Association of Bioelectrical Impedance Analysis Parameters with Malnutrition in Patients Undergoing Maintenance Hemodialysis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
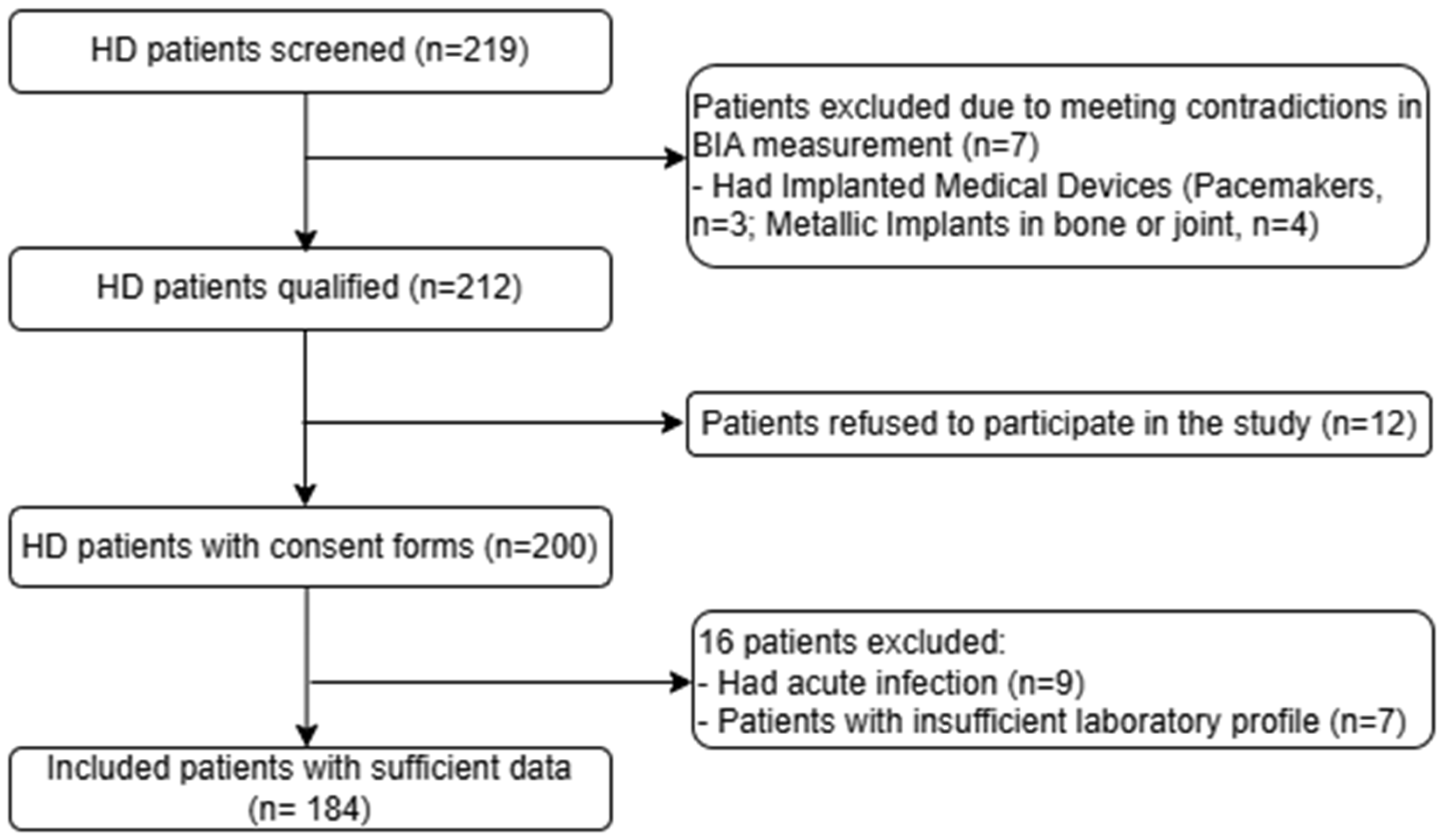
2.1. Study Design and Population
2.2. Measurements
2.2.1. Demographic, Clinical, and Laboratory Parameters
2.2.2. Anthropometric and BIA Parameters
2.3. Nutritional Risk Assessment
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Associations Between the Biomarkers and Malnutrition Risk of the Study Patients
3.3. Associations Between BIA Parameters and the Malnutrition Risk of the Study Patients
3.3.1. Associations Between Muscle-Fat Parameters and Malnutrition Risk
3.3.2. Association Between ECW/TBW Ratio and Malnutrition Risk
3.3.3. Association Between PhA and Malnutrition Risk
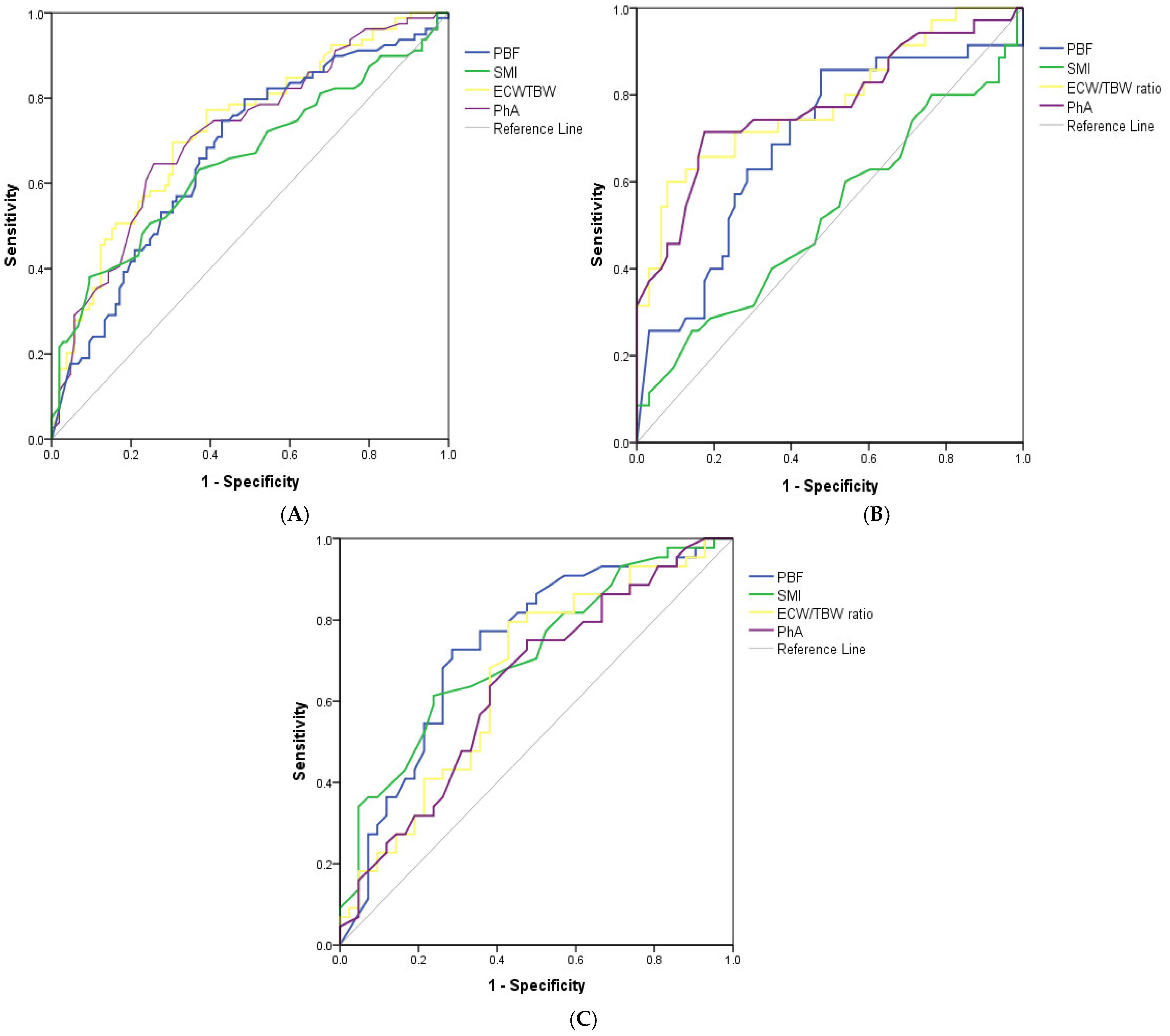
3.4. Cut-Off Values of BIA Parameters to Detect High Risk of Malnutrition in HD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, C.T.; Blankestijn, P.J.; Dember, L.M.; Gallieni, M.; Harris, D.C.H.; Lok, C.E.; Mehrotra, R.; Stevens, P.E.; Wang, A.Y.; Cheung, M.; et al. Dialysis initiation, modality choice, access, and prescription: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 96, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Uhl, S.; Siddique, S.M.; McKeever, L.; Bloschichak, A.; D’Anci, K.; Leas, B.; Mull, N.K.; Tsou, A.Y. AHRQ Comparative Effectiveness Reviews. In Malnutrition in Hospitalized Adults: A Systematic Review; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2021. [Google Scholar]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef]
- Rashid, I.; Sahu, G.; Tiwari, P.; Willis, C.; Asche, C.V.; Bagga, T.K.; Ghule, P.; Bland, A. Malnutrition as a potential predictor of mortality in chronic kidney disease patients on dialysis: A systematic review and meta-analysis. Clin. Nutr. 2024, 43, 1760–1769. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, M.; Ye, T.; Wang, Z.; Yao, Y. Application of bioelectrical impedance analysis in nutritional management of patients with chronic kidney disease. Nutrients 2023, 15, 3941. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Fosbøl, M.Ø.; Zerahn, B. Contemporary methods of body composition measurement. Clin. Physiol. Funct. Imaging 2015, 35, 81–97. [Google Scholar] [CrossRef]
- Duong, T.V.; Wu, P.Y.; Wong, T.C.; Chen, H.H.; Chen, T.H.; Hsu, Y.H.; Peng, S.J.; Kuo, K.L.; Liu, H.C.; Lin, E.T.; et al. Mid-arm circumference, body fat, nutritional and inflammatory biomarkers, blood glucose, dialysis adequacy influence all-cause mortality in hemodialysis patients: A prospective cohort study. Medicine 2019, 98, e14930. [Google Scholar] [CrossRef]
- Sukackiene, D.; Laucyte-Cibulskiene, A.; Vickiene, A.; Rimsevicius, L.; Miglinas, M. Risk stratification for patients awaiting kidney transplantation: Role of bioimpedance derived edema index and nutrition status. Clin. Nutr. 2020, 39, 2759–2763. [Google Scholar] [CrossRef]
- Yajima, T.; Yajima, K. Association of extracellular water/total body water ratio with protein-energy wasting and mortality in patients on hemodialysis. Sci. Rep. 2023, 13, 14257. [Google Scholar] [CrossRef]
- Aatif, T.; Hassani, K.; Alayoud, A.; Maoujoud, O.; Ahid, S.; Benyahia, M.; Oualim, Z. Parameters to assess nutritional status in a Moroccan hemodialysis cohort. Arab. J. Nephrol. Transpl. 2013, 6, 89–97. [Google Scholar]
- Song, H.C.; Shin, J.; Hwang, J.H.; Kim, S.H. Utility of the Global Leadership Initiative on Malnutrition criteria for the nutritional assessment of patients with end-stage renal disease receiving chronic hemodialysis. J. Hum. Nutr. Diet. 2023, 36, 97–107. [Google Scholar] [CrossRef]
- Quoc, N. Vietnam: Over 8.7 Million Afflicted with Chronic Kidney Disease. Available online: https://en.sggp.org.vn/vietnam-over-87-million-afflicted-with-chronic-kidney-disease-post109188.html (accessed on 12 April 2025).
