Prognostic Significance of aVR Lead and QTc Prolongation in Patients with Early Repolarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Inclusion and Exclusion Criteria
2.4. Definitions
2.5. Design of the Laboratory
2.6. Using ECG and QT Interval Computation
2.7. Ethical Considerations
2.8. Statistical Analysis
2.8.1. Power Analysis
2.8.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juang, J.J.; Huang, Y.J.; Chang, I.S.; Chen, C.J.; Wu, I.C.; Hsu, C.C.; Chen, T.Y.; Tseng, W.T.; Yeh, S.S.; Hsiung, C.A. Role of electrocardiographic early repolarization pattern in long-term outcomes of a community-based middle-aged and geriatric ambulatory population: A prospective cohort study. Aging 2020, 12, 26140–26187. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation 2009, 119, e241–e250. [Google Scholar] [PubMed]
- Hemmati, R.; Mohsenzadeh, Y.; Salaminia, S.; Sayehmiri, F. Early repolarization and the severity of coronary artery disease: A case-control study. Caspian. J. Intern. Med. 2021, 12, 526–532. [Google Scholar]
- Haïssaguerre, M.; Derval, N.; Sacher, F.; Jesel, L.; Deisenhofer, I.; de Roy, L.; Pasquié, J.L.; Nogami, A.; Babuty, D.; Yli-Mayry, S.; et al. Sudden cardiac arrest associated with early repolarization. N. Engl. J. Med. 2008, 358, 2016–2023. [Google Scholar] [CrossRef]
- Sinner, M.F.; Reinhard, W.; Müller, M.; Beckmann, B.M.; Martens, E.; Perz, S.; Pfeufer, A.; Winogradow, J.; Stark, K.; Meisinger, C.; et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: A population-based prospective cohort study (MONICA/KORA). PLoS Med. 2010, 7, e1000314. [Google Scholar] [CrossRef]
- Mizusawa, Y.; Bezzina, C.R. Early repolarization pattern: Its ECG characteristics, arrhythmogeneity and heritability. J. Interv. Card. Electrophysiol. 2014, 39, 185–192. [Google Scholar] [CrossRef]
- Tikkanen, J.T.; Junttila, M.J.; Anttonen, O.; Aro, A.L.; Luttinen, S.; Kerola, T.; Sager, S.J.; Rissanen, H.A.; Myerburg, R.J.; Reunanen, A.; et al. Early repolarization: Electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 2011, 123, 2666–2673. [Google Scholar] [CrossRef]
