A Systematic Review and Meta-Analysis of the Success Rate of the Primary Probing in Pediatric Patients with Congenital Nasolacrimal Duct Obstruction in Different Age Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration
2.2. Search Strategy
2.3. Eligibility Criteria, Study Selection and Data Collection
2.4. Meta-Analysis
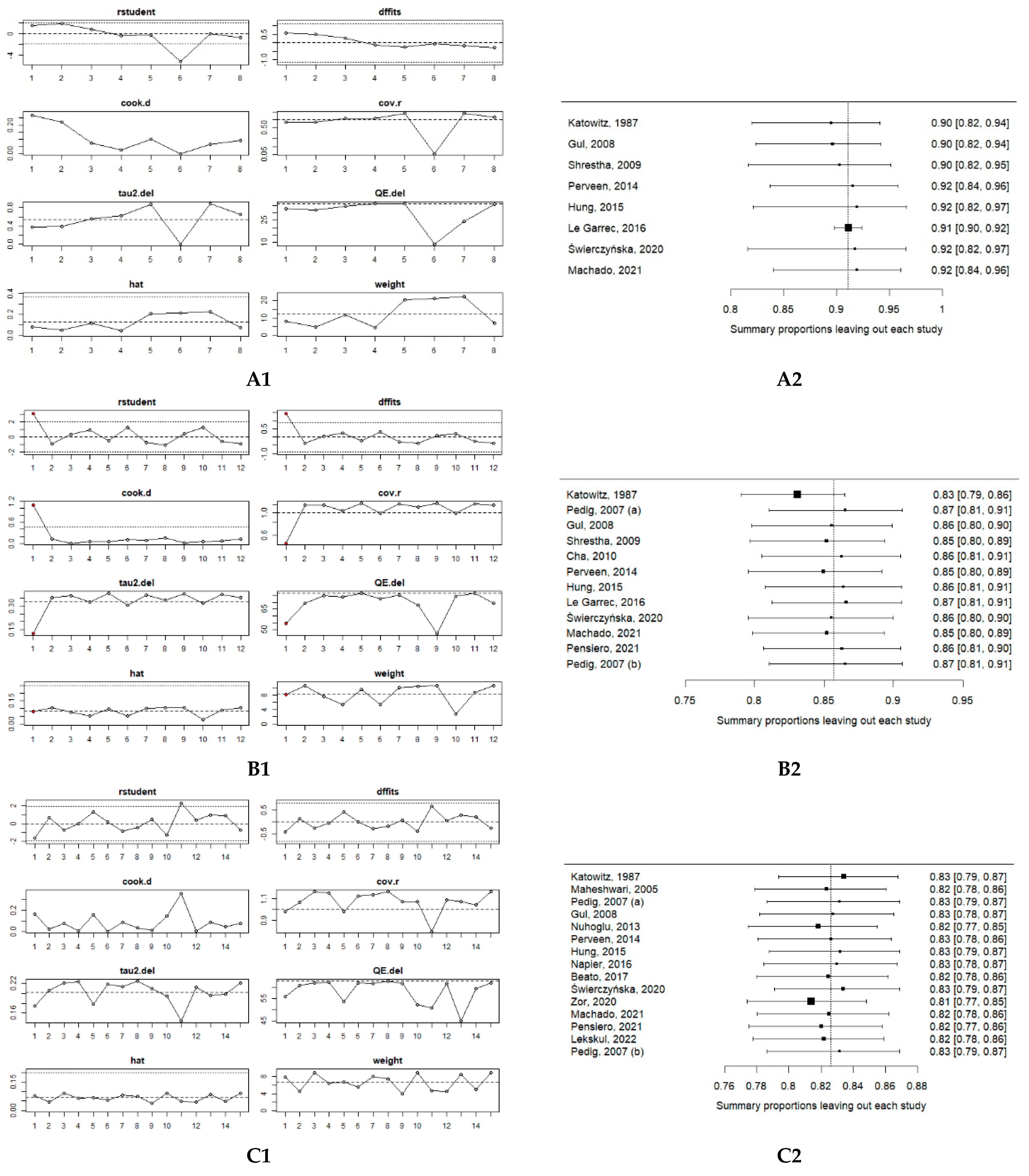
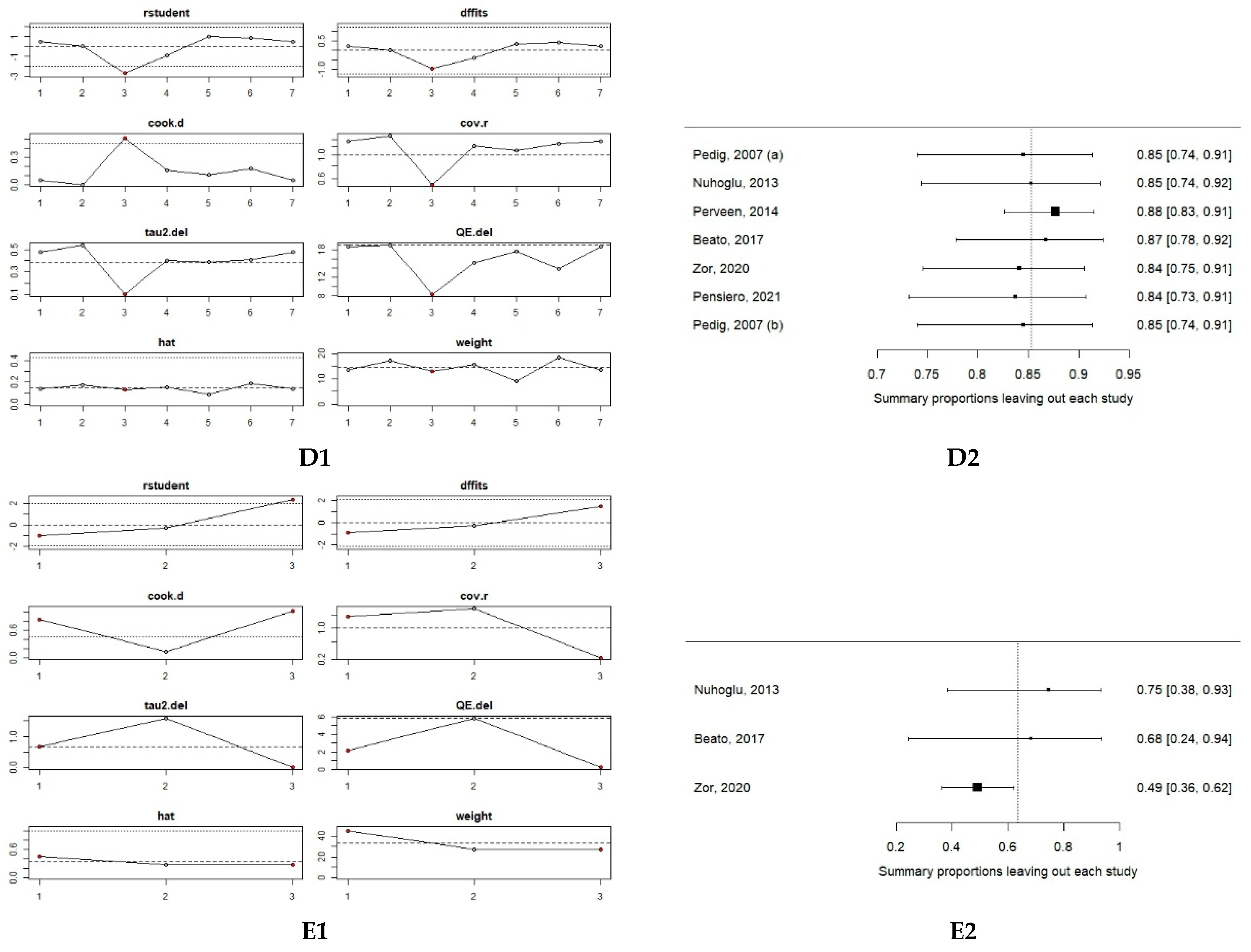
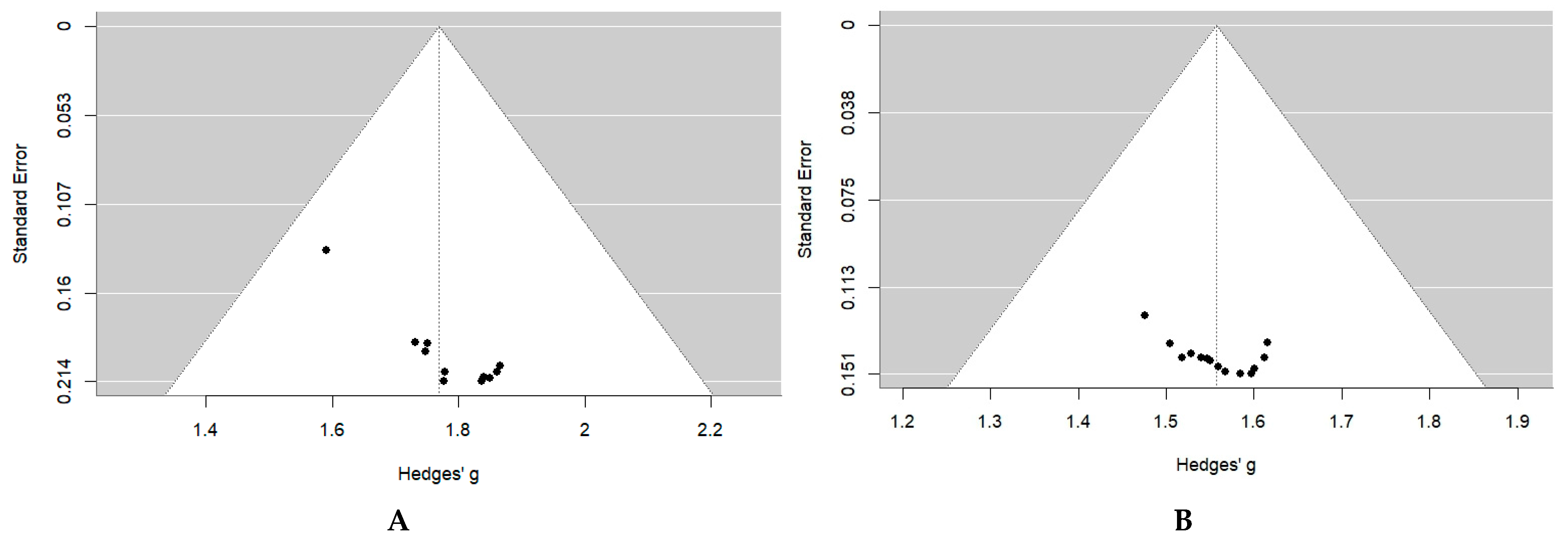
2.5. Risk of Bias and Certainty of Evidence
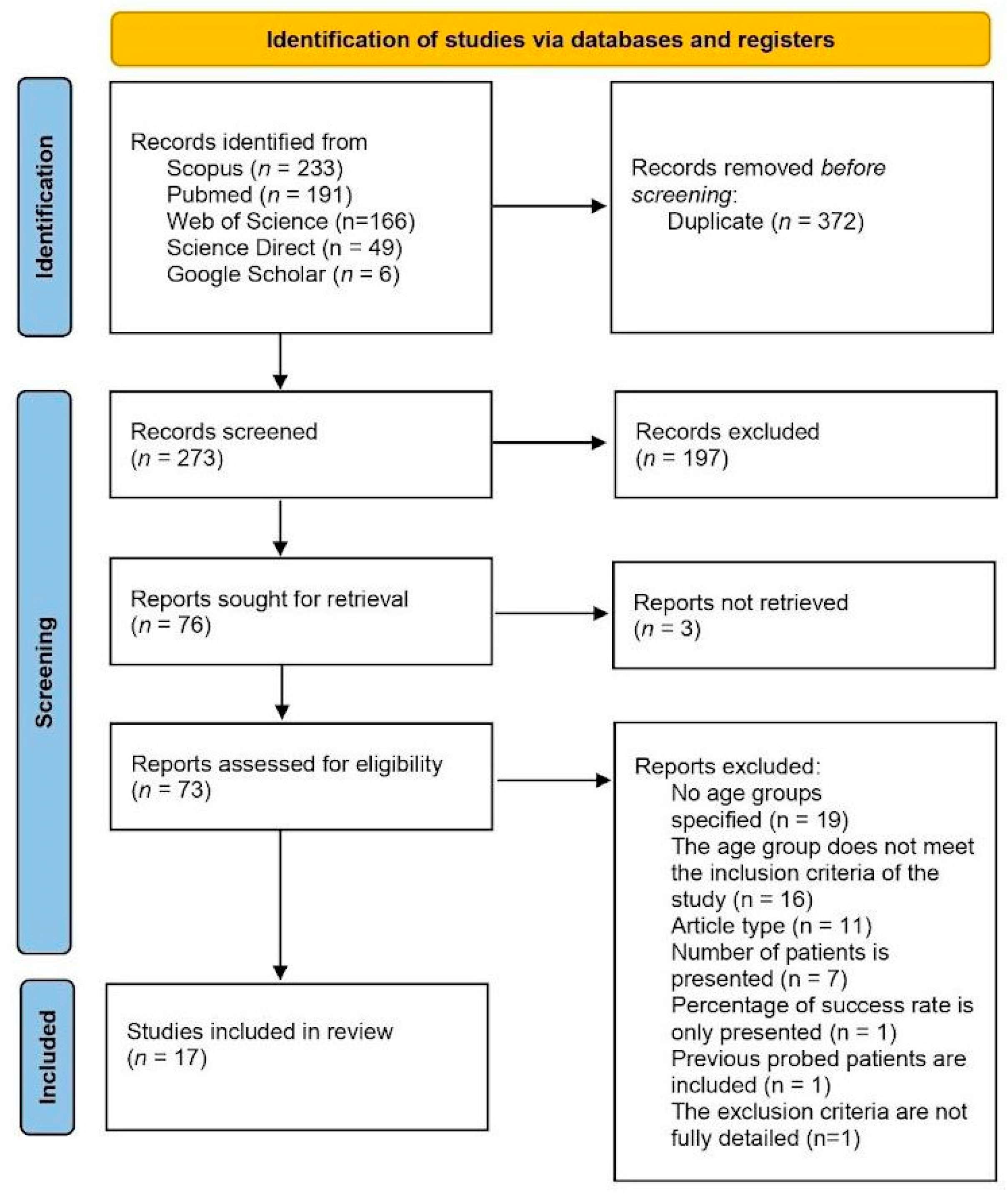
3. Results
3.1. Study Selection and Characteristics of the Included Studies
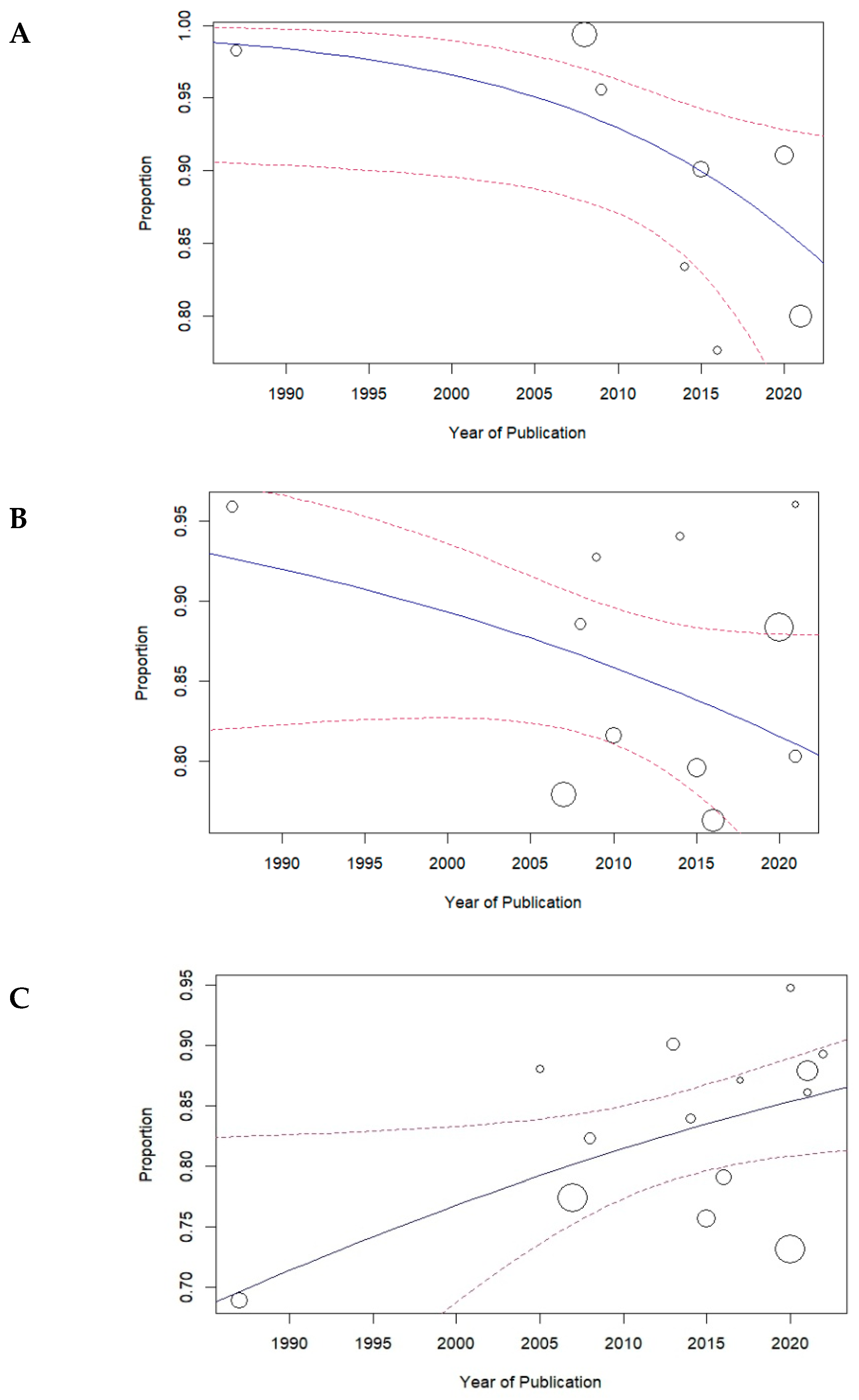
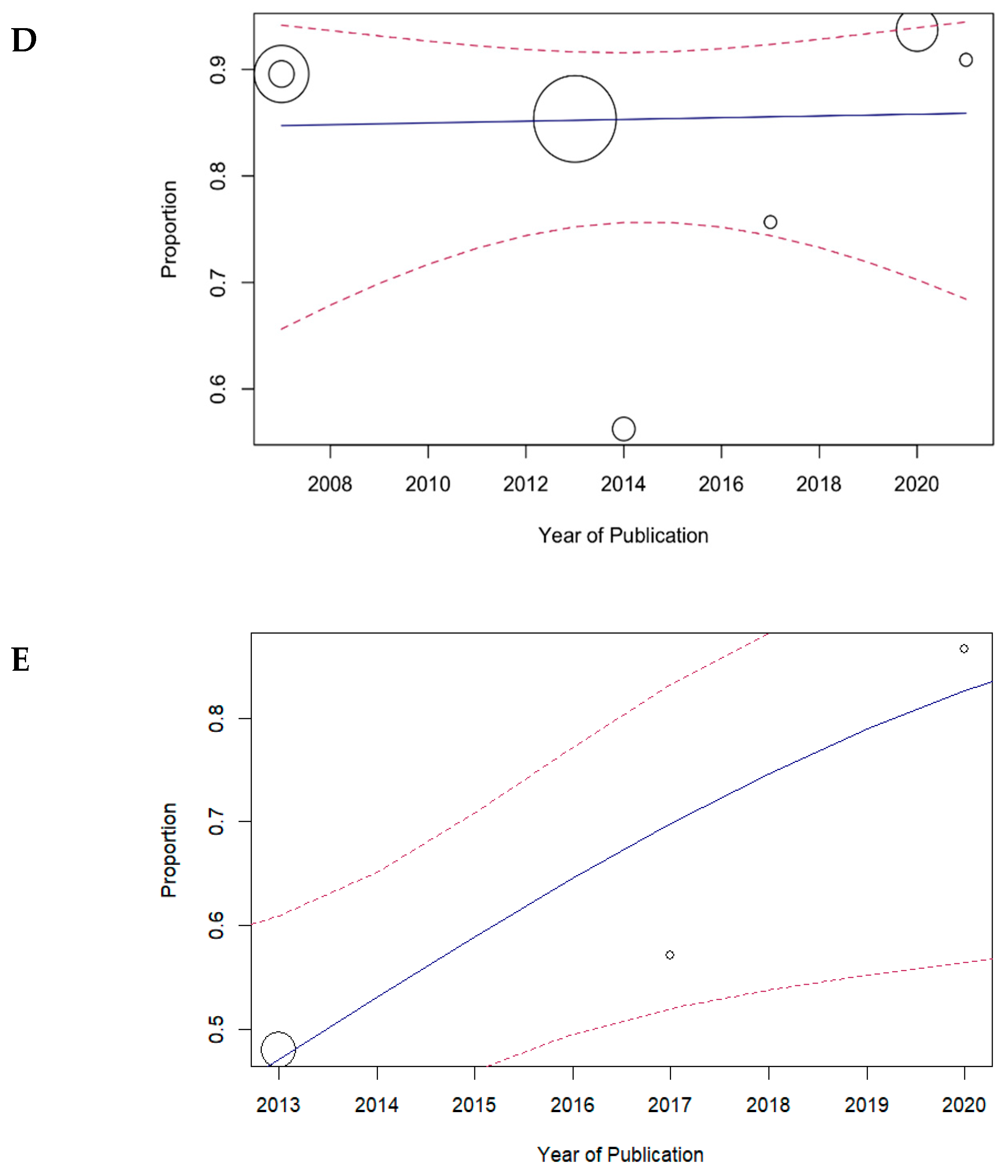
3.2. Meta-Analysis of Primary Probing
4. Discussion
4.1. Limitations
4.2. Future Research Directions
- Conduct larger, prospective, multicenter randomized controlled trials stratified by age, type of anesthesia (general vs. topical), and clinical subtype (simple vs. complex CNLDO) to provide more robust and generalizable data on probing outcomes.
- Investigate long-term outcomes, including recurrence rates, need for secondary procedures, and quality-of-life measures in children undergoing primary probing at various ages.
