1. Introduction
Optimal myocardial protection is one of the cornerstones of surgical success, particularly in cardiac surgery with cardiopulmonary bypass (CPB) and aortic cross-clamp (ACC). Inadequate protection during this critical period may result in ischemia–reperfusion injury, postoperative low cardiac output syndrome (LCOS), arrhythmias, and increased mortality [
1]. This protection is typically provided by cardioplegic solutions during CPB. These solutions decrease metabolic activity and increase ischemic tolerance. The efficacy of myocardial protection is directly related not only to the surgical technique but also to the type, composition, and administration strategy of the cardioplegia solution used.
The prolonged duration of ACC in cardiac surgery remains a critical prognostic indicator as it increases the risk of ischemia–reperfusion injury and subsequent complications [
1]. Conventional cold-blood cardioplegics complicate the surgical workflow with the need for frequent repeat doses because of their short duration of action [
2]. Del Nido (DN) and histidine-tryptophan-ketoglutarate (HTK) are two cardioplegia solutions that are widely used in modern adult cardiac surgery, especially in cases with long ACC duration, can be administered as a single dose, and do not interrupt the surgical flow [
3]. DN, developed for pediatric surgery, is an extracellular solution containing lidocaine and mannitol and is known for its cell membrane stabilization and osmotic protective effects [
4]. In recent years, it has become widespread in adult surgery and an effective and cost-effective option, especially for ischemia durations of up to 90–120 min [
5]. DN provides effective myocardial protection for up to 90 min without the need for re-dosing, especially in short- and medium-term procedures, and is cost-effective [
6]. HTK, on the other hand, is an intracellular solution with high buffering capacity owing to its histidine content and provides metabolic support with ketoglutarate. Although more expensive, they provide longer protection (usually exceeding two hours) and are used in cases requiring long ACC periods [
7,
8]. Both DN and HTK are considered safe for myocardial protection; however, the available literature is limited. DN offers the advantage of reducing intraoperative interruptions due to its simple dosing and efficacy, while HTK provides superior protection in longer procedures [
9]. Although both cardioplegia solutions are widely used in clinical practice, direct studies on the comparative efficacy of DN and HTK solutions in adult cardiac surgery are limited, particularly for ACC times exceeding 150–180 min. Most studies in the literature have insufficient statistical power and tend to focus on smaller and heterogeneous patient groups or address one or a few specific parameters of myocardial protection [
10,
11]. Furthermore, subgroup analyses according to the duration of ischemia, one of the key factors determining cardioplegia efficacy, have generally not been performed [
9]. There is also no consensus in the literature regarding administration protocols, dosing intervals, or biochemical/clinical evaluation criteria [
10]. However, the lack of advanced comparative analyses examining the differential effects of DN and HTK on major clinical outcomes, such as mortality, low cardiac output syndrome (LCOS), and high inotrope requirement, makes it difficult to develop clinical decision algorithms in this field, and the choice of cardioplegia is often based on individual surgeon experience or institutional habits [
9,
10].