- Uberoi, A.; Jain, N.A.; Perez, M.; Weinkopff, A.; Ashley, E.; Hadley, D.; Turakhia, M.P.; Froelicher, V. Early repolarization in an ambulatory clinical population. Circulation 2011, 124, 2208–2214. [Google Scholar] [CrossRef]
- Bronis, K.; Kappos, K.; Manolis, A.S. Early repolarization: Not benign any more-the J-wave syndromes. Hosp. Chron. 2012, 7, 215–228. [Google Scholar]
- Li, G.L.; Yang, L.; Cui, C.C.; Sun, C.F.; Yan, G.X. J wave syndromes: A decade of progress. Chin. Med. J. 2015, 128, 969–975. [Google Scholar] [CrossRef]
- 11. Koncz, I.; Gurabi, Z.; Patocskai, B.; Panama, B.K.; Szél, T.; Hu, D.; Barajas-Martínez, H.; Antzelevitch, C. Mechanisms underlying the development of the electrocardiographic and arrhythmic manifestations of early repolarization syndrome. J. Mol. Cell Cardiol. 2014, 68, 20–28. [Google Scholar] [CrossRef]
- Meijborg, V.M.; Potse, M.; Conrath, C.E.; Belterman, C.N.; De Bakker, J.M.; Coronel, R. Reduced Sodium Current in the Lateral Ventricular Wall Induces Inferolateral J-Waves. Front. Physiol. 2016, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Sato, M.; Kitazawa, H.; Saito, A.; Ikeda, Y.; Fujita, S.; Fuse, K.; Takahashi, M.; Takarada, K.; Oguro, T.; et al. J-waves in patients with an acute ST-elevation myocardial infarction who underwent successful percutaneous coronary intervention: Prevalence, pathogenesis, and clinical implication. Europace 2013, 15, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, K.S.; Güngör, B.; Tatlısu, M.A.; Osmonov, D.; Ekmekçi, A.; Çalık, A.N.; Aruğarslan, E.; Zengin, A.; Bolca, O.; Eren, M.; et al. Presence of early repolarization on admission electrocardiography is associated with long-term mortality and MACE in patients with STEMI undergoing primary percutaneous intervention. J. Cardiol. 2014, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Mun, H.S.; Wi, J.; Uhm, J.S.; Shim, J.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Joung, B. Early repolarization and myocardial scar predict poorest prognosis in patients with coronary artery disease. Yonsei. Med. J. 2014, 55, 928–936. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, M.; Liu, G.; Shi, X.; Zhang, R.; Zhou, X.; Xi, Y.; Sun, J.; Zhu, C.; Chen, Y.; et al. Early Repolarization Pattern Predicts the Increased Risk of Ventricular Arrhythmias in Patients With Acute Anterior ST-Segment Elevation Myocardial Infarction—A Propensity Analysis. Circ. J. 2017, 81, 1346–1353. [Google Scholar] [CrossRef]
- Ahmed, S.; Khatti, S.; Shah, G.A.; Khan, N.; Rai, L.; Khan, M.Z.; Bari, S.A.; Roy, N. Diffuse ST Depression with ST Elevation Avr in Acute Coronary Syndrome and Its Association with Significant Left Main or Three Vessel Coronary Artery Disease and Its Confounders. J. Ayub. Med. Coll. Abbottabad 2023, 35, 43–49. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van de Werf, F.; et al. Fourth universal definition ofmyocardial infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Beinart, R.; Zhang, Y.; Lima, J.A.; Bluemke, D.A.; Soliman, E.Z.; Heckbert, S.R.; Post, W.S.; Guallar, E.; Nazarian, S. The QT interval is associated with incident cardiovascular events: The MESA study. J. Am. Coll. Cardiol. 2014, 64, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Spitaleri, G.; Brugaletta, S.; Scalone, G.; Moscarella, E.; Ortega-Paz, L.; Pernigotti, A.; Gomez-Lara, J.; Cequier, A.; Iñiguez, A.; Serra, A.; et al. Role of ST-Segment Resolution in Patients With ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention (from the 5-Year Outcomes of the EXAMINATION [Evaluation of the Xience-V Stent in Acute Myocardial Infarction] Trial). Am. J. Cardiol. 2018, 121, 1039–1045. [Google Scholar] [PubMed]
- Mahida, S.; Derval, N.; Sacher, F.; Leenhardt, A.; Deisenhofer, I.; Babuty, D.; Schläpfer, J.; de Roy, L.; Frank, R.; Yli-Mayry, S.; et al. Role of electrophysiological studies in predicting risk of ventricular arrhythmia in early repolarization syndrome. J. Am. Coll. Cardiol. 2015, 65, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Singh, V.; Duan, Y.; Su, X.; Su, H.; Zhang, M.; Cao, Y. Prognostic implications of ST-segment elevation in lead aVR in patients with acute coronary syndrome: A meta-analysis. Ann. Noninvasive Electrocardiol. 2021, 26, e12811. [Google Scholar] [CrossRef]
