Glycated Hemoglobin as a Predictor of Postoperative Delirium in Diabetic Patients Undergoing Noncardiac Surgery: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
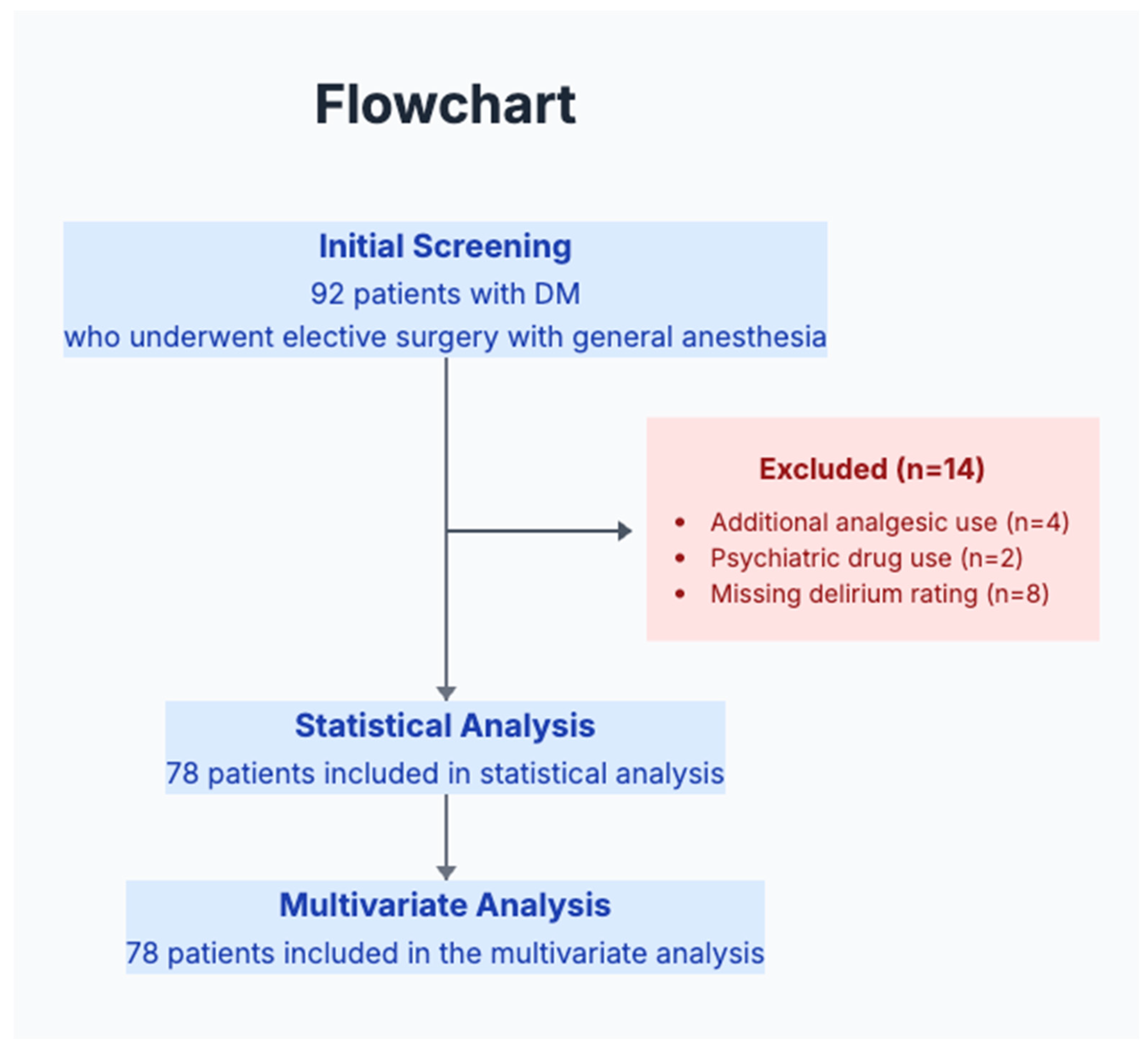
2.2. Study Population and Sample Size
2.3. Inclusion and Exclusion Criteria
2.4. Gathering Information
2.5. Assessment of the Outcomes
2.6. Pilot-Study-Specific Assessments
2.7. Comorbidity Assessment
2.8. Statistical Analysis
3. Results
3.1. Feasibility Outcomes
3.2. Patient Characteristics and Incidence of POD
3.3. Preliminary Clinical Associations
3.4. Post Hoc Power Analysis and Sample Size Calculations for Future Studies
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BBB | Blood–Brain Barrier |
| CAM-ICU | Confusion Assessment Method for the Intensive Care Unit |
| CCI | Charlson Comorbidity Index |
| DM | Diabetes Mellitus |
| ESAIC | European Society of Anaesthesiology and Intensive Care |
| GV | Glycemic Variability |
| HbA1c | Glycated Hemoglobin |
| IQR | Interquartile Range |
| JBDS | Joint British Diabetes Societies |
| OR | Odds Ratio |
| POD | Postoperative Delirium |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| STS | Society of Thoracic Surgeons |
References
- Mossie, A.; Regasa, T.; Neme, D.; Awoke, Z.; Zemedkun, A.; Hailu, S. Evidence-Based Guideline on Management of Postoperative Delirium in Older People for Low Resource Setting: Systematic Review Article. Int. J. Gen. Med. 2022, 15, 4053–4065. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Marcantonio, E.R. Review articles: Postoperative delirium: Acute change with long-term implications. Anesth. Analg. 2011, 112, 1202–1211. [Google Scholar] [CrossRef]