- Saitoh, M.; Ogawa, M.; Kondo, H.; Suga, K.; Takahashi, T.; Itoh, H.; Tabata, Y. Bioelectrical impedance analysis-derived phase angle as a determinant of protein-energy wasting and frailty in maintenance hemodialysis patients: Retrospective cohort study. BMC Nephrol. 2020, 21, 438. [Google Scholar] [CrossRef]
- Tan, R.-s.; Liang, D.-h.; Liu, Y.; Zhong, X.-s.; Zhang, D.-s.; Ma, J. Bioelectrical Impedance Analysis–Derived Phase Angle Predicts Protein–Energy Wasting in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2019, 29, 295–301. [Google Scholar] [CrossRef]
- Lim, C.K.; Lim, J.H.; Ibrahim, I.; Chan, Y.M.; Zakaria, N.F.; Yahya, R.; Daud, Z.A.M. Bioelectrical Impedance Analysis Derived-Phase Angle as a Pragmatic Tool to Detect Protein Energy Wasting among Multi-Ethnic Hemodialysis Patients. Diagnostics 2021, 11, 1745. [Google Scholar] [CrossRef] [PubMed]
- Leal-Escobar, G.; Padilla, I.A.O.; Escobar, K.B.C.; González, B.M.; Grovas, H.P.; Ubaldo, S.R. Phase angle and mid arm circumference as predictors of protein energy wasting in renal replacement therapy patients. Nutr. Hosp. Órgano Of. De La Soc. Española De Nutr. Clínica Y Metab. (SENPE) 2019, 36, 633–639. [Google Scholar]
- Varan, H.D.; Bolayir, B.; Kara, O.; Arik, G.; Kizilarslanoglu, M.C.; Kilic, M.K.; Sumer, F.; Kuyumcu, M.E.; Yesil, Y.; Yavuz, B.B. Phase angle assessment by bioelectrical impedance analysis and its predictive value for malnutrition risk in hospitalized geriatric patients. Aging Clin. Exp. Res. 2016, 28, 1121–1126. [Google Scholar] [CrossRef]
- Jensen, B.; Moritoyo, T.; Kaufer-Horwitz, M.; Peine, S.; Norman, K.; Maisch, M.J.; Matsumoto, A.; Masui, Y.; Velázquez-González, A.; Domínguez-García, J. Ethnic differences in fat and muscle mass and their implication for interpretation of bioelectrical impedance vector analysis. Appl. Physiol. Nutr. Metab. 2019, 44, 619–626. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015.
