Surveillance of Digoxin Concentrations in Critically Ill Individuals with Heart Failure
Abstract
1. Introduction
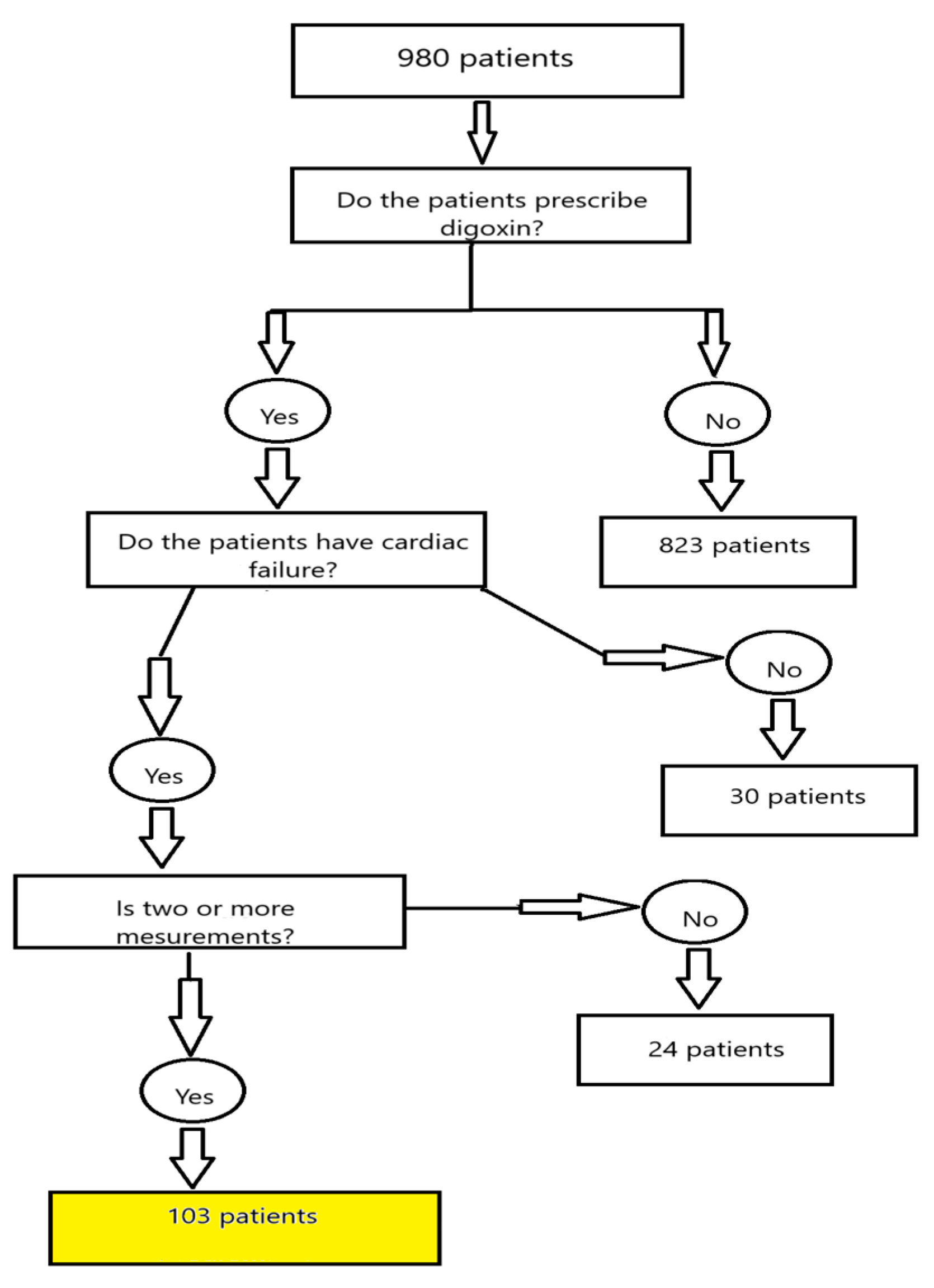
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEI | Angiotensin-converting-enzyme inhibitors |
| ARB | Angiotensin II receptor blocker |
| AF | Atrial fibrillation |
| ß-blocker | Beta blocker |
| Ca-blocker | Calcium blocker |
| CRRT | Continuous renal replacement therapy |
| CYP3A4 | Cytochrome P450 3A4 |
| ECMO | Extracorporeal membrane oxygenation |
| IABP | Intra-aortic balloon pump |
| ICU | Intensive care unit |
| HF | Heart failure |
| HFrEF | Heart failure with reduced ejection fraction |
| LVEF | Left ventricular ejection fraction |
| LVAD | Left ventricular assist device |
| MOF | Multi-organ failure |
| MRA | Mineralocorticoid receptor antagonist |
| Na, K-ATPase | Sodium–potassium ATP pump |
| NO | Nitric oxide |
| NT-proBNP | Natriuretic peptide tests |
| SCD | Sudden cardiac death |
| RRT | Renal replacement therapy |
| RVSP | Right ventricular systolic pressure |
| VF | Ventricular fibrillation |
| VT | Ventricular tachycardia |
References
- Kołodziejczyk, A. Glikozydy nasercowe. In Naturalne Związki Organiczne, 1st ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006; pp. 483–488. [Google Scholar]
- Ritter, J.; Flower, R. Rang and Dale Farmakologia, 9th ed.; Mirowska-Guzel, D., Okopień, B., Eds.; Edra Urban & Partner: Wrocław, Poland, 2021; pp. 304–305. [Google Scholar]
- Charfi, R.; Sassi, M.B. Digoxin therapeutic drug monitoring: Age influence and adverse events. Tunis. Med. 2020, 98, 35–40. [Google Scholar]
- Mc Donagh, T.A.; Metra, M. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Lavalle, C.; Mariani, M.V. Efficacy of Modern Therapies for Heart Failure with Reduced Ejection Fraction in Specific Population Subgroups: A Systematic Review and Network Meta-Analysis. Cardiorenal Med. 2024, 14, 570–580. [Google Scholar] [CrossRef]