- Aldecoa, C.; Bettelli, G.; Bilotta, F.; Sanders, R.D.; Audisio, R.; Borozdina, A.; Cherubini, A.; Jones, C.; Kehlet, H.; MacLullich, A.; et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur. J. Anaesthesiol. 2017, 34, 192–214. [Google Scholar] [CrossRef]
- Chen, H.; Mo, L.; Hu, H.; Ou, Y.; Luo, J. Risk factors of postoperative delirium after cardiac surgery: A meta-analysis. J. Cardiothorac. Surg. 2021, 16, 113. [Google Scholar] [CrossRef]
- Chu, Z.; Wu, Y.; Dai, X.; Zhang, C.; He, Q. The risk factors of postoperative delirium in general anesthesia patients with hip fracture: Attention needed. Medicine 2021, 100, e26156. [Google Scholar] [CrossRef]
- Ntalouka, M.P.; Arnaoutoglou, E.; Vrakas, S.; Staikou, C.; Angelis, F.A.; Papadopoulos, G.; Tzimas, P. The effect of type 2 diabetes mellitus on perioperative neurocognitive disorders in patients undergoing elective noncardiac surgery under general anesthesia. A prospective cohort study. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 252–262. [Google Scholar] [CrossRef]
- Whitlock, E.L.; Vannucci, A.; Avidan, M.S. Postoperative delirium. Minerva Anestesiol. 2011, 77, 448–456. [Google Scholar] [PubMed]
- Fenta, E.; Teshome, D.; Kibret, S.; Hunie, M.; Tiruneh, A.; Belete, A.; Molla, A.; Dessie, B.; Geta, K. Incidence and risk factors of postoperative delirium in elderly surgical patients 2023. Sci. Rep. 2025, 15, 1400. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines Approved by the Guidelines Review Committee. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; World Health Organization Copyright ©; World Health Organization: Geneva, Switzerland, 2011.
