The Value of the Naples Prognostic Score at Diagnosis as a Predictor of Cervical Cancer Progression
Abstract
1. Introduction
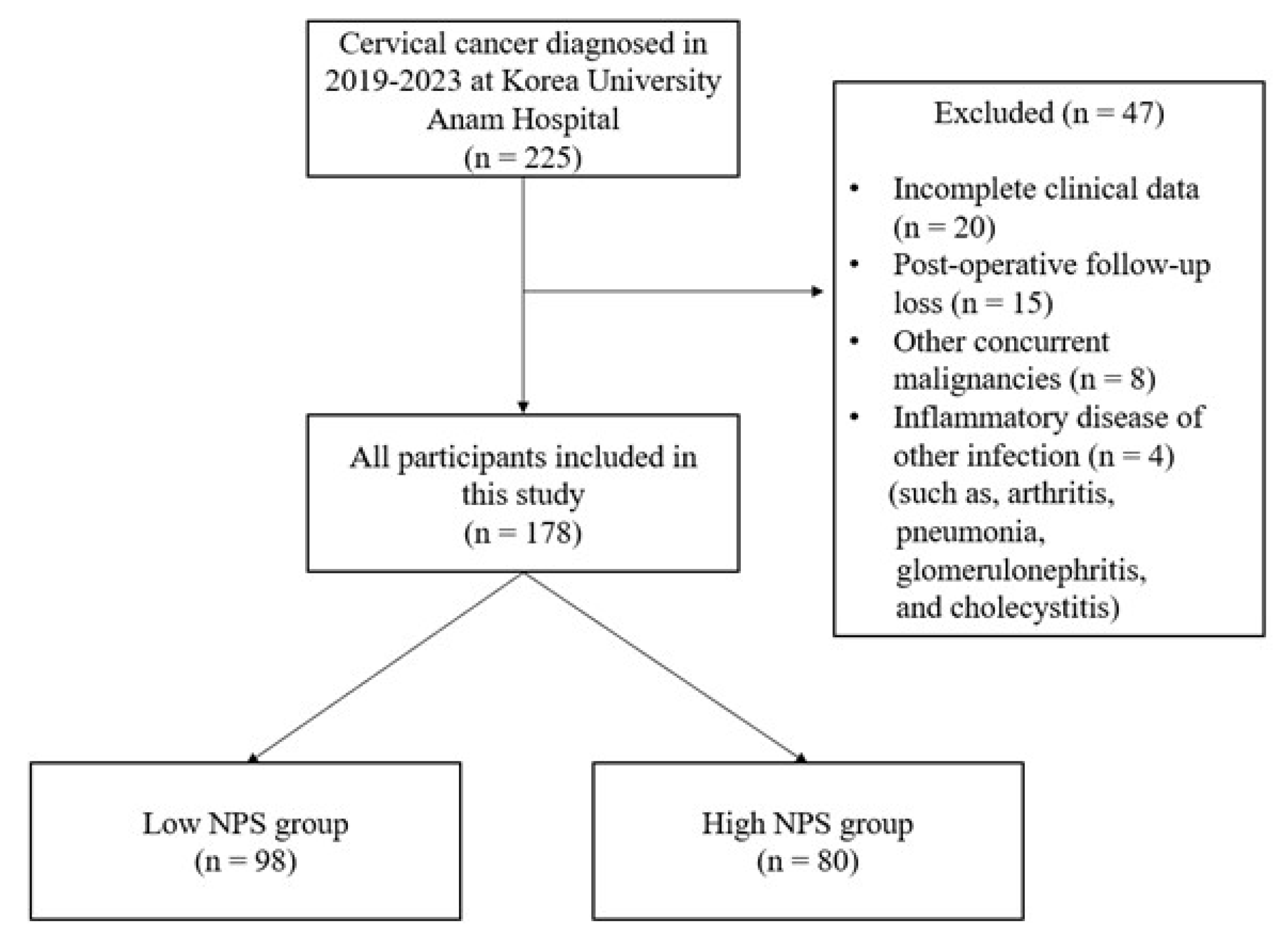
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPV | Human papillomavirus |
| SCC-Ag | Squamous cell carcinoma-Antigen |
| CA 19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| SIS | Systemic inflammation score |
| NLR | Neutrophil to lymphocyte ratio |
| PNI | Prognostic nutritional index |
| CONUT | Controlling nutritional status |
| NPS | Naples prognostic score |
| LMR | Lymphocyte to monocyte ratio |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| PCR | Polymerase chain reaction |
| PET-CT | Positron emission tomography-computed tomography |
| FIGO | International Federation of Gynecology and Obstetrics |
| CCRT | Concurrent chemoradiotherapy |
| DFS | Disease-free survival |
| OS | Overall survival |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| LN | Lymph node |
| HR | Hazard ratio |
| BMI | Body mass index |
| PLND | Pelvic lymph node |
| PALND | Para-aortic lymph node |
| MMP | Metalloproteinases |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| TGF | Transforming growth factor |
| NK | Natural killer |
| PPAR | Peroxisome proliferator-activated receptor |
| CD | Cluster of differentiation |
| FOXP3 | Forkhead box P3 |
| TAMs | Tumor-associated macrophages |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| IL | Interleukin |
| JAK | Janus kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| MAPK | Mitogen-activated protein kinase |
| UK | United kingdom |
| SII | Systemic inflammation index |
| Th | T helper |
| IFN | Interferon |
Appendix A
| Stage | 2018 FIGO Staging of Cervical Cancer |
|---|---|
| I | Confined to the cervix |
| IA | ≤5 mm depth |
| IA1 | ≤3 mm depth |
| IA2 | >3 mm and ≤5 mm depth |
| IB | >5 mm depth |
| IB1 | ≤2 cm maximum diameter |
| IB2 | >2 cm and ≤4 cm maximum diameter |
| IB3 | >4 cm maximum diameter |
| II | Beyond the uterus but not involving the lower one-third of the vagina or pelvic side wall |
| IIA | Upper two-thirds of the vagina |
| IIA1 | Upper two-thirds of the vagina and ≤4 cm |
| IIA2 | Upper two-thirds of the vagina and >4 cm |
| IIB | Parametrial invasion |
| III | Lower vagina, pelvic side wall, ureter, and LNs |
| IIIA | Lower one-third of the vagina |
| IIIB | Pelvic side wall |
