Methylene Blue Alleviates Inflammatory and Oxidative Lung Injury in a Rat Model of Feces-Induced Peritonitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
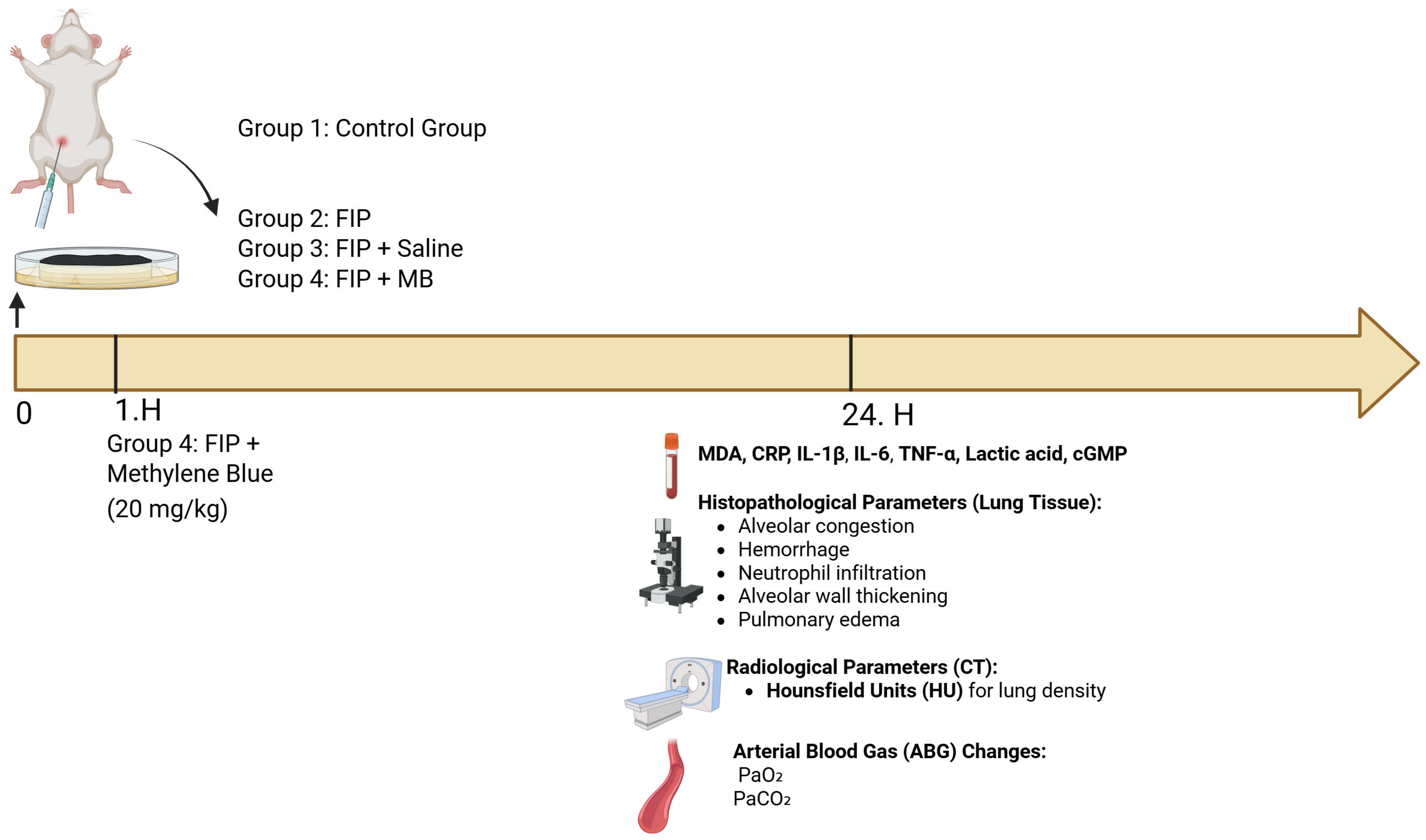
2.2. Experimental Protocol
2.2.1. Sepsis Induction and Experimental Design
2.2.2. Fecal Slurry Preparation and Sepsis Induction
2.2.3. Experimental Groups
2.2.4. Euthanasia and Sample Collection
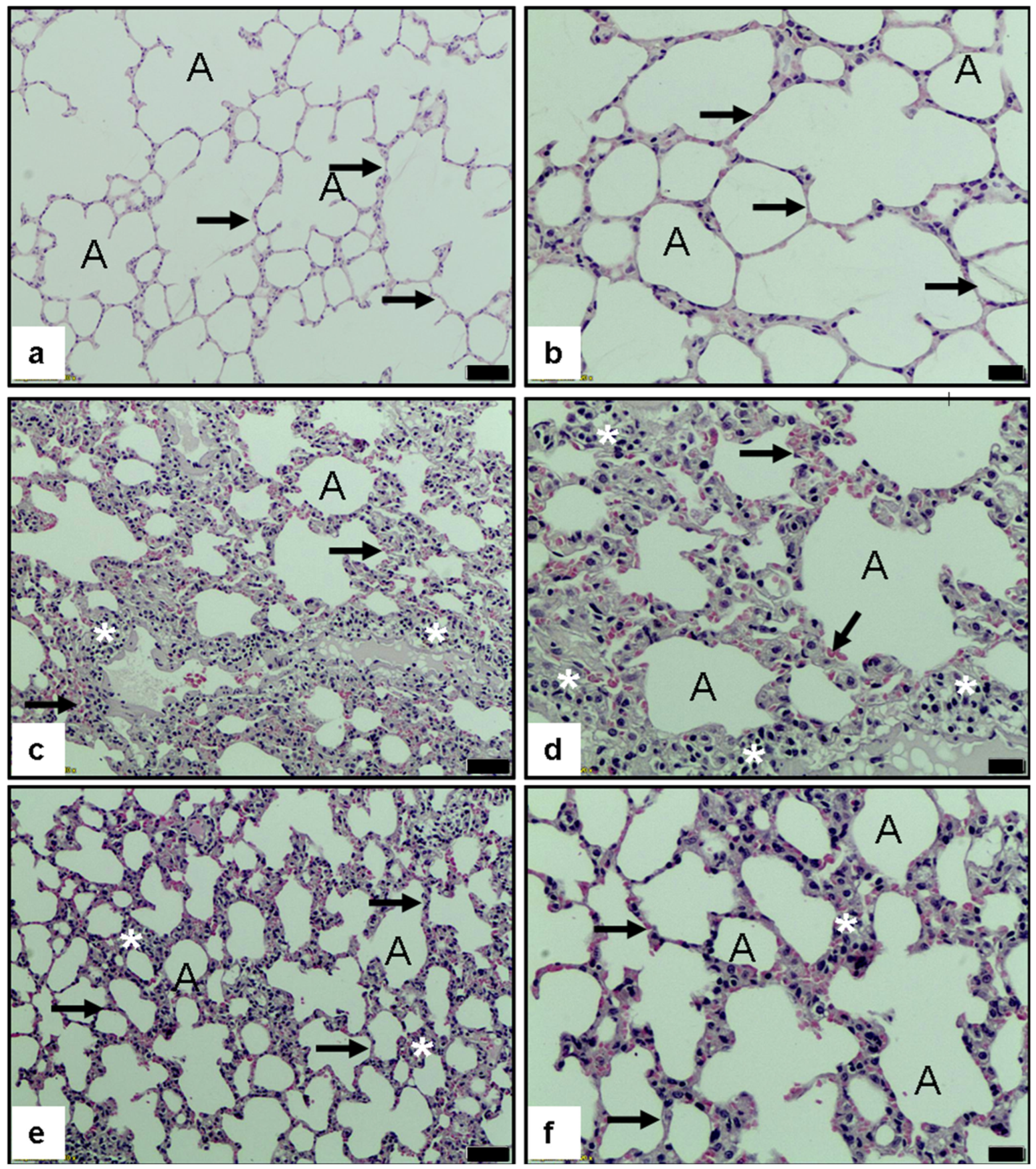
2.3. Histopathological Examination of Lung Tissue
2.3.1. Anesthesia and Tissue Collection
2.3.2. Fixation and Staining
2.3.3. Histopathological Scoring Method
2.4. Computed Tomography (CT) Imaging and Region of Interest (ROI) Analysis
2.5. Arterial Blood Gas Analysis
2.6. Biochemical Analysis
2.6.1. Measurement of Lipid Peroxidation
2.6.2. Measurement of TNF-α Levels
2.6.3. Measurement of C-Reactive Protein (CRP) Levels
2.6.4. Measurement of Lactic Acid Concentrations
2.6.5. Measurement of Cyclic Guanosine Monophosphate (cGMP) Levels
2.6.6. Measurement of Interleukin-6 (IL-6) Levels
2.6.7. Measurement of Interleukin-1 Beta (IL-1β) Levels
2.7. Statistical Analysis
3. Results
3.1. Statistical Power Analysis
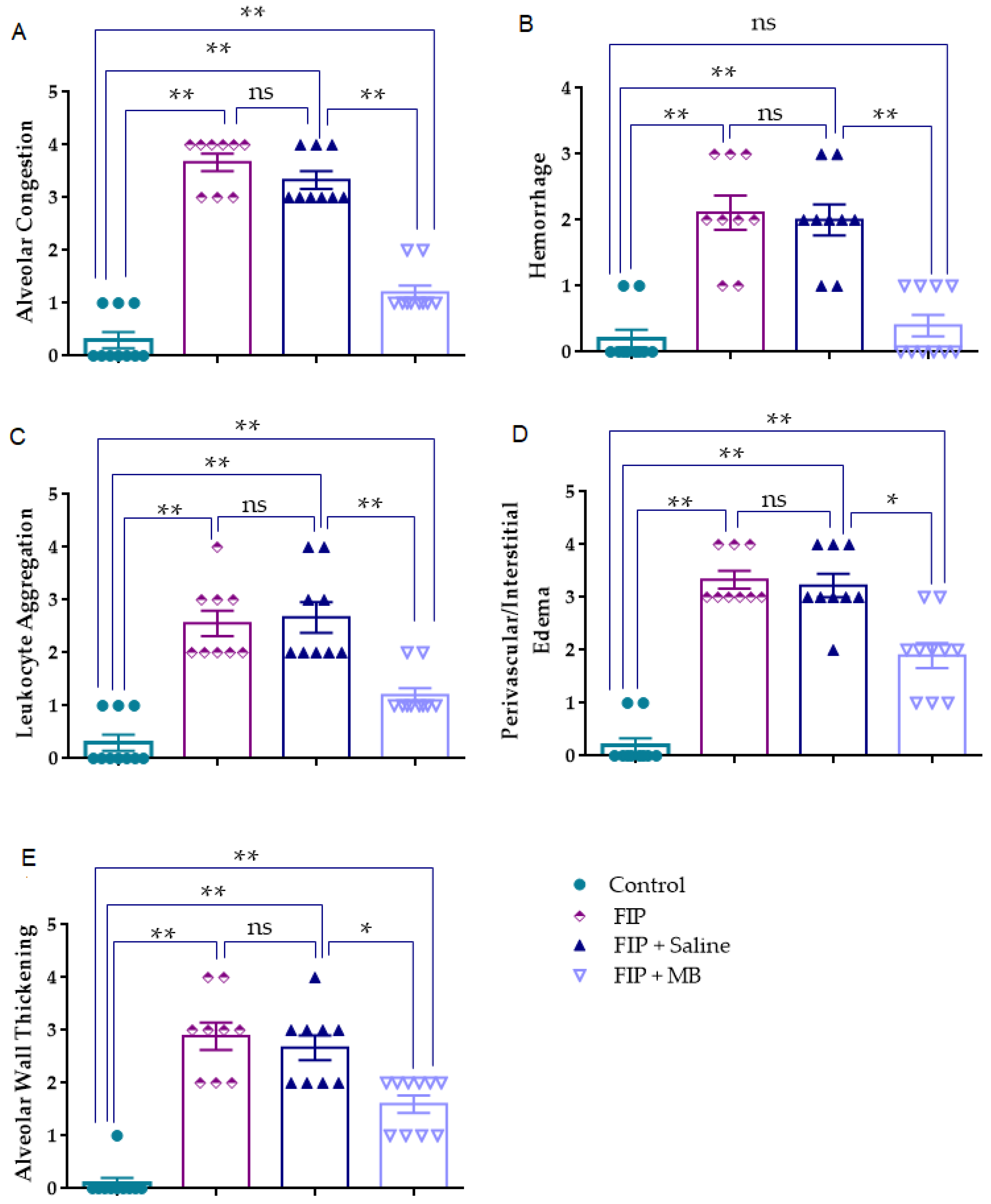
3.2. Evaluation of Histological Lung Injury
3.2.1. Alveolar Congestion (AC)
3.2.2. Hemorrhage (H)
3.2.3. Leukocyte Aggregation (AL)
3.2.4. Perivascular/Interstitial Edema (PE)
3.2.5. Alveolar Wall Thickening (TA)
3.2.6. Histopathological Evaluation of Pulmonary Tissue
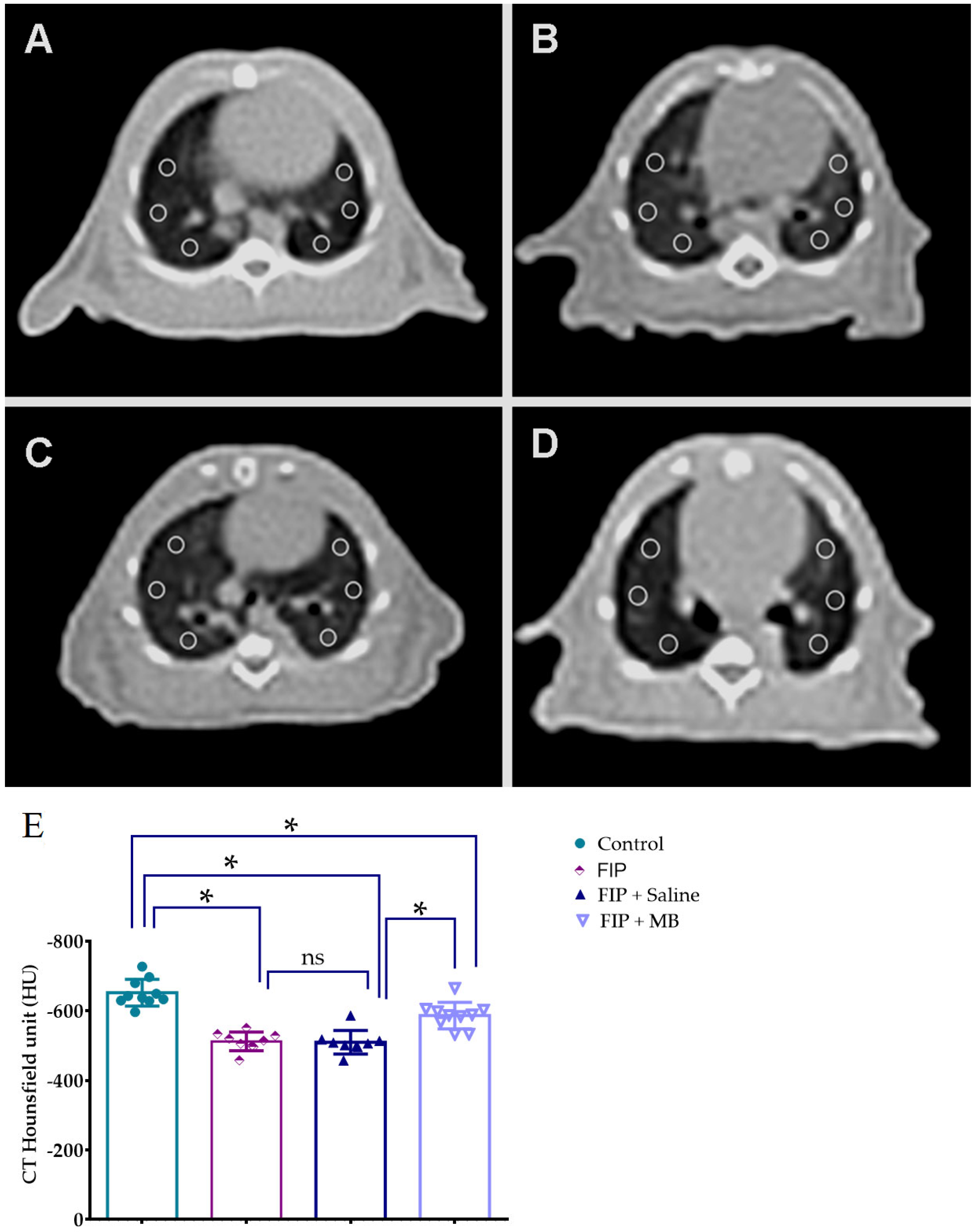
3.3. CT-Based Assessment of Pulmonary Density Using Hounsfield Unit Measurements
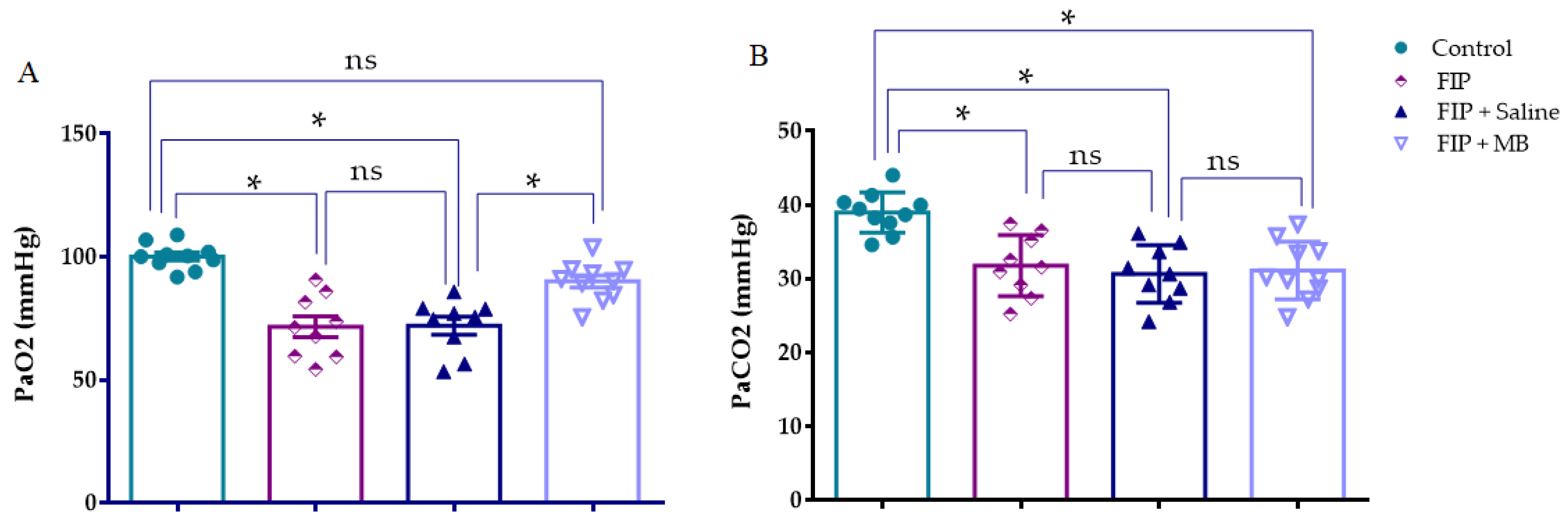
3.4. Arterial Blood Gas Analysis
3.4.1. Arterial Oxygen Pressure (PaO2)
3.4.2. Arterial Carbon Dioxide Pressure (PaCO2)
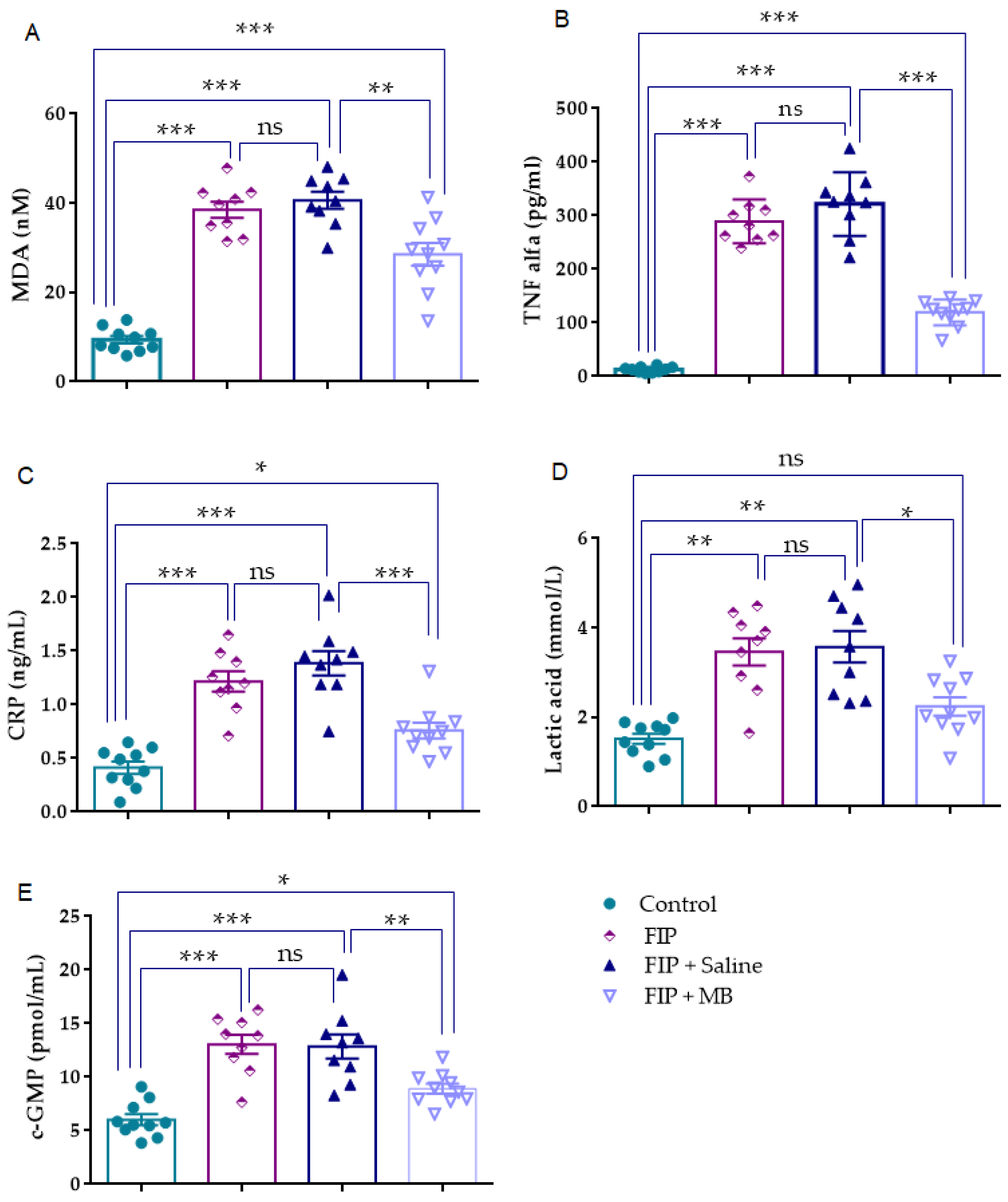
3.5. Assessment of Biochemical Parameters
3.5.1. MDA (nM)
3.5.2. TNF-α (pg/mL)
3.5.3. CRP (ng/mL)
3.5.4. Lactic Acid (mmol/L)
3.5.5. cGMP (pmol/mL)
3.5.6. IL-6 (pg/mL)
3.5.7. IL-1β (pg/mL)
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.K.; Fowler, R.A.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet 2010, 376, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, W.; Dong, Y.; Luo, X.; Miao, H.; Maimaijuma, T.; Xu, X.; Jiang, H.; Huang, Z.; Qi, L.; et al. Early identification and diagnosis, pathophysiology, and treatment of sepsis-related acute lung injury: A narrative review. J. Thorac. Dis. 2024, 16, 5457. [Google Scholar] [CrossRef]