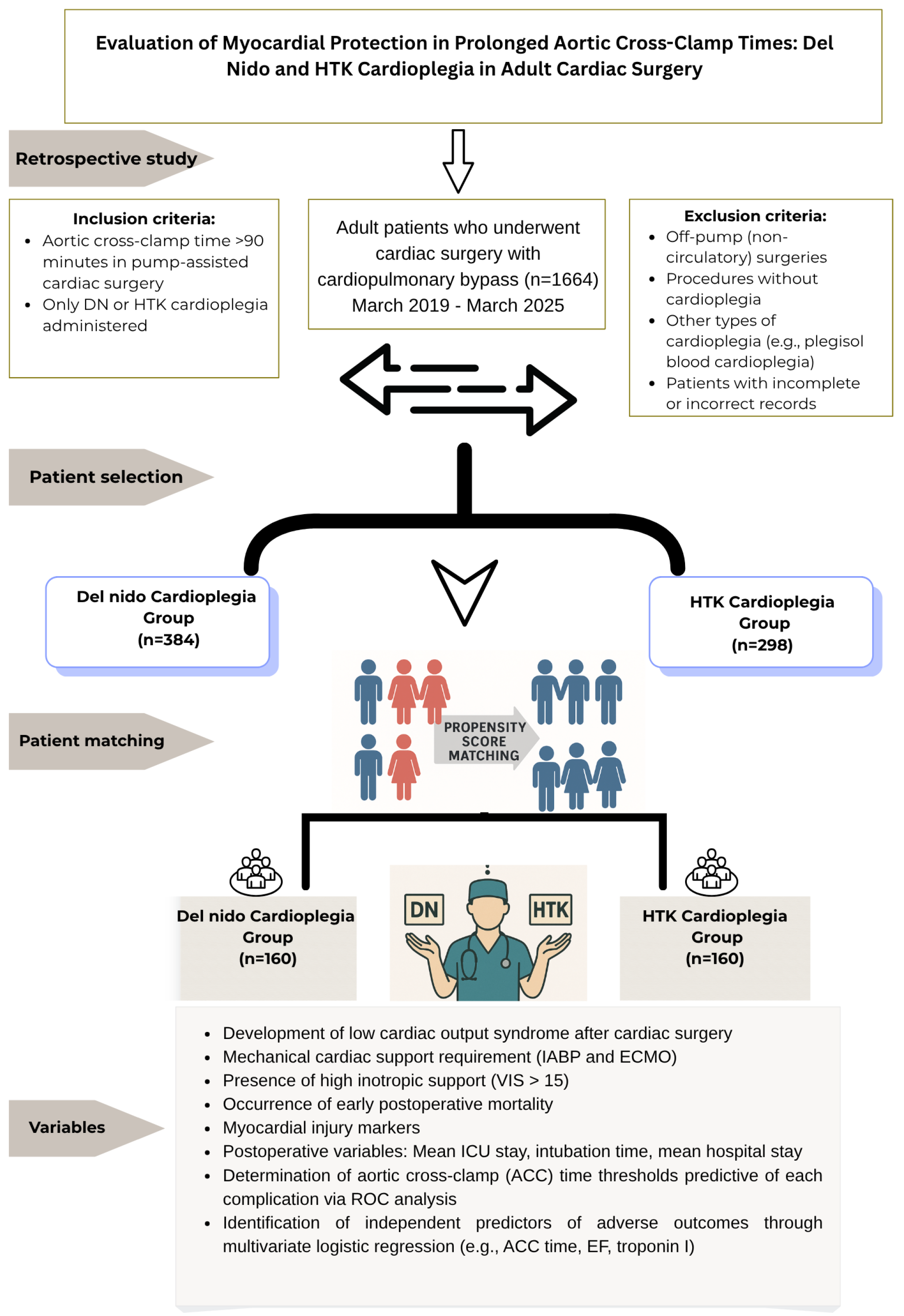
This study aimed to compare the effects of DN and HTK, two different cardioplegia solutions used in adult cardiac surgery, on postoperative clinical outcomes. Biochemical markers (Tn I, CK-MB, and lactate) and clinical outcomes (EF, LCOS, VIS, and arrhythmias) were analyzed together in both cardioplegia groups. In addition, subgroup analyses were performed over time periods defined according to the ACC duration. Thus, the protection profile provided by both cardioplegic solutions at different ischemia durations was revealed multidimensionally. In addition, the predictive value of DN and HTK cardioplegia solutions on major postoperative complications (LCOS, VIS > 15, IABP requirement, and mortality) associated with ACC duration was determined, and the differences in clinical tolerance of both solutions to this duration were revealed. For this purpose, advanced statistical analysis techniques (e.g., ROC analysis, cut-off determination, and multivariate regression) were used to develop the efficacy of cardioplegia in a clinical decision support model.
4. Discussion
Effective myocardial protection is crucial in cardiac surgery, especially in complex and prolonged procedures, to reduce ischemia–reperfusion injury and improve patient outcome. DN and HTK cardioplegia solutions offer different benefits and limitations, depending on the complexity and duration of cardiac surgery. This study compared DN and HTK multifaceted in adult cardiac surgeries with long ACC times, focusing on their efficacy and safety profiles under prolonged ischemic conditions. Our aim was to contribute to clinical decision making by providing clearer insights into the comparative performance of these two solutions under conditions of myocardial ischemia.