| IIIC | Pelvic and para-aortic LN involvement |
| IIIC1 | Pelvic LN involvement |
| IIIC2 | Para-aortic LN involvement |
| IV | Adjacent and distant organs |
| IVA | Rectal or bladder involvement |
| IVB | Distant organs outside the pelvis |
| Risk Factors for OS | ||||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR * | 95% CI | p-Value | |
| Age (years) | 1.03 | (0.999, 1.068) | 0.057 | 1.02 | (0.987, 1.063) | 0.204 |
| Height (cm) | 0.92 | (0.851, 0.990) | 0.026 | 0.93 | (0.861, 1.002) | 0.056 |
| Weight (kg) | 0.94 | (0.893, 0.997) | 0.037 | 0.94 | (0.888, 0.996) | 0.037 |
| BMI | 0.92 | (0.801, 1.059) | 0.248 | 0.93 | (0.784, 1.110) | 0.435 |
| Parity | ||||||
| Nulliparous | 1.00 | Reference | 1.00 | Reference | ||
| Multiparous | 1.27 | (0.360, 4.441) | 0.714 | 0.45 | (0.104, 1.941) | 0.284 |
| Menopausal status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.67 | (0.540, 5.194) | 0.372 | 0.42 | (0.080, 2.209) | 0.305 |
| Comorbidities | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 2.08 | (0.782, 5.553) | 0.142 | 1.05 | (0.317, 3.499) | 0.932 |
| Histology | ||||||
| Squamous | 1.00 | Reference | 1.00 | Reference | ||
| Adenocarcinoma | 3.04 | (1.016, 9.065) | 0.047 | 3.00 | (1.001, 8.996) | 0.050 |
| Others | 18.28 | (4.710, 70.944) | <0.001 | 26.61 | (5.687, 124.525) | <0.001 |
| FIGO stage | ||||||
| Early-stage group | 1.00 | Reference | 1.00 | Reference | ||
| Advanced-stage group | 48.10 | (0.787, 2940.001) | 0.065 | 52.24 | (0.820, 3721.280) | 0.940 |
| Tumor size | ||||||
| ≤4 cm | 1.00 | Reference | 1.00 | Reference | ||
| >4 cm | 2.11 | (0.765, 5.792) | 0.150 | 0.43 | (0.126, 1.496) | 0.186 |
| LN metastasis status | ||||||
| Negative | 1.00 | Reference | 1.00 | Reference | ||
| PLND metastasis | 8.05 | (2.135, 30.385) | 0.002 | 4.68 | (1.159, 18.898) | 0.030 |
| PLDN and PALND | 8.25 | (1.967, 34.571) | 0.004 | 3.51 | (0.746, 16.459) | 0.112 |
| metastases | ||||||
| HPV infection status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.74 | (0.276, 1.991) | 0.553 | 0.33 | (0.107, 1.033) | 0.057 |
| CCRT status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.31 | (0.113, 0.856) | 0.024 | 0.13 | (0.043, 0.389) | <0.001 |
| Adjuvant chemotherapy status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 8.45 | (2.722, 26.229) | <0.001 | 4.89 | (1.437, 16.662) | 0.011 |
