The Role of Myocardial Revascularization in Ischemic Heart Failure in the Era of Modern Optimal Medical Therapy
Abstract
1. Introduction
2. Materials and Methods
3. Pathophysiology of Ischemic Heart Failure and Chronic Total Occlusion
4. Evidence-Based Approaches to Revascularization Strategies in Ischemic Heart Failure with Reduced Ejection Fraction
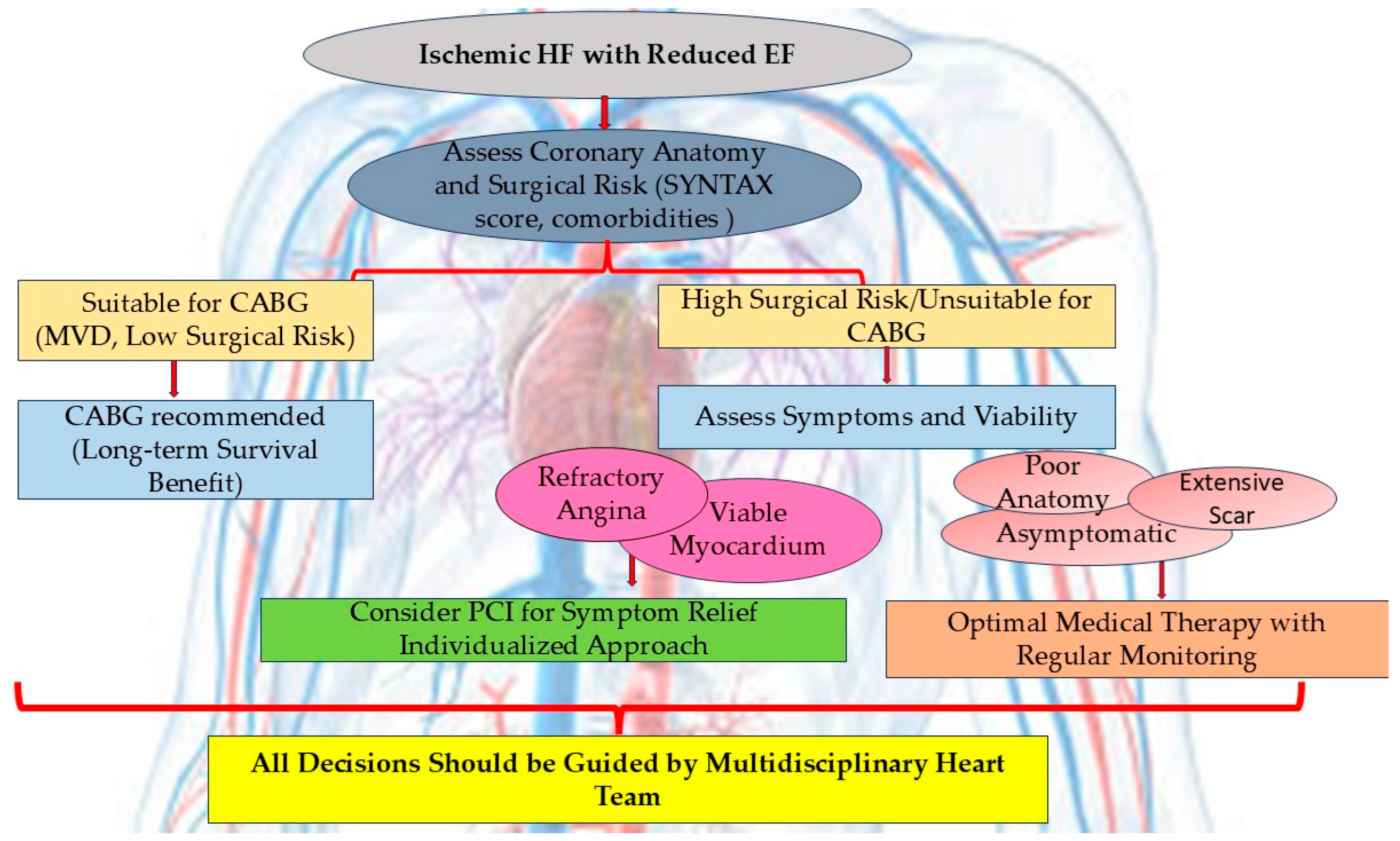
4.1. Patient Selection for Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention
4.1.1. Coronary Artery Bypass Graft Surgery in Ischemic Heart Failure with Reduced Ejection Fraction
4.1.2. Interventional Revascularization in Ischemic Heart Failure with Reduced Ejection Fraction—Revascularization for Chronic Total Occlusion
Revascularization for Chronic Total Occlusion (CTO): Current Evidence and Clinical Implications
4.1.3. Comparative Studies, Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention in Ischemic Heart Failure with Reduced Ejection Fraction
4.2. The Role of Myocardial Viability Assessment in Guiding Revascularization Strategies
4.3. Survival Outcome with Revascularization Compared to Optimal Medical Therapy
5. Coronary Microvascular Dysfunction in Ischemic Heart Failure
6. Contemporary Optimal Medical Therapy in Ischemic Heart Failure
7. Guidelines and Recommendations
8. Future Perspectives
9. Conclusions and Clinical Relevance
- CABG remains the preferred revascularization strategy in patients with MVD, suitable coronary anatomy, and acceptable surgical risk, especially in diabetics, younger patients, and those with concomitant significant mitral regurgitation. These recommendations are derived from trials conducted before the widespread use of contemporary pharmacological HF therapies.
- PCI has not demonstrated a survival benefit over OMT in randomized trials for ischemic HF. However, PCI may be a reasonable revascularization strategy in patients with prohibitive surgical risk, patients with persistent angina despite OMT, particularly when angina predominates over HF-related symptoms, and cases with documented myocardial viability in territories amenable to PCI.
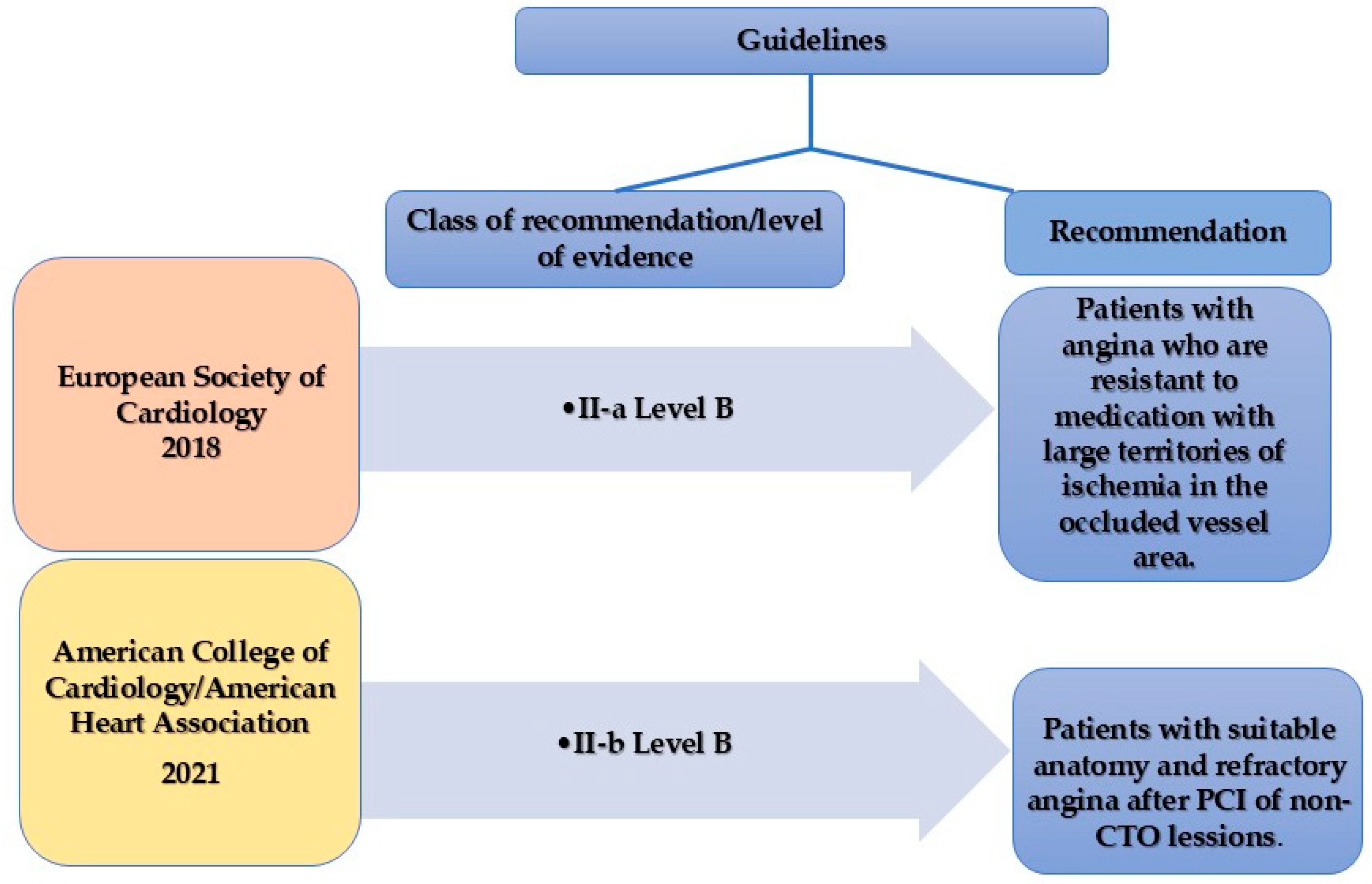
- The role of CTO PCI is not established; the current guidelines recommend it for symptomatic improvement in patients with refractory angina or extensive ischemia (LAD territory) when anatomical factors are favorable.