There is a paucity of studies in the literature that directly compare the myocardial protective performance of DN and HTK at different ACC durations. Many existing studies are limited to small, heterogeneous patient cohorts or focus on only a few specific parameters of myocardial protection [
10,
11]. This study provided a multidimensional profile of the protective efficacy of both solutions over varying ischemic durations by performing subgroup analyses based on ACC timeframes. ROC-based analyses identified ACC time thresholds associated with the development of complications in DN and HTK patients. In our findings, DN cardioplegia was characterized by a volume advantage and lower transfusion requirement when the operation time was <150 min, whereas HTK provided biochemically better myocardial protection in operations lasting ≥180 min. The concept of “gray zone” for ACC duration of 120–150 min was defined and converted into a recommendation.
The mechanism of action of DN cardioplegia involves a sodium channel blockade via lidocaine. It requires re-dosing approximately every 60 min. Currently, the simple administration protocol has made DN a practical, effective, and economical option in cases with moderate ischemic durations. The need for hemodilution and associated transfusion is also reduced because of its lower-volume administration [
19,
20]. In contrast, the requirement for large-volume administration of HTK can lead to significant hemodilution and often requires postoperative transfusions to ensure hemodynamic stability [
20]. In this study, DN cardioplegia showed advantages in reducing intraoperative cardioplegia volume and lowering transfusion requirements compared with HTK, possibly because of the smaller required dose volume and reduced hemodilution effect, in agreement with the literature. On the other hand, more hyponatremia was observed in the HTK group than in the DN group due to its low sodium formulation. However, hyponatremia is clinically mild. None of the patients developed symptomatic hyponatremia, and no neurological complications were reported. These results suggest that the adverse effects associated with HTK, although statistically significant, did not lead to clinically significant outcomes. In this study, although routine ultrafiltration was not systematically performed during CPB, fluid management strategies, such as ultrafiltration, were used in patients who required it. However, the use of ultrafiltration techniques to control intraoperative fluid balance and reduce volume overload and electrolyte disturbances that may occur with large volumes of crystalloid solutions, such as HTK, may alleviate these effects and reduce dependence on transfusions.
In our study, the incidence of ventricular fibrillation (VF) after ACC was higher in the HTK group than in the DN group. This finding is consistent with trends reported in the literature [
21,
22]. The VF episodes observed in this study were transient and effectively controlled using standard defibrillation protocols. Furthermore, these arrhythmias were not associated with adverse clinical outcomes such as increased inotrope requirement, LCOS development, or prolonged intensive care unit stay. The lower incidence of VF in the DN group after ACC may be explained by the protective effects of lidocaine, magnesium, and mannitol on myocardial cell membrane stability and ion regulation [
23,
24]. These components reduce the risk of VF after reperfusion by stabilizing myocardial cell membranes and intracellular ion flow [
25]. In addition to its antiarrhythmic effects, lidocaine has cytoprotective effects, such as anti-apoptotic and anti-hemolytic properties [
26]. This feature of DN cardioplegia may have contributed to the lower VF rate. Although DN is associated with a lower incidence of ventricular fibrillation, VF is inherently multifactorial. It is influenced by factors such as preoperative conduction abnormalities, electrolyte imbalances, ischemia duration, and intraoperative myocardial manipulation. Therefore, these findings should be cautiously interpreted. Future studies should evaluate preoperative ECG findings, intraoperative electrolyte changes, and temperature changes during reperfusion to develop a deeper understanding of the risk of VF.