| Number of chemotherapies | 1.06 | (1.013, 1.105) | 0.011 | 0.96 | (0.868, 1.060) | 0.414 |
| Radical hysterectomy status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.39 | (0.112, 1.382) | 0.146 | 1.28 | (0.302, 5.397) | 0.739 |
| Trachelectomy status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 2.24 | (0.294, 17.042) | 0.437 | 16.55 | (0.868, 315.741) | 0.062 |
| SCC-Ag level | ||||||
| ≤2.0 | 1.00 | Reference | 1.00 | Reference | ||
| >2.0 | 1.88 | (0.697, 5.049) | 0.213 | 0.99 | (0.350, 2.809) | 0.988 |
| CA 19-9 level | ||||||
| ≤37 | 1.00 | Reference | 1.00 | Reference | ||
| >37 | 7.45 | (2.770, 20.014) | <0.001 | 4.83 | (1.744, 13.347) | 0.002 |
| CEA level | ||||||
| ≤4.6 | 1.00 | Reference | 1.00 | Reference | ||
| >4.6 | 1.77 | (0.504, 6.231) | 0.372 | 0.68 | (0.180, 2.590) | 0.575 |
| NPS status | ||||||
| Low NPS | 1.00 | Reference | 1.00 | Reference | ||
| High NPS | 3.52 | (1.135, 10.936) | 0.029 | 1.93 | (0.586, 6.361) | 0.279 |
| Variable | VIF Value |
|---|---|
| Age | 1.36 |
| Comorbidities | 1.20 |
| FIGO stage | 1.96 |
| Tumor size | 1.52 |
| HPV infection status | 1.08 |
| Radical hysterectomy status | 1.48 |
References
- WHO. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 22 January 2025).
- SEER. Cancer of the Cervix Uteri—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 22 January 2025).
- American Cancer Society. Cervical Cancer Statistics—Key Facts About Cervical Cancer. Available online: https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html (accessed on 22 January 2025).
- Chao, X.; Song, X.; Wu, H.; You, Y.; Wu, M.; Li, L. Selection of treatment regimens for recurrent cervical cancer. Front. Oncol. 2021, 11, 618485. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Bae, J.Y. Optimal cutoff level of serum squamous cell carcinoma antigen to detect recurrent cervical squamous cell carcinoma during post-treatment surveillance. Obstet. Gynecol. Sci. 2018, 61, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, B.K.; Seo, J.H.; Choi, J.; Choi, J.W.; Lee, C.K.; Chung, J.B.; Park, Y.; Kim, D.W. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci. Rep. 2020, 10, 8820. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society. Carcinoembryonic Antigen (CEA). Available online: https://cancer.ca/en/treatments/tests-and-procedures/carcinoembryonic-antigen-cea (accessed on 2 February 2025).