- Mc Donagh, T.A.; Metra, M. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [PubMed]
- Sutanto, H.; Lyon, A. Cardiomyocyte calcium handling in health and disease: Insights from in vitro and in silico studies. J. Prog. Biophys. Mol. Biol. 2020, 157, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T. Konturek. Fizjologia Człowieka; Edra Urban&Partner: Wrocław, Poland, 2019; pp. 16–20. [Google Scholar]
- Pagel, P.S.; Freed, J.K. Cardiac Physiology. In Kaplans’s Cardiac Anesthesia for Cardiac and Noncardiac Surgery; Elsevier: Philadelphia, PA, USA, 2017; pp. 143–178. [Google Scholar]
- Kieć-Wilk, B.; Petkow-Dimitrow, P. Role of impaired calcium homeostasis in the development of cardiac hypertrophy. Kardiol. Pol. 2009, 67, 1396–1402. [Google Scholar]
- O’Brien, W.J.; Wallick, E.T. Amino Acid residues of the Na,K-ATPase involved in oubain sensititivity do not bind the sugar moiety of cardiac glycosides. J. Biol. Chem. 1993, 11, 7707–7712. [Google Scholar] [CrossRef]
- Merino, J.L.; Tamargo, J. Practical Compendium of Antiarrhytmic Drugs: A Clinical Consensus Statement of the European Heart Rhythm. Association of the ESC. Europace 2025, euaf076. [Google Scholar] [CrossRef]
- Alkhawam, H.; Abo-salem, E. Effect of digitalis level on readmission and mortality rate among heart failure reduced ejection fraction patients. Heart Lung 2019, 48, 22–27. [Google Scholar] [CrossRef]
- Brunton, L.L.; Lazo, J.S. Farmakologia Goodmana & Gilmana, 11th ed.; Buczko, W., Krzemiński, T.F., Eds.; Wydawnictwo Czelej: Lublin, Poland, 2007; pp. 923–954. [Google Scholar]
- Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych. Available online: https://leki.urpl.gov.pl/files/43_Digoxin_WZF_tabl_250mcg.pdf (accessed on 10 August 2024).