- Support the development of national or regional clinical practice guidelines in countries where standardized recommendations for the management of CNLDO are currently lacking. Such guidelines would help unify treatment approaches and improve patient outcomes across different healthcare settings.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNLO | Congenital Nasolacrimal Duct Obstruction |
| PRISA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| NOS | Newcastle-Ottawa Scale |
| WHO | World Health Organization |
| DDT | Dye disappearance test |
| CRSS | Complete remission of symptoms and signs |
| PEDIG | Pediatric Eye Disease Investigator Group |
| CI | Confidence interval |
References
- Paysse, E.A.; Coats, D.K.; Bernstein, J.M. Congenital nasolacrimal duct obstruction: Diagnosis and management. Clin. Perinatol. 2021, 48, 693–702. [Google Scholar] [CrossRef]
- Sathiamoorthi, S.; Frank, R.D.; Mohney, B.G. Spontaneous Resolution and Timing of Intervention in Congenital Nasolacrimal Duct Obstruction. JAMA Ophthalmol. 2018, 136, 1281–1286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paul, T.O.; Shepherd, R. Congenital nasolacrimal duct obstruction: Natural history and the timing of optimal intervention. J. Pediatr. Ophthalmol. Strabismus 1994, 31, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Schellini, S.A.; Marques-Fernandez, V.; Meneghim, R.L.F.S.; Galindo-Ferreiro, A. Current management strategies of congenital nasolacrimal duct obstructions. Expert Rev. Ophthalmol. 2021, 16, 377–385. [Google Scholar] [CrossRef]
