; Shapiro, S.; Wells, P.S.; Rodger, M.A.; Kovacs, M.J.; Anderson, D.R.; Tagalakis, V.; Houweling, A.H.; Ducruet, T.; Holcroft, C.; et al. Compression stockings to prevent post-thrombotic syndrome: A randomised placebo-controlled trial. Lancet 2014, 383, 880–888. [Google Scholar] [CrossRef]
- Kahn, S.R.; Ginsberg, J.S. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch. Intern. Med. 2004, 164, 17–26. [Google Scholar] [CrossRef]
- Prandoni, P.; Frulla, M.; Sartor, D.; Concolato, A.; Girolami, A. Vein abnormalities and the post-thrombotic syndrome. J. Thromb. Haemost. 2005, 3, 401–402. [Google Scholar] [CrossRef]
- Kahn, S.R.; Partsch, H.; Vedantham, S.; Prandoni, P.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: A recommendation for standardization. J. Thromb. Haemost. 2009, 7, 879–883. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.A.; Prins, M.H.; Bernardi, E.; Marchiori, A.; Bagatella, P.; Frulla, M.; Mosena, L.; Tormene, D.; Piccioli, A.; et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann. Intern. Med. 2002, 137, 955–960. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.A.; Prins, M.H.; Frulla, M.; Marchiori, A.; Bernardi, E.; Tormene, D.; Mosena, L.; Pagnan, A.; Girolami, A. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: A randomized, controlled trial. Ann. Intern. Med. 2004, 141, 249–256. [Google Scholar] [CrossRef]
- Tan, M.; Mos, I.C.M.; Klok, F.A.; Huisman, M.V. Residual venous thrombosis as predictive factor for recurrent venous thromboembolim in patients with proximal deep vein thrombosis: A sytematic review. Br. J. Haematol. 2011, 153, 168–178. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Schellong, S.; Eriksson, H.; Baanstra, D.; Kvamme, A.M.; Friedman, J.; Mismetti, P.; Goldhaber, S.Z.; et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N. Engl. J. Med. 2013, 368, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Kahn, S.R.; Agnelli, G.; Goldhaber, S.; Raskob, G.E.; Comerota, A.J.; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 454S–545S. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014, 123, 1794–1801. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Buring, J.E.; Lipnick, R.J.; Hennekens, C.H. Pooled analyses of randomized trials of streptokinase and heparin in phlebographically documented acute deep venous thrombosis. Am. J. Med. 1984, 76, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Collen, D.; Lijnen, H.R. Tissue-type plasminogen activator: A historical perspective and personal account. J. Thromb. Haemost. 2004, 2, 541–546. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Meyerovitz, M.F.; Green, D.; Vogelzang, R.L.; Citrin, P.; Heit, J.; Sobel, M.; Wheeler, H.B.; Plante, D.; Kim, H. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am. J. Med. 1990, 88, 235–240. [Google Scholar] [CrossRef]
- Arnesen, H.; Høiseth, A.; Ly, B. Streptokinase of heparin in the treatment of deep vein thrombosis. Follow-up results of a prospective study. Acta Med. Scand. 1982, 211, 65–68. [Google Scholar] [CrossRef]
- Comerota, A.J.; Throm, R.C.; Mathias, S.D.; Haughton, S.; Mewissen, M. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J. Vasc. Surg. 2000, 32, 130–137. [Google Scholar] [CrossRef]
- Mewissen, M.W.; Seabrook, G.R.; Meissner, M.H.; Cynamon, J.; Labropoulos, N.; Haughton, S.H. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: Report of a national multicenter registry. Radiology 1999, 211, 39–49. [Google Scholar] [CrossRef]