- Bavendiek, U.; Großhennig, A. Simple and safe digitoxin dosing in heart failure based on data from the DIGITHF trial. Clin. Res. Cardiol. 2023, 112, 1096–1107. [Google Scholar] [CrossRef]
- Adams, K.F.; Patterson, J.H. Relationship of Serum Digoxin Concentration to Mortality and Morbidity in Women in the Digitalis Investigation Group Trial. J. Am. Coll. Cardiol. 2005, 46, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Washam, J.B.; Stevens, S.R. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: A retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Lancet 2015, 385, 2363–2370. [Google Scholar] [CrossRef]
- Lam, P.H.; Packer, M. Digoxin Initiation and Outcomes in Patients with Heart Failure with Preserved Ejection Fraction. Am. J. Med. 2020, 133, 1187–1194. [Google Scholar] [CrossRef]
- Singh, S.; Moore, H. Digoxin Initiation and Outcomes in Patients with Heart Failure (HFrEF and HFpEF) and Atrial Fibrillation. Am. J. Med. 2020, 133, 1460–1470. [Google Scholar] [CrossRef]
- Al-khateeb, M.; Qureshi, W.T. The impact of digoxin on mortality in patients with chronic systolic heart failure: A propensity-matched cohort study. Int. J. Cardiol. 2017, 228, 214–218. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y. Predictors of digoxin use and risk of mortality in ED patients with atrial fibrillation. Am. J. Emerg. Med. 2017, 35, 1589–1594. [Google Scholar] [CrossRef]
- Llàcera, P.; Núñezb, J. Digoxin and prognosis of heart failure in older patients with preserved ejection fraction: Importance of heart rate. Results from an observational and multicenter study. Eur. J. Intern. Med. 2019, 60, 18–23. [Google Scholar] [CrossRef]
- Freeman, J.V.; Reynolds, K. Digoxin and Risk of Death in Adults with Atrial Fibrillation The ATRIA-CVRN Study. Circ. Arrhythm. Electrophysiol. 2015, 8, 49–58. [Google Scholar] [CrossRef]