- Ibarra-Estrada, M.; Kattan, E.; Aguilera-González, P.; Sandoval-Plascencia, L.; Rico-Jauregui, U.; Gómez-Partida, C.A.; Ortiz-Macías, I.X.; López-Pulgarín, J.A.; Chávez-Peña, Q.; Mijangos-Méndez, J.C.; et al. Early adjunctive methylene blue in patients with septic shock: A randomized controlled trial. Crit. Care 2023, 27, 110. [Google Scholar] [CrossRef]
- Spink, J.; Cohen, J.; Evans, T.J. The cytokine responsive vascular smooth muscle cell enhancer of inducible nitric oxide synthase: Activation by nuclear factor-κB. J. Biol. Chem. 1995, 270, 29541–29547. [Google Scholar] [CrossRef]
- Creagh-Brown, B.C.; Griffiths, M.J.; Evans, T.W. Bench-to-bedside review: Inhaled nitric oxide therapy in adults. Crit. Care 2009, 13, 221. [Google Scholar] [CrossRef]
- O’Brien, J.M., Jr.; Ali, N.A.; Aberegg, S.K.; Abraham, E. Sepsis. Am. J. Med. 2007, 120, 1012–1022. [Google Scholar] [CrossRef]
- Pan, P.F.; Wang, Y.; Li, X.P.; Yang, C.B.; Zhong, H.; Du, X.X.; Yu, X. Effects of methylene blue on the nitric oxide-soluble guanylate cyclase-cyclic guanylyl monophosphate pathway and cytokine levels in rats with sepsis. Int. J. Clin. Exp. Med. 2019, 12, 12203–12211. [Google Scholar]
- Ayvaz, S.; Aksu, B.; Karaca, T.; Cemek, M.; Tarladacalisir, Y.-T.; Ayaz, A.; Metin, M.-S.; Basaran, U.; Ayvaz, A.-T.; Aksu, F.; et al. Effects of methylene blue in acute lung injury induced by blunt chest trauma. Hippokratia 2014, 18, 50. [Google Scholar]
- Hetrick, E.M.; Schoenfisch, M.H. Analytical chemistry of nitric oxide. Annu. Rev. Anal. Chem. 2009, 2, 409–433. [Google Scholar] [CrossRef]
- Coneski, P.N.; Schoenfisch, M.H. Nitric oxide release: Part III. Measurement and reporting. Chem. Soc. Rev. 2012, 41, 3753–3758. [Google Scholar] [CrossRef]
- Soop, A.; Sollevi, A.; Weitzberg, E.; Lundberg, J.; Palm, J.; Albert, J. Exhaled NO and plasma cGMP increase after endotoxin infusion in healthy volunteers. Eur. Respir. J. 2003, 21, 594–599. [Google Scholar] [CrossRef]
- Tsikas, D. A critical review and discussion of analytical methods in the L-arginine/nitric oxide area of basic and clinical research. Anal. Biochem. 2008, 379, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Demirbilek, S.; Sizanli, E.; Karadag, N.; Karaman, A.; Bayraktar, N.; Turkmen, E.; Ersoy, M. The effects of methylene blue on lung injury in septic rats. Eur. Surg. Res. 2006, 38, 35–41. [Google Scholar] [CrossRef] [PubMed]