- Viability testing, though not mandatory, can aid in the revascularization decision-making process, especially in selecting borderline surgical candidates and in high-surgical-risk patients. When HF symptoms predominate, evaluation of myocardial scar burden versus viable hibernating myocardium will help in deciding between OMT and a revascularization strategy.
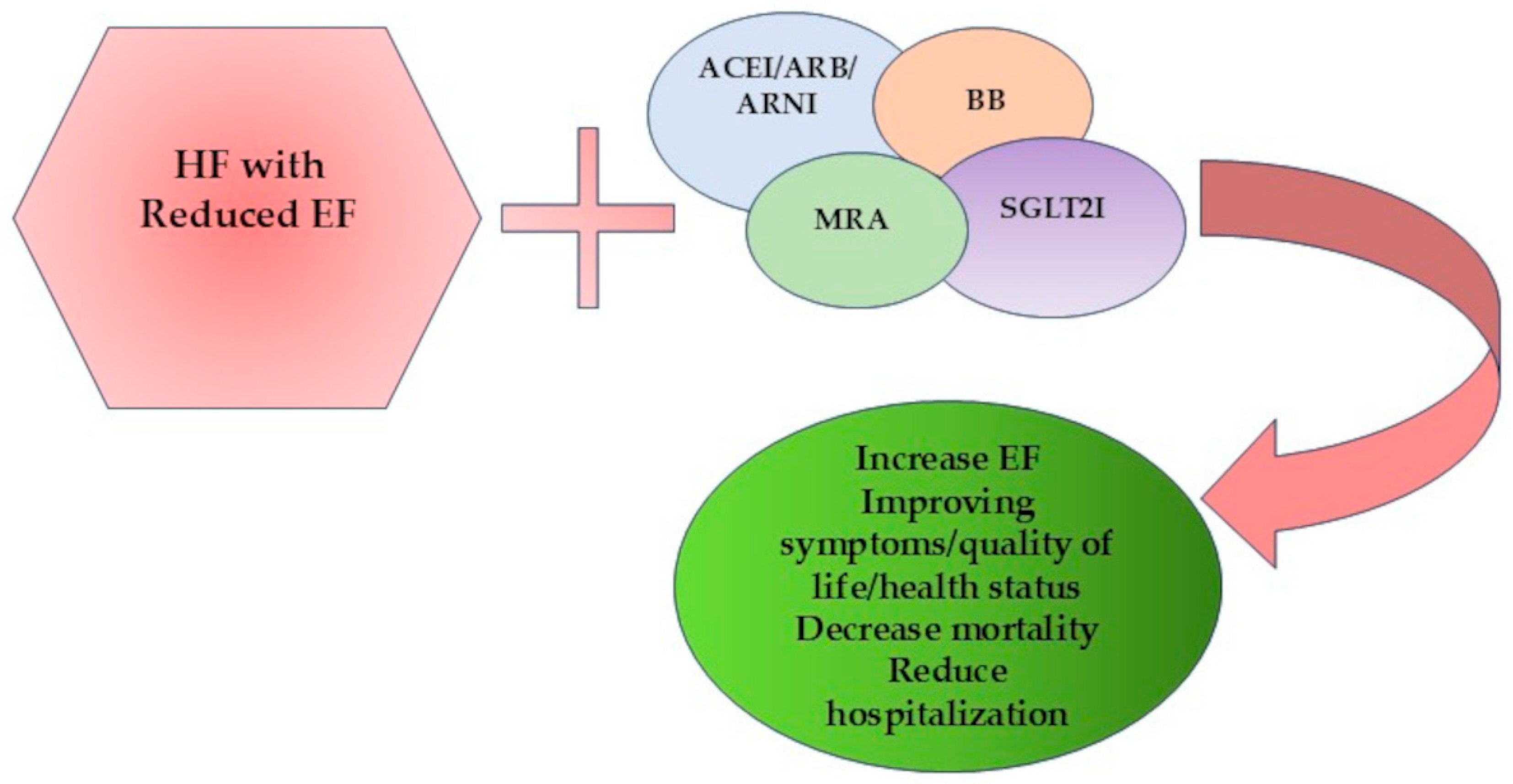
- OMT remains the essential therapeutic element of HF management, with ARNI, BB, MRA, and SGLT2I forming the main pillars, with clear evidence of improving survival and reducing hospitalizations.
- The optimal revascularization strategy in ischemic HF must be individualized. Elements as anatomical complexity, myocardial viability, comorbidities, and patient-centered factors should be assessed within a multidisciplinary Heart Team framework. The current evidence is insufficient to definitively determine the role of PCI in ischemic cardiomyopathy; future research should fill the gap in knowledge with rigorously designed, contemporary trials.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| ACC | American College of Cardiology |
| ACEIs | Angiotensin-converting enzyme inhibitors |
| AHA | American Heart Association |
| ARB | Angiotensin receptor blocker |
| ARNIs | Angiotensin receptor-neprilysin inhibitors |
| BB | Betablocker |
| CAD | Coronary artery disease |
| CABG | Coronary artery bypass grafting |
| CCS | Chronic coronary syndrome |
| CMD | Coronary microvascular dysfunction |
| CMR | Cardiac magnetic resonance |
| COR | Class of recommendation |
| CR | Complete revascularization |
| CTO | Chronic total occlusion |
| CXA | Circumflex artery |
| EF | Ejection fraction |
| ESC | European Society of Cardiology |
| EACTS | European Association for Cardiothoracic Surgery |
| FFR | Fractional flow reserve |
| GLS | Global longitudinal strain |
| GLP-1 Ras | Glucagon-like peptide receptor agonists |
| HCR | Hybrid coronary revascularization |
| HF | Heart failure |
| HFSA | Heart Failure Society of America |
| ICR | Incomplete revascularization |
| IVUS | Intravascular ultrasound |
| J-CTO | Japanese chronic total occlusion |
| LAD | Left arterial descending |
| LOE | Level of evidence |
| LV | Left ventricle |
| MACE | Major adverse cardiovascular event |
| MeSH | Medical Subject Heading |
| MI | Myocardial infarction |
| MVD | Multivessel disease |
| MR | Mitral regurgitation |
| MRA | Mineralocorticoid receptors |
| NO | Nitric oxide |
| NT-proBNP | N terminal proB-type natriuretic peptide |
| Non-IRA | Non-infarct-related artery |
| NSTEMI | Non-ST-elevation myocardial infarction |
| NYHA | New York Heart Association |
| OMT | Optimal medical therapy |
| PCI | Percutaneous coronary intervention |
| PET | Positron emission tomography |
| RCA | Right coronary artery |
| RCTs | Randomized controlled trials |
| ROS | Reactive oxygen species |
| SCAI | Society for Cardiovascular Angiography & Interventions |
| SGLT2Is | Sodium glucose co-transporter 2 inhibitors |
| STEMI | ST elevated myocardial infarction |
References
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the Management of Chronic Coronary Syndromes: Developed by the Task Force for the Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Briceno, N.; Schuster, A.; Lumley, M.; Perera, D. Ischaemic Cardiomyopathy: Pathophysiology, Assessment and the Role of Revascularisation. Heart 2016, 102, 397–406. [Google Scholar] [CrossRef]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- Wolf, S.; Schievano, E.; Amidei, C.B.; Kucher, N.; Valerio, L.; Barco, S.; Fedeli, U. Mortality Trend of Ischemic Heart Disease (2008–2022): A Retrospective Analysis of Epidemiological Data. Int. J. Cardiol. 2024, 406, 132042. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the Epidemiology of Cardiovascular Diseases: A Historical Perspective. Lancet 2013, 383, 999. [Google Scholar] [CrossRef] [PubMed]
- Silverdal, J.; Sjöland, H.; Bollano, E.; Pivodic, A.; Dahlström, U.; Fu, M. Prognostic Impact over Time of Ischaemic Heart Disease vs. Non-ischaemic Heart Disease in Heart Failure. ESC Heart Fail. 2020, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G. Mortality in Heart Failure Patients. Anatol. J. Cardiol. 2014, 15, 63. [Google Scholar] [CrossRef]
- Ryan, M.; Taylor, D.; Dodd, M.; Spertus, J.A.; Kosiborod, M.N.; Shaukat, A.; Docherty, K.F.; Clayton, T.; Perera, D.; Petrie, M.C.; et al. Effect of PCI on Health Status in Ischemic Left Ventricular Dysfunction. JACC Heart Fail. 2024, 12, 1553–1562. [Google Scholar] [CrossRef]
- Ezad, S.M.; McEntegart, M.; Dodd, M.; Didagelos, M.; Sidik, N.; Li Kam Wa, M.; Morgan, H.P.; Pavlidis, A.; Weerackody, R.; Walsh, S.J.; et al. Impact of Anatomical and Viability-Guided Completeness of Revascularization on Clinical Outcomes in Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 84, 340–350. [Google Scholar] [CrossRef]
- Petrie, M.C.; Jhund, P.S.; She, L.; Adlbrecht, C.; Doenst, T.; Panza, J.A.; Hill, J.A.; Lee, K.L.; Rouleau, J.L.; Prior, D.L.; et al. Ten Year Outcomes after Coronary Artery Bypass Grafting According to Age in Patients with Heart Failure and Left Ventricular Systolic Dysfunction: An Analysis of the Extended Follow up of the STICH Trial. Circulation 2016, 134, 1314. [Google Scholar] [CrossRef]
- Murashita, T. The Role of Coronary Artery Bypass Grafting in Patients with Ischemic Cardiomyopathy in the Current Era. J. Thorac. Dis. 2016, 8, E1032. [Google Scholar] [CrossRef]
- Doshi, D.; Ben-Yehuda, O.; Bonafede, M.; Josephy, N.; Karmpaliotis, D.; Parikh, M.A.; Moses, J.W.; Stone, G.W.; Leon, M.B.; Schwartz, A.; et al. Underutilization of Coronary Artery Disease Testing Among Patients Hospitalized With New-Onset Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 450–458. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Raja, V.; Wiegn, P.; Obel, O.; Christakopoulos, G.; Christopoulos, G.; Rangan, B.V.; Roesle, M.; Abdullah, S.M.; Luna, M.; Addo, T.; et al. Impact of Chronic Total Occlusions and Coronary Revascularization on All-Cause Mortality and the Incidence of Ventricular Arrhythmias in Patients with Ischemic Cardiomyopathy: The Study Was Presented at the SCAI 2014 Scientific Sessions, Las Vegas, Nevada. Am. J. Cardiol. 2015, 116, 1358–1362. [Google Scholar] [CrossRef]
- Hao, K.; Takahashi, J.; Sakata, Y.; Miyata, S.; Shiroto, T.; Nochioka, K.; Miura, M.; Oikawa, T.; Abe, R.; Sato, M.; et al. Prognostic Impact of Residual Stenosis after Percutaneous Coronary Intervention in Patients with Ischemic Heart Failure—A Report from the CHART-2 Study. Int. J. Cardiol. 2019, 278, 22–27. [Google Scholar] [CrossRef]