- Mulder, B.A.; Van Veldhuisen, D.J. Digoxin in patients with permanent atrial fibrillation: Data from the RACEII study. Heart Rhythm 2014, 11, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Kutyifa, V. Digoxin therapy and associated clinical outcomes in the MADIT-CRT trial. Heart Rhythm 2015, 12, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Tuszyński, P.K. Uman-Ntuk E.Interakcje leków układu krążenia. In Istotne Interakcje Leków Praktyczny Przewodnik; Wydawnictwo Farmaceutyczne: Kraków, Poland, 2024; pp. 281–286. [Google Scholar]
- Grochla, M.; Saucha, W. Readmissions to General ICUs in a Geographic Area of Poland Are Seemingly Associated with Better Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Weigl, W.; Adamski, J. ICU mortality and variables associated with ICU survival in Poland: A nationwide database study. Eur. J. Anaesthesiol. 2018, 35, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Adamski, J.; Goraj, R. The differences between two selected intensive care units incentral and northern Europe—Preliminary observation. Anaesthesiol. Intensive Ther. 2015, 47, 117–124. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Level | Mixed Level | Above Terap. Level | Below Terap. Level | p | p | p | p | p | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| Digit. serum Con.(ng/mL) | 15 | 1.390 | 0.184 | 73 | 1.203 | 0.501 | 2 | 2.283 | 0.279 | 13 | 0.488 | 0.109 | 0.132 | 0.008 | 0.000 | 0.001 | 0.000 | 0.000 |
| Digoxin dose (mg) | 15 | 0.217 | 0.097 | 73 | 0.203 | 0.070 | 2 | 0.160 | 0.042 | 13 | 0.222 | 0.093 | 0.507 | 0.325 | 0.865 | 0.439 | 0.402 | 0.289 |
| Age (year) | 15 | 70.33 | 13.72 | 73 | 66.33 | 12.14 | 2 | 71.50 | 7.78 | 13 | 72.77 | 11.68 | 0.253 | 0.900 | 0.602 | 0.558 | 0.085 | 0.892 |
| Weight (kg) | 15 | 82.53 | 17.14 | 70 | 90.53 | 28.61 | 2 | 92.00 | 11.31 | 13 | 85.77 | 20.85 | 0.197 | 0.562 | 0.694 | 0.925 | 0.468 | 0.705 |
| ICU stay (day) | 15 | 23.93 | 20.85 | 73 | 28.41 | 18.74 | 2 | 17.00 | 4.24 | 13 | 22.00 | 11.13 | 0.388 | 0.614 | 0.780 | 0.384 | 0.245 | 0.719 |
| LVEF (%) | 14 | 35.14 | 17.50 | 69 | 31.78 | 15.84 | 2 | 55.00 | 0.00 | 11 | 30.64 | 15.58 | 0.423 | 0.069 | 0.435 | 0.026 | 0.805 | 0.029 |