- da Matta Abreu, M.; Pazetti, R.; de Almeida, F.M.; Correia, A.T.; Parra, E.R.; da Silva, L.P.; Vieira, R.d.P.; Pêgo-Fernandes, P.M.; Jatene, F.B. Methylene blue attenuates ischemia–reperfusion injury in lung transplantation. J. Surg. Res. 2014, 192, 635–641. [Google Scholar] [CrossRef]
- Tian, Z.G.; Ji, Y.; Yan, W.J.; Xu, C.Y.; Kong, Q.Y.; Han, F.; Pang, Q.F. Methylene blue protects against paraquat-induced acute lung injury in rats. Int. Immunopharmacol. 2013, 17, 309–313. [Google Scholar] [CrossRef]
- Tian, W.-F.; Zeng, S.; Sheng, Q.; Chen, J.-L.; Weng, P.; Zhang, X.-T.; Yuan, J.-J.; Pang, Q.-F.; Wang, Z.-Q. Methylene Blue Protects the Isolated Rat Lungs from Ischemia–Reperfusion Injury by Attenuating Mitochondrial Oxidative Damage. Lung 2018, 196, 73–82. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Lin, B.; Ye, Y.; Bao, C.; Zhao, X.; Jin, L.; Xiong, X. Methylene blue attenuates lung injury induced by hindlimb ischemia reperfusion in rats. Mediat. Inflamm. 2018, 2018, 2508620. [Google Scholar] [CrossRef]
- Mestriner, F.; Dantas, P.B.; Michelon-Barbosa, J.; Dugaich, V.F.; Luis-Silva, F.; Ribeiro, M.S.; Evora, P.R.; Becari, C. Methylene blue as a potential intervention in sepsis: Effects on survival and microcirculation in rat models of sepsis. Biomed. Pharmacother. 2025, 187, 118131. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Gong, C.; Fu, J.; Liu, X.; Bi, H.; Cheng, Y.; Liu, Y.; Tang, Y.; Wang, D. Evaluation of 2 rat models for sepsis developed by improved cecal ligation/puncture or feces intraperitoneal-injection. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919054. [Google Scholar] [CrossRef] [PubMed]
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.M.; McCormick, J.K.; Mele, T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 2014, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Nacionales, D.C.; Lopez, M.C.; Vanzant, E.; Cuenca, A.; Szpila, B.E.; Cuenca, A.G.; Joseph, A.; Moore, F.A.; Leeuwenburgh, C.; et al. Host responses to sepsis vary in different low-lethality murine models. PLoS ONE 2014, 9, e94404. [Google Scholar] [CrossRef][Green Version]
- Sever, I.H.; Ozkul, B.; Tanriover, D.E.; Ozkul, O.; Elgormus, C.S.; Gur, S.G.; Sogut, I.; Uyanikgil, Y.; Cetin, E.O.; Erbas, O. Protective effect of oxytocin through its anti-inflammatory and antioxidant role in a model of sepsis-induced acute lung injury: Demonstrated by CT and histological findings. Exp. Lung Res. 2021, 47, 426–435. [Google Scholar] [CrossRef]