- Farooq, V.; Serruys, P.W.; Garcia-Garcia, H.M.; Zhang, Y.; Bourantas, C.V.; Holmes, D.R.; MacK, M.; Feldman, T.; Morice, M.C.; Ståhle, E.; et al. The Negative Impact of Incomplete Angiographic Revascularization on Clinical Outcomes and Its Association with Total Occlusions: The SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) Trial. J. Am. Coll. Cardiol. 2013, 61, 282–294. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.S.; Ryu, H.M.; Lee, H.; Bae, M.H.; Lee, J.H.; Yang, D.H.; Cho, Y.; Chae, S.C.; Jun, J.E. Impact of Multivessel Coronary Disease With Chronic Total Occlusion on One-Year Mortality in Patients With Acute Myocardial Infarction. Korean Circ. J. 2012, 42, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Morimoto, T.; Shiomi, H.; Furukawa, Y.; Nakagawa, Y.; Ando, K.; Kadota, K.; Kimura, T. Chronic Total Occlusion in a Non-Infarct-Related Artery Is Closely Associated with Increased Five-Year Mortality in Patients with ST-Segment Elevation Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention (from the CREDO-Kyoto AMI Registry). EuroIntervention 2017, 12, e1874–e1882. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Sakai, K.; Nakano, M.; Ohno, Y.; Nakazawa, G.; Shinozaki, N.; Matsukage, T.; Yoshimachi, F.; Ikari, Y. Impact of the Origin of the Collateral Feeding Donor Artery on Short-Term Mortality in ST-Elevation Myocardial Infarction with Comorbid Chronic Total Occlusion. Int. J. Cardiol. 2016, 218, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Claessen, B.E.; Dangas, G.D.; Weisz, G.; Witzenbichler, B.; Guagliumi, G.; Möckel, M.; Brener, S.J.; Xu, K.; Henriques, J.P.S.; Mehran, R.; et al. Prognostic Impact of a Chronic Total Occlusion in a Non-Infarct-Related Artery in Patients with ST-Segment Elevation Myocardial Infarction: 3-Year Results from the HORIZONS-AMI Trial. Eur. Heart J. 2012, 33, 768–775. [Google Scholar] [CrossRef]
- Braik, N.; Guedeney, P.; Behnes, M.; Desch, S.; Barthélémy, O.; Sandri, M.; de Waha-Thiele, S.; Fuernau, G.; Rouanet, S.; Hauguel-Moreau, M.; et al. Impact of Chronic Total Occlusion and Revascularization Strategy in Patients with Infarct-Related Cardiogenic Shock: A Subanalysis of the Culprit-Shock Trial. Am. Heart J. 2021, 232, 185–193. [Google Scholar] [CrossRef]
- Lexis, C.P.H.; Van Der Horst, I.C.C.; Rahel, B.M.; Lexis, M.A.S.; Kampinga, M.A.; Gu, Y.L.; De Smet, B.J.G.L.; Zijlstra, F. Impact of Chronic Total Occlusions on Markers of Reperfusion, Infarct Size, and Long-Term Mortality: A Substudy from the TAPAS-Trial. Catheter. Cardiovasc. Interv. 2011, 77, 484–491. [Google Scholar] [CrossRef]
- Damluji, A.A.; Pomenti, S.F.; Ramireddy, A.; Al-Damluji, M.S.; Alfonso, C.E.; Schob, A.H.; Marso, S.P.; Gilchrist, I.C.; Moscucci, M.; Kandzari, D.E.; et al. Influence of Total Coronary Occlusion on Clinical Outcomes (from the Bypass Angioplasty Revascularization Investigation 2 DiabetesTrial). Am. J. Cardiol. 2016, 117, 1031–1038. [Google Scholar] [CrossRef]
- Khan, A.A.; Khalid, M.F.; Ayub, M.T.; Murtaza, G.; Sardar, R.; White, C.J.; Mukherjee, D.; Nanjundappa, A.; Paul, T.K. Outcomes of Percutaneous Coronary Intervention Versus Optimal Medical Treatment for Chronic Total Occlusion: A Comprehensive Meta-Analysis. Curr. Probl. Cardiol. 2021, 46, 100695. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, P.H.; Ahn, J.M.; Park, D.W.; Yun, S.C.; Han, S.; Kang, H.; Kang, S.J.; Kim, Y.H.; Lee, C.W.; et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation 2019, 139, 1674–1683. [Google Scholar] [CrossRef]
- Henriques, J.P.S.; Hoebers, L.P.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Bax, M.; Ioanes, D.; Suttorp, M.J.; Strauss, B.H.; Barbato, E.; et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients With STEMI: The EXPLORE Trial. J. Am. Coll. Cardiol. 2016, 68, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A Randomized Multicentre Trial to Compare Revascularization with Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Obedinskiy, A.A.; Kretov, E.I.; Boukhris, M.; Kurbatov, V.P.; Osiev, A.G.; Ibn Elhadj, Z.; Obedinskaya, N.R.; Kasbaoui, S.; Grazhdankin, I.O.; Prokhorikhin, A.A.; et al. The IMPACTOR-CTO Trial. JACC Cardiovasc. Interv. 2018, 11, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520. [Google Scholar] [CrossRef]
- Babes, E.E.; Tit, D.M.; Bungau, A.F.; Bustea, C.; Rus, M.; Bungau, S.G.; Babes, V.V. Myocardial Viability Testing in the Management of Ischemic Heart Failure. Life 2022, 12, 1760. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Bustea, C.; Tit, D.M.; Bungau, A.F.; Bungau, S.G.; Pantea, V.A.; Babes, E.E.; Pantea-Roșan, L.R. Predictors of Readmission after the First Acute Coronary Syndrome and the Risk of Recurrent Cardiovascular Events—Seven Years of Patient Follow-Up. Life 2023, 13, 950. [Google Scholar] [CrossRef]
- Liao, R.; Li, Z.; Wang, Q.; Lin, H.; Sun, H. Revascularization of Chronic Total Occlusion Coronary Artery and Cardiac Regeneration. Front. Cardiovasc. Med. 2022, 9, 940808. [Google Scholar] [CrossRef]
- Galassi, A.R.; Vadalà, G.; Werner, G.S.; Cosyns, B.; Sianos, G.; Hill, J.; Dudek, D.; Picano, E.; Novo, G.; Andreini, D.; et al. Evaluation and Management of Patients with Coronary Chronic Total Occlusions Considered for Revascularisation. A Clinical Consensus Statement of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC, the European Association of Cardiovascular Imaging (EACVI) of the ESC, and the ESC Working Group on Cardiovascular Surgery. EuroIntervention 2024, 20, e174–e184. [Google Scholar] [CrossRef]
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current Perspectives on Coronary Chronic Total Occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef]
- Drescher, C.S.; Chamis, A.L.; Patolia, H.; Tanaka, J.; Tannu, M.; Othman, I.; Jones, W.S.; Rymer, J.A. Abstract 12820: Utilization of Cardiac Magnetic Resonance Imaging for Management of Coronary Chronic Total Occlusions. Circulation 2022, 146, A12820. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Teruel, L.; Muntane, G.; Campbell, N.G.; Fox, D.J.; Brown, B.; Skene, C.; Davidson, N.; Leon, V.; et al. Chronic Total Occlusion in an Infarct-Related Coronary Artery and the Risk of Appropriate ICD Therapies. J. Cardiovasc. Electrophysiol. 2017, 28, 1169–1178. [Google Scholar] [CrossRef]
- Kamalesh, M.; Sharp, T.G.; Tang, X.C.; Shunk, K.; Ward, H.B.; Walsh, J.; King, S.; Colling, C.; Moritz, T.; Stroupe, K.; et al. Percutaneous Coronary Intervention versus Coronary Bypass Surgery in United States Veterans with Diabetes. J. Am. Coll. Cardiol. 2013, 61, 808–816. [Google Scholar] [CrossRef]
- Myers, W.O.; Blackstone, E.H.; Davis, K.; Foster, E.D.; Kaiser, G.C. CASS Registry: Long Term Surgical Survival. J. Am. Coll. Cardiol. 1999, 33, 488–498. [Google Scholar] [CrossRef]
- Panza, J.A.; Velazquez, E.J.; She, L.; Smith, P.K.; Nicolau, J.C.; Favaloro, R.R.; Gradinac, S.; Chrzanowski, L.; Prabhakaran, D.; Howlett, J.G.; et al. Extent of Coronary and Myocardial Disease and Benefit from Surgical Revascularization in Patients with Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2014, 64, 553. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; Castelvecchio, S.; Panza, J.A.; Berman, D.S.; Velazquez, E.J.; Michler, R.E.; She, L.; Holly, T.A.; Desvigne-Nickens, P.; Kosevic, D.; et al. Severity of Remodeling, Myocardial Viability, and Survival in Ischemic LV Dysfunction After Surgical Revascularization. JACC Cardiovasc. Imaging 2015, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.; Wertheimer, J.; Miller, A.; O’Connor, C.M.; Pina, I.L.; Selzman, C.; Sueta, C.; She, L.; Greene, D.; Lee, K.L.; et al. The STICH Trial (Surgical Treatment for Ischemic Heart Failure): Mode-of-Death Results. JACC Heart Fail. 2013, 1, 400–408. [Google Scholar] [CrossRef]