| RVSP (mmHg) | 10 | 34.70 | 19.12 | 39 | 40.44 | 22.17 | 1 | 70.00 | 49.50 | 4 | 45.25 | 22.70 | 0.187 | 0.008 | 0.146 | 0.019 | 0.452 | 0.073 |
| NT-proBNP (pg/mL) | 11 | 16,020 | 13,907 | 53 | 14,615 | 12,643 | 0 | 8 | 11,282 | 8480 | 0.736 | 0.419 | 0.486 | |||||
| N | n | % | N | n | % | N | n | % | N | n | % | |||||||
| Women | 15 | 10 | 66.7% | 73 | 21 | 28.8% | 2 | 2 | 100.0% | 13 | 1 | 7.7% | 0.012 | 0.884 | 0.005 | 0.168 | 0.208 | 0.037 |
| Death | 15 | 7 | 46.7% | 73 | 30 | 41.1% | 2 | 2 | 100.0% | 13 | 5 | 38.5% | 0.912 | 0.506 | 0.956 | 0.349 | 0.898 | 0.388 |
| SCD | 15 | 2 | 13.3% | 73 | 25 | 34.2% | 2 | 1 | 50.0% | 13 | 5 | 38.5% | 0.196 | 0.772 | 0.274 | 0.771 | 0.982 | 0.642 |
| AF | 15 | 15 | 100.0% | 73 | 66 | 90.4% | 2 | 2 | 100.0% | 13 | 11 | 84.6% | 0.468 | - | 0.400 | 0.440 | 0.891 | 0.602 |
| Liver failure | 15 | 4 | 26.7% | 73 | 33 | 45.2% | 2 | 1 | 50.0% | 13 | 3 | 23.1% | 0.299 | 0.884 | 0.827 | 0.558 | 0.236 | 0.954 |
| Kidney failure | 15 | 15 | 100.0% | 73 | 68 | 93.2% | 2 | 2 | 100.0% | 13 | 13 | 100.0% | 0.666 | - | - | 0.292 | 0.742 | - |
| Respiratory failure | 15 | 15 | 100.0% | 73 | 68 | 93.2% | 2 | 2 | 100.0% | 13 | 12 | 92.3% | 0.666 | - | 0.942 | 0.292 | 0.631 | 0.264 |
| CRRT | 15 | 9 | 60.0% | 73 | 50 | 68.5% | 2 | 2 | 100.0% | 13 | 8 | 61.5% | 0.737 | 0.746 | 0.761 | 0.860 | 0.864 | 0.788 |
| Dobutamine | 15 | 7 | 46.7% | 73 | 51 | 69.9% | 2 | 1 | 50.0% | 13 | 7 | 53.8% | 0.154 | 0.506 | 1.000 | 0.860 | 0.416 | 0.509 |
| Norepinephrine | 15 | 15 | 100.0% | 73 | 67 | 91.8% | 2 | 2 | 100.0% | 13 | 10 | 76.9% | 0.557 | - | 0.175 | 0.369 | 0.262 | 0.849 |
| Epinephrine | 15 | 8 | 53.3% | 73 | 50 | 68.5% | 2 | 2 | 100.0% | 13 | 5 | 38.5% | 0.407 | 0.621 | 0.684 | 0.860 | 0.078 | 0.388 |
| Milrinone | 15 | 6 | 40.0% | 73 | 15 | 20.5% | 2 | 1 | 50.0% | 13 | 1 | 7.7% | 0.202 | 0.621 | 0.126 | 0.898 | 0.477 | 0.602 |
| Vasopressin | 15 | 1 | 6.7% | 73 | 14 | 19.2% | 2 | 2 | 100.0% | 13 | 3 | 23.1% | 0.426 | 0.024 | 0.486 | 0.060 | 0.958 | 0.179 |
