The Prevalence of and Factors Associated with Sarcopenic Obesity, Sarcopenia, and Obesity Among Korean Adults: Findings from the 2022–2023 Korea National Health and Nutrition Examination Survey
Abstract
1. Introduction
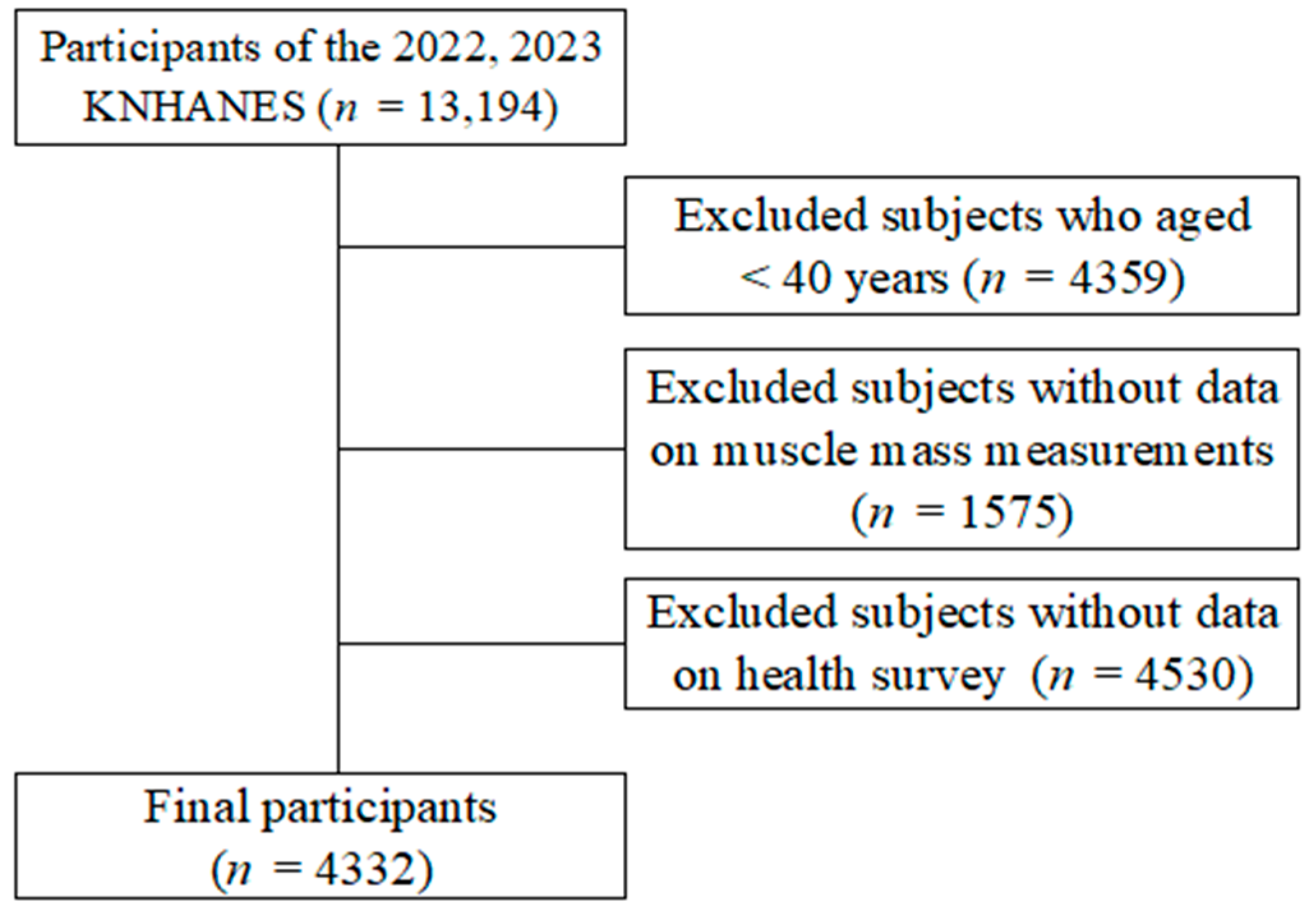
2. Materials and Methods
2.1. Study Design and Participants
2.2. Definitions of Sarcopenic Obesity, Sarcopenia, and Obesity Sarcopenia
2.2.1. Sociodemographic Factors
2.2.2. Health-Related Factors
2.2.3. Comorbid Health Conditions
2.3. Statistical Analysis
3. Results
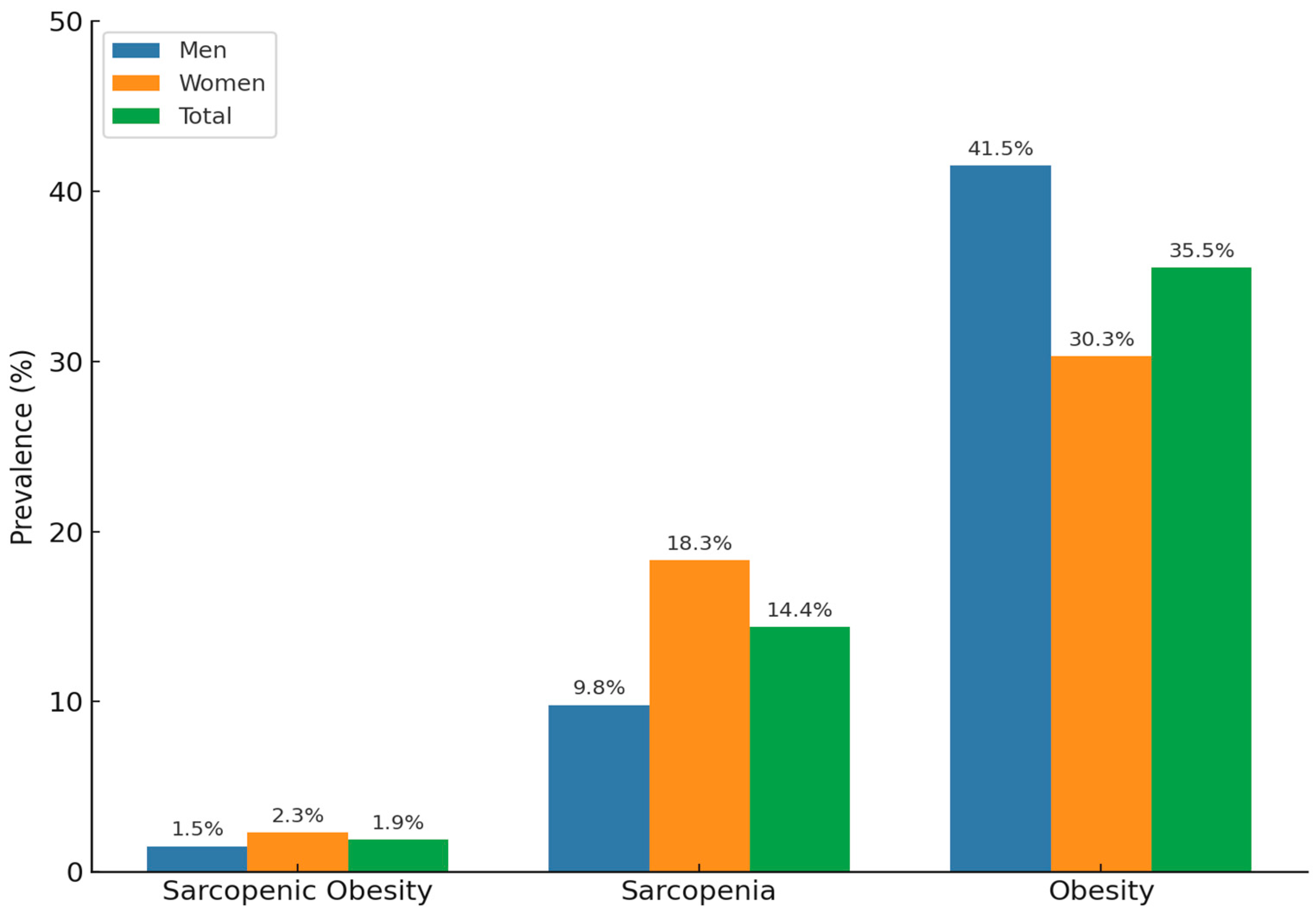
3.1. Prevalence of Sarcopenic Obesity, Sarcopenia, and Obesity
3.2. Sociodemographic Characteristics of the Study Population
3.3. Health-Related Characteristics of the Study Population
3.4. Factors Associated with Sarcopenic Obesity, Sarcopenia, and Obesity
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Yang, Y.S.; Han, B.D.; Han, K.; Jung, J.H.; Son, J.W. Obesity Fact Sheet in Korea, 2021: Trends in Obesity Prevalence and Obesity-Related Comorbidity Incidence Stratified by Age from 2009 to 2019. J. Obes. Metab. Syndr. 2022, 31, 169–177. [Google Scholar] [CrossRef]
- Silveira, E.A.; da Silva Filho, R.R.; Spexoto, M.C.B.; Haghighatdoost, F.; Sarrafzadegan, N.; de Oliveira, C. The Role of Sarcopenic Obesity in Cancer and Cardiovascular Disease: A Synthesis of the Evidence on Pathophysiological Aspects and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 4339. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clinical cases in mineral and bone metabolism. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2014, 11, 177–180. [Google Scholar]
- Makizako, H.; Nakai, Y.; Tomioka, K.; Taniguchi, Y. Prevalence of sarcopenia defined using the Asia Working Group for Sarcopenia criteria in Japanese community-dwelling older adults: A systematic review and meta-analysis. Phys. Ther. Res. 2019, 22, 53–57. [Google Scholar] [CrossRef]
- Meng, S.; He, X.; Fu, X.; Zhang, X.; Tong, M.; Li, W.; Zhang, W.; Shi, X.; Liu, K. The prevalence of sarcopenia and risk factors in the older adult in China: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1415398. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Yoon, H.; Won, J. Prevalence of Sarcopenia and its Risk Factors in Community-dwelling Older People during the COVID-19 Pandemic. J. Korean Acad. Fundam. Nurs. 2024, 31, 90–99. [Google Scholar] [CrossRef]