- Brandjes, D.P.; Büller, H.R.; Heijboer, H.; Huisman, M.V.; de Rijk, M.; Jagt, H.; ten Cate, J.W. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997, 349, 759–762. [Google Scholar] [CrossRef]
- Watson, L.; Broderick, C.; Armon, M.P. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst. Rev. 2016, 11, CD002783. [Google Scholar] [CrossRef]
- PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: The PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 2005, 112, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Mismetti, P.; Laporte, S.; Pellerin, O.; Ennezat, P.-V.; Couturaud, F.; Elias, A.; Falvo, N.; Meneveau, N.; Quere, I.; Roy, P.-M.; et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: A randomized clinical trial. JAMA 2015, 313, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, S.; Vesely, T.M.; Sicard, G.A.; Brown, D.; Rubin, B.; Sanchez, L.A.; Parti, N.; Picus, D. Pharmacomechanical thrombolysis and early stent placement for iliofemoral deep vein thrombosis. J. Vasc. Interv. Radiol. 2004, 15, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Haig, Y.; Enden, T.; Grøtta, O.; Kløw, N.-E.; Slagsvold, C.-E.; Ghanima, W.; Sandvik, L.; Hafsahl, G.; Holme, P.A.; Holmen, L.O.; et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016, 3, e64–e71. [Google Scholar] [CrossRef]
- Comerota, A.J.; Kearon, C.; Gu, C.-S.; Julian, J.A.; Goldhaber, S.Z.; Kahn, S.R.; Jaff, M.R.; Razavi, M.K.; Kindzelski, A.L.; Bashir, R.; et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation 2019, 139, 1162–1173. [Google Scholar] [CrossRef]
- Notten, P.; Ten Cate-Hoek, A.J.; Arnoldussen, C.W.K.P.; Strijkers, R.H.W.; de Smet, A.A.E.A.; Tick, L.W.; van de Poel, M.H.W.; Wikkeling, O.R.M.; Vleming, L.-J.; Koster, A.; et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): A single-blind, multicentre, randomised trial. Lancet Haematol. 2020, 7, e40–e49. [Google Scholar] [CrossRef]
- Sharifi, M.; Bay, C.; Mehdipour, M.; Sharifi, J.; TORPEDO Investigators. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: Midterm results. J. Endovasc. Ther. 2012, 19, 273–280. [Google Scholar] [CrossRef]
- Bashir, R.; Zack, C.J.; Zhao, H.; Comerota, A.J.; Bove, A.A. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern. Med. 2014, 174, 1494–1501. [Google Scholar] [CrossRef]
- Kim, H.S.; Preece, S.R.; Black, J.H.; Pham, L.D.; Streiff, M.B. Safety of catheter-directed thrombolysis for deep venous thrombosis in cancer patients. J. Vasc. Surg. 2008, 47, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.S.; Konig, G.; Leers, S.A.; Cho, J.; Rhee, R.Y.; Makaroun, M.S.; Chaer, R.A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis: An alternative in patients with contraindications to thrombolysis. J. Vasc. Surg. 2009, 50, 1092–1098. [Google Scholar] [CrossRef]
- Dumantepe, M.; Tarhan, I.A.; Ozler, A. Treatment of chronic deep vein thrombosis using ultrasound accelerated catheter-directed thrombolysis. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Engelberger, R.P.; Spirk, D.; Willenberg, T.; Alatri, A.; Do, D.-D.; Baumgartner, I.; Kucher, N. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ. Cardiovasc. Interv. 2015, 8, e002027. [Google Scholar] [CrossRef]
- Sista, A.K.; Vedantham, S.; Kaufman, J.A.; Madoff, D.C. Endovascular interventions for acute and chronic lower extremity deep venous disease: State of the art. Radiology 2015, 276, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, S.; Thorpe, P.E.; Cardella, J.F.; Grassi, C.J.; Patel, N.H.; Ferral, H.; Hofmann, L.V.; Janne d’Othée, B.M.; Antonaci, V.P.; Brountzos, E.N.; et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J. Vasc. Interv. Radiol. 2006, 17, 435–447; quiz 448. [Google Scholar] [CrossRef]