- Rimsevicius, L.; Gincaite, A.; Vicka, V.; Sukackiene, D.; Pavinic, J.; Miglinas, M. Malnutrition Assessment in Hemodialysis Patients: Role of Bioelectrical Impedance Analysis Phase Angle. J. Ren. Nutr. 2016, 26, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Redondo, E.; Morgado-Pérez, A.; Pérez-Sáez, M.-J.; Faura, A.; Sánchez-Rodríguez, D.; Tejero-Sánchez, M.; Meza-Valderrama, D.; Muns, M.D.; Pascual, J.; Marco, E. Low Phase Angle Values Are Associated with Malnutrition according to the Global Leadership Initiative on Malnutrition Criteria in Kidney Transplant Candidates: Preliminary Assessment of Diagnostic Accuracy in the FRAILMar Study. Nutrients 2023, 15, 1084. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, M.; Barril, G. Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients—A Case–Control Study. Nutrients 2023, 15, 5036. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakashima, A.; Doi, S.; Maeda, K.; Ishiuchi, N.; Naito, T.; Masaki, T. Lower Geriatric Nutritional Risk Index (GNRI) Is Associated with Higher Risk of Fractures in Patients Undergoing Hemodialysis. Nutrients 2021, 13, 2847. [Google Scholar] [CrossRef]
- Takahashi, H.; Ito, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kasuga, H.; Yasuda, K.; Maruyama, S.; Matsuo, S.; Murohara, T.; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J. Cardiol. 2014, 64, 32–36. [Google Scholar] [CrossRef]
- Hirose, S.; Nakajima, T.; Nozawa, N.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Matsumoto, K.; Nishikawa, K.; Toyama, Y.; Takahashi, R.; et al. Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2554. [Google Scholar] [CrossRef]
- Castel, H.; Shahar, D.; Harman-Boehm, I. Gender differences in factors associated with nutritional status of older medical patients. J. Am. Coll. Nutr. 2006, 25, 128–134. [Google Scholar] [CrossRef]
- Darling, A.M.; Sunguya, B.; Ismail, A.; Manu, A.; Canavan, C.; Assefa, N.; Sie, A.; Fawzi, W.; Sudfeld, C.; Guwattude, D. Gender differences in nutritional status, diet and physical activity among adolescents in eight countries in sub-Saharan Africa. Trop. Med. Int. Health 2020, 25, 33–43. [Google Scholar] [CrossRef]
- Hamdan, Z.; Nazzal, Z.; Al-Amouri, F.M.; Ishtayah, S.; Sammoudi, S.; Bsharat, L.; Badrasawi, M. Factors associated with malnutrition inflammation score among hemodialysis patients: A cross-sectional investigation in tertiary care hospital, Palestine. PLoS ONE 2025, 20, e0317132. [Google Scholar] [CrossRef]
- Badrasawi, M.; Zidan, S.; Sharif, I.; Qaisiyha, J.; Ewaida, S.; Jaradat, T.; Samamra, Y. Prevalence and correlates of malnutrition among hemodialysis patients at hebron governmental hospital, Palestine: Cross-sectional study. BMC Nephrol. 2021, 22, 214. [Google Scholar] [CrossRef]
- Pham Thi Lan, A.; Truong Thanh, A.; Luong Ngoc, Q.; Pham Nhat, T.; Doan Duy, T. Prevalence and factors associated with malnutrition among hemodialysis patients in a single hemodialysis center in Vietnam: A cross-sectional study. Medicine 2024, 103, e37679. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The Inconsistency of “Optimal” Cutpoints Obtained using Two Criteria based on the Receiver Operating Characteristic Curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [CrossRef]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Kadoshi, H.; Shapiro, G.; Feldman, L.; Averbukh, Z.; Weissgarten, J. Comparison analysis of nutritional scores for serial monitoring of nutritional status in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Kuo, K.L.; Peng, C.H.; Wu, C.H.; Wang, Y.C.; Tarng, D.C. Association of Fluid Retention With Anemia and Clinical Outcomes Among Patients With Chronic Kidney Disease. J. Am. Heart Assoc. 2015, 4, e001480. [Google Scholar] [CrossRef]