- Mirvis, M.D.; Goldberger, L.A. Electrocardiografía. Braunwald E. In Tratado de Cardiología, 7th ed.; Elsevier: Madrid, Spain, 2006; pp. 107–149. [Google Scholar]
- Mantri, N.; Lu, M.; Zaroff, J.G.; Risch, N.; Hoffmann, T.; Oni-Orisan, A.; Lee, C.; Jorgenson, E.; Iribarren, C. QT Interval Dynamics and Cardiovascular Outcomes: A Cohort Study in an Integrated Health Care Delivery System. J. Am. Heart Assoc. 2021, 10, e018513. [Google Scholar] [CrossRef]
- Sut, N. Sample size determination and power analysis in clinical trials. Ulus. Romatol. Derg. 2011, 3, 29–33. [Google Scholar]
- Jones, R.L.; Rubal, B.; Jones, S.; Steel, K.; Davenport, E.; Nasir, J. Prevalence of early repolarization in a large cohort of young adults. J. Am. Coll. Cardiol. 2015, 65, A371. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Yan, G.X. J wave syndromes. Heart Rhythm 2010, 7, 549–558. [Google Scholar] [CrossRef]
- Rosso, R.; Kogan, E.; Belhassen, B.; Rozovski, U.; Scheinman, M.M.; Zeltser, D.; Halkin, A.; Steinvil, A.; Heller, K.; Glikson, M.; et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects. J. Am. Coll. Cardiol. 2008, 52, 1231–1238. [Google Scholar] [CrossRef]
- Casado Arroyo, R.; Sieira, J.; Kubala, M.; Latcu, D.G.; Maeda, S.; Brugada, P. Electrophysiological Basis for Early Repolarization Syndrome. Front. Cardiovasc. Med. 2018, 5, 161. [Google Scholar] [CrossRef]
- Nunn, L.M.; Bhar-Amato, J.; Lowe, M.D.; Macfarlane, P.W.; Rogers, P.; McKenna, W.J.; Elliott, P.M.; Lambiase, P.D. Prevalence of J-point elevation in sudden arrhythmic death syndrome families. J. Am. Coll. Cardiol. 2011, 58, 286–290. [Google Scholar] [CrossRef]