- Kotfis, K.; Szylińska, A.; Listewnik, M.; Brykczyński, M.; Ely, E.W.; Rotter, I. Diabetes and elevated preoperative HbA1c level as risk factors for postoperative delirium after cardiac surgery: An observational cohort study. Neuropsychiatr. Dis. Treat. 2019, 15, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, K.; Szylińska, A.; Listewnik, M.; Drożdżal, S.; Tomska, N.; Rotter, I.; Kotfis, K. Cardiac Delirium Index for Predicting the Occurrence of Postoperative Delirium in Adult Patients After Coronary Artery Bypass Grafting. Clin. Interv. Aging 2021, 16, 487–495. [Google Scholar] [CrossRef] [PubMed]
- WHO. Handbook for Good Clinical Research Practice (GCP): Guidance for Implementation; World Health Organization: Geneva, Switzerland, 2005. Available online: https://iris.who.int/handle/10665/43392 (accessed on 13 August 2025).
- Ely, E.W.; Margolin, R.; Francis, J.; May, L.; Truman, B.; Dittus, R.; Speroff, T.; Gautam, S.; Bernard, G.R.; Inouye, S.K. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit. Care Med. 2001, 29, 1370–1379. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Copeland, G.P.; Jones, D.; Walters, M. POSSUM: A scoring system for surgical audit. Br. J. Surg. 1991, 78, 355–360. [Google Scholar] [CrossRef]
- Blakoe, M.; Olsen, D.B.; Noergaard, M.W. Preoperative prediction models for postoperative delirium in cardiac surgery patients—A scoping review. Contemp. Nurse 2025, 61, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, X.; Jin, X.; Lv, M.; Li, X.; Dou, J.; Zeng, J.; An, P.; Chen, Y.; Chen, K.; et al. Effects of Preoperative HbA1c Levels on the Postoperative Outcomes of Coronary Artery Disease Surgical Treatment in Patients with Diabetes Mellitus and Nondiabetic Patients: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2020, 2020, 3547491. [Google Scholar] [CrossRef]
- Park, S.J.; Oh, A.R.; Lee, J.H.; Yang, K.; Park, J. Association of preoperative blood glucose level with delirium after non-cardiac surgery in diabetic patients. Korean J. Anesthesiol. 2024, 77, 226–235. [Google Scholar] [CrossRef]
- Windmann, V.; Spies, C.; Knaak, C.; Wollersheim, T.; Piper, S.K.; Vorderwülbecke, G.; Kurpanik, M.; Kuenz, S.; Lachmann, G. Intraoperative hyperglycemia increases the incidence of postoperative delirium. Minerva Anestesiol. 2019, 85, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Chew, S.; Seet, E.; Wong, W.Y.; Lim, V.; Chua, N.; Zhang, J.; Lim, B.; Chua, V.; Loh, N.H.W.; et al. Incidence and risk factors of delirium in post-anaesthesia care unit. Ann. Acad. Med. Singap. 2022, 51, 87–95. [Google Scholar] [CrossRef]