| Levosimendan | 15 | 1 | 6.7% | 73 | 6 | 8.2% | 2 | 0 | 0.0% | 13 | 1 | 7.7% | 0.748 | 0.221 | 0.528 | 0.369 | 0.627 | 0.264 |
| IABP | 15 | 2 | 13.3% | 73 | 20 | 27.4% | 2 | 0 | 0.0% | 13 | 2 | 15.4% | 0.413 | 0.536 | 0.699 | 0.957 | 0.569 | 0.602 |
| LVAD | 15 | 0 | 0.0% | 73 | 5 | 6.8% | 2 | 0 | 0.0% | 13 | 0 | 0.0% | 0.666 | - | - | 0.292 | 0.742 | - |
| ECMO | 15 | 2 | 13.3% | 73 | 7 | 9.6% | 2 | 0 | 0.0% | 13 | 1 | 7.7% | 0.975 | 0.536 | 0.896 | 0.440 | 0.763 | 0.264 |
| NO | 15 | 2 | 13.3% | 73 | 12 | 16.4% | 2 | 1 | 50.0% | 13 | 1 | 7.7% | 0.930 | 0.772 | 0.896 | 0.772 | 0.696 | 0.602 |
| Readmission to ICU | 15 | 1 | 6.7% | 73 | 13 | 17.8% | 2 | 0 | 0.0% | 13 | 2 | 15.4% | 0.492 | 0.221 | 0.896 | 0.772 | 0.854 | 0.602 |
| Yes | No | p | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |||
| Concentration (ng/mL) | 34 | 1.25 | 0.47 | 69 | 1.12 | 0.55 | 0.249 | Women |
| Digoxin dose (mg) | 34 | 0.171 | 0.053 | 69 | 0.224 | 0.080 | 0.000 | |
| Concentration (ng/mL) | 44 | 1.33 | 0.59 | 59 | 1.03 | 0.43 | 0.003 | Death |
| Digoxin dose (mg) | 44 | 0.207 | 0.096 | 59 | 0.206 | 0.058 | 0.976 | |
| Concentration (ng/mL) | 33 | 1.05 | 0.49 | 70 | 1.21 | 0.54 | 0.155 | SCD |
| Digoxin dose (mg) | 33 | 0.205 | 0.075 | 70 | 0.207 | 0.078 | 0.921 | |
| Concentration (ng/mL) | 41 | 1.31 | 0.58 | 62 | 1.06 | 0.46 | 0.016 | Liver failure |
| Digoxin dose (mg) | 41 | 0.214 | 0.075 | 62 | 0.202 | 0.077 | 0.415 | |
| Concentration (ng/mL) | 13 | 0.49 | 0.11 | 90 | 1.26 | 0.49 | 0.000 | Below therapeutic level |
| Digoxin dose (mg) | 13 | 0.222 | 0.093 | 90 | 0.204 | 0.074 | 0.430 | |
| Concentration (ng/mL) | 15 | 1.39 | 0.18 | 88 | 1.12 | 0.55 | 0.067 | Therapeutic level |
| Digoxin dose (mg) | 15 | 0.217 | 0.097 | 88 | 0.205 | 0.073 | 0.557 | |
| Concentration (ng/mL) | 2 | 2.28 | 0.28 | 101 | 1.14 | 0.50 | 0.002 | Above therapeutic level |
| Digoxin dose (mg) | 2 | 0.160 | 0.042 | 101 | 0.207 | 0.077 | 0.387 | |
| Concentration (ng/mL) | 73 | 1.20 | 0.50 | 30 | 1.06 | 0.57 | 0.207 | Mixed level |