- Higashino, A.; Tsuruta, Y.; Moriyama, S.; Miura, S.; Taketani, T.; Ohno, T. Does Cardiac Function Improvement With Coronary Artery Bypass Grafting Reduce All-Cause Mortality? Ann. Thorac. Surg. Short Rep. 2024, 2, 737–741. [Google Scholar] [CrossRef]
- Gaba, P.; Gersh, B.J.; Ali, Z.A.; Moses, J.W.; Stone, G.W. Complete versus Incomplete Coronary Revascularization: Definitions, Assessment and Outcomes. Nat. Rev. Cardiol. 2021, 18, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Complete Coronary Revascularization∗: A New Strategy to Improve Clinical Outcomes for Stable Coronary Artery Disease? J. Am. Coll. Cardiol. 2023, 82, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, H.Y.; Dou, K.; Yin, D.; Zhu, C.; Feng, L.; Zhou, Y.; Pu, J.; Zhang, Q.; Pan, H.; et al. Outcomes of Functionally Complete vs Incomplete Revascularization: Insights From the FAVOR III China Trial. Cardiovasc. Interv. 2022, 15, 2490–2502. [Google Scholar] [CrossRef]
- Garcia, S.; Sandoval, Y.; Roukoz, H.; Adabag, S.; Canoniero, M.; Yannopoulos, D.; Brilakis, E.S. Outcomes After Complete Versus Incomplete Revascularization of Patients With Multivessel Coronary Artery Disease: A Meta-Analysis of 89,883 Patients Enrolled in Randomized Clinical Trials and Observational Studies. J. Am. Coll. Cardiol. 2013, 62, 1421–1431. [Google Scholar] [CrossRef]
- Takahashi, K.; Serruys, P.W.; Gao, C.; Ono, M.; Wang, R.; Thuijs, D.J.F.M.; Mack, M.J.; Curzen, N.; Mohr, F.W.; Davierwala, P.; et al. Ten-Year All-Cause Death According to Completeness of Revascularization in Patients With Three-Vessel Disease or Left Main Coronary Artery Disease: Insights From the SYNTAX Extended Survival Study. Circulation 2021, 144, 96–109. [Google Scholar] [CrossRef]
- Fearon, W.F.; Zimmermann, F.M.; De Bruyne, B.; Piroth, Z.; van Straten, A.H.M.; Szekely, L.; Davidavičius, G.; Kalinauskas, G.; Mansour, S.; Kharbanda, R.; et al. Fractional Flow Reserve-Guided PCI as Compared with Coronary Bypass Surgery. N. Engl. J. Med. 2022, 386, 128–137. [Google Scholar] [CrossRef]
- Maestre-Luque, L.C.; Gonzalez-Manzanares, R.; Suárez de Lezo, J.; Hidalgo, F.; Barreiro-Mesa, L.; de Juan, J.; Gallo, I.; Perea, J.; Alvarado, M.; Romero, M.; et al. Complete vs. Incomplete Percutaneous Revascularization in Patients with Chronic Total Coronary Artery Occlusion. Front. Cardiovasc. Med. 2024, 11, 1443258. [Google Scholar] [CrossRef]
- Nagaraja, V.; Ooi, S.Y.; Nolan, J.; Large, A.; De Belder, M.; Ludman, P.; Bagur, R.; Curzen, N.; Matsukage, T.; Yoshimachi, F.; et al. Impact of Incomplete Percutaneous Revascularization in Patients with Multivessel Coronary Artery Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e004598. [Google Scholar] [CrossRef]
- Pantea-Roșan, L.R.; Bungau, S.G.; Radu, A.F.; Pantea, V.A.; Moisi, M.I.; Vesa, C.M.; Behl, T.; Nechifor, A.C.; Babes, E.E.; Stoicescu, M.; et al. A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon. Diagnostics 2022, 12, 932. [Google Scholar] [CrossRef]
- Alidoosti, M.; Saroukhani, S.; Lotfi-Tokaldany, M.; Jalali, A.; Sobh-Rakhshankhah, A. Objectifying the Level of Incomplete Revascularization by the Residual SYNTAX Score and Evaluating Its Impact on the One-Year Outcome of Percutaneous Coronary Intervention in Patients with Multi-Vessel Disease. Cardiovasc. Revasc. Med. 2016, 17, 308–312. [Google Scholar] [CrossRef]
- Rumiz, E.; Berenguer, A.; Vilar, J.V.; Valero, E.; Facila, L.; Cubillos, A.; Sanmiguel, D.; Almela, P.; Morell, S. Long-Term Outcomes and Predictors of Morbi-Mortality According to Age in Stemi Patients with Multivessel Disease: Impact of an Incomplete Revascularization. Catheter. Cardiovasc. Interv. 2018, 92, E512–E517. [Google Scholar] [CrossRef]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’Ascenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-Year Prognostic Differences and Management Strategies between ST-Elevation and Non-ST-Elevation Myocardial Infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs 2025, 1–11. [Google Scholar] [CrossRef]
- Gallinoro, E.; Paolisso, P.; Di Gioia, G.; Bermpeis, K.; Fernandez-Peregrina, E.; Candreva, A.; Esposito, G.; Fabbricatore, D.; Bertolone, D.T.; Bartunek, J.; et al. Deferral of Coronary Revascularization in Patients With Reduced Ejection Fraction Based on Physiological Assessment: Impact on Long-Term Survival. J. Am. Heart Assoc. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Kaltenbach, M.; Hartmann, A.; Vallbracht, C. Procedural Results and Patient Selection in Recanalization of Chronic Coronary Occlusions by Low Speed Rotational Angioplasty. Eur. Heart J. 1993, 14, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Kandzari, D.E.; Mehran, R.; Colombo, A.; Schwartz, R.S.; Bailey, S.; Moussa, I.; Teirstein, P.S.; Dangas, G.; Baim, D.S.; et al. Percutaneous Recanalization of Chronically Occluded Coronary Arteries: A Consensus Document—Part I. Circulation 2005, 112, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Brilakis, E.S.; Grantham, J.A.; Rinfret, S.; Wyman, R.M.; Burke, M.N.; Karmpaliotis, D.; Lembo, N.; Pershad, A.; Kandzari, D.E.; Buller, C.E.; et al. A Percutaneous Treatment Algorithm for Crossing Coronary Chronic Total Occlusions. JACC Cardiovasc. Interv. 2012, 5, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ramanath, V.S.; Thompson, C.A. Coronary Chronic Total Occlusion Recanalisation—Current Techniques and Approaches. Interv. Cardiol. Rev. 2013, 8, 41. [Google Scholar] [CrossRef]
- Ramanathan, K.; Abel, J.G.; Park, J.E.; Fung, A.; Mathew, V.; Taylor, C.M.; Mancini, G.B.J.; Gao, M.; Ding, L.; Verma, S.; et al. Surgical Versus Percutaneous Coronary Revascularization in Patients With Diabetes and Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2017, 70, 2995–3006. [Google Scholar] [CrossRef]
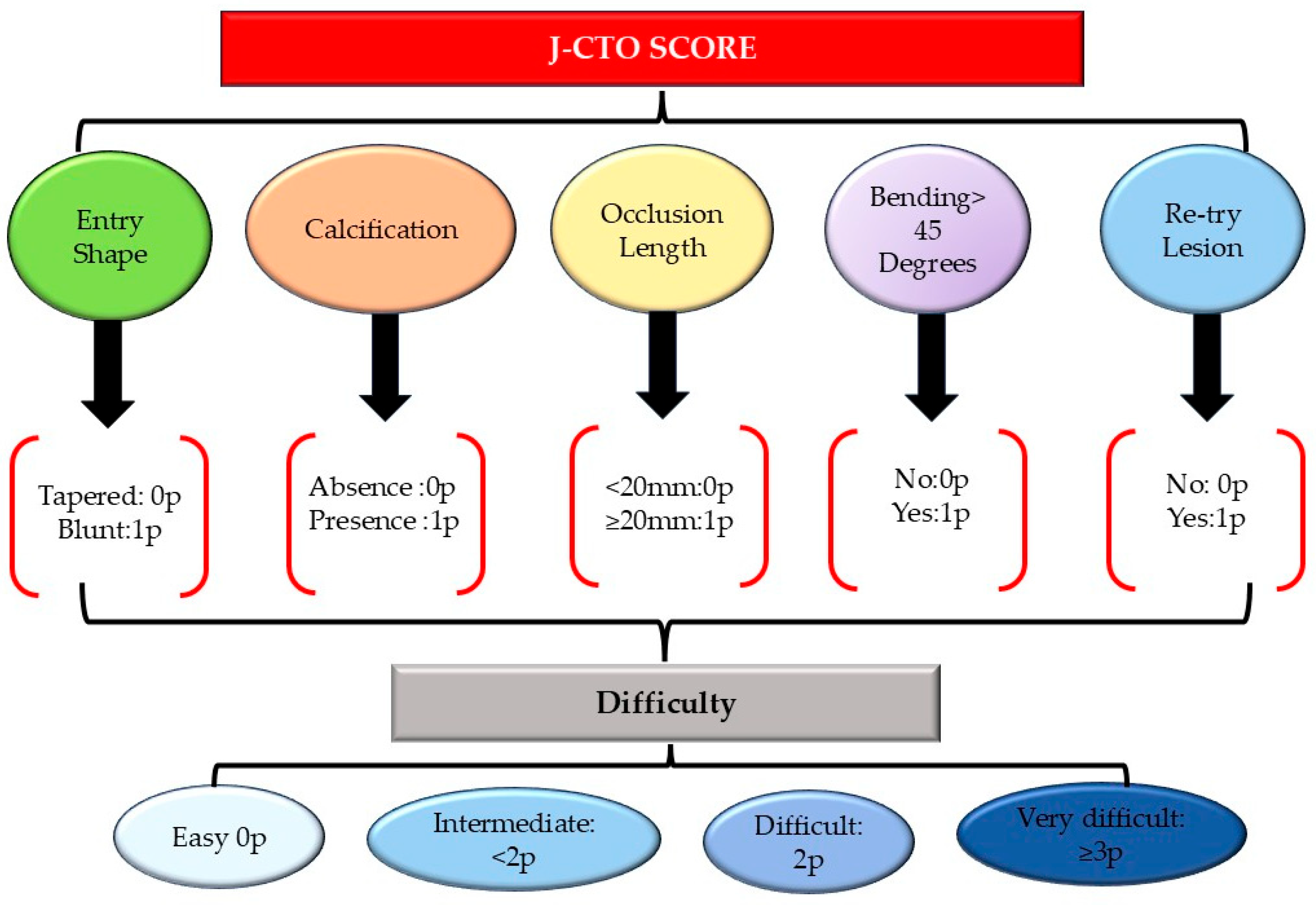
- Christopoulos, G.; Wyman, M.R.; Alaswad, K.; Karmpaliotis, D.; Lombardi, W.; Aaron Grantham, J.; Yeh, R.W.; Jaffer, F.A.; Cipher, D.J.; Rangan, B.V.; et al. Clinical Utility of the J-CTO Score in Coronary Chronic Total Occlusion Interventions: Results from a Multicenter Registry. Circ. Cardiovasc. Interv. 2015, 8, 10–1161. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Abi Rafeh, N.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention: A Global Expert Consensus Document. Circulation 2019, 140, 420–433. [Google Scholar] [CrossRef]