- Badura, K.; Janc, J.; Wąsik, J.; Gnitecki, S.; Skwira, S.; Młynarska, E.; Rysz, J.; Franczyk, B. Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines 2024, 12, 1191. [Google Scholar] [CrossRef]
- Azzeh, F.S.; Turkistani, W.M.; Ghaith, M.M.; Bahubaish, L.A.; Kensara, O.A.; Almasmoum, H.A.; Aldairi, A.F.; Khan, A.A.; Alghamdi, A.A.; Shamlan, G.; et al. Factors associated with the prevalence of malnutrition among adult hemodialytic patients: A two-center study in the Jeddah region, Saudi Arabia. Medicine 2022, 101, e30757. [Google Scholar] [CrossRef]
- Zaki, D.; Mohamed, R.R.; Mohammed, N.; Abdel-Zaher, R.B. Assessment of malnutrition status in hemodialysis patients. Clin. Med. Diagn. 2019, 9, 8–13. [Google Scholar]
- González-Ortiz, A.J.; Arce-Santander, C.V.; Vega-Vega, O.; Correa-Rotter, R.; Espinosa-Cuevas, M.A. Assessment of the reliability and consistency of the “Malnutrition Inflammation Score”(MIS) in Mexican adults with chronic kidney disease for diagnosis of protein-energy wasting syndrome (PEW). Nutr. Hosp. 2015, 31, 1352–1358. [Google Scholar]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Mahillo, I.; Ortiz, A. Criteria for classification of protein–energy wasting in dialysis patients: Impact on prevalence. Br. J. Nutr. 2019, 121, 1271–1278. [Google Scholar] [CrossRef]
- Wang, W.; Meng, X.; Liu, J.; Lou, X.; Zhang, P.; He, P.; Chen, J.; Yuan, J. Study on the correlation between bioelectrical impedance analysis index and protein energy consumption in maintenance dialysis patients. Nutr. J. 2023, 22, 56. [Google Scholar] [CrossRef]
- Demir, M.; Kucuk, A.; Sezer, M.T.; Altuntas, A.; Kaya, S. Malnutrition-inflammation score and endothelial dysfunction in hemodialysis patients. J. Ren. Nutr. 2010, 20, 377–383. [Google Scholar] [CrossRef]
- Macedo, C.; Amaral, T.F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Malnutrition and Sarcopenia Combined Increases the Risk for Mortality in Older Adults on Hemodialysis. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Mori, K.; Miyabe, M.; Matsufuji, S.; Ichii, M.; Morioka, T.; Kizu, A.; Tsujimoto, Y.; Emoto, M. Nutritional Status Association With Sarcopenia in Patients Undergoing Maintenance Hemodialysis Assessed by Nutritional Risk Index. Front. Nutr. 2022, 9, 896427. [Google Scholar] [CrossRef]
- Landen, S.; Hiam, D.; Voisin, S.; Jacques, M.; Lamon, S.; Eynon, N. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J. Physiol. 2023, 601, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Ando, M.; Suminaka, T.; Shimada, N.; Asano, K.; Ono, J.-i.; Jikuya, K.; Mochizuki, S. Body water balance in hemodialysis patients reflects nutritional, circulatory, and body fluid status. J. Biorheol. 2018, 32, 46–55. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Garcia-Almeida, J.M. Phase angle in applications of bioimpedance in health and disease. Rev. Endocr. Metab. Disord. 2023, 24, 367–370. [Google Scholar] [CrossRef]