| Digoxin dose (mg) | 73 | 0.203 | 0.070 | 30 | 0.216 | 0.092 | 0.441 | |
| Concentration (ng/mL) | 69 | 1.20 | 0.54 | 34 | 1.07 | 0.48 | 0.245 | CRRT |
| Digoxin dose (mg) | 69 | 0.203 | 0.083 | 34 | 0.214 | 0.062 | 0.484 | |
| Concentration (ng/mL) | 10 | 1.01 | 0.34 | 93 | 1.18 | 0.54 | 0.335 | ECMO |
| Digoxin dose (mg) | 10 | 0.218 | 0.063 | 93 | 0.205 | 0.078 | 0.621 | |
| Concentration (ng/mL) | 16 | 1.23 | 0.52 | 87 | 1.15 | 0.53 | 0.568 | Readmission to ICU |
| Digoxin dose (mg) | 16 | 0.196 | 0.063 | 87 | 0.208 | 0.079 | 0.560 | |
| Age | Weight | ICU Days Hospitalization | Digit. Serum Concentr. (ng/mL) | Digitalis Dose (mg) | NT-ProBNP | ||
|---|---|---|---|---|---|---|---|
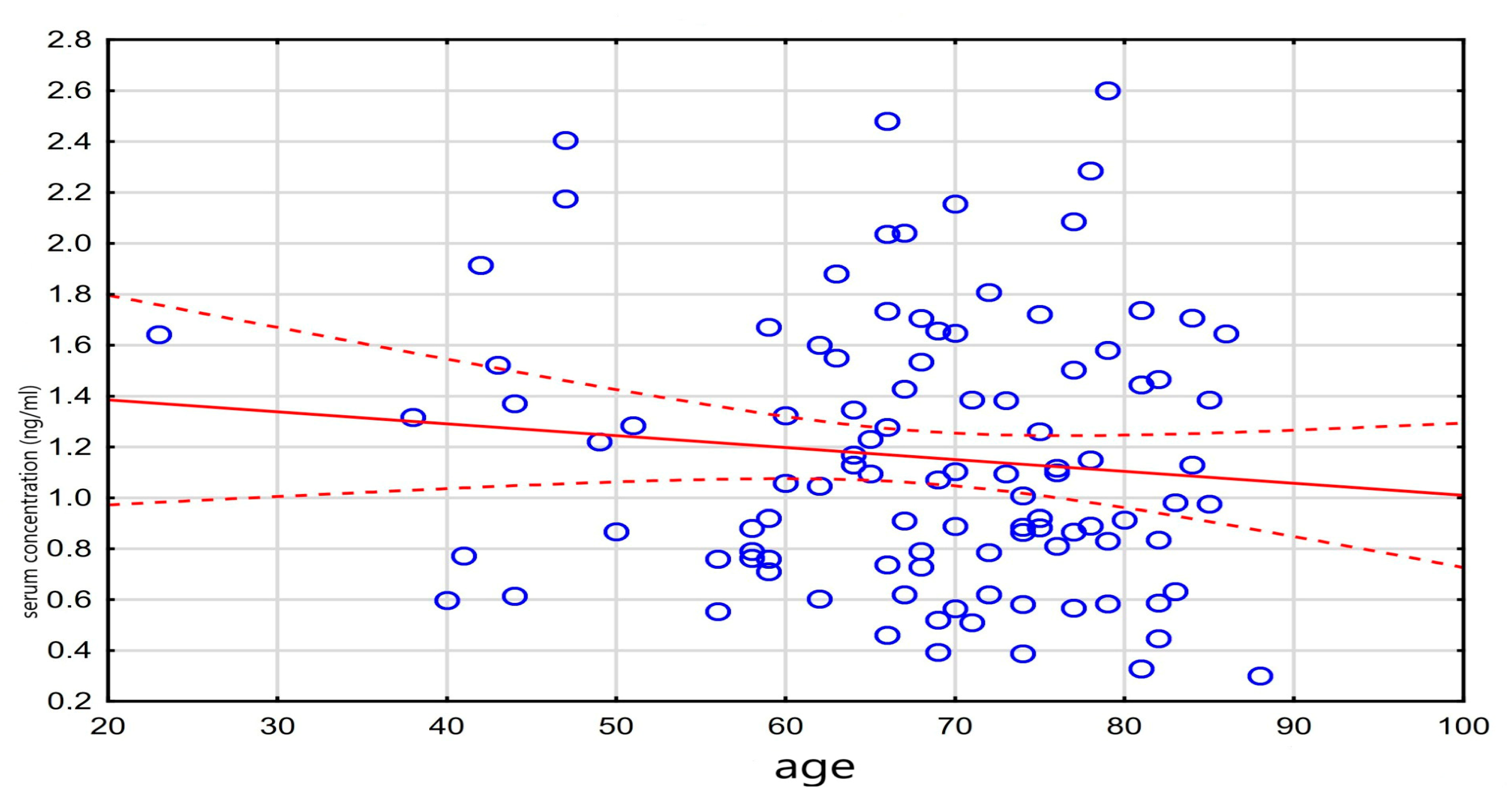
| Age | r | −0.21 | −0.10 | −0.11 | −0.20 | −0.18 | |
| n | 100 | 103 | 103 | 103 | 72 | ||
| p | 0.037 | 0.300 | 0.269 | 0.048 | 0.129 | ||
| Weight | r | −0.21 | −0.12 | -0.03 | 0.16 | −0.20 | |
| n | 100 | 100 | 100 | 100 | 69 | ||
| p | 0.037 | 0.220 | 0.760 | 0.111 | 0.098 | ||
| ICU days hospitalization | r | −0.10 | −0.12 | −0.05 | -0.11 | −0.19 | |
| n | 103 | 100 | 103 | 103 | 72 | ||
| p | 0.300 | 0.220 | 0.594 | 0.253 | 0.106 | ||
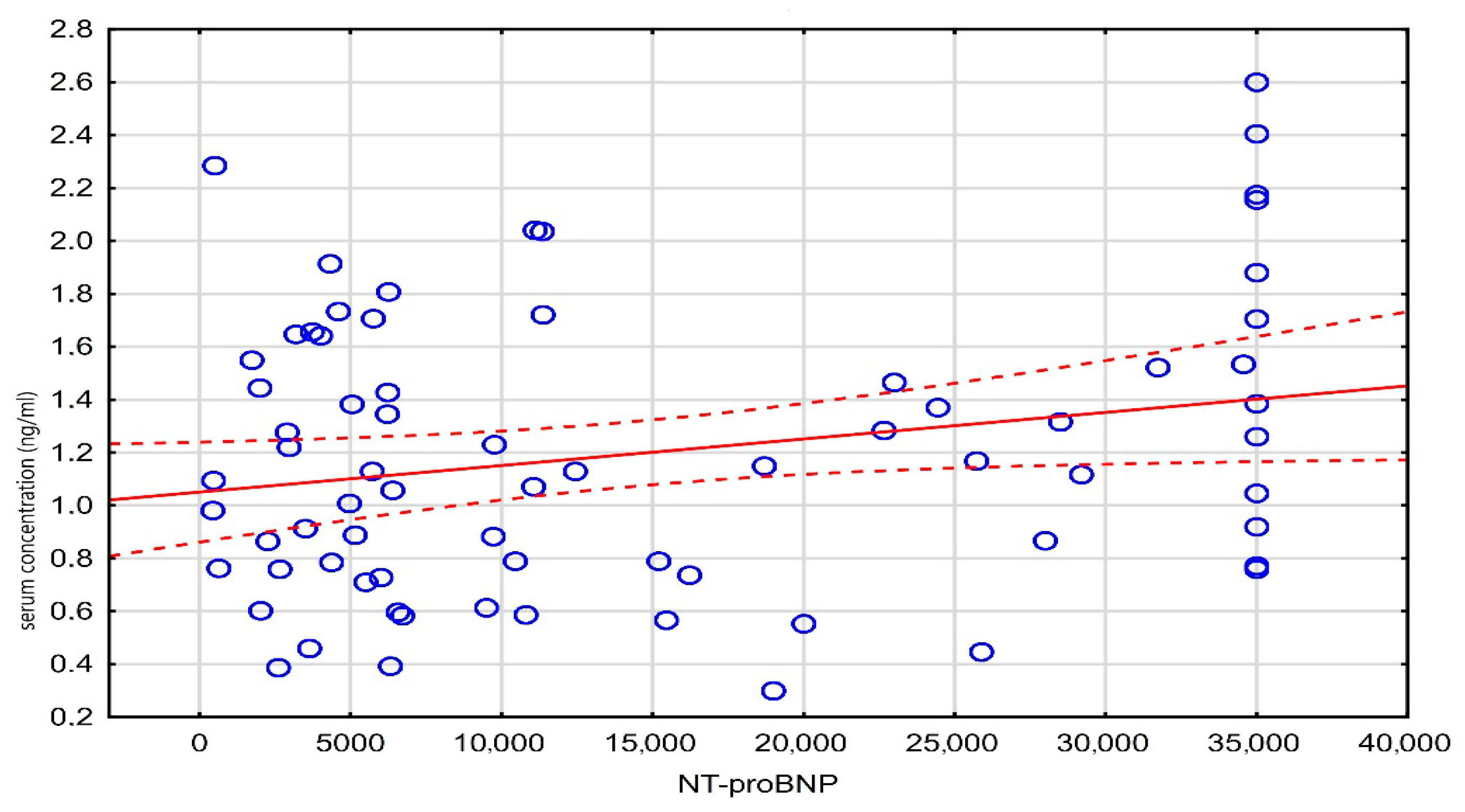
| Digit. serum concentr. (ng/mL) | r | −0.11 | −0.03 | −0.05 | 0.04 | 0.23 | |
| n | 103 | 100 | 103 | 103 | 72 | ||
| p | 0.269 | 0.760 | 0.594 | 0.672 | 0.048 | ||
| Digitalis dose (mg) | r | −0.20 | 0.16 | −0.11 | 0.04 | −0.03 | |
| n | 103 | 100 | 103 | 103 | 72 | ||
| p | 0.048 | 0.111 | 0.253 | 0.672 | 0.801 | ||
| NT-proBNP | r | −0.18 | −0.20 | -0.19 | 0.23 | −0.03 | |
| n | 72 | 69 | 72 | 72 | 72 | ||
| p | 0.129 | 0.098 | 0.106 | 0.048 | 0.801 |
| Full Model | |||
|---|---|---|---|
| N = 93 | coefficient | SD | p |
| Intercept | 0.8797 | 0.6178 | 0.1588 |
| Age (year) | −0.0037 | 0.0055 | 0.5047 |
| Weight (kg) | −0.0027 | 0.0028 | 0.3537 |
| LVEF (%) | 0.0066 | 0.0051 | 0.1992 |
| Digoxin dose (mg) | 1.0157 | 0.8243 | 0.2220 |
| Women | 0.1898 | 0.1351 | 0.1644 |