- Tanaka, H.; Morino, Y.; Abe, M.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Impact of J-CTO Score on Procedural Outcome and Target Lesion Revascularisation after Percutaneous Coronary Intervention for Chronic Total Occlusion: A Substudy of the J-CTO Registry (Multicentre CTO Registry in Japan). EuroIntervention 2016, 11, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.; Gonzalo, N.; Moreno, V.H.; Fuentes, M.; Santos-Martinez, S.; Fernandez-Diaz, J.A.; Amat-Santos, I.J.; Ojeda, F.B.; Borrego, J.C.; Cuesta, J.; et al. Choice of CTO Scores to Predict Procedural Success in Clinical Practice. A Comparison of 4 Different CTO PCI Scores in a Comprehensive National Registry Including Expert and Learning CTO Operators. PLoS ONE 2021, 16, e0245898. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting Successful Guidewire Crossing Through Chronic Total Occlusion of Native Coronary Lesions Within 30 Minutes: The J-CTO (Multicenter CTO Registry in Japan) Score as a Difficulty Grading and Time Assessment Tool. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Mares, A.; Mukherjee, D. Management of Chronic Total Occlusion of Coronary Artery. Int. J. Angiol. 2020, 30, 48. [Google Scholar] [CrossRef]
- Elias, J.; van Dongen, I.M.; Hoebers, L.P.; Ouweneel, D.M.; Claessen, B.E.P.M.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Piek, J.J.; van der Schaaf, R.J.; et al. Recovery and Prognostic Value of Myocardial Strain in ST-Segment Elevation Myocardial Infarction Patients with a Concurrent Chronic Total Occlusion. Eur. Radiol. 2020, 30, 600–608. [Google Scholar] [CrossRef]
- van Veelen, A.; van Dongen, I.M.; Elias, J.; Råmunddal, T.; Eriksen, E.; van der Schaaf, R.J.; Claessen, B.E.P.M.; Postema, P.G.; Henriques, J.P.S. Exercise Testing after Chronic Total Coronary Occlusion Revascularization in Patients with STEMI and a Concurrent CTO: A Subanalysis of the EXPLORE-Trial. Catheter. Cardiovasc. Interv. 2019, 94, 536–545. [Google Scholar] [CrossRef]
- van Dongen, I.M.; Kolk, M.Z.H.; Elias, J.; Meijborg, V.M.F.; Coronel, R.; de Bakker, J.M.T.; Claessen, B.E.P.M.; Delewi, R.; Ouweneel, D.M.; Scheunhage, E.M.; et al. The Effect of Revascularization of a Chronic Total Coronary Occlusion on Electrocardiographic Variables. A Sub-Study of the EXPLORE Trial. J. Electrocardiol. 2018, 51, 906–912. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; van Veelen, A.; Agostoni, P.; van Houwelingen, G.K.; Ouweneel, D.M.; Hoebers, L.P.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Bax, M.; et al. Predictors and Outcomes of Procedural Failure of Percutaneous Coronary Intervention of a Chronic Total Occlusion-A Subanalysis of the EXPLORE Trial. Catheter. Cardiovasc. Interv. 2021, 97, 1176–1183. [Google Scholar] [CrossRef]
- Allahwala, U.K.; Ward, M.R.; Brieger, D.; Weaver, J.C.; Bhindi, R. Indications for Percutaneous Coronary Intervention (PCI) in Chronic Total Occlusion (CTO): Have We Reached a DECISION or Do We Continue to EXPLORE After EURO-CTO? Heart Lung Circ. 2019, 28, 1484–1489. [Google Scholar] [CrossRef]
- Elias, J.; Van Dongen, I.M.; Hoebers, L.P.; Ouweneel, D.M.; Claessen, B.E.P.M.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Van Der Schaaf, R.J.; Ioanes, D.; et al. Improved Recovery of Regional Left Ventricular Function after PCI of Chronic Total Occlusion in STEMI Patients: A Cardiovascular Magnetic Resonance Study of the Randomized Controlled EXPLORE Trial. J. Cardiovasc. Magn. Reson. 2017, 19, 53. [Google Scholar] [CrossRef]
- Werner, G.S.; Hildick-Smith, D.; Yuste, V.M.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. Three-Year Outcomes of A Randomized Multicentre Trial Comparing Revascularization and Optimal Medical Therapy for Chronic Total Coronary Occlusions (EuroCTO). EuroIntervention 2023, 19, 571–579. [Google Scholar] [CrossRef]
- Park, S.-J. Optimal Medical Therapy With or Without Stenting For Coronary Chronic Total Occlusion—American College of Cardiology. Available online: https://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2017/03/17/08/40/DECISION-CTO (accessed on 6 March 2025).
- Brilakis, E.S.; Mashayekhi, K.; Burke, M.N. How DECISION-CTO Can Help Guide the Decision to Perform Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019, 139, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K.; Nührenberg, T.G.; Toma, A.; Gick, M.; Ferenc, M.; Hochholzer, W.; Comberg, T.; Rothe, J.; Valina, C.M.; Löffelhardt, N.; et al. A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion: The REVASC Trial. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Claessen, B.E.; Dangas, G.D.; Godino, C.; Henriques, J.P.S.; Leon, M.B.; Park, S.J.; Stone, G.W.; Moses, J.W.; Colombo, A.; Mehran, R. Impact of Target Vessel on Long-Term Survival after Percutaneous Coronary Intervention for Chronic Total Occlusions. Catheter. Cardiovasc. Interv. 2013, 82, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Safley, D.M.; House, J.A.; Marso, S.P.; Grantham, J.A.; Rutherford, B.D. Improvement in Survival Following Successful Percutaneous Coronary Intervention of Coronary Chronic Total Occlusions: Variability by Target Vessel. JACC Cardiovasc. Interv. 2008, 1, 295–302. [Google Scholar] [CrossRef]
- Will, M.; Schwarz, K.; Aufhauser, S.; Leibundgut, G.; Schmidt, E.; Mayer, D.; Vock, P.; Borovac, J.A.; Kwok, C.S.; Lamm, G.; et al. The Impact of Successful Chronic Total Occlusion Percutaneous Coronary Intervention on Clinical Outcomes: A Tertiary Single-Center Analysis. Front. Cardiovasc. Med. 2024, 11, 1447829. [Google Scholar] [CrossRef]
- Khan, M.S.; Sami, F.; Singh, H.; Ullah, W.; Ma’en, A.D.; Changal, K.H.; Mir, T.; Ali, Z.; Kabour, A. Medical Therapy vs Early Revascularization in Diabetics with Chronic Total Occlusions: A Meta-Analysis and Systematic Review. World J. Cardiol. 2020, 12, 559–570. [Google Scholar] [CrossRef]
- Almarzooq, Z.I.; Tamez, H.; Wang, Y.; Curtis, J.P.; Kirtane, A.J.; Secemsky, E.A.; Valsdottir, L.R.; Yeh, R.W. Long-Term Outcomes of Chronic Total Occlusion Percutaneous Coronary Intervention Among Medicare Beneficiaries. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100584. [Google Scholar] [CrossRef]
- Megaly, M.; Saad, M.; Tajti, P.; Burke, M.N.; Chavez, I.; Gössl, M.; Lips, D.; Mooney, M.; Poulose, A.; Sorajja, P.; et al. Meta-Analysis of the Impact of Successful Chronic Total Occlusion Percutaneous Coronary Intervention on Left Ventricular Systolic Function and Reverse Remodeling. J. Interv. Cardiol. 2018, 31, 562–571. [Google Scholar] [CrossRef]
- Bondarenko, O.; Beek, A.M.; Twisk, J.W.R.; Visser, C.A.; Van Rossum, A.C. Time Course of Functional Recovery after Revascularization of Hibernating Myocardium: A Contrast-Enhanced Cardiovascular Magnetic Resonance Study. Eur. Heart J. 2008, 29, 2000–2005. [Google Scholar] [CrossRef]
- Råmunddal, T.; Holck, E.N.; Karim, S.; Eftekhari, A.; Escaned, J.; Ioanes, D.; Walsh, S.; Spratt, J.; Veien, K.; Jensen, L.O.; et al. International Randomized Trial on the Effect of Revascularization or Optimal Medical Therapy of Chronic Total Coronary Occlusions with Myocardial Ischemia—ISCHEMIA-CTO Trial—Rationale and Design. Am. Heart J. 2023, 257, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, C.; Sorini Dini, C.; Werner, G.S. Thousand Registries Are Not Worth a Randomized Trial: Also True for Chronic Total Occlusions? JACC Cardiovasc. Interv. 2017, 10, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.R.; Brilakis, E.S.; Boukhris, M.; Tomasello, S.D.; Sianos, G.; Karmpaliotis, D.; Di Mario, C.; Strauss, B.H.; Rinfret, S.; Yamane, M.; et al. Appropriateness of Percutaneous Revascularization of Coronary Chronic Total Occlusions: An Overview. Eur. Heart J. 2016, 37, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, A.; Coerkamp, C.F.; Somsen, Y.B.O.; Råmunddal, T.; Ioanes, D.; Laanmets, P.; van der Schaaf, R.J.; Eriksen, E.; Bax, M.; Suttorp, M.J.; et al. Ten-Year Outcome of Recanalization or Medical Therapy for Concomitant Chronic Total Occlusion After Myocardial Infarction. J. Am. Heart Assoc. 2024, 13, 33556. [Google Scholar] [CrossRef]