- Grommes, J.; Strijkers, R.; Greiner, A.; Mahnken, A.H.; Wittens, C.H.A. Safety and feasibility of ultrasound-accelerated catheter-directed thrombolysis in deep vein thrombosis. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 526–532. [Google Scholar] [CrossRef]
- Cynamon, J.; Stein, E.G.; Dym, R.J.; Jagust, M.B.; Binkert, C.A.; Baum, R.A. A new method for aggressive management of deep vein thrombosis: Retrospective study of the power pulse technique. J. Vasc. Interv. Radiol. 2006, 17, 1043–1049. [Google Scholar] [CrossRef]
- Parikh, S.; Motarjeme, A.; McNamara, T.; Raabe, R.; Hagspiel, K.; Benenati, J.F.; Sterling, K.; Comerota, A. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: Initial clinical experience. J. Vasc. Interv. Radiol. 2008, 19, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, S.; Vesely, T.M.; Parti, N.; Darcy, M.; Hovsepian, D.M.; Picus, D. Lower extremity venous thrombolysis with adjunctive mechanical thrombectomy. J. Vasc. Interv. Radiol. 2002, 13, 1001–1008. [Google Scholar] [CrossRef]
- Comerota, A.J.; Grewal, N.; Martinez, J.T.; Chen, J.T.; Disalle, R.; Andrews, L.; Sepanski, D.; Assi, Z. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J. Vasc. Surg. 2012, 55, 768–773. [Google Scholar] [CrossRef]
- Neglén, P.; Hollis, K.C.; Olivier, J.; Raju, S. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J. Vasc. Surg. 2007, 46, 979–990. [Google Scholar] [CrossRef]
- Garcia, M.J.; Lookstein, R.; Malhotra, R.; Amin, A.; Blitz, L.R.; Leung, D.A.; Simoni, E.J.; Soukas, P.A. Endovascular Management of Deep Vein Thrombosis with Rheolytic Thrombectomy: Final Report of the Prospective Multicenter PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) Registry. J. Vasc. Interv. Radiol. 2015, 26, 777–785; quiz 786. [Google Scholar] [CrossRef]
- Lin, P.H.; Zhou, W.; Dardik, A.; Mussa, F.; Kougias, P.; Hedayati, N.; Naoum, J.J.; El Sayed, H.; Peden, E.K.; Huynh, T.T. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am. J. Surg. 2006, 192, 782–788. [Google Scholar] [CrossRef]
- Shi, H.-J.; Huang, Y.-H.; Shen, T.; Xu, Q. Percutaneous mechanical thrombectomy combined with catheter-directed thrombolysis in the treatment of symptomatic lower extremity deep venous thrombosis. Eur. J. Radiol. 2009, 71, 350–355. [Google Scholar] [CrossRef]
- Abramowitz, S. Effective Removal of Chronic Thrombus with the ClotTriever System: Results from the CLOUT Registry. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 544–545. [Google Scholar] [CrossRef]
- Tu, T.; Toma, C.; Tapson, V.F.; Adams, C.; Jaber, W.A.; Silver, M.; Khandhar, S.; Amin, R.; Weinberg, M.; Engelhardt, T.; et al. A Prospective, Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: The FLARE Study. JACC Cardiovasc. Interv. 2019, 12, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Gould, M.K.; Louie, J.D.; Rosenberg, J.K.; Sze, D.Y.; Hofmann, L.V. Catheter-directed therapy for the treatment of massive pulmonary embolism: Systematic review and meta-analysis of modern techniques. J. Vasc. Interv. Radiol. 2009, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, G.J.; Lohan, D.G.; Gough, N.; Cronin, C.G.; Kee, S.T. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J. Vasc. Interv. Radiol. 2007, 18, 715–724. [Google Scholar] [CrossRef]