- Dumitru, C.A.; Lang, S.; Brandau, S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin. Cancer Biol. 2013, 23, 141–148. [Google Scholar] [CrossRef]
- Halazun, K.J.; Aldoori, A.; Malik, H.Z.; Al-Mukhtar, A.; Prasad, K.R.; Toogood, G.J.; Lodge, J.P.A. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur. J. Surg. Oncol. (EJSO) 2008, 34, 55–60. [Google Scholar] [CrossRef]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C.; Luyten, E.J.; Mahadev, S.; Witkowski, P.; Siegel, A.B.; Brown, R.S.; Emond, J.C. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef]
- Shimada, H.; Takiguchi, N.; Kainuma, O.; Soda, H.; Ikeda, A.; Cho, A.; Miyazaki, A.; Gunji, H.; Yamamoto, H.; Nagata, M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010, 13, 170–176. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Dai, Y.; Li, J. Preoperative systemic inflammation score (SIS) is superior to neutrophil to lymphocyte ratio (NLR) as a predicting indicator in patients with esophageal squamous cell carcinoma. BMC Cancer 2019, 19, 721. [Google Scholar] [CrossRef]
- Itoh, S.; Tsujita, E.; Fukuzawa, K.; Sugimachi, K.; Iguchi, T.; Ninomiya, M.; Maeda, T.; Kajiyama, K.; Adachi, E.; Uchiyama, H.; et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: A multi-institutional retrospective study. Pancreatology 2021, 21, 1356–1363. [Google Scholar] [CrossRef]
- Kuroda, D.; Sawayama, H.; Kurashige, J.; Iwatsuki, M.; Eto, T.; Tokunaga, R.; Kitano, Y.; Yamamura, K.; Ouchi, M.; Nakamura, K.; et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018, 21, 204–212. [Google Scholar] [CrossRef]
- Li, Q.; Cong, R.; Wang, Y.; Kong, F.; Ma, J.; Wu, Q.; Ma, X. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: Results from a retrospective cohort study. Gynecol. Oncol. 2021, 160, 91–98. [Google Scholar] [CrossRef]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis. Colon Rectum 2017, 60, 1273–1284. [Google Scholar] [CrossRef]
- Yang, J.; Lv, L.; Zhao, F.; Mei, X.; Zhou, H.; Yu, F. The value of the preoperative Naples prognostic score in predicting prognosis in gallbladder cancer surgery patients. World J. Surg. Oncol. 2023, 21, 303. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-M.; Lu, W.; Cheng, J.; Dai, M.; Liu, S.-Y.; Wang, D.-D.; Fu, T.-W.; Ye, T.-W.; Liu, J.-W.; Zhang, C.-W.; et al. Naples prognostic score is an independent prognostic factor in patients undergoing hepatectomy for hepatocellular carcinoma. J. Hepatocell. Carcinoma 2023, 10, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Hu, H.; Kang, W.; Liu, H.; Ma, F.; Ma, S.; Li, Y.; Jin, P.; Tian, Y. Prognostic impact of preoperative Naples prognostic score in gastric cancer patients undergoing surgery. Front. Surg. 2021, 8, 617744. [Google Scholar] [CrossRef]
- Gulturk, I.; Yilmaz, M.; Tacar, S.Y.; Bakkaloglu, O.K.; Sonmezoz, G.B.; Erdal, G.S.; Ozmen, A.; Tural, D. Naples prognostic score may predict overall survival in metastatic pancreatic cancer. J. Cancer Res. Ther. 2024, 20, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Salib, M.Y.; Russell, J.H.B.; Stewart, V.R.; Sudderuddin, S.A.; Barwick, T.D.; Rockall, A.G.; Bharwani, N. 2018 FIGO staging classification for cervical cancer: Added benefits of imaging. Radiographics 2020, 40, 1807–1822. [Google Scholar] [CrossRef]
- Liang, W.; Ferrara, N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol. Res. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love–hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef]