- Morrison, D.G.; Binenbaum, G.; Chang, M.Y.; Heidary, G.; Trivedi, R.H.; Galvin, J.A.; Pineles, S.L. Office- or Facility-Based Probing for Congenital Nasolacrimal Duct Obstruction: A Report by the American Academy of Ophthalmology. Ophthalmology 2021, 128, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Trott, S.; Colgrove, N.; Westgate, P.; Bush, M.; Iverson, K. Systematic review of endoscopic-assisted surgical management for congenital nasolacrimal duct obstruction. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110448. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, S.; Sutherland, M.S.; Haddow, K.; Blaikie, A. Success rates of endoscopic-assisted probing for congenital nasolacrimal duct obstruction in children. J. Laryngol. Otol. 2013, 127, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Puvanachandra, N.; Trikha, S.; MacEwen, C.J.; Morris, R.J.; Hodgkins, P.R. A National Survey of the Management of Congenital Nasolacrimal Duct Obstruction in the United Kingdom. J. Pediatr. Ophthalmol. Strabismus 2010, 47, 76–80. [Google Scholar] [CrossRef]
- Vagge, A.; Giannaccare, G.; Nucci, P.; Serafino, M. Management of congenital nasolacrimal duct obstruction: A narrative review of the literature. J. Clin. Med. 2022, 11, 1010. [Google Scholar] [CrossRef]