- Reinhard, W.; Kaess, B.M.; Debiec, R.; Nelson, C.P.; Stark, K.; Tobin, M.D.; Macfarlane, P.W.; Tomaszewski, M.; Samani, N.J.; Hengstenberg, C. Heritability of early repolarization: A population-based study. Circ. Cardiovasc. Genet. 2011, 4, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.J.; Ackerman, M.J.; Funk, M.; Gibler, W.B.; Kligfield, P.; Menon, V.; Philippides, G.J.; Roden, D.M.; Zareba, W.; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; et al. Prevention of torsade de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010, 121, 1047–1060. [Google Scholar] [CrossRef]
- Gadaleta, F.L.; Llois, S.C.; Lapuente, A.R.; Batchvarov, V.N.; Kaski, J.C. Prognostic value of corrected QT-interval prolongation in patients with unstable angina pectoris. Am. J. Cardiol. 2003, 92, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Nabati, M.; Emadi, M.; Mollaalipour, M.; Bagheri, B.; Nouraei, M. ST-segment elevation in lead aVR in the setting of acute coronary syndrome. Acta Cardiol. 2016, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Misumida, N.; Kobayashi, A.; Fox, J.T.; Hanon, S.; Schweitzer, P.; Kanei, Y. Predictive Value of ST-Segment Elevation in Lead aVR for Left Main and/or Three-Vessel Disease in Non-ST-Segment Elevation Myocardial Infarction. Ann. Noninvasive Electrocardiol. 2016, 21, 91–97. [Google Scholar] [CrossRef]
- Rathi, N.; Baloch, M.Z.; Sachdewani, R.K.; Memon, F. Frequency of left main artery/three vessel disease predicted through ECG changes in patients presenting with non-ST segment acute coronary syndrome. J. Liaquat. Uni. Med. Health Sci. 2016, 15, 93–97. [Google Scholar]
- Demirci, B.; Caltili, C.; Coskun, A. The Relationship of Early Repolarization Morphology with Prognosis and Mortality Accompanied by Angiography Results. Konuralp Med. J. 2022, 14, 329–336. [Google Scholar] [CrossRef]
- Kenigsberg, D.N.; Khanal, S.; Kowalski, M.; Krishnan, S.C. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J. Am. Coll. Cardiol. 2007, 49, 1299–1305. [Google Scholar] [CrossRef]