- Mohr, F.W.; Morice, M.C.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; MacK, M.J.; Holmes, D.R.; Morel, M.A.; Van Dyck, N.; et al. Coronary Artery Bypass Graft Surgery versus Percutaneous Coronary Intervention in Patients with Three-Vessel Disease and Left Main Coronary Disease: 5-Year Follow-up of the Randomised, Clinical SYNTAX Trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- Serruys, P.W.; Revaiah, P.C.; Ninomiya, K.; Masuda, S.; Kotoku, N.; Kageyama, S.; Onuma, Y.; Morel, M.A.; Garg, S.; Feldman, T.; et al. 10 Years of SYNTAX: Closing an Era of Clinical Research After Identifying New Outcome Determinants. JACC Asia 2023, 3, 409–430. [Google Scholar] [CrossRef]
- Sedlis, S.P.; Ramanathan, K.B.; Morrison, D.A.; Sethi, G.; Sacks, J.; Henderson, W. Outcome of Percutaneous Coronary Intervention versus Coronary Bypass Grafting for Patients with Low Left Ventricular Ejection Fractions, Unstable Angina Pectoris, and Risk Factors for Adverse Outcomes with Bypass (the AWESOME Randomized Trial and Registry). Am. J. Cardiol. 2004, 94, 118–120. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M.; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef]
- Farkouh, M.E.; Domanski, M.; Sleeper, L.A.; Siami, F.S.; Dangas, G.; Mack, M.; Yang, M.; Cohen, D.J.; Rosenberg, Y.; Solo-mon, S.D.; et al. Strategies for Multivessel Revascularization in Patients with Diabetes. N. Engl. J. Med. 2012, 367, 2375–2384. [Google Scholar] [CrossRef]
- Råmunddal, T.; Hoebers, L.; Henriques, J.P.S.; Dworeck, C.; Angerås, O.; Odenstedt, J.; Ioanes, D.; Olivecrona, G.; Harnek, J.; Jensen, U.; et al. Chronic Total Occlusions in Sweden--a Report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS ONE 2014, 9, e103850. [Google Scholar] [CrossRef]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Bader Eddeen, A.; Ruel, M. Long-Term Outcomes in Patients With Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting. JAMA Cardiol. 2020, 5, 631–641. [Google Scholar] [CrossRef]
- Nagendran, J.; Norris, C.M.; Graham, M.M.; Ross, D.B.; Macarthur, R.G.; Kieser, T.M.; Maitland, A.M.; Southern, D.; Meyer, S.R. Coronary Revascularization for Patients with Severe Left Ventricular Dysfunction. Ann. Thorac. Surg. 2013, 96, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhang, D.; Lyu, S.; Song, X.; Yuan, F.; Xu, F.; Zhang, M. Meta-Analysis Comparing Percutaneous Coronary Revascularization Using Drug-Eluting Stent Versus Coronary Artery Bypass Grafting in Patients With Left Ventricular Systolic Dysfunction. Am. J. Cardiol. 2018, 122, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Ricci, F.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; De Stasio, V.; Di Donna, C.; Spiritigliozzi, L.; Chiocchi, M.; Lee, S.J.; et al. Cardiac Magnetic Resonance Tissue Characterization in Ischemic Cardiomyopathy. J. Thorac. Imaging 2022, 37, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli-Ducci, C.; Auger, D.; Di Mario, C.; Locca, D.; Petryka, J.; O’Hanlon, R.; Grasso, A.; Wright, C.; Symmonds, K.; Wage, R.; et al. CMR Guidance for Recanalization of Coronary Chronic Total Occlusion. JACC Cardiovasc. Imaging 2016, 9, 547–556. [Google Scholar] [CrossRef]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Myocardial Viability Testing and Impact of Revascularization on Prognosis in Patients with Coronary Artery Disease and Left Ventricular Dysfunction: A Meta-Analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Calvert, M.; Freemantle, N.; Arrow, Y.; Ball, S.G.; Bonser, R.S.; Chattopadhyay, S.; Norell, M.S.; Pennell, D.J.; Senior, R. The Heart Failure Revascularisation Trial (HEART). Eur. J. Heart Fail. 2011, 13, 227–233. [Google Scholar] [CrossRef]
- Beanlands, R.S.B.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; Gulenchyn, K.Y.; Garrard, L.; deKemp, R.; Guo, A.; et al. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction and Suspected Coronary Disease. A Randomized, Controlled Trial (PARR-2). J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef]
- Mc Ardle, B.; Shukla, T.; Nichol, G.; Dekemp, R.A.; Bernick, J.; Guo, A.; Lim, S.P.; Davies, R.A.; Haddad, H.; Duchesne, L.; et al. Long-Term Follow-Up of Outcomes With F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction Secondary to Coronary Disease. Circ. Cardiovasc. Imaging 2016, 9, e004331. [Google Scholar] [CrossRef]
- Liga, R.; Colli, A.; Taggart, D.P.; Boden, W.E.; De Caterina, R. Myocardial Revascularization in Patients With Ischemic Cardiomyopathy: For Whom and How. J. Am. Heart Assoc. 2023, 12, 26943. [Google Scholar] [CrossRef]
- Stone, G.W.; Ali, Z.A.; O’Brien, S.M.; Rhodes, G.; Genereux, P.; Bangalore, S.; Mavromatis, K.; Horst, J.; Dressler, O.; Poh, K.K.; et al. Impact of Complete Revascularization in the ISCHEMIA Trial. J. Am. Coll. Cardiol. 2023, 82, 1175–1188. [Google Scholar] [CrossRef]
- de Vos, A.; Jansen, T.P.J.; van ’t Veer, M.; Dimitriu-Leen, A.; Konst, R.E.; Elias-Smale, S.; Paradies, V.; Rodwell, L.; van den Oord, S.; Smits, P.; et al. Microvascular Resistance Reserve to Assess Microvascular Dysfunction in ANOCA Patients. JACC Cardiovasc. Interv. 2023, 16, 470–481. [Google Scholar] [CrossRef]
- Moliner-Abós, C.; Calvo-Barceló, M.; Solé-Gonzalez, E.; Borrellas Martín, A.; Fluvià-Brugués, P.; Sánchez-Vega, J.; Vime-Jubany, J.; Vallverdú, M.F.; Taurón Ferrer, M.; Tobias-Castillo, P.E.; et al. Revascularization and Outcomes in Ischaemic Left Ventricular Dysfunction after Heart Failure Admission: The RevascHeart Study. Eur. J. Heart Fail. 2024, 27, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuki, R.T.; Shrive, F.M.; Galbraith, P.D.; Knudtson, M.L.; Graham, M.M. Revascularization in Patients with Heart Failure. CMAJ 2006, 175, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Gautier, S.V.; Mironkov, A.B.; Sakhovsky, S.A.; Koloskova, N.N.; Muminov, I.I.; Spirina, E.A.; Tunyaeva, I.Y.; Mironkov, B.L. Efficacy of Myocardial Revascularization in Potential Recipients of Heart with the Chronic Ischemic Heart Failure. Vestn. Transplantologii Iskusstv. Organov. 2018, 20, 39–44. [Google Scholar] [CrossRef]
- Revascularization Versus Medical Treatment in Patients With Ischemic Left Ventricular Dysfunction|Clinical Research Trial Listing. Available online: https://www.centerwatch.com/clinical-trials/listings/NCT05828719/revascularization-versus-medical-treatment-in-patients-with-ischemic-left-ventricular-dysfunction (accessed on 16 April 2025).