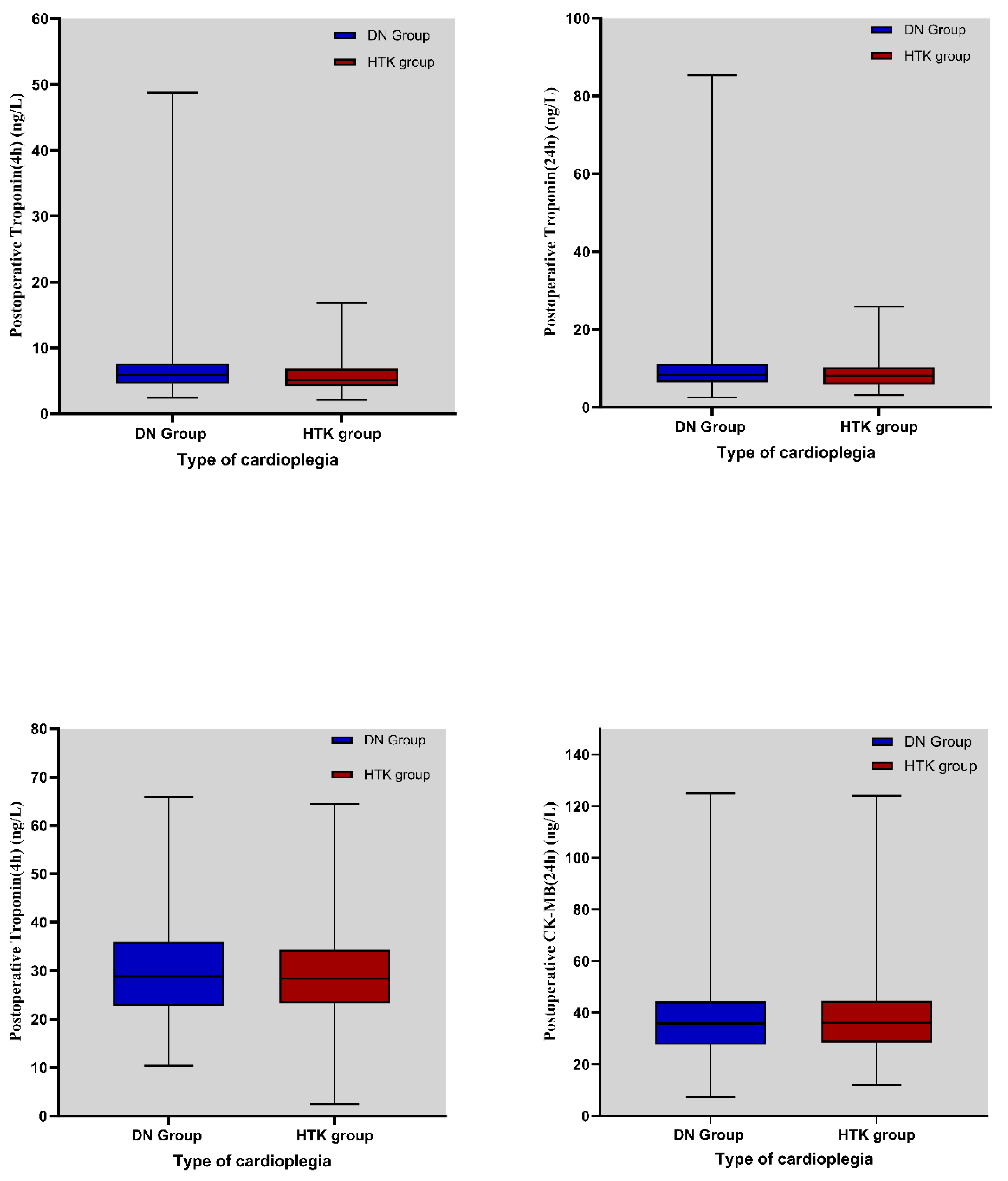
Troponin I (TnI) and CK-MB levels are critical markers of myocardial injury and provide insight into the efficacy of cardioplegia [
27]. In the literature, no significant difference was observed in TnI and CK-MB levels between the DN and HTK cardioplegia groups at ACC times of 120–150 min, representing a moderate ischemic period [
28]. In this study, the myocardial protection profiles of DN and HTK were similar at this time interval. No significant differences were observed in terms of clinical outcomes. However, this does not mean that the groups are equivalent. However, the limited sample size and lack of statistical power should be taken into consideration. Although effect size analyses (Cohen’s d < 0.2) are supportive of non-inferiority by indicating clinically small differences, the ACC duration range of 120–150 min may be considered a “gray zone” that should be specifically examined in prospective studies with larger samples and homogeneous surgical procedures. In this critical interval, the decision between DN and HTK may enter the realm of *clinical equilibrium* or *therapeutic uncertainty*, where no single solution is unequivocally superior to others for all patients. In clinical practice, this highlights the need to individualize the choice of cardioplegia in cases in this gray area according to patient characteristics and surgical predictions. For example, in patients with sensitivity to electrolyte balance or the risk of repeat aortic clamping, the flexibility of DN redosability may be advantageous. In contrast, if the operation can be completed with a single dose of cardioplegia, HTK may be a more appropriate option. Therefore, this interval stands out as a decision point where individualized cardioprotective strategies can be developed, considering the unique advantages of both cardioplegia solutions.