- Nedergaard, B.S.; Ladekarl, M.; Nyengaard, J.R.; Nielsen, K. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol. Oncol. 2008, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Wenzel, K.; Schulz, T.; Hofmann, S.; Neef, H.; Lautenschläger, C.; Langner, J. Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int. Arch. Allergy Immunol. 2009, 114, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 2013, 228, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, E.; Li, Z. Neutrophil-to-lymphocyte ratio is an independent predictor for survival outcomes in cervical cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 21917. [Google Scholar] [CrossRef]
- Du, J.-Q.; Zhang, F.; Wang, C.-Q.; Zhu, J.-F.; Xu, L.-X.; Yang, Y.-H.; Han, M.-F.; Hu, Y. Effects of peripheral blood neutrophil/lymphocyte ratio levels and their changes on the prognosis of patients with early cervical cancer. Front. Oncol. 2023, 13, 1139809. [Google Scholar] [CrossRef]
- Li, Y.-X.; Chang, J.-Y.; He, M.-Y.; Wang, H.-R.; Luo, D.-Q.; Li, F.-H.; Li, J.-H.; Ran, L.; Izadpanah, R. Neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR) predict clinical outcome in patients with stage IIB cervical cancer. J. Oncol. 2021, 2021, 2939162. [Google Scholar] [CrossRef]
- Liang, C.; Xu, Z.; Shen, X.; Wu, K.; Hanprasertpong, J. Correlation between neutrophil-to-lymphocyte ratio and pretreatment magnetic resonance imaging and their predictive significance in cervical carcinoma patients referred for radiotherapy. J. Oncol. 2022, 2022, 3409487. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Zhang, F.; Sheng, X.-G.; Chen, L. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1–IIa cervical cancer patients who undergo radical surgery. OncoTargets Ther. 2015, 8, 1355–1362. [Google Scholar] [CrossRef][Green Version]
- Trinh, H.; Dzul, S.P.; Hyder, J.; Jang, H.; Kim, S.; Flowers, J.; Vaishampayan, N.; Chen, J.; Winer, I.; Miller, S. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT). Clin. Chim. Acta 2020, 510, 711–716. [Google Scholar] [CrossRef]
- Königsbrügge, O.; Posch, F.; Riedl, J.; Reitter, E.-M.; Zielinski, C.; Pabinger, I.; Ay, C. Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist 2016, 21, 252–257. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, L.; Peng, F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics 2013, 68, 686–693. [Google Scholar] [CrossRef]
- Lei, J.; Wang, Y.; Guo, X.; Yan, S.; Ma, D.; Wang, P.; Li, B.; Du, W.; Guo, R.; Kan, Q. Low preoperative serum ALB level is independently associated with poor overall survival in endometrial cancer patients. Future Oncol. 2020, 16, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, G.; Cho, S.H.; Oh, R.; Kim, J.Y.; Lee, Y.-B.; Jin, S.-M.; Hur, K.Y.; Kim, J.H. Association between total cholesterol levels and all-cause mortality among newly diagnosed patients with cancer. Sci. Rep. 2024, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The role of IL-6 in cancer cell invasiveness and metastasis—Overview and therapeutic opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Li, P.; Wang, T.; Li, X.; Wei, Z.; Zhang, Z.; Zhong, L.; Cao, L.; Heckman, M.G.; Zhang, Y.-W.; et al. Apolipoprotein E epsilon 2 allele and low serum cholesterol as risk factors for gastric cancer in a Chinese Han population. Sci. Rep. 2016, 6, 19930. [Google Scholar] [CrossRef]
- Kitahara, C.M.; de González, A.B.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef]