- Danielewicz, A.L.; Marra, A.; Tringali, G.; Micheli, R.D.; Abbruzzese, L.; Fanari, P.; Codecasa, F.; Lazzer, S.; Mendonça, V.A.; Lacerda, A.C.R. Analysis of sarcopenic obesity prevalence and diagnostic agreement according to the 2022 ESPEN and EASO Consensus in hospitalized older adults with severe obesity. Front. Endocrinol. 2024, 15, 1366229. [Google Scholar] [CrossRef]
- Gao, Q.; Mei, F.; Shang, Y.; Hu, K.; Chen, F.; Zhao, L.; Ma, B. Global prevalence of sarcopenic obesity in older adults: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 4633–4641. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Lee, D.-Y. Sex-specific Sarcopenia prevalence and risk factors in the Korean Population: A cross-sectional epidemiological study. Medicina 2024, 60, 899. [Google Scholar] [CrossRef]
- Lee, D.-Y. Association Between Chronic Obstructive Pulmonary Disease and Low Muscle Mass in Korean Adults. J. Clin. Med. 2025, 14, 1134. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Kim, S.-G. The association between pulmonary function and metabolic syndrome in Koreans: A cross-sectional study. Int. J. Gerontol. 2021, 15, 228–232. [Google Scholar]
- Lee, D.Y.; Nam, S.M. Association between restrictive pulmonary disease and type 2 diabetes in Koreans: A cross-sectional study. World J. Diabetes 2020, 11, 425. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Brunelli, A.; Saatchi, T.; Urbani, S.; Giani, A.; Rossi, A.P.; Zoico, E.; Fantin, F. The Role of Crosstalk between Adipose Cells and Myocytes in the Pathogenesis of Sarcopenic Obesity in the Elderly. Cells 2022, 11, 3361. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; He, C.; Chen, J.; Deng, D.; Lu, W.; Wang, Y. Prevalence of sarcopenia was higher in women than in men: A cross-sectional study from a rural area in eastern China. PeerJ 2022, 10, e13678. [Google Scholar] [CrossRef]