- Han, B.-G.; Lee, J.Y.; Kim, J.-S.; Yang, J.-W. Decreased Bioimpedance Phase Angle in Patients with Diabetic Chronic Kidney Disease Stage 5. Nutrients 2019, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Franco-Oliva, A.; Ávila-Nava, A.; Rodríguez-Aguilar, E.A.; Trujillo-Mercado, A.; García-Guzmán, A.D.; Pinzón-Navarro, B.A.; Fuentes-Servín, J.; Guevara-Cruz, M.; Medina-Vera, I. Association between phase angle and the nutritional status in pediatric populations: A systematic review. Front. Nutr. 2023, 10, 1142545. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, J.; Cheng, S.; Liang, B. The Role of Standardized Phase Angle in the Assessment of Nutritional Status and Clinical Outcomes in Cancer Patients: A Systematic Review of the Literature. Nutrients 2023, 15, 50. [Google Scholar] [CrossRef]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Chesworth, B.; Madill, J. Is phase angle an appropriate indicator of malnutrition in different disease states? A systematic review. Clin. Nutr. ESPEN 2019, 29, 1–14. [Google Scholar] [CrossRef]
- Chua, H.-R.; Xiang, L.; Chow, P.-Y.; Xu, H.; Shen, L.; Lee, E.; Teo, B.-W. Quantifying acute changes in volume and nutritional status during haemodialysis using bioimpedance analysis. Nephrology 2012, 17, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Don, B.R.; Kaysen, G. Poor Nutritional Status and Inflammation: Serum Albumin: Relationship to Inflammation and Nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Total (n = 184) | GNRI | p-Value | |
|---|---|---|---|---|
| GNRI ≥ 92 (n = 105) | GNRI < 92 (n = 79) | |||
| Age | 0.025 | |||
| <60 years | 95 (51.6) | 62 (59.0) | 33 (41.8) | |
| ≥60 years | 89 (48.4) | 43 (41.0) | 46 (58.2) | |
| Gender | 0.035 | |||
| Male | 119 (64.7) | 63 (60.0) | 35 (44.3) | |
| Female | 65 (35.3) | 42 (40.0) | 44 (55.7) | |
| Comorbidity | 0.514 | |||
| No | 119 (64.7) | 70 (66.7) | 49 (62.0) | |
| One or more | 65 (35.3) | 35 (33.3) | 30 (38.0) | |
| Height (cm) | 159.8 (7.2) | 160.5 (7.4) | 159.0 (7.6) | 0.192 |
| Weight (kg) | 55.1 (9.4) | 58.8 (8.7) | 50.2 (7.9) | <0.001 |
| BMI (kg/m2) | 21.5 (2.8) | 22.8 (2.5) | 19.8 (2.2) | <0.001 |
| HD vintage (months) | 36 (12–60) | 36 (12–72) | 24 (4–60) | 0.023 |
| Laboratory findings | ||||
| WBC (×103/µL) | 6.1 (5.0–7.7) | 6.7 (5.5–7.9) | 5.3 (4.5–7.2) | 0.001 |
| Neutrophils (%) | 65.2 (10.2) | 65.5 (11.0) | 64.7 (9.0) | 0.603 |
| Lymphocytes (%) | 20.6 (7.9) | 20.3 (8.3) | 20.8 (7.2) | 0.676 |
| RBC (×106/µL) | 2.85 (0.63) | 2.93 (0.64) | 2.75 (0.61) | 0.160 |
| Hemoglobin (g/L) | 87.4 (19.1) | 90.3 (20.6) | 83.6 (16.3) | 0.019 |
| Hematocrit (%) | 26.2 (5.7) | 27.1 (6.1) | 25.2 (5.1) | 0.025 |
| Platelet (×103/µL) | 195.5 (152.0–233.0) | 199.0 (157.0–231.0) | 187.0 (142.0–236.0) | 0.547 |
| AST (IU/L) | 17.6 (13.8–26.4) | 16.6 (13.0–23.7) | 18.8 (15.8–28.5) | 0.055 |
| ALT (IU/L) | 13.1 (8.8–20.0) | 11.9 (8.9–19.2) | 13.9 (8.9–20.9) | 0.578 |
| Serum albumin (g/L) | 39.0 (36.9–41.7) | 40.2 (38.8–43.0) | 37.1 (34.8–38.8) | <0.001 |
| Urea (mmol/L) | 23.7 (17.0–28.4) | 24.7 (18.8–30.0) | 20.1 (16.6–27.2) | 0.013 |