- Canbolat, N.; Ozkul, B.; Sever, I.H.; Sogut, I.; Eroglu, E.; Uyanikgil, Y.; Erbas, O. Vitamins C and E protect from sepsis-induced lung damage in rat and CT correlation. Bratisl. Med. J. Bratisl. Lek. Listy 2022, 123, 828–832. [Google Scholar] [CrossRef]
- Sorgun, O.; Çakır, A.; Bora, E.; Erdoğan, M.; Uyanıkgil, Y.; Erbaş, O. Anti-inflammatory and antioxidant properties of betaine protect against sepsis-induced acute lung injury: CT and histological evidence. Braz. J. Med. Biol. Res. 2023, 56, e12906. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Youness, E.R.; Morsy, F.A.; Sleem, A.A. Methylene Blue Protects Against Acute Ethanol-Induced Oxidative Stress and Organ Damage. Int. J. Halal Res. 2021, 3, 88–102. [Google Scholar] [CrossRef]
- Haouzi, P.; Gueguinou, M.; Sonobe, T.; Judenherc-Haouzi, A.; Tubbs, N.; Trebak, M.; Cheung, J.; Bouillaud, F. Revisiting the physiological effects of methylene blue as a treatment of cyanide intoxication. Clin. Toxicol. 2018, 56, 828–840. [Google Scholar] [CrossRef]
- Shomer, N.H.; Allen-Worthington, K.H.; Hickman, D.L.; Jonnalagadda, M.; Newsome, J.T.; Slate, A.R.; Valentine, H.; Williams, A.M.; Wilkinson, M. Review of rodent euthanasia methods. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 242–253. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Peng, Z.; Hu, B.; Rao, X.; Li, J. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 2020, 45, 61–80. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Angus, D.C.; Bellomo, R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.J.; Kraskauskas, D.; Martin, E.J.; Farkas, D.; Wegelin, J.A.; Brophy, D.; Ward, K.R.; Voelkel, N.F.; Fowler, A.A.; Natarajan, R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 303, L20–L32. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Adrover, J.M.; Felice, C.; Christensen, L.N.; He, X.-Y.; Merrill, J.R.; Wilkinson, J.E.; Janowitz, T.; Lyons, S.K.; Egeblad, M.; et al. PTP1B inhibitors protect against acute lung injury and regulate CXCR4 signaling in neutrophils. JCI Insight 2022, 7, e158199. [Google Scholar] [CrossRef] [PubMed]
- Landry, D.W.; Oliver, J.A.; Epstein, F.H. The pathogenesis of vasodilatory shock. N. Engl. J. Med. 2001, 345, 588–595. [Google Scholar] [CrossRef]
- Levy, B.; Collin, S.; Sennoun, N.; Ducrocq, N.; Kimmoun, A.; Asfar, P.; Perez, P.; Meziani, F. Vascular hyporesponsiveness to vasopressors in septic shock: From bench to bedside. Intensive Care Med. 2010, 36, 2019–2029. [Google Scholar] [CrossRef]