- Hilleman, D.E.; Razavi, M.K. Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J. Vasc. Interv. Radiol. 2008, 19, 377–383. [Google Scholar] [CrossRef]
- Raju, S.; Neglen, P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: A permissive role in pathogenicity. J. Vasc. Surg. 2006, 44, 136–143; discussion 144. [Google Scholar] [CrossRef]
- Mousa, A.Y.; AbuRahma, A.F. May-Thurner syndrome: Update and review. Ann. Vasc. Surg. 2013, 27, 984–995. [Google Scholar] [CrossRef]
- Raju, S.; Darcey, R.; Neglén, P. Unexpected major role for venous stenting in deep reflux disease. J. Vasc. Surg. 2010, 51, 401–408; discussion 408. [Google Scholar] [CrossRef]
- Razavi, M.K.; Jaff, M.R.; Miller, L.E. Safety and Effectiveness of Stent Placement for Iliofemoral Venous Outflow Obstruction: Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2015, 8, e002772. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Ward, M.; Kirk, O. A modification of iliac vein stent technique. Ann. Vasc. Surg. 2014, 28, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Hager, E.S.; Naddaf, A.; Dillavou, E.; Singh, M.; Chaer, R.A. Outcomes and predictors of failure of thrombolysis for iliofemoral deep venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Marston, W.; Black, S.; Bentley, D.; Neglén, P. The initial report on 1-year outcomes of the feasibility study of the VENITI VICI VENOUS STENT in symptomatic iliofemoral venous obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 192–200. [Google Scholar] [CrossRef]
- Enden, T.; Resch, S.; White, C.; Wik, H.S.; Kløw, N.E.; Sandset, P.M. Cost-effectiveness of additional catheter-directed thrombolysis for deep vein thrombosis. J. Thromb. Haemost. 2013, 11, 1032–1042. [Google Scholar] [CrossRef]
- Magnuson, E.A.; Chinnakondepalli, K.; Vilain, K.; Kearon, C.; Julian, J.A.; Kahn, S.R.; Goldhaber, S.Z.; Jaff, M.R.; Kindzelski, A.L.; Herman, K.; et al. Cost-Effectiveness of Pharmacomechanical Catheter-Directed Thrombolysis Versus Standard Anticoagulation in Patients with Proximal Deep Vein Thrombosis: Results From the ATTRACT Trial. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005659. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacała, A.; Dorochowicz, M.; Fischer, J.; Korbecki, A.; Truszyński, A.; Madura, A.; Dyś, K.; Szuba, A.; Guziński, M. Interventional Procedures in Deep Venous Thrombosis Treatment: A Review of Techniques, Outcomes, and Patient Selection. Medicina 2025, 61, 1476. https://doi.org/10.3390/medicina61081476
Kacała A, Dorochowicz M, Fischer J, Korbecki A, Truszyński A, Madura A, Dyś K, Szuba A, Guziński M. Interventional Procedures in Deep Venous Thrombosis Treatment: A Review of Techniques, Outcomes, and Patient Selection. Medicina. 2025; 61(8):1476. https://doi.org/10.3390/medicina61081476
Chicago/Turabian StyleKacała, Arkadiusz, Mateusz Dorochowicz, Jędrzej Fischer, Adrian Korbecki, Aleksander Truszyński, Anna Madura, Krzysztof Dyś, Andrzej Szuba, and Maciej Guziński. 2025. "Interventional Procedures in Deep Venous Thrombosis Treatment: A Review of Techniques, Outcomes, and Patient Selection" Medicina 61, no. 8: 1476. https://doi.org/10.3390/medicina61081476
APA StyleKacała, A., Dorochowicz, M., Fischer, J., Korbecki, A., Truszyński, A., Madura, A., Dyś, K., Szuba, A., & Guziński, M. (2025). Interventional Procedures in Deep Venous Thrombosis Treatment: A Review of Techniques, Outcomes, and Patient Selection. Medicina, 61(8), 1476. https://doi.org/10.3390/medicina61081476