- Lin, A.E.; Chang, Y.C.; Lin, M.Y.; Tam, K.W.; Shen, Y.D. Comparison of treatment for congenital nasolacrimal duct obstruction: A systematic review and meta-analysis. Can. J. Ophthalmol. 2016, 51, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Petris, C.; Liu, D. Probing for congenital nasolacrimal duct obstruction. Cochrane Database Syst. Rev. 2017, 7, CD011109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farat, J.G.; Schellini, S.A.; Dib, R.E.; Santos, F.G.D.; Meneghim, R.L.F.S.; Jorge, E.C. Probing for congenital nasolacrimal duct obstruction: A systematic review and meta-analysis of randomized clinical trials. Arq. Bras. Oftalmol. 2021, 84, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, B.; Babaei, L.; Moradi, M.; Chaibakhsh, S.; Aghajani, A. Assessing the success rate of treatment in simple and complex congenital nasolacrimal duct obstruction: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, R.; Alanazi, F.; Alrashidi, A.; Hazazi, M.; Alenezi, M. Comparative Analysis of Silicone Tube Intubation Versus Probing and Balloon Dilation for Congenital Nasolacrimal Duct Obstruction: A Systematic Review and Meta-Analysis. J. Craniofac. Surg. 2024, 35, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Research PROSPERO International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 27 March 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 November 2024).