| Creatinine (µmol/L) | 854.5 (698.1–1049.3) | 919.0 (790.4–1114.7) | 750.2 (662.3–936.5) | <0.001 |
| CRP (mg/L) | 3.9 (1.5–10.9) | 3.4 (1.6–13.5) | 5.5 (1.6–9.5) | 0.933 |
| Cholesterol (mmol/L) | 4.57 (1.09) | 4.64 (1.14) | 4.48 (1.01) | 0.360 |
| Triglyceride (mmol/L) | 1.70 (1.08–2.56) | 2.04 (1.31–2.80) | 1.29 (0.89–1.96) | <0.001 |
| LDL-C (mmol/L) | 2.88 (0.79) | 2.96 (0.92) | 2.77 (0.75) | 0.128 |
| HDL-C (mmol/L) | 0.98 (0.81–1.18) | 0.92 (0.79–1.05) | 1.10 (0.90–1.23) | 0.002 |
| Ferritin (ng/mL) | 182.5 (78.5–434.2) | 123.9 (77.1–443.4) | 210.6 (113.9–406.6) | 0.399 |
| BIA parameters | ||||
| SLM (kg) | 42.4 (37.3–49.3) | 44.5 (39.1–50.8) | 38.8 (34.5–47.0) | <0.001 |
| FFM (kg) | 45.0 (40.0–52.3) | 47.1 (41.7–53.8) | 41.4 (37.4–49.6) | <0.001 |
| SMM (kg) | 24.6 (21.3–29.2) | 25.9 (22.7–29.7) | 22.1 (19.5–27.1) | <0.001 |
| PBF (%) | 16.9 (10.3–24.3) | 20.5 (14.0–25.5) | 14.5 (7.4–18.1) | <0.001 |
| BCM (kg) | 29.2 (25.6–34.3) | 30.7 (27.1–34.8) | 26.5 (23.6–32.0) | <0.001 |
| VFA (cm2) | 39.9 (23.5–57.2) | 44.1 (26.1–65.7) | 33.6 (19.0–46.7) | 0.003 |
| SMI (kg/m2) | 7.2 (6.4–8.0) | 7.4 (6.9–8.1) | 6.8 (5.9–7.7) | <0.001 |
| ICW (L) | 20.4 (17.9–23.9) | 21.4 (18.9–24.3) | 18.5 (16.5–22.3) | <0.001 |
| ECW (L) | 12.8 (11.3–14.6) | 13.2 (11.6–14.9) | 12.0 (10.8–14.0) | 0.005 |
| TBW (L) | 33.2 (29.1–38.3) | 34.7 (30.5–39.6) | 30.4 (27.5–36.7) | <0.001 |
| ECW/TBW (Total,%) | 38.6 (37.3–39.6) | 39.2 (38.4–40.2) | 38.2 (37.1–38.9) | <0.001 |
| PhA (°) | 5.40 (4.63–6.40) | 4.80 (4.15–5.70) | 5.90 (5.10–6.70) | <0.001 |
| Parameters | High Risk of Malnutrition | |||||
|---|---|---|---|---|---|---|
| Overall Sample | Males | Females | ||||
| aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | P-Value | |
| Laboratory parameters | ||||||
| Hemoglobin, 1 g/L increase | 0.980 (0.964–0.997) | 0.022 | 0.974 (0.951–0.997) | 0.025 | 0.993 (0.969–1.019) | 0.613 |
| Hematocrit, 1% increase | 0.941 (0.890–0.994) | 0.031 | 0.925 (0.858–0.996) | 0.039 | 0.978 (0.896–1.067) | 0.620 |
| Urea, 1 mmol/L increase | 0.958 (0.922–0.995) | 0.026 | 0.928 (0.873–0.988) | 0.019 | 0.977 (0.930–1.026) | 0.347 |
| Creatinine, 1 µmol/L increase | 0.998 (0.996–0.999) | 0.001 | 0.997 (0.995–0.999) | 0.008 | 0.999 (0.997–1.001) | 0.195 |
| Triglyceride, 1 mmol/L increase | 0.619 (0.463–0.828) | 0.001 | 0.628 (0.433–0.912) | 0.014 | 0.595 (0.363–0.977) | 0.040 |
| HDL-C, 1 mmol/L increase | 11.731 (2.897–47.506) | 0.001 | 16.099 (7.139–81.124) | <0.001 | 3.055 (0.519–17.983) | 0.217 |
| BIA parameters | ||||||
| SLM, 1 kg increase | 0.923 (0.884–0.962) | <0.001 | 0.931 (0.865–1.001) | 0.055 | 0.851 (0.764–0.948) | 0.003 |
| FFM, 1 kg increase | 0.926 (0.890–0.964) | <0.001 | 0.934 (0.872–1.001) | 0.051 | 0.857 (0.774–0.950) | 0.003 |
| SMM, 1 kg increase | 0.870 (0.813–0.931) | <0.001 | 0.868 (0.769–0.980) | 0.023 | 0.764 (0.640–0.911) | 0.003 |
| PBF, 1 percent increase | 0.921 (0.885–0.959) | <0.001 | 0.914 (0.861–0.970) | 0.003 | 0.885 (0.824–0.950) | 0.001 |
| BCM, 1 kg increase | 0.880 (0.828–0.936) | <0.001 | 0.878 (0.786–0.981) | 0.022 | 0.781 (0.665–0.918) | 0.003 |
| VFA, 1 cm2 increase | 0.974 (0.960–0.989) | 0.001 | 0.975 (0.956–0.995) | 0.014 | 0.961 (0.934–0.989) | 0.012 |
| SMI, 1 kg/m2 increase | 0.647 (0.500–0.837) | 0.001 | 0.845 (0.549–1.302) | 0.445 | 0.436 (0.238–0.799) | 0.007 |