- Mann, T.; Moses, A.; Yesaulov, A.; Hochstadt, A.; Granot, Y.; Rosso, R.; Shacham, Y.; Chorin, E. QT interval dynamics in patients with ST-elevation MI. Front. Cardiovasc. Med. 2023, 9, 1056456. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, A.E.; Cruz-Inerarity, H.; Negrín-Valdés, T.; Fardales-Rodríguez, R.; Chávez-González, E. Corrected QT-Interval Dispersion: An Electrocardiographic Tool to Predict Recurrence of Myocardial Infarction. MEDICC Rev. 2019, 21, 22–28. [Google Scholar] [CrossRef]
- Lee, G.K.; Hsieh, Y.P.; Hsu, S.W.; Lan, S.J.; Soni, K. Value of ST-segment change in lead aVR in diagnosing left main disease in Non-ST-elevation acute coronary syndrome-A meta-analysis. Ann. Noninvasive Electrocardiol. 2019, 24, e12692. [Google Scholar] [CrossRef]
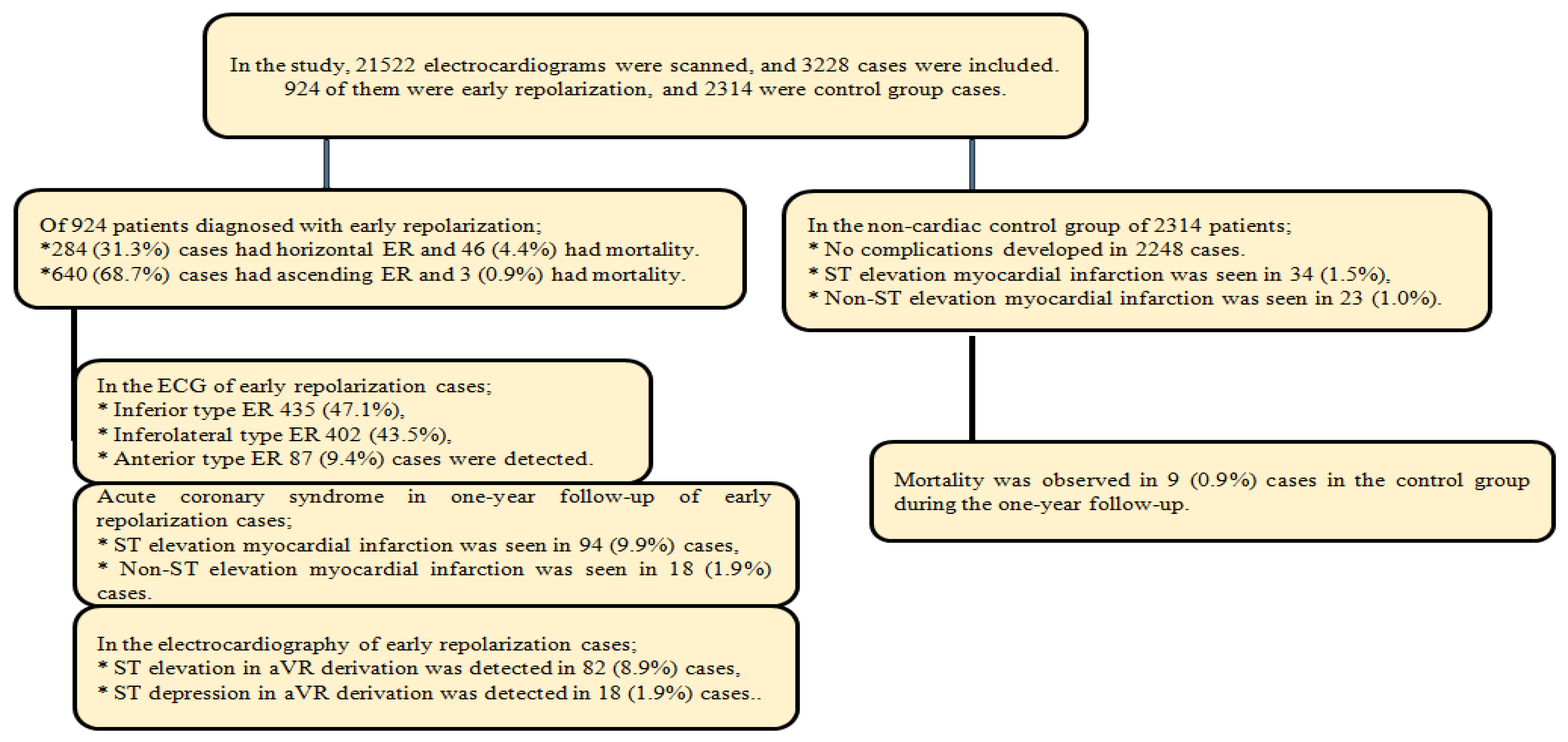
| Early Repolarization | Non-Cardiac Follow-Up Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Patient Mean (SD), n (%) | Horizontal Mean (SD), n (%) | Ascending Mean (SD), n (%) | Mean (SD), n (%) | p-Value | ||||||
| Age, year | 48.43 (9.65) | 53.52 (8.65) | 46.16 (9.2) | 51.67 (7.98) | <0.001 | |||||
| Gender | Female | 284 (30.7) | 84 (29.6) | 278 (43.4) | 738 (31.9) | 0.001 | ||||
| Male | 640 (69.3) | 200 (70.4) | 362 (56.6) | 1576 (68.1) | ||||||
| Corrected QT interval, ms | 409.54 (33.46) | 434.05 (37.74) | 398.67 (26.41) | 411.57 (27.91) | <0.001 | |||||
| Time, day | 335.2 (83.27) | 279.65 (123.95) | 359.85 (35.1) | 364.87 (54.38) | <0.001 | |||||
| Lab | Troponin I, pg/dL | 0.08 (0.19) | 0.13 (0.23) | 0.07 (0.16) | 0.05 (0.09) | 0.003 | ||||
| Blood sugar, mg/dL | 112.7 (34.49) | 127.45 (42.95) | 106.16 (27.28) | 114.83 (29.72) | <0.001 | |||||
| Triglyceride, mg/dL | 88.41 (25.93) | 90.24 (256.97) | 87.5 (25.38) | 96.38 (21.67) | 0.005 | |||||
| Cholesterol, mg/dL | 151.84 (41.5) | 155.64 (40.12) | 149.94 (42.11) | 164.31 (44.73) | 0.057 | |||||
| VLDL, mg/dL | 97.74 (34.26) | 99.82 (33.81) | 96.7 (34.5) | 94.64 (35.92) | 0.274 | |||||
| HDL, mg/dL | 35.19 (19.11) | 36.89 (30.49) | 34.33 (9.11) | 36.93 (21.85) | 0.564 | |||||
| Mortality | 49 (5.3) | 46 (93.9) | 3 (6.1) | 9 (0.39) | ||||||
| Comorbidity | Hypertension | 106 (11.5) | 319 (13.8) | |||||||
| Diabetes mellitus | 132 (14.3) | 287 (12.4) | ||||||||