- Li, L.; Yu, Z.; Ren, J.; Niu, T. Low cholesterol levels are associated with increasing risk of plasma cell neoplasm: A UK biobank cohort study. Cancer Med. 2023, 12, 20964–20975. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, M.; Zhu, J.; Gu, R.; Yang, B.; Ji, S.; Zhao, Y.; Gu, K. Prognostic value of Naples prognostic score in locally advanced cervical cancer patients undergoing concurrent chemoradiotherapy. Biomol. Biomed. 2025, 25, 986–999. [Google Scholar] [CrossRef]
- Ma, M.; Weng, M.; Chen, F.; Hu, Y.; Lai, J.; Wang, Y.; Zhou, Y. Systemic inflammation score is a prognostic marker after curative resection in gastric cancer. ANZ J. Surg. 2019, 89, 377–382. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ohki, S.; Kaneta, A.; Matsuishi, A.; Maruyama, Y.; Yamada, L.; Tada, T.; Hanayama, H.; Watanabe, Y.; Hayase, S.; et al. Systemic inflammation score as a preoperative prognostic factor for patients with pT2–T4 resectable gastric cancer: A retrospective study. BMC Surg. 2023, 23, 8. [Google Scholar] [CrossRef]
- Liu, S.; Yu, X.; Ye, F.; Jiang, L. Can the systemic inflammation score be used to predict prognosis in gastric cancer patients undergoing surgery? A systematic review and meta-analysis. Front. Surg. 2022, 9, 971326. [Google Scholar] [CrossRef] [PubMed]
- Maßmann, M.; Treckmann, J.; Markus, P.; Schumacher, B.; Albers, D.; Ting, S.; Mende, B.; Roehrle, J.; Virchow, I.; Rosery, V.; et al. A prognostic systemic inflammation score (SIS) in patients with advanced intrahepatic cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 5085–5094. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xiao, X.; Dong, Z.; Wang, Q. The systemic inflammation score is associated with the survival of patients with prostate cancer. J. Inflamm. Res. 2023, 16, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Ju, M.; Komori, K.; Tamagawa, H.; Tamagawa, A.; Maezawa, Y.; Hashimoto, I.; Kano, K.; Hara, K.; Cho, H.; et al. The systemic inflammation score is an independent prognostic factor for esophageal cancer patients who receive curative treatment. Anticancer Res. 2022, 42, 2711–2717. [Google Scholar] [CrossRef]
- Chen, L.; Bai, P.; Kong, X.; Huang, S.; Wang, Z.; Wang, X.; Fang, Y.; Wang, J. Prognostic nutritional index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front. Cell Dev. Biol. 2021, 9, 656741. [Google Scholar] [CrossRef]
- Tobing, E.; Tansol, C.; Tania, C.; Sihombing, A.T. Prognostic nutritional index (PNI) as independent predictor of poor survival in prostate cancer: A systematic review and meta-analysis. Clin. Genitourin. Cancer 2024, 22, 102142. [Google Scholar] [CrossRef]
- Ellez, H.I.; Keskinkilic, M.; Semiz, H.S.; Arayici, M.E.; Kısa, E.; Oztop, I. The prognostic nutritional index (PNI): A new biomarker for determining prognosis in metastatic castration-sensitive prostate carcinoma. J. Clin. Med. 2023, 12, 5434. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, H.-P.; Kim, H.S.; Park, S. Preoperative prognostic nutritional index is a prognostic indicator of cancer-specific survival in patients undergoing endometrial cancer surgery. J. Korean Med. Sci. 2023, 38, e163. [Google Scholar] [CrossRef]
- Buglio, A.L.; Bellanti, F.; Capurso, C.; Vendemiale, G. Controlling nutritional status (CONUT) score as a predictive marker in hospitalized frail elderly patients. J. Pers. Med. 2023, 13, 1119. [Google Scholar] [CrossRef]
- Miano, N.; Di Marco, M.; Alaimo, S.; Coppolino, G.; L’episcopo, G.; Leggio, S.; Scicali, R.; Piro, S.; Purrello, F.; Di Pino, A. Controlling nutritional status (CONUT) score as a potential prognostic indicator of in-hospital mortality, sepsis and length of stay in an internal medicine department. Nutrients 2023, 15, 1554. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J. Gastrointest. Oncol. 2019, 10, 965–978. [Google Scholar] [CrossRef]