- Buckinx, F.; Aubertin-Leheudre, M. Sarcopenia in Menopausal Women: Current Perspectives. Int. J. Women’s Health 2022, 14, 805–819. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Habib, S.S.; Alkahtani, S.; Alhussain, M.; Aljuhani, O. Sarcopenia Coexisting with High Adiposity Exacerbates Insulin Resistance and Dyslipidemia in Saudi Adult Men. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3089–3097. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Beaudart, C.; Demonceau, C.; Reginster, J.Y.; Locquet, M.; Cesari, M.; Cruz Jentoft, A.J.; Bruyère, O. Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1228–1243. [Google Scholar] [CrossRef]
- Mitkin, N.A.; Kirilkin, G.E.; Unguryanu, T.N.; Malyutina, S.; Cook, S.; Kudryavtsev, A.V. The relationship between physical performance and alcohol consumption levels in Russian adults. Sci. Rep. 2024, 14, 1417. [Google Scholar] [CrossRef]
- Skinner, J.; Shepstone, L.; Hickson, M.; Welch, A.A. Alcohol Consumption and Measures of Sarcopenic Muscle Risk: Cross-Sectional and Prospective Associations Within the UK Biobank Study. Calcif. Tissue Int. 2023, 113, 143–156. [Google Scholar] [CrossRef]
- Bu, Y.L.; Wang, C.; Zhao, C.; Lu, X.; Gao, W. The association of alcohol consumption with the risk of sarcopenia: A dose-response meta-analysis. Am. J. Drug Alcohol Abus. 2024, 50, 305–320. [Google Scholar] [CrossRef]
- Swan, L.; Warters, A.; O’Sullivan, M. Socioeconomic Inequality and Risk of Sarcopenia in Community-Dwelling Older Adults. Clin. Interv. Aging 2021, 16, 1119–1129. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 1541–1554. [Google Scholar] [CrossRef]
- Locquet, M.; Bruyère, O.; Lengelé, L.; Reginster, J.Y.; Beaudart, C. Relationship between smoking and the incidence of sarcopenia: The SarcoPhAge cohort. Public Health 2021, 193, 101–108. [Google Scholar] [CrossRef]
- Yang, L.; Ran, Q.; Yeo, Y.H.; Wen, Z.; Tuo, S.; Li, Y.; Yuan, J.; Dai, S.; Wang, J.; Ji, F.; et al. Sex disparity in the association between alcohol consumption and sarcopenia: A population-based study. Front. Nutr. 2025, 12, 1536488. [Google Scholar] [CrossRef]
- Scott, D.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.; Waite, L.M.; Hirani, V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2018, 108, 99–105. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef]