- Oh, A.R.; Lee, D.Y.; Lee, S.; Lee, J.H.; Yang, K.; Choi, B.; Park, J. Association between Preoperative Glucose Dysregulation and Delirium after Non-Cardiac Surgery. J. Clin. Med. 2024, 13, 932. [Google Scholar] [CrossRef]
- Choi, H.; Park, C.S.; Huh, J.; Koo, J.; Jeon, J.; Kim, E.; Jung, S.; Kim, H.W.; Lim, J.Y.; Hwang, W. Intraoperative Glycemic Variability and Mean Glucose are Predictors for Postoperative Delirium After Cardiac Surgery: A Retrospective Cohort Study. Clin. Interv. Aging 2022, 17, 79–95. [Google Scholar] [CrossRef]
- Martocchia, A.; Scarienzi, M.; Prunas, P.; Bentivegna, E.; Cacciafesta, M.; Martelletti, P.; Sesti, G. The effects of the glycaemic control on the severity of the delirium in the advanced phase of Alzheimer’s disease. F1000Research 2020, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.T.; Chen, T.J.; Shah, N.M.; Agrest, B.; Grotticelli, J. Anesthesia-mediated neuroinflammatory sequelae in post operative cognitive dysfunction: Mechanisms and therapeutic implications. Front. Anesthesiol. 2024, 3, 1281034. [Google Scholar] [CrossRef]
- Alam, A.; Hana, Z.; Jin, Z.; Suen, K.C.; Ma, D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018, 37, 547–556. [Google Scholar] [CrossRef]
- Ravi, B.; Pincus, D.; Choi, S.; Jenkinson, R.; Wasserstein, D.N.; Redelmeier, D.A. Association of Duration of Surgery with Postoperative Delirium Among Patients Receiving Hip Fracture Repair. JAMA Netw. Open 2019, 2, e190111. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Xu, F.; Ding, Y.; Zhao, S.; Chen, X. The effect of anesthetic depth on postoperative delirium in older adults: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 719. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Feng, X.; Liu, H.; Shan, X.; Ji, F.; Peng, K. Effects of anesthetic depth on postoperative pain and delirium: A meta-analysis of randomized controlled trials with trial sequential analysis. Chin. Med. J. 2022, 135, 2805–2814. [Google Scholar] [CrossRef]
- Pérez-Otal, B.; Aragón-Benedí, C.; Pascual-Bellosta, A.; Ortega-Lucea, S.; Martínez-Ubieto, J.; Ramírez-Rodríguez, J.M. Neuromonitoring depth of anesthesia and its association with postoperative delirium. Sci. Rep. 2022, 12, 12703. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Terrando, N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth. Analg. 2019, 128, 781–788. [Google Scholar] [CrossRef]