| ICW, 1 L increase | 0.833 (0.763–0.910) | <0.001 | 0.830 (0.708–0.973) | 0.021 | 0.703 (0.558–0.885) | 0.003 |
| ECW, 1 L increase | 0.823 (0.718–0.942) | 0.005 | 0.907 (0.727–1.131) | 0.386 | 0.660 (0.472–0.921) | 0.015 |
| TBW, 1 L increase | 0.905 (0.857–0.955) | <0.001 | 0.919 (0.837–1.008) | 0.072 | 0.817 (0.712–0.937) | 0.004 |
| ECW/TBW, 1% increase | 2.162 (1.575–2.968) | <0.001 | 3.510 (1.928–6.388) | <0.001 | 1.630 (1.120–2.371) | 0.011 |
| PhA, 1 degree increase | 0.481 (0.338–0.686) | <0.001 | 0.385 (0.218–0.680) | 0.001 | 0.612 (0.382–0.980) | 0.041 |
| Cut-Off Value * | Cut-Off Value # | AUC | 95%CI | Sensitivity (%) | Specificity (%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Overall sample (n = 184) | |||||||
| PBF, % | 20.45 | 20.45 | 0.672 | 0.593–0.751 | 79.7 | 50.5 | <0.001 |
| SMI, kg/m2 | 7.75 | 7.75 | 0.654 | 0.572–0.736 | 77.2 | 36.2 | <0.001 |
| ECW/TBW, % | 38.63 | 38.63 | 0.728 | 0.654–0.801 | 69.6 | 69.5 | <0.001 |
| PhA, ° | 5.45 | 5.45 | 0.717 | 0.642–0.792 | 70.9 | 64.8 | <0.001 |
| Males (n = 98) | |||||||
| PBF, % | 17.45 | 17.45 | 0.691 | 0.577–0.805 | 85.7 | 47.6 | 0.002 |
| SMI, kg/m2 | 8.25 | 8.25 | 0.523 | 0.397–0.648 | 65.7 | 31.7 | 0.711 |
| ECW/TBW, % | 39.31 | 39.31 | 0.782 | 0.681–0.883 | 60 | 92.1 | <0.001 |
| PhA, ° | 5.15 | 5.15 | 0.775 | 0.670–0.879 | 71.4 | 82.5 | <0.001 |
| Females (n = 86) | |||||||
| PBF, % | 21.1 | 21.1 | 0.733 | 0.624–0.841 | 72.7 | 64.3 | <0.001 |
| SMI, kg/m2 | 6.7 | 6.7 | 0.712 | 0.604–0.820 | 77.3 | 47.6 | 0.001 |
| ECW/TBW, % | 38.39 | 38.39 | 0.660 | 0.543–0.776 | 79.5 | 57.1 | 0.011 |
| PhA, ° | 5.65 | 5.65 | 0.640 | 0.523–0.757 | 75.0 | 52.4 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, M.D.; Dao, T.V.; Vu, A.T.X.; Bui, H.T.Q.; Nguyen, B.T.; Nguyen, A.T.T.; Ta, T.T.T.; Cap, D.M.; Le, T.D.; Phan, P.H.; et al. Association of Bioelectrical Impedance Analysis Parameters with Malnutrition in Patients Undergoing Maintenance Hemodialysis: A Cross-Sectional Study. Medicina 2025, 61, 1396. https://doi.org/10.3390/medicina61081396
Pham MD, Dao TV, Vu ATX, Bui HTQ, Nguyen BT, Nguyen ATT, Ta TTT, Cap DM, Le TD, Phan PH, et al. Association of Bioelectrical Impedance Analysis Parameters with Malnutrition in Patients Undergoing Maintenance Hemodialysis: A Cross-Sectional Study. Medicina. 2025; 61(8):1396. https://doi.org/10.3390/medicina61081396
Chicago/Turabian StylePham, Minh D., Thang V. Dao, Anh T. X. Vu, Huong T. Q. Bui, Bon T. Nguyen, An T. T. Nguyen, Thuy T. T. Ta, Duc M. Cap, Toan D. Le, Phuc H. Phan, and et al. 2025. "Association of Bioelectrical Impedance Analysis Parameters with Malnutrition in Patients Undergoing Maintenance Hemodialysis: A Cross-Sectional Study" Medicina 61, no. 8: 1396. https://doi.org/10.3390/medicina61081396
APA StylePham, M. D., Dao, T. V., Vu, A. T. X., Bui, H. T. Q., Nguyen, B. T., Nguyen, A. T. T., Ta, T. T. T., Cap, D. M., Le, T. D., Phan, P. H., Vu, H. N., Le, T. D., Pham, T. Q., Le, T. V., Luong, T. C., Ta, T. B., & Duong, T. V. (2025). Association of Bioelectrical Impedance Analysis Parameters with Malnutrition in Patients Undergoing Maintenance Hemodialysis: A Cross-Sectional Study. Medicina, 61(8), 1396. https://doi.org/10.3390/medicina61081396