- Reyhan, A. Probing success in congenital nasolacrimal duct obstruction: An evaluation of age-related outcomes and associated sociodemographic, clinical, maternal, and neonatal variables. Ann. Clin. Anal. Med. 2025, 16, 146–151. [Google Scholar] [CrossRef]
- Baarah, B.T.; Abu-Laban, W. Management of congenital nasolacrimal duct obstruction: Comparison of probing vs conservative medical approach. Bahrain Med. Bull. 2000, 22, 21–23. [Google Scholar]
- Al-Faky, Y.H.; Al-Sobaie, N.; Mousa, A.; Al-Odan, H.; Al-Huthail, R.; Osman, E.; Al-Mosallam, A.R. Evaluation of treatment modalities and prognostic factors in children with congenital nasolacrimal duct obstruction. J. AAPOS 2012, 16, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Katowitz, J.A.; Welsh, M.G. Timing of initial probing and irrigation in congenital nasolacrimal duct obstruction. Ophthalmology 1987, 94, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari Rajat, M.S. Results of Probing for Congenital Nasolacrimal Duct Obstruction in Children Older than 13 Months of Age. Indian J. Ophthalmol. 2005, 53, 49–51. [Google Scholar] [CrossRef]
- Pediatric Eye Disease Investigator Group; Repka, M.X.; Chandler, D.L.; Beck, R.W.; Crouch, E.R., 3rd; Donahue, S.; Holmes, J.M.; Lee, K.; Melia, M.; Quinn, G.E.; et al. Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 years. Ophthalmology 2008, 115, 577–584.e3. [Google Scholar] [CrossRef]
- Gul, S.; Dabir, S.A.; Jatoi, S.M.; Narsani, A.; Alam, M. Efficacy of probing in the treatment of congenital nasolacrimal duct obstruction in three age groups. Int. J. Ophthalmol. 2008, 8, 864–866. [Google Scholar]
- Shrestha, J.B.; Bajimaya, S.; Hennig, A. Outcome of probing under topical anesthesia in children below 18 months of age with congenital nasolacrimal duct obstruction. Nepal. Med. Coll. J. 2009, 11, 46–49. [Google Scholar] [PubMed]
- Cha, D.S.; Lee, H.; Park, M.S.; Lee, J.M.; Baek, S.H. Clinical outcomes of initial and repeated nasolacrimal duct office-based probing for congenital nasolacrimal duct obstruction. Korean J. Ophthalmol. 2010, 24, 261–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuhoglu, F.; Buyrukcu, A.T.; Ozdemir, F.E.; Eltutar, K. Effectivity of nasolacrimal probing for the treatment of congenital nasolacrimal duct obstruction: A retrospective study. Guoji Yanke Zazhi (Int. Eye Sci.) 2013, 13, 652–655. [Google Scholar]
- Perveen, S.; Sufi, A.R.; Rashid, S.; Khan, A. Success rate of probing for congenital nasolacrimal duct obstruction at various ages. J. Ophthalmic Vis. Res. 2014, 9, 60–69. [Google Scholar] [PubMed] [PubMed Central]
- Hung, C.H.; Chen, Y.C.; Lin, S.L.; Chen, W.L. Nasolacrimal Duct Probing under Topical Anesthesia for Congenital Nasolacrimal Duct Obstruction in Taiwan. Pediatr. Neonatol. 2015, 56, 402–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Napier, M.L.; Armstrong, D.J.; McLoone, S.F.; McLoone, E.M. Congenital Nasolacrimal Duct Obstruction: Comparison of Two Different Treatment Algorithms. J. Pediatr. Ophthalmol. Strabismus 2016, 53, 285–291. [Google Scholar] [CrossRef]
- Le Garrec, J.; Abadie-Koebele, C.; Parienti, J.J.; Molgat, Y.; Degoumois, A.; Mouriaux, F. Nasolacrimal duct office probing in children under the age of 12 months: Cure rate and cost evaluation. J. Fr. Ophtalmol. 2016, 39, 171–177. [Google Scholar] [CrossRef]