| SCD | −0.1456 | 0.1284 | 0.2607 |
| AF | −0.0536 | 0.2125 | 0.8016 |
| Liver failure | 0.3189 | 0.1290 | 0.0158 |
| Kidney failure | 0.1001 | 0.2955 | 0.7357 |
| Respiratory failure | 0.0427 | 0.2354 | 0.8567 |
| CRRT | −0.0547 | 0.1327 | 0.6815 |
| Dobutamine | −0.0657 | 0.1307 | 0.6168 |
| Norepinephrine | 0.1412 | 0.2092 | 0.5021 |
| Epinephrine | −0.0546 | 0.1312 | 0.6786 |
| Milrinone | 0.1317 | 0.1639 | 0.4245 |
| Vasopressin | 0.1051 | 0.1572 | 0.5060 |
| Levosimendan | 0.1233 | 0.2506 | 0.6241 |
| IABP | 0.2352 | 0.1631 | 0.1538 |
| LVAD | −0.0623 | 0.2813 | 0.8255 |
| ECMO | −0.5607 | 0.2313 | 0.0179 |
| Reduced Model | |||
| N = 93 | coefficient | SD | p |
| Intercept | 0.9407 | 0.0812 | 0.0000 |
| Liver failure | 0.3434 | 0.1085 | 0.0021 |
| Women | 0.2201 | 0.1072 | 0.0431 |
| ECMO | −0.4397 | 0.1803 | 0.0167 |
| NO | 0.2774 | 0.1506 | 0.0689 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochla, M.; Basiak, M.; Sztohryn, E.; Szczepańska-Gumulak, A.; Chylak, M.; Okopień, B.; Knapik, P. Surveillance of Digoxin Concentrations in Critically Ill Individuals with Heart Failure. Medicina 2025, 61, 1365. https://doi.org/10.3390/medicina61081365
Grochla M, Basiak M, Sztohryn E, Szczepańska-Gumulak A, Chylak M, Okopień B, Knapik P. Surveillance of Digoxin Concentrations in Critically Ill Individuals with Heart Failure. Medicina. 2025; 61(8):1365. https://doi.org/10.3390/medicina61081365
Chicago/Turabian StyleGrochla, Marek, Marcin Basiak, Ewa Sztohryn, Anna Szczepańska-Gumulak, Maciej Chylak, Bogusław Okopień, and Piotr Knapik. 2025. "Surveillance of Digoxin Concentrations in Critically Ill Individuals with Heart Failure" Medicina 61, no. 8: 1365. https://doi.org/10.3390/medicina61081365
APA StyleGrochla, M., Basiak, M., Sztohryn, E., Szczepańska-Gumulak, A., Chylak, M., Okopień, B., & Knapik, P. (2025). Surveillance of Digoxin Concentrations in Critically Ill Individuals with Heart Failure. Medicina, 61(8), 1365. https://doi.org/10.3390/medicina61081365