| Tobacco | 244 (26.4) | 502 (21.7) | ||||||||
| Early Repolarization | Mortality | |||||
|---|---|---|---|---|---|---|
| Horizontal n (%) | Ascending n (%) | p-Value | n (%) | p-Value | ||
| Early repolarization | Inferior | 148 (52.1) | 287 (44.8) | 0.048 | 35 (71.4) | 0.002 |
| Inferolateral | 113 (39.8) | 289 (45.2) | 12 (24.5) | |||
| Anterior | 23 (8.1) | 64 (10) | 2 (4.1) | |||
| Acute coronary syndrome | No | 186 (65.5) | 628 (98.1) | 0.001 | 3 (6.1) | 0.001 |
| STEMI | 86 (30.3) | 6 (0.9) | 40 (81.6) | |||
| NSTEMI | 12 (4.2) | 6 (0.9) | 6 (12.3) | |||
| aVR Derivation | No | 203 (71.5) | 621 (97) | 0.001 | 2 (4.1) | 0.001 |
| ST Elevation | 69 (24.3) | 13 (2.1) | 43 (87.8) | |||
| STDepression | 12 (4.2) | 6 (0.9) | 4 (8.1) | |||
| Early Repolarization | aVR Derivation | Acute Coronary Syndrome | Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Age, year | −0.361 | <0.001 | 0.426 | <0.001 | 0.494 | <0.001 | 0.342 | 0.001 |
| Corrected QT interval, ms | −0.440 | <0.001 | 0.489 | <0.001 | 0.549 | <0.001 | 0.367 | 0.001 |
| Cardiac troponin I, pg/dL | −0.096 | 0.003 | 0.099 | 0.003 | 0.110 | 0.001 | 0.141 | 0.001 |
| Blood sugar, mg/dL | −0.307 | <0.001 | 0.304 | <0.001 | 0.357 | <0.001 | 0.210 | 0.001 |
| Early Repolarization | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Early Repolarization | Age, year | 0.910 | 0.893–0.927 | <0.001 | 0.968 | 0.947–0.989 | 0.003 |
| Corrected QT interval, ms | 0.964 | 0.959–0.969 | <0.001 | 0.972 | 0.965–0.978 | <0.001 | |
| Cardiac troponin I, pg/dL | 0.199 | 0.098–0.401 | 0.001 | 0.225 | 0.104–0.489 | 0.001 | |
| Blood sugar, mg/dL | 0.981 | 0.976–0.986 | <0.001 | 0.991 | 0.983–0.999 | 0.022 | |
| aVR Derivation | Age, year | 1.320 | 1.249–1.395 | <0.001 | 1.091 | 1.026–1.160 | 0.005 |
| Corrected QT interval, ms | 1.083 | 1.068–1.098 | <0.001 | 1071 | 1.053–1.090 | <0.001 | |
| Cardiac troponin I, pg/dL | 8.232 | 3.705–18.289 | <0.001 | 25.410 | 6.095–105.928 | <0.001 | |
| Blood sugar, mg/dL | 1.017 | 1.011–1.022 | 0.001 | ||||
| Mortality | Age, year | 1.409 | 1.298–1.530 | <0.001 | 1.230 | 1.086–1.393 | 0.001 |