- Mitaka, C.; Hirata, Y.; Yokoyama, K.; Makita, K.; Imai, T. A selective inhibitor for inducible nitric oxide synthase improves hypotension and lactic acidosis in canine endotoxic shock. Crit. Care Med. 2001, 29, 2156–2161. [Google Scholar] [CrossRef]
- Mallat, J.; Rahman, N.; Hamed, F.; Hernandez, G.; Fischer, M.O. Pathophysiology, mechanisms, and managements of tissue hypoxia. Anaesth. Crit. Care Pain Med. 2022, 41, 101087. [Google Scholar] [CrossRef]
- McMullan, R.R.; McAuley, D.F.; O’kAne, C.M.; Silversides, J.A. Vascular leak in sepsis: Physiological basis and potential therapeutic advances. Crit. Care 2024, 28, 97. [Google Scholar] [CrossRef]
- Liao, M.-H.; Chen, S.-J.; Tsao, C.-M.; Shih, C.-C.; Wu, C.-C. Possible biomarkers of early mortality in peritonitis-induced sepsis rats. J. Surg. Res. 2013, 183, 362–370. [Google Scholar] [CrossRef]
- Galili, Y.; Kluger, Y.; Mianski, Z.; Iaina, A.; Wollman, Y.; Marmur, S.; Soffer, D.; Chernikovsky, T.; Klausner, J.P.; Rabau, M.Y. Methylene blue–a promising treatment modality in sepsis induced by bowel perforation. Eur. Surg. Res. 1997, 29, 390–395. [Google Scholar] [CrossRef]
- Paya, D.; Gray, G.A.; Stoclet, J.C. Effects of methylene blue on blood pressure and reactivity to norepinephrine in endotoxemic rats. J. Cardiovasc. Pharmacol. 1993, 21, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Marczin, N.; Ryan, U.S.; Catravas, J.D. Methylene blue inhibits nitrovasodilator-and endothelium-derived relaxing factor-induced cyclic GMP accumulation in cultured pulmonary arterial smooth muscle cells via generation of superoxide anion. J. Pharmacol. Exp. Ther. 1992, 263, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb. Haemost. 2019, 17, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef]
- Szabó, C.; Módis, K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock 2010, 34, 4–14. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Abreu-González, P.; Domínguez-Rodriguez, A.; Labarta, L.; Díaz, C.; Solé-Violán, J.; Ferreres, J.; Cabrera, J.; Igeño, J.C.; et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit. Care 2013, 17, R290. [Google Scholar] [CrossRef]