| Factors | Categories | Total (n = 4332) | Sarcopenic Obesity (n = 101) | Sarcopenia (n = 704) | Obesity (n = 1515) | Normal (n = 2012) | p |
|---|---|---|---|---|---|---|---|
| Age | Middle | 70.8 | 10.8 | 55.4 | 68.6 | 79.5 | <0.001 |
| Older | 29.2 | 89.2 | 44.6 | 31.4 | 20.5 | ||
| Sex | Male | 46.0 | 35.1 | 31.3 | 53.9 | 45.0 | <0.001 |
| Female | 54.0 | 64.9 | 68.7 | 46.1 | 55.0 | ||
| Education level | elementary | 11.8 | 48.6 | 19.4 | 12.8 | 7.4 | <0.001 |
| middle | 10.1 | 21.1 | 14.5 | 11.6 | 7.31 | ||
| high | 32.8 | 18.4 | 32 | 32 | 34.2 | ||
| university | 45.4 | 11.8 | 34.1 | 43.7 | 51.3 | ||
| Marital status | with | 84.6 | 64.1 | 80.6 | 83.0 | 87.8 | <0.001 |
| without | 15.4 | 35.9 | 19.4 | 17.0 | 12.2 | ||
| Individual income | Q1 (Lowest) | 20.2 | 31.5 | 21.2 | 21.0 | 18.9 | 0.004 |
| Q2 | 24.6 | 19.4 | 26.1 | 24.0 | 24.7 | ||
| Q3 | 26.7 | 26.2 | 22.0 | 29.7 | 26.0 | ||
| Q4 (Highest) | 28.5 | 22.9 | 30.7 | 25.2 | 30.4 | ||
| Residential area | Urban | 85.0 | 85.0 | 85.7 | 84.3 | 85.3 | 0.820 |
| Rural | 15.0 | 15.0 | 14.3 | 15.7 | 14.7 |
| Factors | Categories | Total (n = 4332) | Sarcopenic Obesity (n = 101) | Sarcopenia (n = 704) | Obesity (n = 1515) | Normal (n = 2012) | p |
|---|---|---|---|---|---|---|---|
| Subjective health status | Good | 34.7 | 21.3 | 29.0 | 30.4 | 40.2 | <0.001 |
| Moderate | 48.8 | 42.0 | 48.8 | 49.3 | 48.8 | ||
| Bad | 16.4 | 36.7 | 22.2 | 20.3 | 11.0 | ||
| Stress level | High | 20.7 | 20.0 | 18.2 | 23.3 | 19.6 | 0.038 |
| Low | 79.3 | 80.0 | 81.8 | 76.7 | 80.4 | ||
| Smoking status | Current | 15.5 | 9.0 | 13.1 | 17.6 | 15.0 | <0.001 |
| Past | 25.6 | 21.9 | 15.3 | 29.8 | 25.7 | ||
| Non | 58.9 | 69.1 | 71.6 | 52.6 | 59.3 | ||
| Alcohol status | Yes | 52.7 | 29.3 | 36.7 | 56.4 | 55.7 | <0.001 |
| No | 47.3 | 70.7 | 63.3 | 43.6 | 44.3 | ||
| Physical activity | Low | 89.9 | 98.7 | 95.7 | 90.6 | 87.4 | <0.001 |
| Moderate-vigorous | 10.1 | 1.3 | 4.3 | 9.4 | 12.6 | ||
| Comorbidity conditions | |||||||
| Diabetes | 42.9 | 62.6 | 39.0 | 56.3 | 33.3 | <0.001 | |