- Cikes, M.; Solomon, S.D. Beyond Ejection Fraction: An Integrative Approach for Assessment of Cardiac Structure and Function in Heart Failure. Eur. Heart J. 2016, 37, 1642–1650. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Merz, C.N.B. Coronary Microvascular Dysfunction: An Update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 Inhibitors: Mechanisms of Cardiovascular Benefit beyond Glycaemic Control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- La Vecchia, G.; Fumarulo, I.; Caffè, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. [Google Scholar] [CrossRef]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation Position Paper on “Coronary Microvascular Dysfunction in Cardiovascular Disease”. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef]
- Mancini, G.B.J.; Henry, G.C.; Macaya, C.; O’Neill, B.J.; Pucillo, A.L.; Carere, R.G.; Wargovich, T.J.; Mudra, H.; Lüscher, T.F.; Klibaner, M.I.; et al. Angiotensin-Converting Enzyme Inhibition with Quinapril Improves Endothelial Vasomotor Dysfunction in Patients with Coronary Artery Disease: The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 1996, 94, 258–265. [Google Scholar] [CrossRef]
- Bruen, R.; Curley, S.; Kajani, S.; Lynch, G.; O’Reilly, M.E.; Dillon, E.T.; Brennan, E.P.; Barry, M.; Sheehan, S.; McGillicuddy, F.C.; et al. Liraglutide Attenuates Preestablished Atherosclerosis in Apolipoprotein E-Deficient Mice via Regulation of Immune Cell Phenotypes and Proinflammatory Mediators. J. Pharmacol. Exp. Ther. 2019, 370, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Malgie, J.; Clephas, P.R.D.; Brunner-La Rocca, H.P.; de Boer, R.A.; Brugts, J.J. Guideline-Directed Medical Therapy for HFrEF: Sequencing Strategies and Barriers for Life-Saving Drug Therapy. Heart Fail. Rev. 2023, 28, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Riegger, A.J.G. ACE Inhibitors in Congestive Heart Failure. Cardiology 1989, 76 (Suppl. S2), 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.C. Angiotensin-Converting Enzyme Inhibitors or β-Blockers in Heart Failure. Circulation 2005, 112, 2380–2382. [Google Scholar] [CrossRef]
- Khalil, M.E.; Basher, A.W.; Brown, E.J.; Alhaddad, I.A. A Remarkable Medical Story: Benefits of Angiotensin-Converting Enzyme Inhibitors in Cardiac Patients. J. Am. Coll. Cardiol. 2001, 37, 1757–1764. [Google Scholar] [CrossRef]
- Desai, A.S.; McMurray, J.J.V.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Chen, F.; Gong, J.; Rizkala, A.R.; Brahimi, A.; Claggett, B.; et al. Effect of the Angiotensin-Receptor-Neprilysin Inhibitor LCZ696 Compared with Enalapril on Mode of Death in Heart Failure Patients. Eur. Heart J. 2015, 36, 1990–1997. [Google Scholar] [CrossRef]
- Chauhan, M.; Patel, J.B.; Ahmad, F. Ramipril. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Wilson, S.; Colucci, M. Renal Effects of ACE Inhibitors in Heart Failure—UpToDate. Available online: https://www.uptodate.com/contents/renal-effects-of-ace-inhibitors-in-heart-failure/print (accessed on 24 January 2025).
- Arnold, J.M.O.; Yusuf, S.; Young, J.; Mathew, J.; Johnstone, D.; Avezum, A.; Lonn, E.; Pogue, J.; Bosch, J. Prevention of Heart Failure in Patients in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2003, 107, 1284–1290. [Google Scholar] [CrossRef]
- Ambrosioni, E.; Borghi, C.; Magnani, B. The Effect of the Angiotensin-Converting-Enzyme Inhibitor Zofenopril on Mortality and Morbidity after Anterior Myocardial Infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N. Engl. J. Med. 1995, 332, 80–85. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Nair, A.P.; Misra, A.; Scott, C.Z.; Mahar, J.H.; Fedson, S. Neprilysin Inhibitors in Heart Failure: The Science, Mechanism of Action, Clinical Studies, and Unanswered Questions. JACC Basic Transl. Sci. 2022, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Rakugi, H. Angiotensin Receptor-Neprilysin Inhibitors: Comprehensive Review and Implications in Hypertension Treatment. Hypertens. Res. 2021, 44, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.S.; Lang, C.C. Angiotensin Receptor-Neprilysin Inhibitors: Clinical Potential in Heart Failure and Beyond. Vasc. Health Risk Manag. 2015, 11, 283. [Google Scholar] [CrossRef]
- Solomon, S.D.; Claggett, B.; Packer, M.; Desai, A.; Zile, M.R.; Swedberg, K.; Rouleau, J.; Shi, V.; Lefkowitz, M.; McMurray, J.J.V. Efficacy of Sacubitril/Valsartan Relative to a Prior Decompensation: The PARADIGM-HF Trial. JACC Heart Fail. 2016, 4, 816–822. [Google Scholar] [CrossRef]
- Carnicelli, A.P.; Li, Z.; Greiner, M.A.; Lippmann, S.J.; Greene, S.J.; Mentz, R.J.; Hardy, N.C.; Blumer, V.; Shen, X.; Yancy, C.W.; et al. Sacubitril/Valsartan Adherence and Postdischarge Outcomes Among Patients Hospitalized for Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2021, 9, 876–886. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E. Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef]
- Wachter, R.; Senni, M.; Belohlavek, J.; Straburzynska-Migaj, E.; Witte, K.K.; Kobalava, Z.; Fonseca, C.; Goncalvesova, E.; Cavusoglu, Y.; Fernandez, A.; et al. Initiation of Sacubitril/Valsartan in Haemodynamically Stabilised Heart Failure Patients in Hospital or Early after Discharge: Primary Results of the Randomised TRANSITION Study. Eur. J. Heart Fail. 2019, 21, 998–1007. [Google Scholar] [CrossRef]
- Masarone, D.; Martucci, M.L.; Errigo, V.; Pacileo, G. The Use of β-Blockers in Heart Failure with Reduced Ejection Fraction. J. Cardiovasc. Dev. Dis. 2021, 8, 101. [Google Scholar] [CrossRef]
- Arnold, S.V.; Silverman, D.N.; Gosch, K.; Nassif, M.E.; Infeld, M.; Litwin, S.; Meyer, M.; Fendler, T.J. Beta-Blocker Use and Heart Failure Outcomes in Mildly Reduced and Preserved Ejection Fraction. JACC Heart Fail. 2023, 11, 893–900. [Google Scholar] [CrossRef]
- Marti, H.P.; Pavía López, A.A.; Schwartzmann, P. Safety and Tolerability of β-Blockers: Importance of Cardioselectivity. Curr. Med. Res. Opin. 2024, 40, 55–62. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A.; Swedberg, K.; Cleland, J.G.F.; Di Lenarda, A.; Hanrath, P.; Komajda, M.; Lubsen, J.; Lutiger, B.; Metra, M.; Remme, W.J.; et al. Comparison of Carvedilol and Metoprolol on Clinical Outcomes in Patients with Chronic Heart Failure in the Carvedilol Or Metoprolol European Trial (COMET): Randomised Controlled Trial. Lancet 2003, 362, 7–13. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A.; Dargie, H.J.; Lechat, P.; Drummond, G.A.; Squire, I.B.; Segev, A.; Mekori, Y.A. The Cardiac Insufficiency Bisoprolol Study II. Lancet 1999, 353, 1360–1361. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarson, Å.; Goldstein, S.; Fagerberg, B.; Wedel, H.; Waagstein, F.; Kjekshus, J.; Wikstrand, J.; El Allaf, D.; Vítovec, J.; Aldershvile, J.; et al. Effects of Controlled-Release Metoprolol on Total Mortality, Hospitalizations, and Well-Being in Patients with Heart Failure: The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000, 283, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Edelmann, F. The Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone and Heart Failure with Preserved Ejection Fraction. Cardiovasc. Diabetol. 2023, 22, 162. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.Y.; Zannad, F. Safety and Tolerability of the Novel Non-Steroidal Mineralocorticoid Receptor Antagonist BAY 94-8862 in Patients with Chronic Heart Failure and Mild or Moderate Chronic Kidney Disease: A Randomized, Double-Blind Trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef]
- Bhinder, J.; Patibandla, S.; Gupta, C.A.; Levine, A.; Gass, A.L.; Lanier, G.M. Mineralocorticoid Receptor Antagonist Use in Heart Failure With Reduced Ejection Fraction and End-Stage Renal Disease Patients on Dialysis: A Literature Review. Cardiol. Rev. 2020, 28, 107–115. [Google Scholar] [CrossRef]
- Duran, J.M.; Gad, S.; Brann, A.; Greenberg, B. Mineralocorticoid Receptor Antagonist Use Following Heart Failure Hospitalization. ESC Heart Fail. 2020, 7, 482–492. [Google Scholar] [CrossRef]
- Chang, J.; Ambrosy, A.P.; Vardeny, O.; Van Spall, H.G.C.; Mentz, R.J.; Sauer, A.J. Mineralocorticoid Antagonism in Heart Failure: Established and Emerging Therapeutic Role. JACC Heart Fail. 2024, 12, 1979–1993. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- Glick, H.A.; Orzol, S.M.; Tooley, J.F.; Remme, W.J.; Sasayama, S.; Pitt, B. Economic Evaluation of the Randomized Aldactone Evaluation Study (RALES): Treatment of Patients with Severe Heart Failure. Cardiovasc. Drugs Ther. 2002, 16, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.V.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Williams, G.; Remme, W.; Martinez, F.; Lopez-Sendon, J.; Zannad, F.; Neaton, J.; Roniker, B.; Hurley, S.; Burns, D.; et al. The EPHESUS Trial: Eplerenone in Patients with Heart Failure Due to Systolic Dysfunction Complicating Acute Myocardial Infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc. Drugs Ther. 2001, 15, 79–87. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 Inhibitors in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Spertus, J.A.; Birmingham, M.C.; Nassif, M.; Damaraju, C.V.; Abbate, A.; Butler, J.; Lanfear, D.E.; Lingvay, I.; Kosiborod, M.N.; Januzzi, J.L. The SGLT2 Inhibitor Canagliflozin in Heart Failure: The CHIEF-HF Remote, Patient-Centered Randomized Trial. Nat. Med. 2022, 28, 809–813. [Google Scholar] [CrossRef]