- Abdelnaser, M.; Alaaeldin, R.; Attya, M.E.; Fathy, M. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 947–958. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Y.; Feng, Y.; Cui, R.; Miao, R.; Zhang, X.; Qu, K.; Liu, C.; Zhang, J. Methane alleviates sepsis-induced injury by inhibiting pyroptosis and apoptosis: In vivo and in vitro experiments. Aging 2019, 11, 1226. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, H.; Wan, X. Protective effect of Poria cocos polysaccharides on fecal peritonitis-induced sepsis in mice through inhibition of oxidative stress, inflammation, apoptosis, and reduction of Treg cells. Front. Microbiol. 2022, 13, 887949. [Google Scholar] [CrossRef]
- Dinc, S.; Caydere, M.; Akgul, G.; Yenidogan, E.; Hucumenoglu, S.; Rajesh, M. Methylene blue inhibits the inflammatory process of the acetic acid-induced colitis in rat colonic mucosa. Int. Surg. 2015, 100, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Liu, C.; Fu, B.; Ji, X.; Chen, M.; Zhang, Y.; Lin, Y.; Chen, J.; Song, Y.; Gu, S. Methylene blue reduces retinal cell inflammation, apoptosis, and oxidative stress in a rat model of diabetic retinopathy via sirtuin 1 activation. Altern. Ther. Health Med. 2023, 29, 156–165. [Google Scholar] [PubMed]
- Ahn, H.; Kang, S.G.; Yoon, S.-I.; Ko, H.-J.; Kim, P.-H.; Hong, E.-J.; An, B.-S.; Lee, E.; Lee, G.-S. MBinhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci. Rep. 2017, 7, 12409. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, H.; Qiao, G.; Zhang, Z. Methylene blue mitigates lung injury in HCA rats by regulating macrophage pyroptosis via Nrf2/HO-1 and NLRP3 pathways. Biomol. Biomed. 2025, 25, 2020–2034. [Google Scholar] [CrossRef]
- Li, Y.; Ying, W. Methylene blue reduces the serum levels of interleukin-6 and inhibits STAT3 activation in the brain and the skin of lipopolysaccharide-administered mice. Front. Immunol. 2023, 14, 1181932. [Google Scholar] [CrossRef]
- Lee, S.W.; Han, H.C. Methylene blue application to lessen pain: Its analgesic effect and mechanism. Front. Neurosci. 2021, 15, 663650. [Google Scholar] [CrossRef]
- Memis, D.; Karamanlioglu, B.; Yuksel, M.; Gemlik, I.; Pamukcu, Z. The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth. Intensive Care 2002, 30, 755–762. [Google Scholar] [CrossRef]
- Kirov, M.Y.; Evgenov, O.V.; Evgenov, N.V.; Egorina, E.M.; Sovershaev, M.A.; Sveinbjørnsson, B.; Nedashkovsky, E.V.; Bjertnaes, L.J. Infusion of methylene blue in human septic shock: A pilot, randomized, controlled study. Crit. Care Med. 2001, 29, 1860–1867. [Google Scholar] [CrossRef]
- Park, B.-K.; Shim, T.-S.; Lim, C.-M.; Lee, S.-D.; Kim, W.-S.; Kim, D.-S.; Kim, W.-D.; Koh, Y. The effects of methylene blue on hemodynamic parameters and cytokine levels in refractory septic shock. Korean J. Intern. Med. 2005, 20, 123–128. [Google Scholar] [CrossRef]