- Pian, G.; Oh, S.Y. Comparison of nutritional and immunological scoring systems predicting prognosis in T1-2N0 colorectal cancer. Int. J. Color. Dis. 2022, 37, 179–188. [Google Scholar] [CrossRef]
- Alvarez, K.L.F.; Beldi, M.; Sarmanho, F.; Rossetti, R.A.M.; Silveira, C.R.F.; Mota, G.R.; Andreoli, M.A.; Caruso, E.D.d.C.; Kamillos, M.F.; Souza, A.M.; et al. Local and systemic immunomodulatory mechanisms triggered by Human Papillomavirus transformed cells: A potential role for G-CSF and neutrophils. Sci. Rep. 2017, 7, 9087. [Google Scholar] [CrossRef]



| Low-NPS Group (N = 98) | High-NPS Group (N = 80) | p-Value | |
|---|---|---|---|
| Age (years) | 53.05 ± 14.09 | 58.60 ± 13.35 | 0.013 |
| Height (cm) | 158.52 ± 5.89 | 154.33 ± 7.16 | <0.001 |
| Weight (kg) | 60.44 ± 8.84 | 56.53 ± 11.91 | 0.016 |
| BMI (kg/m2) | 24.33 ± 4.45 | 23.66 ± 4.30 | 0.311 |
| Parity | 0.080 | ||
| Nulliparous | 26 (26.5%) | 12 (15.0%) | |
| Multiparous | 72 (73.5%) | 68 (85.0%) | |
| Menopausal status | 0.026 | ||
| No | 40 (40.8%) | 20 (25.0%) | |
| Yes | 58 (59.2%) | 60 (75.0%) | |
| Comorbidities | 0.016 | ||
| No | 72 (73.5%) | 45 (56.3%) | |
| Yes | 26 (26.5%) | 35 (43.7%) | |
| Histology | 0.010 | ||
| Squamous | 69 (71.1%) | 63 (78.8%) | |
| Adenocarcinoma | 28 (28.9%) | 13 (16.2%) | |
| Others | 0 (0.0%) | 4 (5.0%) | |
| FIGO stage | 0.002 | ||
| Early-stage group | 48 (49.0%) | 21 (26.3%) | |
| Advanced-stage group | 50 (51.0%) | 59 (73.7%) | |
| Tumor size | 0.031 | ||
| ≤4 cm | 60 (61.2%) | 36 (45.0%) | |
| >4 cm | 38 (38.8%) | 44 (55.0%) | |
| LN metastasis status | 0.002 | ||
| Negative | 70 (71.4%) | 39 (48.8%) | |
| PLND metastasis | 20 (20.4%) | 21 (26.2%) | |
| PLND and PALND | 8 (8.2%) | 20 (25.0%) | |
| metastases | |||
| HPV infection status | 0.025 | ||
| No | 54 (55.7%) | 31 (38.7%) | |
| Yes | 43 (44.3%) | 49 (61.3%) | |
| CCRT status | 0.511 | ||
| No | 39 (39.8%) | 28 (35.0%) | |
| Yes | 59 (60.2%) | 52 (65.0%) | |
| Adjuvant chemotherapy status | 0.001 | ||
| No | 81 (82.7%) | 48 (60.0%) | |
| Yes | 17 (17.3%) | 32 (40.0%) | |
| Number of chemotherapies | 1.09 ± 2.59 | 4.36 ± 7.71 | <0.001 |
| Radical hysterectomy status | 0.222 | ||
| No | 60 (61.2%) | 56 (70.0%) | |
| Yes | 38 (38.8%) | 24 (30.0%) | |
| Trachelectomy status | 0.381 | ||
| No | 94 (95.9%) | 79 (98.8%) | |
| Yes | 4 (4.1%) | 1 (1.2%) | |
| SCC-Ag level | 0.334 | ||
| ≤2.0 | 68 (69.4%) | 50 (62.5%) | |
| >2.0 | 30 (30.6%) | 30 (37.5%) | |
| CA 19-9 level | 0.016 | ||
| ≤37 | 87 (88.8%) | 60 (75.0%) | |
| >37 | 11 (11.2%) | 20 (25.0%) | |
| CEA level | 0.232 | ||
| ≤4.6 | 88 (89.8%) | 67 (83.8%) | |
| >4.6 | 10 (10.2%) | 13 (16.3%) |
| Risk Factors for DFS | ||||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR * | 95% CI | p-Value | |
| Age (years) | 1.02 | (0.999, 1.036) | 0.058 | 0.99 | (0.969, 1.012) | 0.375 |
| Height (cm) | 0.97 | (0.927, 1.004) | 0.078 | 1.01 | (0.954, 1.050) | 0.960 |
| Weight (kg) | 0.99 | (0.963, 1.015) | 0.409 | 0.99 | (0.973, 1.027) | 0.985 |
| BMI | 0.99 | (0.929, 1.049) | 0.671 | 0.99 | (0.926, 1.065) | 0.852 |
| Parity | ||||||
| Nulliparous | 1.00 | Reference | 1.00 | Reference | ||
| Multiparous | 2.21 | (1.001, 4.880) | 0.058 | 1.66 | (0.670, 4.087) | 0.275 |
| Menopausal status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.60 | (0.886, 2.897) | 0.119 | 0.96 | (0.401, 2.298) | 0.926 |