| Hypertension | 29.5 | 39.4 | 28.4 | 37.1 | 23.9 | <0.001 | |
| High triglyceride | 25.9 | 29.2 | 17.0 | 38.0 | 19.5 | <0.001 | |
| Low HDL-C | 21.6 | 40.6 | 16.1 | 29.6 | 16.5 | <0.001 | |
| Categories | Sarcopenic Obesity | Sarcopenia | Obesity | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Age | Middle | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Older | 14.42 (7.03–29.57) | <0.001 | 2.31 (1.77–3.01) | <0.001 | 1.30 (1.05–1.61) | 0.016 | |
| Sex | Male | 1.15 (0.56–2.37) | 0.697 | 0.78 (0.57–1.06) | 0.112 | 1.35 (1.03–1.77) | 0.032 |
| Female | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Education level | Elementary | 3.43 (1.31–8.97) | 0.012 | 1.58 (1.10–2.29) | 0.015 | 1.33 (0.98–1.81) | 0.069 |
| Middle | 2.53 (0.94–6.78) | 0.065 | 1.68 (1.18–2.40) | 0.004 | 1.35 (1.02–1.79) | 0.038 | |
| High | 1.24 (0.56–2.74) | 0.597 | 1.13 (0.88–1.46) | 0.338 | 0.94 (0.78–1.12) | 0.488 | |
| University | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Marital status | With | 0.70 (0.42–1.16) | 0.161 | 0.98 (0.75–1.28) | 0.866 | 0.85 (0.68–1.06) | 0.153 |
| Without | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Individual income | Q1 (Lowest) | 1.37 (0.71–2.65) | 0.348 | 0.91 (0.69–1.21) | 0.521 | 1.08 (0.85–1.38) | 0.511 |
| Q2 | 0.87 (0.43–1.77) | 0.698 | 0.97 (0.72–1.29) | 0.811 | 1.04 (0.81–1.34) | 0.746 | |
| Q3 | 1.20 (0.59–2.46) | 0.614 | 0.80 (0.59–1.08) | 0.144 | 1.29 (1.03–1.60) | 0.026 | |
| Q4 (Highest) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Residential area | Urban | 1.71 (0.77–3.82) | 0.186 | 1.26 (0.96–1.66) | 0.093 | 1.16 (0.93–1.44) | 0.188 |
| Rural | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Subjective health status | Good | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Moderate | 1.20 (0.64–2.24) | 0.565 | 1.24 (0.99–1.54) | 0.056 | 1.20 (1.00–1.44) | 0.048 | |
| Bad | 2.97 (1.50–5.85) | 0.002 | 2.12 (1.51–2.98) | <0.001 | 1.97 (1.54–2.52) | <0.001 | |
| Stress level | High | 1.43 (0.80–2.57) | 0.225 | 0.95 (0.72–1.26) | 0.734 | 1.20 (0.97–1.47) | 0.086 |
| Low | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Smoking status | Current | 1.21 (0.82–1.78) | 0.327 | 1.21 (0.82–1.78) | 0.327 | 0.81 (0.59–1.12) | 0.210 |