In this study, both TnI levels and IABP utilization rates were significantly lower in the HTK cardioplegia group than in the DN group in patients with ACC duration exceeding 180 min. These findings suggest that HTK may provide more effective myocardial protection than DN during prolonged ischemic periods. This superiority may be related not only to the high buffering capacity of HTK but also to its synergistic components such as alpha-ketoglutarate (promoting ATP production) and tryptophan (membrane stabilization and reduction in oxidative stress) [
29]. Furthermore, HTK has the potential to prevent ischemia–reperfusion injury by reducing intracellular edema and calcium overload owing to its low sodium and calcium contents [
30]. The possibility of a single-dose administration does not interrupt the surgical flow and avoids the cumulative dilutional effects that can be seen in DN. These physiological and operational advantages make HTK a more effective cardioprotective agent in procedures exceeding 180 min. However, although higher troponin I levels at 4 h in the DN group suggest the possibility of biochemically increased myocardial damage, this difference was not reflected in clinical outcomes, such as postoperative EF, LCOS, need for mechanical support, or mortality. This biomarker-clinical outcome divergence reflects the uncertainties encountered in the clinical interpretation of the troponin levels. TnI elevation may be related to cardioplegia efficacy, reperfusion properties, myocardial edema, or transient metabolic stress. Furthermore, short-term troponin elevation may not represent permanent myocardial damage unless left ventricular function is impaired. Furthermore, the fundamental biochemical differences between DN and HTK cardioplegia solutions—DN being extracellular and HTK being intracellular—lead to unique effects on protective mechanisms [
9]. These pathophysiological differences may explain the changes in troponin kinetics and myocardial responses at different durations.
Other important indicators of myocardial protection efficacy are EF and inotropic support requirements [
31]. Both cardioplegia groups experienced postoperative decreases in EF, but subgroup analysis showed a more pronounced decrease in EF in the DN group at ACC times > 180 min. These findings are consistent with those of previous studies suggesting that HTK may contribute to myocardial stability under prolonged ischemic conditions [
32]. In contrast, some animal model studies have found that DN cardioplegia better preserved diastolic and systolic left ventricular function than HTK, but these differences usually disappeared after reperfusion [
33].
The vasoactive–inotropic score (VIS) is an important parameter indicating the need for postoperative cardiovascular support [
34]. In this study, although the dobutamine doses were higher in the HTK group, the total VISs were similar between the two groups. This apparent discrepancy is likely due to differences in clinical preference or patient-specific hemodynamic goals and does not directly indicate myocardial dysfunction. VIS is a scoring system based on the total effect of multiple doses of inotropic and vasoactive agents (dopamine, adrenaline, noradrenaline, and milrinone). Therefore, a higher dose of a single agent may not significantly change the total VIS if the dose of other agents is low. The higher use of dobutamine in the HTK group may have been due to the need for right ventricular support, particularly in multivalvular and complex surgeries. This suggests that composite scoring systems, such as the VIS, may mask drug-based details and individualized treatment strategies.
Serum lactate levels are another reliable and indirect measure of metabolic stress and ischemia–reperfusion injury [
35]. Elevated serum lactate levels after cardiac surgery reflect impaired tissue perfusion and an increased anaerobic metabolism. Excluding hypovolemia, elevated lactate levels after surgery are associated with low cardiac output. Studies have reported that DN cardioplegia results in lower serum lactate levels, possibly because of its formulation that stabilizes cellular metabolism and limits anaerobic processes [
35]. Although HTK enables prolonged cardiac arrest, intracellular effects of prolonged ischemia may increase serum lactate levels. In our study, while the total ACC durations did not reveal statistically significant lactate differences, we observed an increase in lactate levels for DN beyond 150 min of ACC, suggesting that lactate trends should be carefully considered when using DN for prolonged ischemic durations.