| Comorbidities | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.91 | (1.130, 3.237) | 0.016 | 1.63 | (0.919, 2.893) | 0.094 |
| Histology | ||||||
| Squamous | 1.00 | Reference | 1.00 | Reference | ||
| Adenocarcinoma | 1.96 | (1.112, 3.468) | 0.020 | 2.56 | (1.442, 4.550) | 0.001 |
| Others | 2.40 | (0.575, 10.001) | 0.230 | 1.78 | (0.423, 7.524) | 0.430 |
| FIGO stage | ||||||
| Early-stage group | 1.00 | Reference | 1.00 | Reference | ||
| Advanced-stage group | 6.79 | (2.909, 15.855) | <0.001 | 5.84 | (2.483, 13.723) | <0.001 |
| Tumor size | ||||||
| ≤4 cm | 1.00 | Reference | 1.00 | Reference | ||
| >4 cm | 2.08 | (1.213, 3.551) | 0.008 | 0.87 | (0.462, 1.642) | 0.669 |
| LN metastasis status | ||||||
| Negative | 1.00 | Reference | 1.00 | Reference | ||
| PLND metastasis | 3.74 | (2.010, 6.975) | <0.001 | 2.32 | (1.157, 4.633) | 0.018 |
| PLDN and PALND | 5.54 | (2.833, 10.848) | <0.001 | 3.72 | (1.725, 8.033) | 0.001 |
| metastases | ||||||
| HPV infection status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 2.02 | (1.161, 3.511) | 0.013 | 1.53 | (0.844, 2.764) | 0.162 |
| CCRT status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 1.69 | (0.921, 3.092) | 0.090 | 0.54 | (0.266, 1.076) | 0.079 |
| Adjuvant chemotherapy status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 20.55 | (10.472, 40.322) | <0.001 | 15.42 | (7.426, 32.020) | <0.001 |
| Number of chemotherapies | 1.10 | (1.078, 1.131) | <0.001 | 1.09 | (1.059, 1.115) | <0.001 |
| Radical hysterectomy status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.54 | (0.295, 0.991) | 0.047 | 1.43 | (0.673, 3.015) | 0.355 |
| Trachelectomy status | ||||||
| No | 1.00 | Reference | 1.00 | Reference | ||
| Yes | 0.63 | (0.087, 4.583) | 0.651 | 2.69 | (0.309, 23.456) | 0.370 |
| SCC-Ag level | ||||||
| ≤2.0 | 1.00 | Reference | 1.00 | Reference | ||
| >2.0 | 1.43 | (0.833, 2.463) | 0.194 | 0.80 | (0.428, 1.493) | 0.483 |
| CA 19-9 level | ||||||
| ≤37 | 1.00 | Reference | 1.00 | Reference | ||
| >37 | 2.46 | (1.378, 4.405) | 0.002 | 1.55 | (0.851, 2.834) | 0.151 |
| CEA level | ||||||
| ≤4.6 | 1.00 | Reference | 1.00 | Reference | ||
| >4.6 | 1.54 | (0.751, 3.138) | 0.240 | 0.96 | (0.458, 2.010) | 0.913 |
| NPS status | ||||||
| Low NPS | 1.00 | Reference | 1.00 | Reference | ||
| High NPS | 1.89 | (1.422, 2.465) | <0.001 | 1.98 | (1.131, 3.455) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-M.; Seo, H.; Kim, S.; Cho, H.-W.; Min, K.-J.; Lee, S.; Hong, J.-H.; Song, J.-Y.; Lee, J.-K.; Lee, N.-W. The Value of the Naples Prognostic Score at Diagnosis as a Predictor of Cervical Cancer Progression. Medicina 2025, 61, 1381. https://doi.org/10.3390/medicina61081381
Lee S-M, Seo H, Kim S, Cho H-W, Min K-J, Lee S, Hong J-H, Song J-Y, Lee J-K, Lee N-W. The Value of the Naples Prognostic Score at Diagnosis as a Predictor of Cervical Cancer Progression. Medicina. 2025; 61(8):1381. https://doi.org/10.3390/medicina61081381
Chicago/Turabian StyleLee, Seon-Mi, Hyunkyoung Seo, Seongmin Kim, Hyun-Woong Cho, Kyung-Jin Min, Sanghoon Lee, Jin-Hwa Hong, Jae-Yun Song, Jae-Kwan Lee, and Nak-Woo Lee. 2025. "The Value of the Naples Prognostic Score at Diagnosis as a Predictor of Cervical Cancer Progression" Medicina 61, no. 8: 1381. https://doi.org/10.3390/medicina61081381
APA StyleLee, S.-M., Seo, H., Kim, S., Cho, H.-W., Min, K.-J., Lee, S., Hong, J.-H., Song, J.-Y., Lee, J.-K., & Lee, N.-W. (2025). The Value of the Naples Prognostic Score at Diagnosis as a Predictor of Cervical Cancer Progression. Medicina, 61(8), 1381. https://doi.org/10.3390/medicina61081381