- Butler, J.; Usman, M.S.; Khan, M.S.; Greene, S.J.; Friede, T.; Vaduganathan, M.; Filippatos, G.; Coats, A.J.S.; Anker, S.D. Efficacy and Safety of SGLT2 Inhibitors in Heart Failure: Systematic Review and Meta-Analysis. ESC Heart Fail. 2020, 7, 3298–3309. [Google Scholar] [CrossRef]
- Jhalani, N.B. Clinical Considerations for Use of SGLT2 Inhibitor Therapy in Patients with Heart Failure and Reduced Ejection Fraction: A Review. Adv. Ther. 2022, 39, 3472–3487. [Google Scholar] [CrossRef]
- Kaplinsky, E. DAPA-HF Trial: Dapagliflozin Evolves from a Glucose-Lowering Agent to a Therapy for Heart Failure. Drugs Context 2020, 9, 2019-11-3. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef]
- Talano, J.V.; Scanlon, P.J.; Meadows, W.R.; Kahn, M.; Pifarre, R.; Gunnar, R.M. Influence of Surgery on Survival in 145 Patients with Left Main Coronary Artery Disease. Circulation 1975, 52, I105–I111. [Google Scholar]
- European Coronary Surgery Study Group. Long-Term Results of Prospective Randomised Study of Coronary Artery Bypass Surgery in Stable Angina Pectoris. Lancet 1982, 320, 1173–1180. [Google Scholar] [CrossRef]
- The Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. Eleven-Year Survival in the Veterans Administration Randomized Trial of Coronary Bypass Surgery for Stable Angina. N. Engl. J. Med. 1984, 311, 1333–1339. [Google Scholar] [CrossRef]
- Head, S.J.; Milojevic, M.; Daemen, J.; Ahn, J.M.; Boersma, E.; Christiansen, E.H.; Domanski, M.J.; Farkouh, M.E.; Flather, M.; Fuster, V.; et al. Mortality after Coronary Artery Bypass Grafting versus Percutaneous Coronary Intervention with Stenting for Coronary Artery Disease: A Pooled Analysis of Individual Patient Data. Lancet 2018, 391, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Bonow, R.O.; Bhatt, D.L.; Falk, V.; Gaudino, M. Improving Terminology to Describe Coronary Artery Procedures: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 180–188. [Google Scholar] [CrossRef]
- Vedin, O.; Lam, C.S.P.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.O.; Savarese, G.; Dahlström, U.; Lund, L.H. Significance of Ischemic Heart Disease in Patients with Heart Failure and Preserved, Midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Anderson, M.; Burkhoff, D.; Grines, C.L.; Kapur, N.K.; Lansky, A.J.; Mannino, S.; McCabe, J.M.; Alaswad, K.; Daggubati, R.; et al. Improved Outcomes in Patients with Severely Depressed LVEF Undergoing Percutaneous Coronary Intervention with Contemporary Practices. Am. Heart J. 2022, 248, 139–149. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Kleiman, N.S.; Moses, J.; Henriques, J.P.S.; Dixon, S.; Massaro, J.; Palacios, I.; Maini, B.; Mulukutla, S.; Džavík, V.; et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support with Impella 2.5 versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention: The PROTECT II Study. Circulation 2012, 126, 1717–1727. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; Dimaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E18–E114. [Google Scholar] [CrossRef]
- Patel, M.R.; Calhoon, J.H.; Dehmer, G.J.; Grantham, J.A.; Maddox, T.M.; Maron, D.J.; Smith, P.K. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Th. J. Am. Coll. Cardiol. 2017, 69, 2212–2241. [Google Scholar] [CrossRef]
- Thielmann, M.; Bonaros, N.; Barbato, E.; Barili, F.; Folliguet, T.; Friedrich, G.; Gottardi, R.; Legutko, J.; Parolari, A.; Punjabi, P.; et al. Hybrid Coronary Revascularization: Position Paper of the European Society of Cardiology Working Group on Cardiovascular Surgery and European Association of Percutaneous Cardiovascular Interventions. Eur. J. Cardio-Thoracic. Surg. 2024, 66, ezae271. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Wu, Y.; Cozzens, K.; Sundt, T.M.; Girardi, L.; Chikwe, J.; Wechsler, A.; Smith, C.R.; Gold, J.P.; Lahey, S.J.; et al. Hybrid Coronary Revascularization Versus Conventional Coronary Artery Bypass Surgery: Utilization and Comparative Outcomes. Circ. Cardiovasc. Interv. 2020, 13, E009386. [Google Scholar] [CrossRef]
- Hage, A.; Giambruno, V.; Jones, P.; Chu, M.W.; Fox, S.; Teefy, P.; Lavi, S.; Bainbridge, D.; Harle, C.; Iglesias, I.; et al. Hybrid Coronary Revascularization Versus Off-Pump Coronary Artery Bypass Grafting: Comparative Effectiveness Analysis With Long-Term Follow-up. J. Am. Heart Assoc. 2019, 8, e014204. [Google Scholar] [CrossRef]
- Hannan, E.L.; Wu, Y.F.; Cozzens, K.; Tamis-Holland, J.; Ling, F.S.K.; Jacobs, A.K.; Venditti, F.J.; Berger, P.B.; Walford, G.; King, S.B. Hybrid Coronary Revascularization vs. Percutaneous Coronary Interventions for Multivessel Coronary Artery Disease. J. Geriatr. Cardiol. 2021, 18, 159. [Google Scholar] [CrossRef]
- Abubakar, M.; Javed, I.; Rasool, H.F.; Raza, S.; Basavaraju, D.; Abdullah, R.M.; Ahmed, F.; Salim, S.S.; Faraz, M.A.; Hassan, K.M.; et al. Advancements in Percutaneous Coronary Intervention Techniques: A Comprehensive Literature Review of Mixed Studies and Practice Guidelines. Cureus 2023, 15, e41311. [Google Scholar] [CrossRef]
- Sabouret, P.; Manzo-Silberman, S.; Alasnag, M.; Fysekidis, M.; Gulati, M.; Galati, G.; Spadafora, L.; Banach, M.; Biondi-Zoccai, G.; Bhatt, D.L. New Approaches to Reduce Recurrent PCI: To Angioplasty and Beyond! Eur. Heart J. Open 2023, 3, oead049. [Google Scholar] [CrossRef]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef] [PubMed]
- ul Haq, M.A.; Wong, C.; Mutha, V.; Anavekar, N.; Lim, K.; Barlis, P.; Hare, D.L. Therapeutic Interventions for Heart Failure with Preserved Ejection Fraction: A Summary of Current Evidence. World J. Cardiol. 2014, 6, 67. [Google Scholar] [CrossRef] [PubMed]


| Study | Time | Inclusion Criteria | Exclusion Criteria | No. Pts. | Symptoms/ EF | Mortality/ Outcome/ MACE | Ref. |
|---|---|---|---|---|---|---|---|
| DECISION-CTO | 2010–2016 | Silent ischemia Stable angina ACS CTO > 2.5 mm | EF < 30% CTO 3 different vessels Severe comorbidities | 834 | 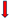 Angina Angina Quality of life Quality of life EF EF | No difference | [83] |
| EURO-CTO | 2012–2015 | CTO > 2.5 mm CTO > 3 months | Intolerance to dual antiplatelet therapy Need for elective non-cardiac surgery | 396 | NYHA class improved at 12 months in PCI groups | No difference | [32] |
| EXPLORE-CTO | 2007–2015 | CTO > 2.5 mm CTO in non-infarcted related artery after PCI for STEMI | Hemodynamic instability Valvular disease requiring surgery AF Severe renal insufficiency | 304 | CTO-LAD EF at 4 months follow-up EF at 4 months follow-up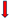 Angina Angina | No difference | [31] |
| IMPACTOR-CTO | 2010–2014 | RCA-CTO | Unsuccessful PCI-CTO | 94 | 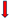 Angina AnginaImproved symptoms | No difference | [33] |
 —decrease and
—decrease and  —increase.
—increase.| Treatment | Mortality | EF Improvement | MACE | Symptoms Relief And QOL | Hospitalization Rate |
|---|---|---|---|---|---|
| PCI | No consistent benefit over OMT | Limited/no significant improvement over OMT | No benefits over OMT | QOL improved at 6–12 months/no difference at 24 months | No significant reduction over OMT |
| CABG | Consistent long-term benefit over OMT Long-term benefit over PCI in MVD/SYNTAX score > 23 | Improvement in selected groups with severe LV dysfunction | Reduced in MVD/SYNTAX score > 23 | Improved | Reduced in the long term |
| Modern OMT | Consistent benefit | Improvement | Reduced | Improved/limited in refractory angina | Reduced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaj-Tunduc, I.-P.; Brisc, C.M.I.; Brisc, C.M.; Zaha, D.-C.; Buştea, C.-M.; Babeş, V.-V.; Sirca-Tirla, T.; Muste, F.-A.; Babeş, E.-E. The Role of Myocardial Revascularization in Ischemic Heart Failure in the Era of Modern Optimal Medical Therapy. Medicina 2025, 61, 1451. https://doi.org/10.3390/medicina61081451
Blaj-Tunduc I-P, Brisc CMI, Brisc CM, Zaha D-C, Buştea C-M, Babeş V-V, Sirca-Tirla T, Muste F-A, Babeş E-E. The Role of Myocardial Revascularization in Ischemic Heart Failure in the Era of Modern Optimal Medical Therapy. Medicina. 2025; 61(8):1451. https://doi.org/10.3390/medicina61081451
Chicago/Turabian StyleBlaj-Tunduc, Ioana-Paula, Ciprian Marcel Ioan Brisc, Cristina Mihaela Brisc, Dana-Carmen Zaha, Cristiana-Magdalena Buştea, Vlad-Victor Babeş, Teodora Sirca-Tirla, Francesca-Andreea Muste, and Elena-Emilia Babeş. 2025. "The Role of Myocardial Revascularization in Ischemic Heart Failure in the Era of Modern Optimal Medical Therapy" Medicina 61, no. 8: 1451. https://doi.org/10.3390/medicina61081451
APA StyleBlaj-Tunduc, I.-P., Brisc, C. M. I., Brisc, C. M., Zaha, D.-C., Buştea, C.-M., Babeş, V.-V., Sirca-Tirla, T., Muste, F.-A., & Babeş, E.-E. (2025). The Role of Myocardial Revascularization in Ischemic Heart Failure in the Era of Modern Optimal Medical Therapy. Medicina, 61(8), 1451. https://doi.org/10.3390/medicina61081451