In clinical studies, a higher incidence of VF was observed in the HTK group [
21]. In our study, a higher incidence of VF was observed in the HTK group than in the DN group after ACC. Therefore, this situation should be clinically considered. However, these episodes are usually transient and controlled using standard defibrillation protocols. Furthermore, this was not associated with adverse clinical outcomes, such as higher inotrope requirement, development of LCOS, or prolonged intensive care unit stay.
In a subgroup analysis of patients who underwent isolated CABG, HTK cardioplegia was associated with lower postoperative TnI and CK-MB levels. On the other hand, while parameters such as LCOS and IABP requirement were statistically similar, the HTK group consistently showed lower injury markers, indicating that HTK exhibits a favorable profile in surgeries more prone to ischemia, such as combined CABG.
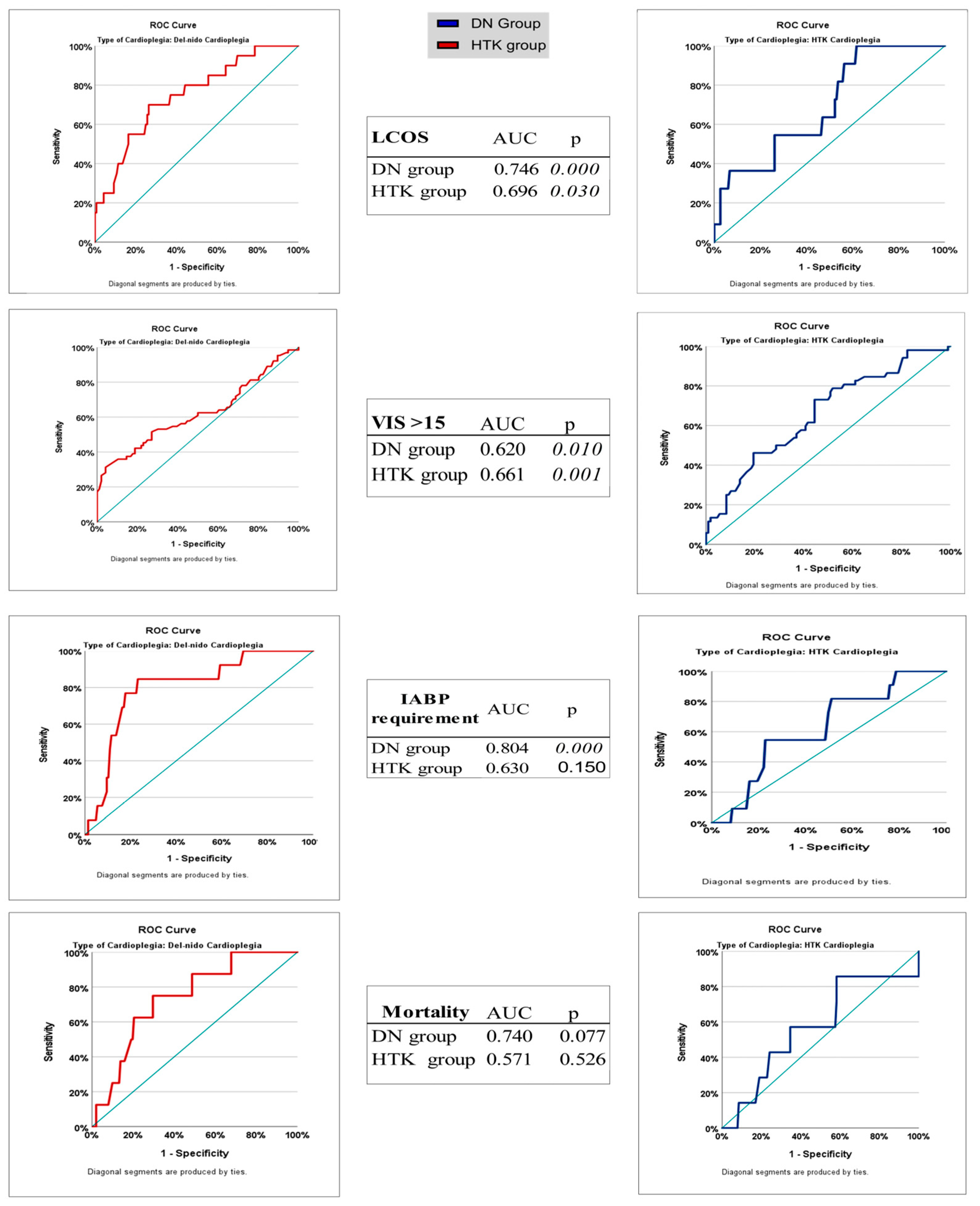
Receiver operating characteristic (ROC) analysis is a powerful method for evaluating the predictability of postoperative complications with certain clinical parameters. In our study, we sought to answer the question “When does the risk of complications become apparent if I use which agent?” by estimating the ACC duration using ROC analysis. In the current literature, no direct study has compared the cut-off thresholds that can predict postoperative complications of DN and HTK cardioplegia types with ROC analysis over ACC duration. Willekes and Duan et al. compared the clinical outcomes of DN and HTK with ACC durations of 90–150 min and longer, but ROC-based cut-offs and sensitivity-specificity analyses were not discriminatively presented in these studies [
9,
36]. Similarly, although there are observations on the safe use of DN as a single dose for a dosing duration of more than 90 min, these studies did not include an ACC duration cut-off analysis [
6]. In this study, for the first time, ROC-based cut-off values were determined for important postoperative outcomes such as LCOS, VIS > 15, mortality, and IABP according to ACC duration in the DN and HTK groups, and the statistical power (AUC, sensitivity, specificity) of operational ACC tolerance thresholds as the threshold duration of both cardioplegics was measured. In our study, ROC analyses of ACC durations showed high diagnostic accuracy in predicting outcomes such as LCOS and IABP requirement in the DN group (AUC: 0.746 for LCOS; cut-off: 161 min; AUC: 0.804 for IABP; cut-off: 168.5 min). In contrast, although these cutoff times were longer in the HTK group, the AUC values were low and did not reach statistical significance. Similarly, in terms of the need for high inotropic support, such as