- Anckarsäter, R.; Zetterberg, H.; Månsson, J.E.; Blennow, K.; Anckarsäter, H. Non-neurological surgery results in a neurochemical stress response. J. Neural Transm. 2008, 115, 397–399. [Google Scholar] [CrossRef]
- Koch, S.; Blankertz, B.; Windmann, V.; Spies, C.; Radtke, F.M.; Röhr, V. Desflurane is risk factor for postoperative delirium in older patients’ independent from intraoperative burst suppression duration. Front. Aging Neurosci. 2023, 15, 1067268. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, C.H.; LaHue, S.C.; Oldham, M.A.; Josephson, S.A.; Whitaker, E.; Douglas, V.C. Predisposing and Precipitating Factors Associated with Delirium: A Systematic Review. JAMA Netw. Open 2023, 6, e2249950. [Google Scholar] [CrossRef]
- An, Z.; Xiao, L.; Chen, C.; Wu, L.; Wei, H.; Zhang, X.; Dong, L. Analysis of risk factors for postoperative delirium in middle-aged and elderly fracture patients in the perioperative period. Sci. Rep. 2023, 13, 13019. [Google Scholar] [CrossRef]
- Komici, K.; Fantini, C.; Santulli, G.; Bencivenga, L.; Femminella, G.D.; Guerra, G.; Mone, P.; Rengo, G. The role of diabetes mellitus on delirium onset: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2025, 24, 216. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, C.; Bettelli, G.; Bilotta, F.; Sanders, R.D.; Aceto, P.; Audisio, R.; Cherubini, A.; Cunningham, C.; Dabrowski, W.; Forookhi, A.; et al. Update of the European Society of Anaesthesiology and Intensive Care Medicine evidence-based and consensus-based guideline on postoperative delirium in adult patients. Eur. J. Anaesthesiol. 2024, 41, 81–108. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.; Viola, L.; Navaratnarajah, M.; Thirukumaran, D.; Velissaris, T. Glycated Haemoglobin (HbA(1C)) in Cardiac Surgery: A Narrative Review. J. Clin. Med. 2024, 14, 23. [Google Scholar] [CrossRef]
- Wong, J.K.L.; Ke, Y.; Ong, Y.J.; Li, H.; Wong, T.H.; Abdullah, H.R. The impact of preoperative glycated hemoglobin (HbA1c) on postoperative complications after elective major abdominal surgery: A meta-analysis. Korean J. Anesthesiol. 2022, 75, 47–60. [Google Scholar] [CrossRef]
| POD (−) (n = 71) | POD (+) (n = 7) | Total | p-Value | p-Value § | |