- Beato, J.; Mota, Á.; Gonçalves, N.; Santos-Silva, R.; Magalhães, A.; Breda, J.; Falcão-Reis, F. Factors Predictive of Success in Probing for Congenital Nasolacrimal Duct Obstruction. J. Pediatr. Ophthalmol. Strabismus 2017, 54, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Świerczyńska, M.; Tobiczyk, E.; Rodak, P.; Barchanowska, D.; Filipek, E. Success rates of probing for congenital nasolacrimal duct obstruction at various ages. BMC Ophthalmol. 2020, 20, 403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zor, K.R.; Küçük, E.; Yılmaz Öztorun, Z. Outcomes and comparison of nasolacrimal probing for patients older than 12 months. Ther. Adv. Ophthalmol. 2020, 12, 2515841420927138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Machado, D.C.S.; Castro, R.C.F.; Souza BAAde Dias Mde, M.; Lima AFde, O.; Léda, R.M. Efficacy of probing for congenital nasolacrimal duct obstruction in a private tertiary hospital: 10-year experience. Rev. Brasoftalmol. [Internet] 2021, 80, 133–135. [Google Scholar] [CrossRef]
- Pensiero, S.; Diplotti, L.; Visalli, G.; Ronfani, L.; Giangreco, M.; Barbi, E. Minimally-Invasive Surgical Approach to Congenital Dacryostenosis: Proposal for a New Protocol. Front. Pediatr. 2021, 9, 569262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lekskul, A.; Preechaharn, P.; Jongkhajornpong, P.; Wuthisiri, W. Age-Specific Outcomes of Conservative Approach and Probing for Congenital Nasolacrimal Duct Obstruction. Clin. Ophthalmol. 2022, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Błaszczyk, K.; Biedka, K.; Estreicher, A.; Wesołowski, M.; Bulski, J.; Sobaś, A.; Ziobro, O.; Maj, F.; Sornat, K.; Klasa, A.; et al. Congenital Nasolacrimal Duct Obstruction: Natural Course, Diagnosis and Therapeutic Strategies. J. Clin. Med. 2025, 14, 3716. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.D.; Hariharan, L.; Repka, M.X.; Chandler, D.; Melia, B.M.; Beck, R.W.; Pediatric Eye Disease Investigator Group (PEDIG). Cost-effectiveness of 2 approaches to managing nasolacrimal duct obstruction in infants: The importance of the spontaneous resolution rate. Arch Ophthalmol. 2011, 129, 603–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Vagge, A.; Ferro Desideri, L.; Nucci, P.; Serafino, M.; Giannaccare, G.; Lembo, A.; Traverso, C.E. Congenital Nasolacrimal Duct Obstruction (CNLDO): A Review. Diseases 2018, 6, 96. [Google Scholar] [CrossRef]
- Pediatric Eye Disease Investigator Group. A randomized trial comparing the cost-effectiveness of 2 approaches for treating unilateral nasolacrimal duct obstruction. Arch. Ophthalmol. 2012, 130, 1525–1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumura, N.; Suzuki, T.; Goto, S.; Fujita, T.; Yamane, S.; Maruyama-Inoue, M.; Kadonosono, K. Transcanalicular endoscopic primary dacryoplasty for congenital nasolacrimal duct obstruction. Eye 2019, 33, 1008–1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Last Name, Year | Design | Country/WHO Region | Age Group Range (in Months) | Total Eyes Treated | Success Rate After Primary Probing (Eyes) | Type of Anesthesia | Postoperative Evaluation Measures | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|
| Katowitz, 1987 [21] | Retrospective cohort study | USA/America | 0 < a < 6 6 < a < 12 12 < a < 24 | 58 219 106 | 57 210 73 | General | Observation DDT | 1 week–6 months |