| Corrected QT interval, ms | 1.124 | 1.086–1.164 | <0.000 | 1.094 | 1.057–1.133 | <0.001 | |
| Cardiac troponin I, pg/dL | 27.800 | 11.112–69.548 | <0.001 | 1784.34 | 123.32–25818.44 | <0.001 | |
| Blood sugar, mg/dL | 1.013 | 1.008–1.019 | 0.001 | ||||
| Early Repolarization | ||||||
|---|---|---|---|---|---|---|
| AUC | 95% CI | p | Sensitivity (%) | Specificity (%) | ||
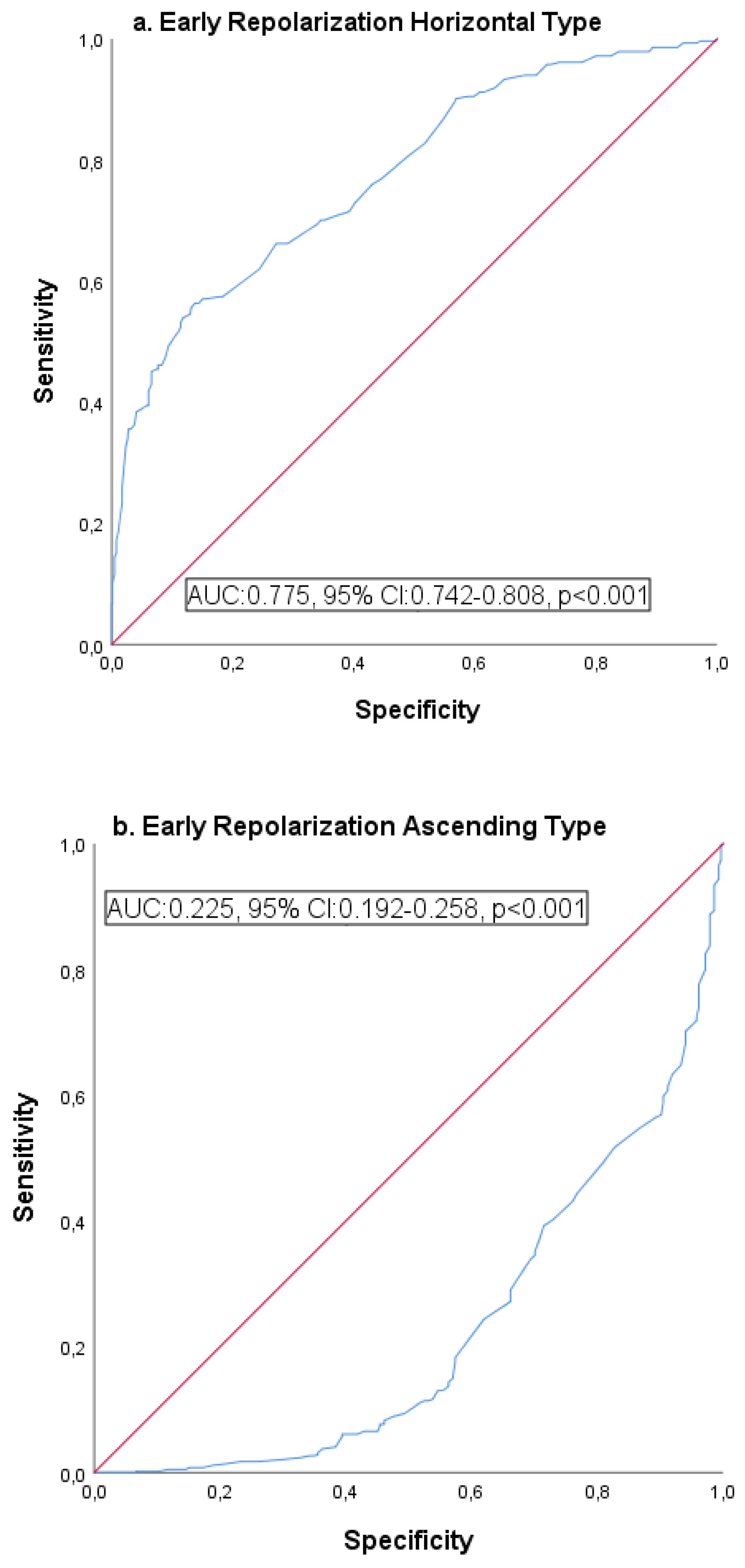
| ROC curve analysis | Horizontal | 0.775 | 0.742–0.808 | <0.001 | 83.1 | 81.7 |
| Ascending | 0.225 | 0.192–0.258 | <0.001 | 78.7 | 76.9 | |
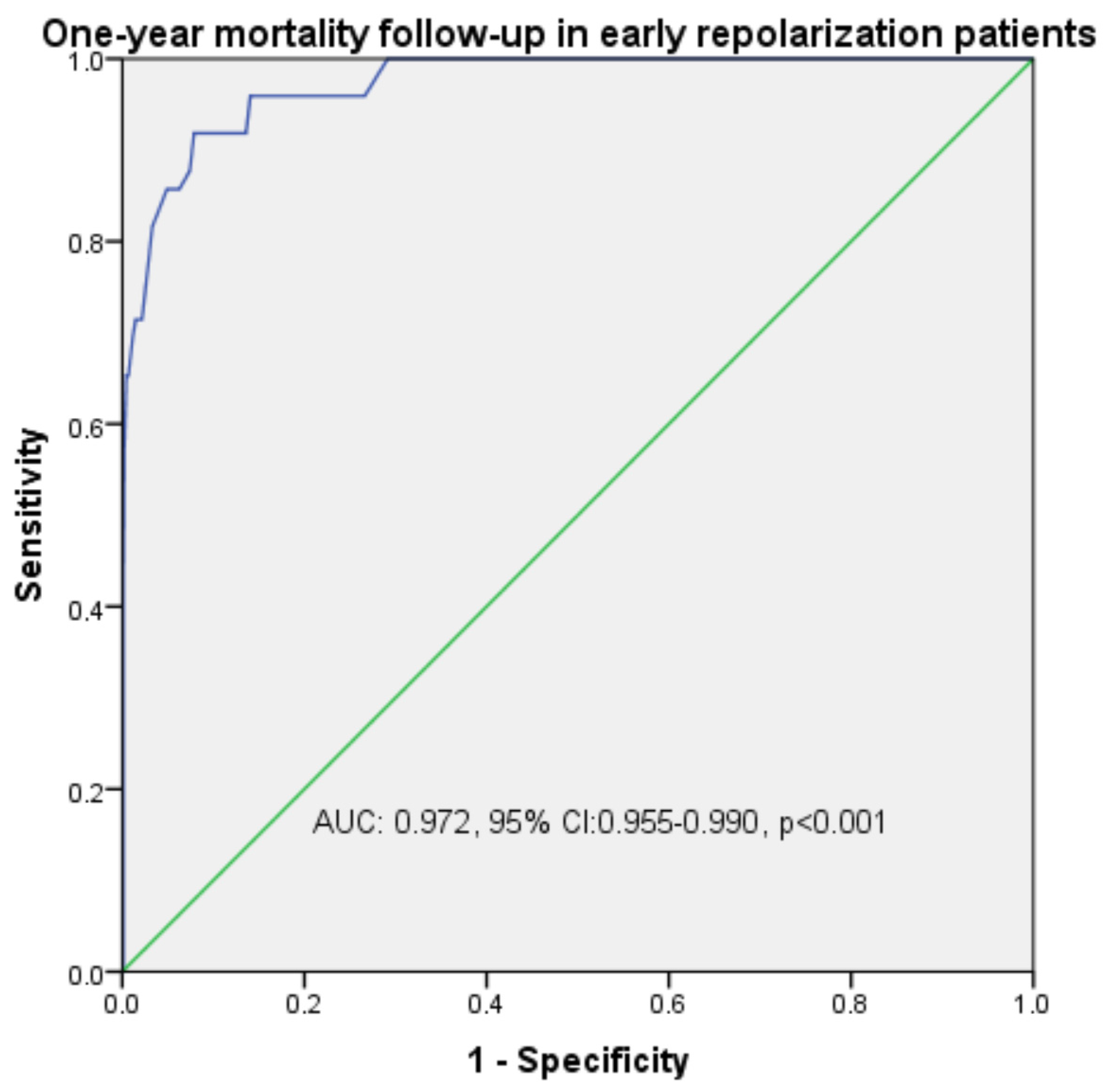
| Mortality | 0.972 | 0.955–0.990 | <0.001 | 98.4 | 97.1 | |
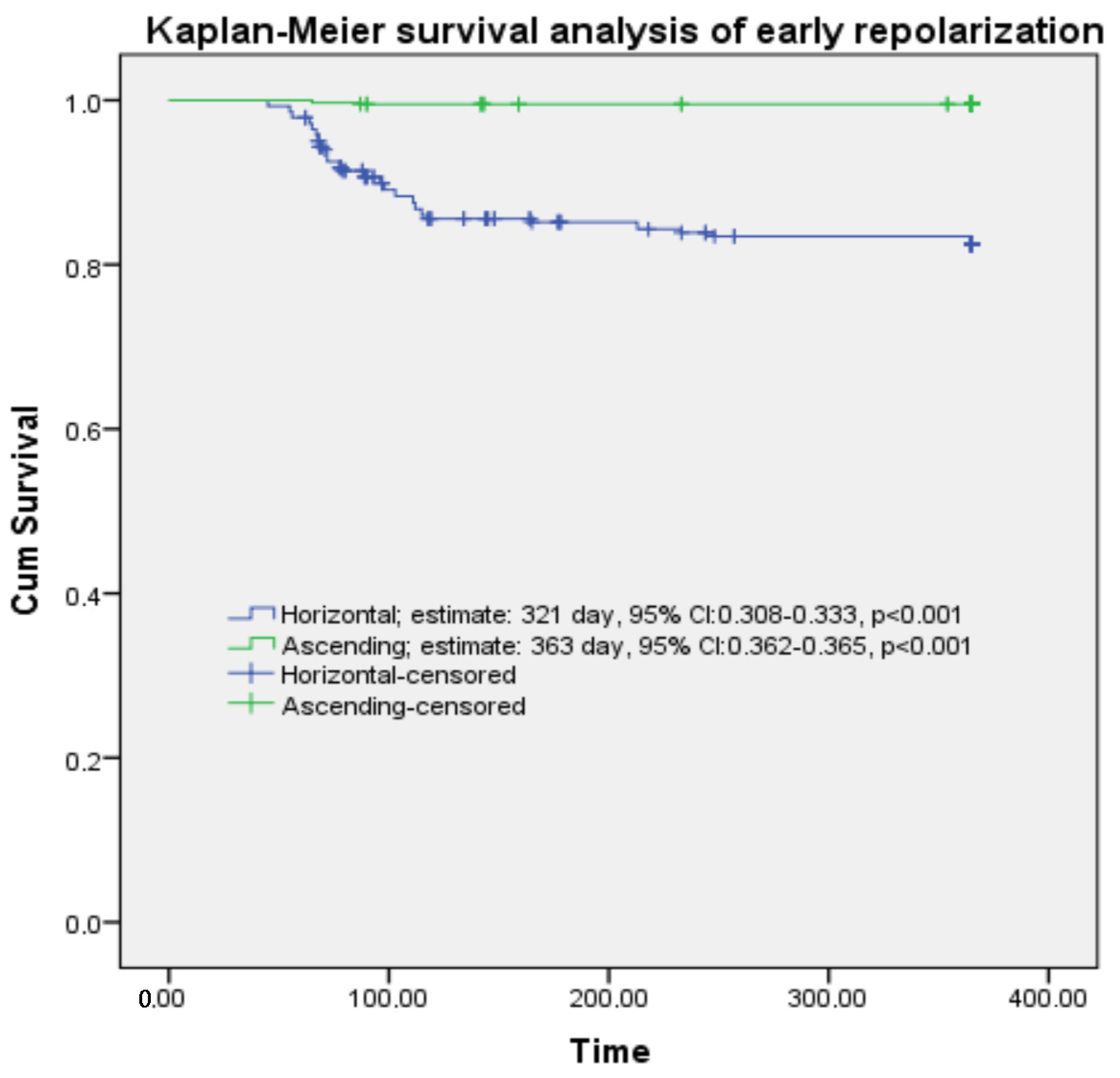
| Kaplan–Meier survival analysis | Estimate, day | 95% CI | p | |||
| Horizontal | 321 | 308–333 | <0.001 | |||
| Ascending | 363 | 362–364 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altinbilek, E.; Coskun, A.; Demirci, B.; Oymak, I.; Calik, M.; Öztürk, D.; Afacan, M.A.; Bayramoglu, B. Prognostic Significance of aVR Lead and QTc Prolongation in Patients with Early Repolarization. Medicina 2025, 61, 1466. https://doi.org/10.3390/medicina61081466
Altinbilek E, Coskun A, Demirci B, Oymak I, Calik M, Öztürk D, Afacan MA, Bayramoglu B. Prognostic Significance of aVR Lead and QTc Prolongation in Patients with Early Repolarization. Medicina. 2025; 61(8):1466. https://doi.org/10.3390/medicina61081466
Chicago/Turabian StyleAltinbilek, Ertugrul, Abuzer Coskun, Burak Demirci, Ismail Oymak, Mustafa Calik, Derya Öztürk, Mustafa Ahmet Afacan, and Burcu Bayramoglu. 2025. "Prognostic Significance of aVR Lead and QTc Prolongation in Patients with Early Repolarization" Medicina 61, no. 8: 1466. https://doi.org/10.3390/medicina61081466
APA StyleAltinbilek, E., Coskun, A., Demirci, B., Oymak, I., Calik, M., Öztürk, D., Afacan, M. A., & Bayramoglu, B. (2025). Prognostic Significance of aVR Lead and QTc Prolongation in Patients with Early Repolarization. Medicina, 61(8), 1466. https://doi.org/10.3390/medicina61081466