- El-Lakany, M.A.; Fouda, M.A.; El-Gowelli, H.M.; El-Gowilly, S.M.; El-Mas, M.M. Gonadal hormone receptors underlie the resistance of female rats to inflammatory and cardiovascular complications of endotoxemia. Eur. J. Pharmacol. 2018, 823, 41–48. [Google Scholar] [CrossRef]
- Diodato, M.D.; Knöferl, M.W.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 2001, 14, 162–169. [Google Scholar] [CrossRef]
- Kennedy, L.H.; Hwang, H.; Wolfe, A.M.; Hauptman, J.; Nemzek-Hamlin, J.A. Effects of buprenorphine and estrous cycle in a murine model of cecal ligation and puncture. Comp. Med. 2014, 64, 270–282. [Google Scholar]
- Zellweger, R.; Wichmann, M.W.; Ayala, A.; Stein, S.; DeMaso, C.M.; Chaudry, I.H. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit. Care Med. 1997, 25, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Chwastek, D.; Dwivedi, D.J.; Schlechte, J.; Yu, I.-L.; McDonald, B.; Arora, J.; Cani, E.; Eng, M.; Engelberts, D.; et al. Development and characterization of a fecal-induced peritonitis model of murine sepsis: Results from a multi-laboratory study and iterative modification of experimental conditions. Intensive Care Med. Exp. 2023, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Chen, A.; Heinen, L.; Liu, R.; Dwivedi, D.J.; Zhou, J.; Lalu, M.M.; Mendelson, A.A.; McDonald, B.; Kretz, C.A.; et al. Impact of age on the host response to sepsis in a murine model of fecal-induced peritonitis. Intensive Care Med. Exp. 2024, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Shaked, G.; Czeiger, D.; Dukhno, O.; Levy, I.; Artru, A.A.; Shapira, Y.; Douvdevani, A. Ketamine improves survival and suppresses IL-6 and TNFalpha production in a model of Gram-negative bacterial sepsis in rats. Resuscitation 2004, 62, 237–242. [Google Scholar] [CrossRef]
- Alva, N.; Palomeque, J.; Carbonell, T. Nitric oxide induced by ketamine/xylazine anesthesia maintains hepatic blood flow during hypothermia. Nitric Oxide 2006, 15, 64–69. [Google Scholar] [CrossRef]
- Galley, H.F.; Le Cras, A.E.; Logan, S.D.; Webster, N.R. Differential nitric oxide synthase activity, cofactor availability and cGMP accumulation in the central nervous system during anaesthesia. Br. J. Anaesth. 2001, 86, 388–394. [Google Scholar] [CrossRef]

| Control | FIP | FIP + Saline | FIP + MB | |
|---|---|---|---|---|
| AC | 0.0 [0.0–1.0] | 4.0 [3.0–4.0] * | 3.0 [3.0–4.0] * | 1.0 [1.0–1.25] *,## |