| Maheshwari, 2005 [22] | Retrospective cohort study | India/South-East Asia | 12 < a < 24 | 42 | 37 | General | CRSS | 1 week–3 months |
| PEDIG, 2007 [23] | Prospective cohort study | USA/America | 6 < a< 12 12 < a < 24 24 < a < 48 | 421 421 48 | 328 326 43 | General/ Topical | CRSS The questionnaire DDT | 1 month ± 1 week |
| Gul, 2008 [24] | Prospective cohort study | Pakistan/Eastern Mediterranean | 0 < a < 6 6 < a < 12 12 < a < 24 | 76 70 68 | 76 62 56 | General | CRSS | 1 week–3 months |
| Shrestha, 2009 [25] | Prospective cohort study | Nepal/South-East Asia | 0 < a < 6 6 < a < 12 | 45 41 | 43 38 | Topical | CRSS | 3–6 weeks |
| Cha, 2010 [26] | Retrospective comparative case series | South Korea/Western Pacific | 6 < a < 12 | 136 | 111 | Topical | CRSS | 1 week–2 months |
| Nuhoglu, 2013 [27] | Retrospective cohort study | Turkey/European | 12 < a < 24 24 < a < 48 a > 48 | 131 82 48 | 118 70 23 | General | The questionnaire DDT | 1 day–3 months |
| Perveen, 2014 [28] | Prospective cohort study | India/South-East Asia | 0 < a < 6 6 < a < 12 12 < a < 24 24 < a < 48 | 2 50 50 16 | 2 47 42 9 | General | CRSS | 2 weeks–6 months |
| Hung, 2015 [29] | Prospective cohort study | Taiwan/Western Pacific | 0 < a < 6 6 < a< 12 12 < a < 24 | 181 206 144 | 163 164 109 | Topical | CRSS | 1 week |
| Napier, 2016 [30] | Retrospective comparative study | United Kingdom/European | 12 < a < 24 | 110 | 87 | General | CRSS | 6–12 weeks |
| Le Garrec, 2016 [31] | Retrospective cohort study | France/European | 0 < a < 6 6 < a < 12 | 156 287 | 156 287 | Topical | CRSS | 4 weeks |
| Beato, 2017 [32] | Retrospective cohort study | Portugal/European | 12 < a < 24 24 < a < 48 a > 48 | 31 37 7 | 27 28 4 | General | CRSS | 1–82 months |
| Świerczyńska, 2020 [33] | Retrospective cohort study | Poland/European | 0 < a < 6 6 < a < 12 12 < a < 24 | 1571 969 391 | 1431 856 286 | Topical | CRSS DDT | 3 weeks–6 months |
| Zor, 2020 [34] | Retrospective cohort study | Turkey/Europaen | 12 < a < 24 24 < a < 48 a > 48 | 96 32 15 | 91 30 13 | General | CRSS DDT | 6–36 months |
| Machado, 2021 [35] | Retrospective cohort study | Brazil/America | 0 < a < 6 6 < a < 12 12 < a < 24 | 5 25 36 | 4 24 31 | General | CRSS The questionnaire DDT | 4, 81 months |
| Pensiero, 2021 [36] | Retrospective cohort study | Italy/European | 6 < a < 12 12 < a < 24 24 < a < 48 | 71 356 198 | 57 313 180 | General | CRSS | 6 months |
| Lekskul, 2022 [37] | Retrospective cohort study | Thailand/South-East Asia | 12 < a < 24 | 56 | 50 | General | CRSS | 1 month |
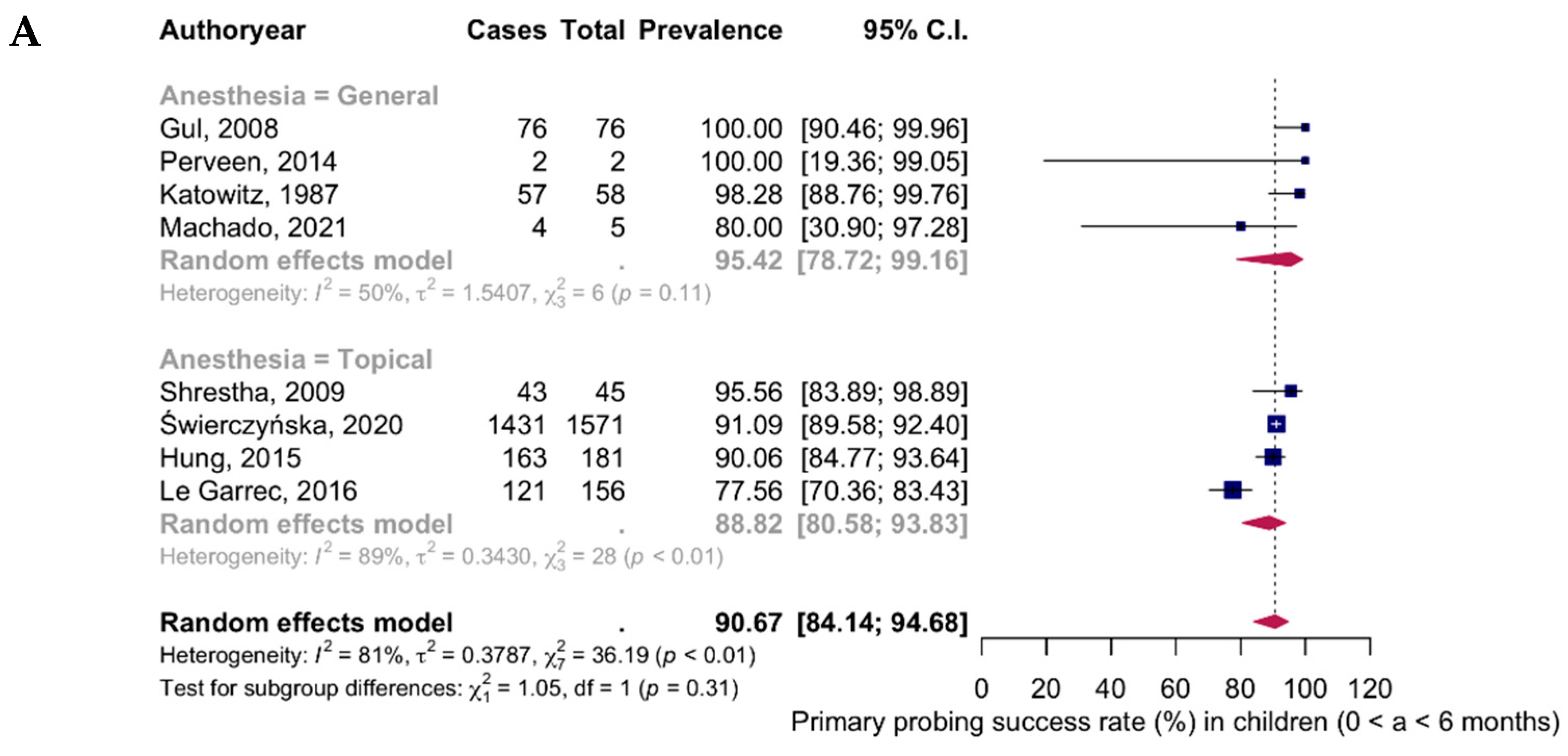
| Age Group | Eyes Assessed (n) | Successful Cases (n) | Pooled Mean Success Rate (%, 95% CI, I2) |
|---|---|---|---|
| 0–6 months (8 studies) | |||
| General anesthesia | 141 | 139 | 95.42% (95% CI: 78.72–99.16; I2 = 50%) |
| Topical anesthesia | 1953 | 1758 | 88.82% (95% CI: 80.58–93.83; I2 = 89%) |
| Total | 2094 | 1897 | 90.67% (95% CI: 84.14–94.68; I2 = 81%) |
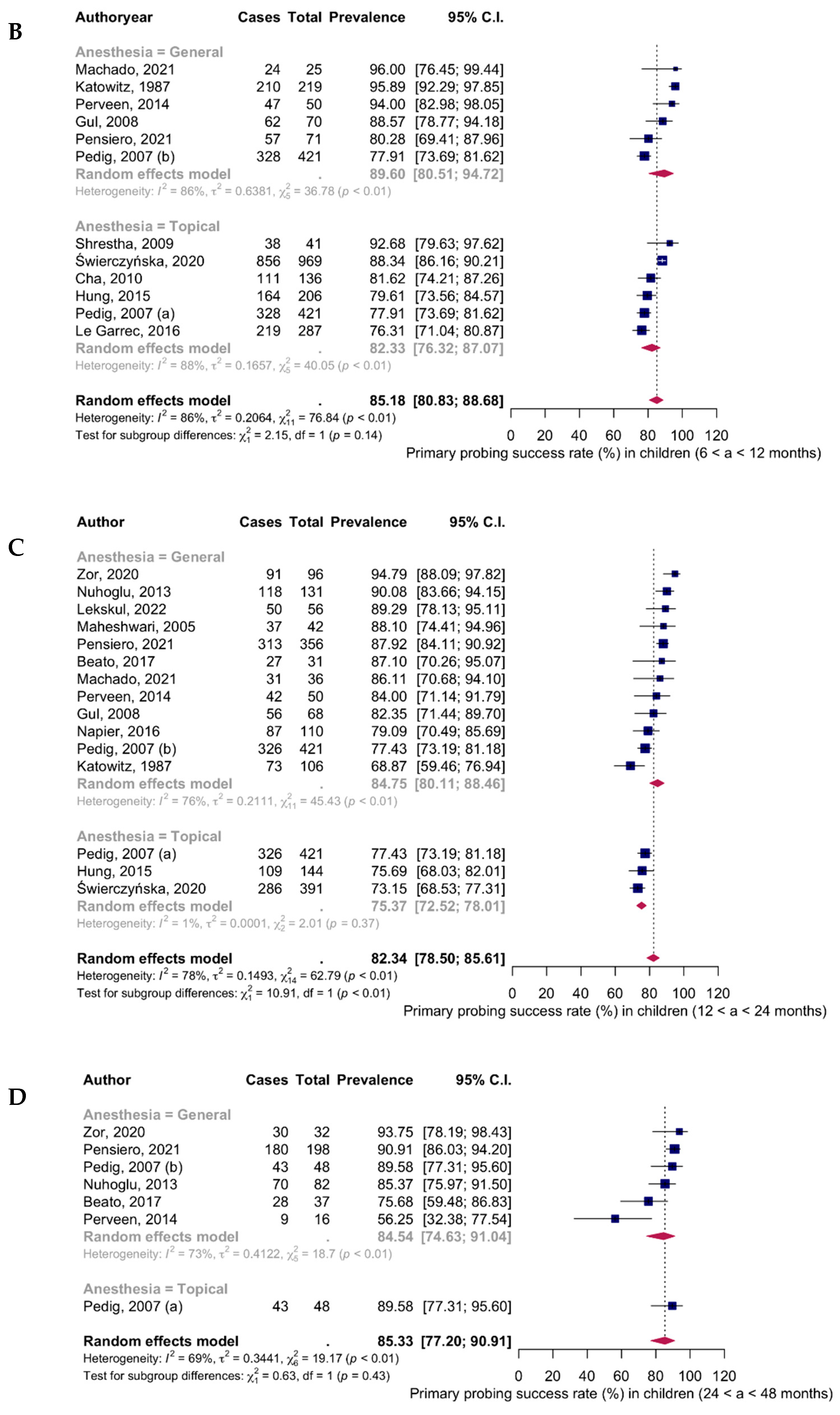
| 6–12 months (11 studies) | |||
| General anesthesia | 856 | 728 | 89.60% (95% CI: 80.51–94.72; I2 = 86%) |
| Topical anesthesia | 2060 | 1716 | 82.33% (95% CI: 76.32–87.07; I2 = 88%) |