VIS > 15, the HTK group had a higher sensitivity of 73.1% (the rate of catching patients who will develop the need for inotropes when using HTK), while the DN group had a higher specificity of 95.8% (the power to say that if VIS > 15 did not develop in patients with DN, there were no complications). These findings suggest that DN may more accurately exclude the risk of complications, whereas HTK is more sensitive in terms of early diagnosis. However, both groups showed limited diagnostic accuracy in terms of mortality prediction; the AUC was clinically remarkable at 0.740 only in the DN group, but statistical significance was not achieved. These results suggest that the cut-off values determined according to the duration of ACC do not reflect the ischemia tolerance limit of cardioplegia but rather the threshold at which the risk of complications becomes apparent. Thus, a higher cut-off value does not necessarily imply better ischemia tolerance; this value should always be evaluated in combination with diagnostic accuracy parameters such as AUC, sensitivity, and specificity. The predictive power of DN cardioplegia with higher AUC values, especially for outcomes such as LCOS and the need for IABP, suggests that the clinical predictability and confidence interval of this strategy are more consistent. Furthermore, the ability of HTK to demonstrate a higher physiological tolerance to ischemia and to tolerate longer ACC times before the onset of these complications provides an important guide for complex cases in which prolonged clamp times are anticipated. Conversely, the lower intraoperative cardioplegia volume and reduced need for transfusion with DN provide compelling arguments for its use in less complex, shorter procedures, where minimizing exposure to blood products and optimizing resource utilization are priorities.
In light of this information, for clinical practice, DN cardioplegia may be preferred for ACC duration < 150 min because of its proven efficacy, lower intraoperative volume, less transfusion requirement, and favorable effect on VF after ACC. For ACC duration > 180 min, HTK cardioplegia is recommended because of its better biochemical stability, lower TnI levels, and reduced need for IABP during prolonged ischemic periods. Its higher physiological tolerance to ischemia makes it a more robust choice for highly complex and lengthy procedures. In the ACC 120–150 min range, the choice of solution should be based on operational foresight, dosing flexibility, and patient-specific risk factors. The ACC 150–180 min is an area of treatment uncertainty. For this specific period, further research is needed to refine the recommendations, taking into account the findings of this study.