|---|---|---|---|---|---|
| Anesthesia duration (min) | 110 (70–150) | 225 (162–255) | 135.5 ± 82.2 | 0.024 | 0.040 |
| Surgery duration (min) | 100 (65–140) | 210 (150–240) | 124.7 ± 80.7 | 0.026 | 0.044 |
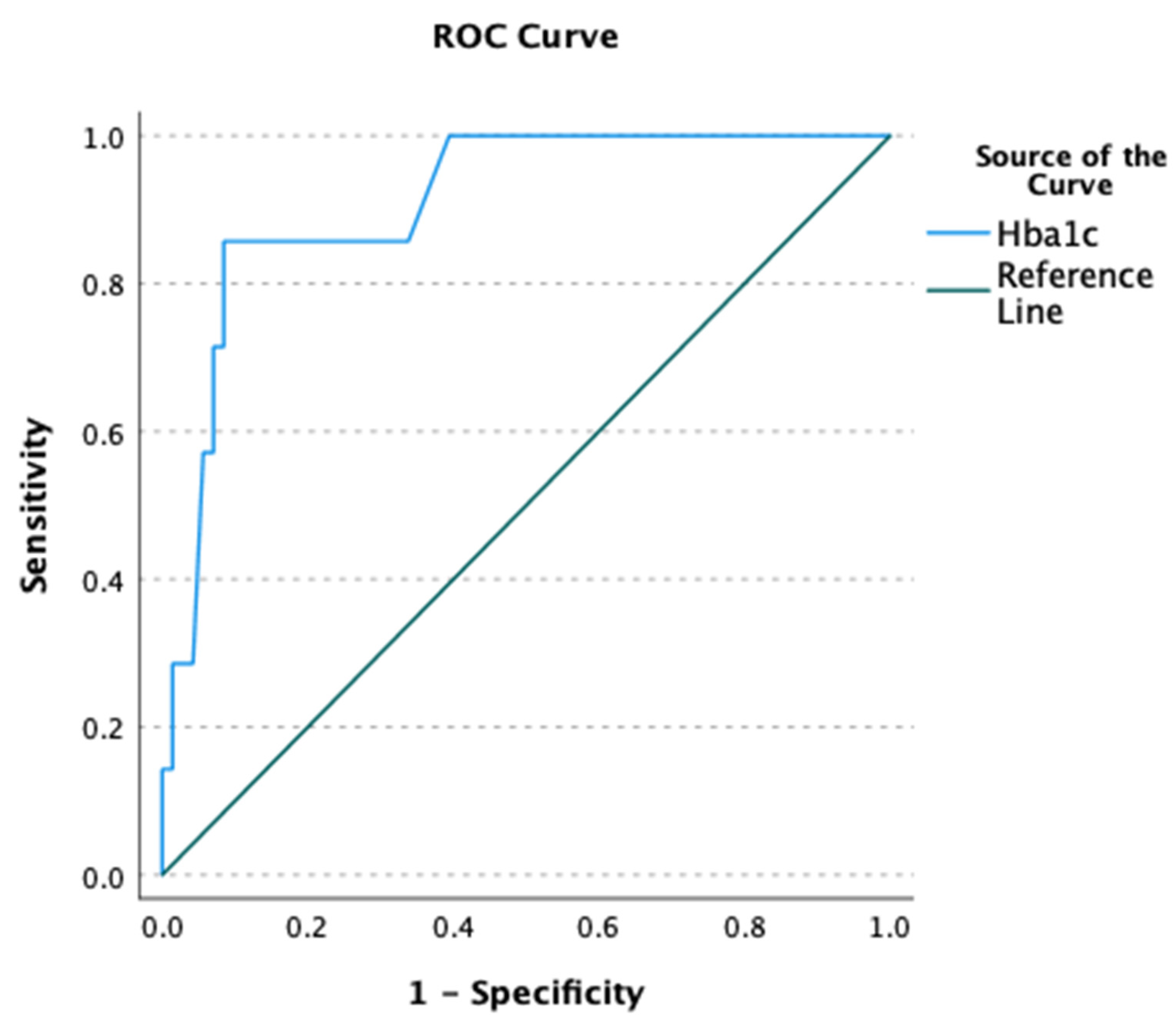
| HbA1c (%) | 6.6 ± 1.1 | 9.0 ± 2.2 | 6.8 ± 1.4 | 0.027 | 0.005 |
| <7% | 53 (74.6) | 1 (14.3) | 54 (69.2) | ||
| ≥7% | 18 (25.4) | 6 (85.7) | 24 (30.8) | 0.003 | 0.003 |
| Fasting blood glucose (mg/dL) | 128 (111–158) | 194 (182–251) | 142.8 ± 45.9 | 0.008 | 0.005 |
| Number of blood transfusions | 0 (0–0) | 0 (0–1) | 1.9 ± 0.7 | 0.069 | 0.228 |
| Hb (g/dL) | 12.6 ± 2.0 | 11.5 ± 1.5 | 12.5 ± 2.0 | 0.170 | 0.157 |
| Hematocrit (%) | 37.4 ± 5.7 | 33.5 ± 4.1 | 37.0 ± 5.7 | 0.087 | 0.095 |
| BUN (mg/dL) | 14.9 (12.6–19.0) | 19.0 (12.5–27.7) | 17.8 ± 12.5 | 0.441 | 0.243 |
| Creatine (mg/dL) | 0.8 (0.7–1.1) | 1.2 (0.6–1.3) | 1.1 ± 1.4 | 0.358 | 0.657 |
| AST (U/L) | 19.9 ± 5.4 | 19.0 ± 6.0 | 19.8 ± 5.5 | 0.689 | 0.684 |
| ALT (U/L) | 20.1 ± 7.9 | 19.0 (15.0–32.0) | 20.8 ± 7.9 | 0.649 | 0.625 |
| Sodium (mmol/L) | 138.9 ± 2.8 | 140 ± 2.9 | 139.0 ± 2.8 | 0.555 | 0.314 |
| Potassium (mmol/L) | 4.4 (4.2–4.7) | 4.2 (4.1–4.9) | 4.4 ± 0.5 | 0.827 | 0.970 |
| Age (yr) | 57.8 ± 10.0 | 59.4 ± 9.9 | 57.9 ± 9.9 | 0.677 | 0.673 |
| CCI | 3 (2–4) | 4 (2–4) | 3 (2–4) | 0.247 | 0.213 |
| BMI (kg/m2) | 29.3 ± 5.0 | ||||
| Normal | 11 (15.5) | 4 (57.1) | 15 (19.2) | 0.058 | |
| Overweight | 28 (39.4) | 2 (28.6) | 30 (38.5) | 0.082 | |
| Obese | 32 (45.1) | 1 (14.3) | 33 (42.3) | 0.052 | 0.036 |
| Gender | |||||
| Female | 44 (62.0) | 2 (28.6) | 46 (59.0) | 0.116 | |
| Male | 27 (38.0) | 5 (71.4) | 32 (41.0) | 0.107 | |
| Alcohol use | |||||
| Yes | 2 (2.8) | 1 (14.3) | 3 (3.8) | 0.249 | 0.177 |
| No | 69 (97.2) | 6 (85.7) | 75 (96.2) | ||
| Smoking | |||||
| Yes | 22 (31.0) | 1 (14.3) | 23 (29.5) | 0.667 | 0.372 |
| No | 49 (69.0) | 6 (85.7) | 55 (70.5) | ||
| Antidiabetic treatment | |||||
| Insulin | 13 (18.3) | 3 (42.9) | 16 (20.5) | 0.345 | |
| Oral antidiabetic | 53 (74.6) | 1 (14.3) | 54 (69.2) | 0.002 | 0.014 |
| Both | 4 (5.6) | 2 (28.6) | 6 (7.7) | 0.676 | |
| None | 1 (1.4) | 1 (14.3) | 2 (2.6) | 0.047 | |
| Antidiabetic medication use | |||||
| Yes | 41 (57.7) | 5 (71.4) | 46 (59.0) | 0.694 | 0.488 |
| No | 30 (42.3) | 2 (28.6) | 32 (41.0) | ||
| Additional comorbidity | |||||
| None | 25 (35.2) | 2 (28.6) | 27 (34.6) | 0.561 | |