| Past | 0.80 (0.41–1.57) | 0.796 | 0.66 (0.47–0.92) | 0.014 | 0.94 (0.70–1.25) | 0.645 | |
| Non | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Alcohol status | Yes | 0.83 (0.47–1.45) | 0.511 | 0.65 (0.47–0.92) | 0.014 | 1.06 (0.89–1.26) | 0.520 |
| No | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Physical activity | Low | 5.30 (0.70–40.01) | 0.105 | 2.24 (1.44–3.48) | <0.001 | 1.29 (0.98–1.71) | 0.070 |
| Moderate-vigorous | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Comorbidity conditions | |||||||
| Diabetes | 1.84 (1.14–2.96) | 0.012 | 1.15 (0.93–1.42) | 0.202 | 2.02 (1.71–2.35) | <0.001 | |
| Hypertension | 1.11 (0.66–1.86) | 0.703 | 1.07 (0.85–1.35) | 0.564 | 1.50 (1.26–1.78) | <0.001 | |
| High triglyceride | 1.49 (0.87–2.56) | 0.146 | 1.01 (0.76–1.36) | 0.918 | 1.97 (1.60–2.42) | <0.001 | |
| Low HDL-C | 1.98 (1.17–3.36) | 0.011 | 0.72 (0.54–0.96) | 0.028 | 1.44 (1.18–1.76) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-Y. The Prevalence of and Factors Associated with Sarcopenic Obesity, Sarcopenia, and Obesity Among Korean Adults: Findings from the 2022–2023 Korea National Health and Nutrition Examination Survey. Medicina 2025, 61, 1424. https://doi.org/10.3390/medicina61081424
Lee D-Y. The Prevalence of and Factors Associated with Sarcopenic Obesity, Sarcopenia, and Obesity Among Korean Adults: Findings from the 2022–2023 Korea National Health and Nutrition Examination Survey. Medicina. 2025; 61(8):1424. https://doi.org/10.3390/medicina61081424
Chicago/Turabian StyleLee, Do-Youn. 2025. "The Prevalence of and Factors Associated with Sarcopenic Obesity, Sarcopenia, and Obesity Among Korean Adults: Findings from the 2022–2023 Korea National Health and Nutrition Examination Survey" Medicina 61, no. 8: 1424. https://doi.org/10.3390/medicina61081424
APA StyleLee, D.-Y. (2025). The Prevalence of and Factors Associated with Sarcopenic Obesity, Sarcopenia, and Obesity Among Korean Adults: Findings from the 2022–2023 Korea National Health and Nutrition Examination Survey. Medicina, 61(8), 1424. https://doi.org/10.3390/medicina61081424