| Total | 2495 | 2116 | 85.18% (95% CI: 80.83–88.68; I2 = 86%) |
| 12–24 months (14 studies) | |||
| General anesthesia | 1503 | 1251 | 84.75% (95% CI: 80.11–88.46; I2 = 76%) |
| Topical anesthesia | 956 | 721 | 75.37% (95% CI: 72.52–78.01; I2 = 1%) |
| Total | 2038 | 1646 | 82.34% (95% CI: 78.50–85.61; I2 = 78%) |
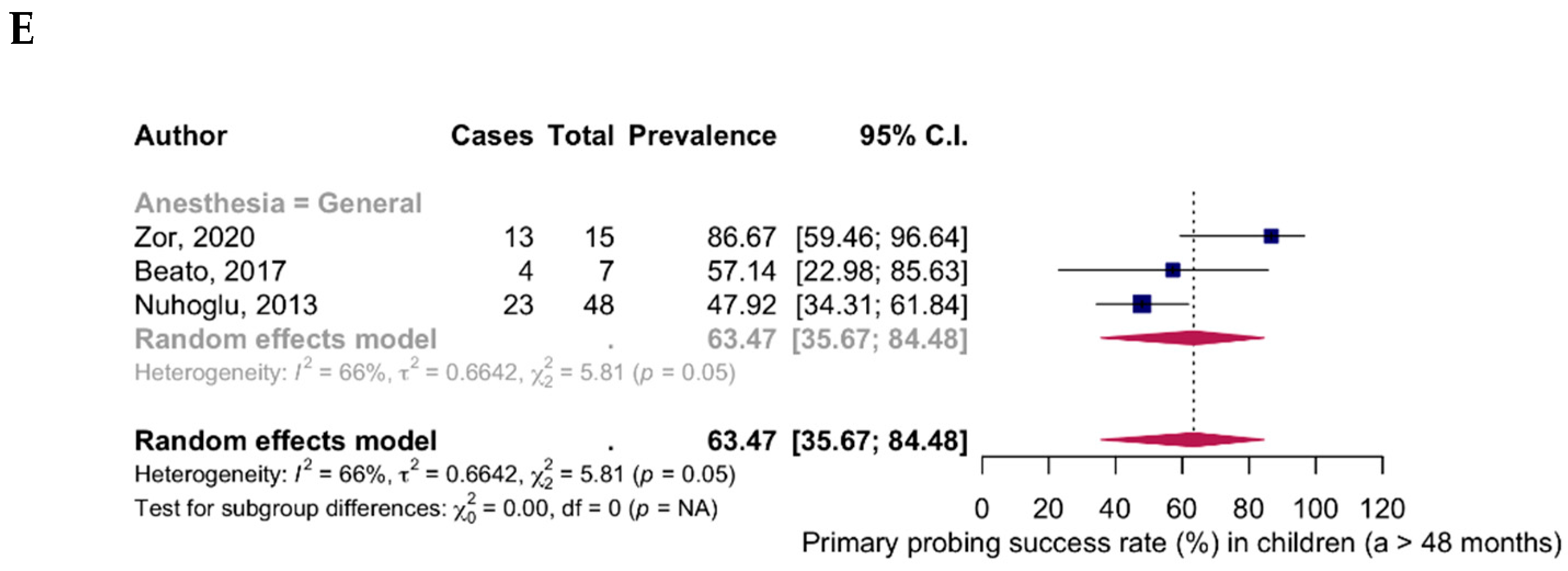
| 24–48 months (6 studies) | |||
| General anesthesia | 413 | 360 | 84.54% (95% CI: 74.63–91.04; I2 = 73%) |
| Topical anesthesia | 48 | 43 | 89.58% (95% CI: 77.31–95.60) |
| Total | 413 | 360 | 85.33% (95% CI: 77.20–90.91; I2 = 69%) |
| >48 months (3 studies) | |||
| General anesthesia | 70 | 40 | 63.47% (95% CI: 35.67–84.48; I2 = 66%) |
| Topical anesthesia | 0 | 0 | |
| Total | 70 | 40 | 63.47% (95% CI: 35.67–84.48; I2 = 66%) |
| Outcome | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence |
|---|---|---|---|---|---|---|---|
| Pooled success rate of nasolacrimal duct probing (0–6 months) | Meta-analysis of observational studies | Low | Serious | Not serious | Not serious | Not assessed (n < 10) | Low |
| Pooled success rate of nasolacrimal duct probing (6–12 months) | Meta-analysis of observational studies | Low | Serious | Not serious | Not serious | Not detected | Low |
| Pooled success rate of nasolacrimal duct probing (12–24 months) | Meta-analysis of observational studies | Low | Serious | Not serious | Not serious | Not detected | Low |
| Pooled success rate of nasolacrimal duct probing (24–48 months) | Meta-analysis of observational studies | Low | Serious | Not serious | Not serious | Not assessed (n < 10) | Low |
| Pooled success rate of nasolacrimal duct probing (≥48 months) | Meta-analysis of observational studies | Low | Serious | Not serious | Serious | Not assessed (n < 10) | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultanbayeva, Z.; Dzhumabekov, A.; Aldasheva, N.; Issergepova, B.; Kuanyshbekov, Y.; Taushanova, M.; Karibayeva, I. A Systematic Review and Meta-Analysis of the Success Rate of the Primary Probing in Pediatric Patients with Congenital Nasolacrimal Duct Obstruction in Different Age Groups. Medicina 2025, 61, 1432. https://doi.org/10.3390/medicina61081432
Sultanbayeva Z, Dzhumabekov A, Aldasheva N, Issergepova B, Kuanyshbekov Y, Taushanova M, Karibayeva I. A Systematic Review and Meta-Analysis of the Success Rate of the Primary Probing in Pediatric Patients with Congenital Nasolacrimal Duct Obstruction in Different Age Groups. Medicina. 2025; 61(8):1432. https://doi.org/10.3390/medicina61081432
Chicago/Turabian StyleSultanbayeva, Zhansaya, Auyeskhan Dzhumabekov, Neilya Aldasheva, Botagoz Issergepova, Yerzhan Kuanyshbekov, Maiya Taushanova, and Indira Karibayeva. 2025. "A Systematic Review and Meta-Analysis of the Success Rate of the Primary Probing in Pediatric Patients with Congenital Nasolacrimal Duct Obstruction in Different Age Groups" Medicina 61, no. 8: 1432. https://doi.org/10.3390/medicina61081432
APA StyleSultanbayeva, Z., Dzhumabekov, A., Aldasheva, N., Issergepova, B., Kuanyshbekov, Y., Taushanova, M., & Karibayeva, I. (2025). A Systematic Review and Meta-Analysis of the Success Rate of the Primary Probing in Pediatric Patients with Congenital Nasolacrimal Duct Obstruction in Different Age Groups. Medicina, 61(8), 1432. https://doi.org/10.3390/medicina61081432