| H | 0.0 [0.0–0.25] | 2.0 [1.5–3.0] * | 2.0 [1.5–2.5] * | 0.0 [0.0–1.0] ## |
| AL | 0.0 [0.0–1.0] | 2.0 [2.0–3.0] * | 2.0 [2.0–3.5] * | 1.0 [1.0–1.25] *,## |
| PE | 0.0 [0.0–0.25] | 3.0 [3.0–4.0] * | 3.0 [3.0–4.0] * | 2.0 [1.0–2.25] *,# |
| TA | 0.0 [0.0–0.0] | 3.0 [2.0–3.5] * | 3.0 [2.0–3.0] * | 2.0 [1.0–2.0] *,# |
| Control | FIP | FIP + Saline | FIP + MB | |
|---|---|---|---|---|
| CT Hounsfield unit (HU) | −652 ± 12.13 | −512 ± 8.9 * | −509 ± 11.27 * | −586 ± 12.06 *,# |
| Control | FIP | FIP + Saline | FIP + MB | |
|---|---|---|---|---|
| PaO2 (mmHg) | 100.2 ± 1.645 | 71.64 ± 4.219 * | 72.09 ± 3.614 * | 90.04 ± 2.473 # |
| PaCO2 (mmHg) | 38.98 ± 0.8624 | 31.78 ± 1.379 * | 30.65 ± 1.293 * | 31.12 ± 1.237 * |
| Control | FIP | FIP + Saline | FIP + MB | |
|---|---|---|---|---|
| MDA (nM) | 9.3 ± 0.8 | 38.52 ± 1.8 *** | 40.62 ± 1.8 *** | 28.53 ± 2.5 ***,## |
| TNF alfa (pg/mL) | 12.56 ± 1.61 | 289.1 ± 13.69 *** | 321.7 ± 19.80 *** | 119.3 ± 7.646 ***,### |
| CRP (ng/mL) | 0.4 ± 0.05 | 1.2 ± 0.09 *** | 1.3 ± 0.11 *** | 0.7 ± 0.07 *,### |
| Lactic acid (mmol/L) | 1.5 ± 0.1 | 3.4 ± 0.3 ** | 3.5 ± 0.3 ** | 2.2 ± 0.2 # |
| c-GMP (pmol/mL) | 6.01 ± 0.5 | 13.06 ± 0.9 *** | 12.87 ± 1.12 *** | 8.9 ± 0.47 *,## |
| Control | FIP | FIP + Saline | FIP + MB | |
|---|---|---|---|---|
| IL-6 (pg/mL) | 5.5 ± 1.07 | 41,523 ± 3406 * | 39,864 ± 1836 * | 21,612 ± 1294 *,## |
| IL 1-Beta (pg/mL) | 3.34 ± 0.52 | 2520 ± 185.1 * | 3052 ± 177.7 * | 2244 ± 70.06 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibekoğlu, C.; Kemertaş, K.; Aygun, H.; Erbas, O. Methylene Blue Alleviates Inflammatory and Oxidative Lung Injury in a Rat Model of Feces-Induced Peritonitis. Medicina 2025, 61, 1456. https://doi.org/10.3390/medicina61081456
Dibekoğlu C, Kemertaş K, Aygun H, Erbas O. Methylene Blue Alleviates Inflammatory and Oxidative Lung Injury in a Rat Model of Feces-Induced Peritonitis. Medicina. 2025; 61(8):1456. https://doi.org/10.3390/medicina61081456
Chicago/Turabian StyleDibekoğlu, Cengiz, Kubilay Kemertaş, Hatice Aygun, and Oytun Erbas. 2025. "Methylene Blue Alleviates Inflammatory and Oxidative Lung Injury in a Rat Model of Feces-Induced Peritonitis" Medicina 61, no. 8: 1456. https://doi.org/10.3390/medicina61081456
APA StyleDibekoğlu, C., Kemertaş, K., Aygun, H., & Erbas, O. (2025). Methylene Blue Alleviates Inflammatory and Oxidative Lung Injury in a Rat Model of Feces-Induced Peritonitis. Medicina, 61(8), 1456. https://doi.org/10.3390/medicina61081456