| Comorbidity | 29 (40.8) | 2 (28.6) | 31 (39.7) | 0.886 | |
| Multiple comorbidities | 17 (23.9) | 3 (42.9) | 20 (25.6) | 0.606 | 0.413 |
| ASA | |||||
| Score 2 | 65 (91.5) | 5 (71.4) | 70 (89.7) | ||
| Score 3 | 6 (8.5) | 2 (28.6) | 8 (10.3) | 0.149 | 0.118 |
| Surgical severity | |||||
| Minor | 26 (36.6) | 1 (14.3) | 27 (34.6) | 0.243 | |
| Moderate | 33 (46.5) | 3 (42.9) | 36 (46.2) | 0.468 | |
| Major | 8 (11.3) | 1 (14.3) | 9 (11.5) | 0.423 | |
| Major+ | 4 (5.6) | 2 (28.6) | 6 (7.7) | 0.147 | 0.055 |
| Postoperative analgesics | |||||
| Tramadol | 32 (45.1) | 1 (14.3) | 33 (42.3) | 0.127 | |
| Tramadol + paracetamol | 18 (25.4) | 1 (14.3) | 19 (24.4) | 0.690 | |
| Morphine | 21 (29.6) | 5 (71.4) | 26 (33.3) | 0.114 | 0.073 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | OR | CI %95 | p-Value | Adjusted OR | CI %95 | p-Value |
| HbA1c (%) | 2.19 | 1.27–3.78 | 0.005 | 2.96 | 1.34–6.52 | 0.007 |
| Blood glucose (g/dL) | 1.02 | 1.01–1.04 | 0.005 | 1.04 | 1.01–1.07 | 0.013 |
| Anesthesia duration (min) | 1.01 | 1.00–1.02 | 0.039 | 1.02 | 1.00–1.04 | 0.019 |
| Surgery duration (min) | 1.01 | 1.00–1.02 | 0.043 | - | - | - |
| Oral antidiabetic drugs | 0.038 | 0.003–0.511 | 0.014 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahceci, M.; Gulec, E.; Turktan, M.; Hatipoglu, Z.; Ozcengiz, D. Glycated Hemoglobin as a Predictor of Postoperative Delirium in Diabetic Patients Undergoing Noncardiac Surgery: A Retrospective Study. Medicina 2025, 61, 1474. https://doi.org/10.3390/medicina61081474
Bahceci M, Gulec E, Turktan M, Hatipoglu Z, Ozcengiz D. Glycated Hemoglobin as a Predictor of Postoperative Delirium in Diabetic Patients Undergoing Noncardiac Surgery: A Retrospective Study. Medicina. 2025; 61(8):1474. https://doi.org/10.3390/medicina61081474
Chicago/Turabian StyleBahceci, Mahir, Ersel Gulec, Mediha Turktan, Zehra Hatipoglu, and Dilek Ozcengiz. 2025. "Glycated Hemoglobin as a Predictor of Postoperative Delirium in Diabetic Patients Undergoing Noncardiac Surgery: A Retrospective Study" Medicina 61, no. 8: 1474. https://doi.org/10.3390/medicina61081474
APA StyleBahceci, M., Gulec, E., Turktan, M., Hatipoglu, Z., & Ozcengiz, D. (2025). Glycated Hemoglobin as a Predictor of Postoperative Delirium in Diabetic Patients Undergoing Noncardiac Surgery: A Retrospective Study. Medicina, 61(8), 1474. https://doi.org/10.3390/medicina61081474






