Mortality Risk of Colistin vs. Non-Colistin Use in Cancer Patients with Multidrug-Resistant Gram-Negative Bacterial Infections: Stratified by Resistance Profile and Concomitant Medications
Abstract
1. Introduction
2. Materials and Methods
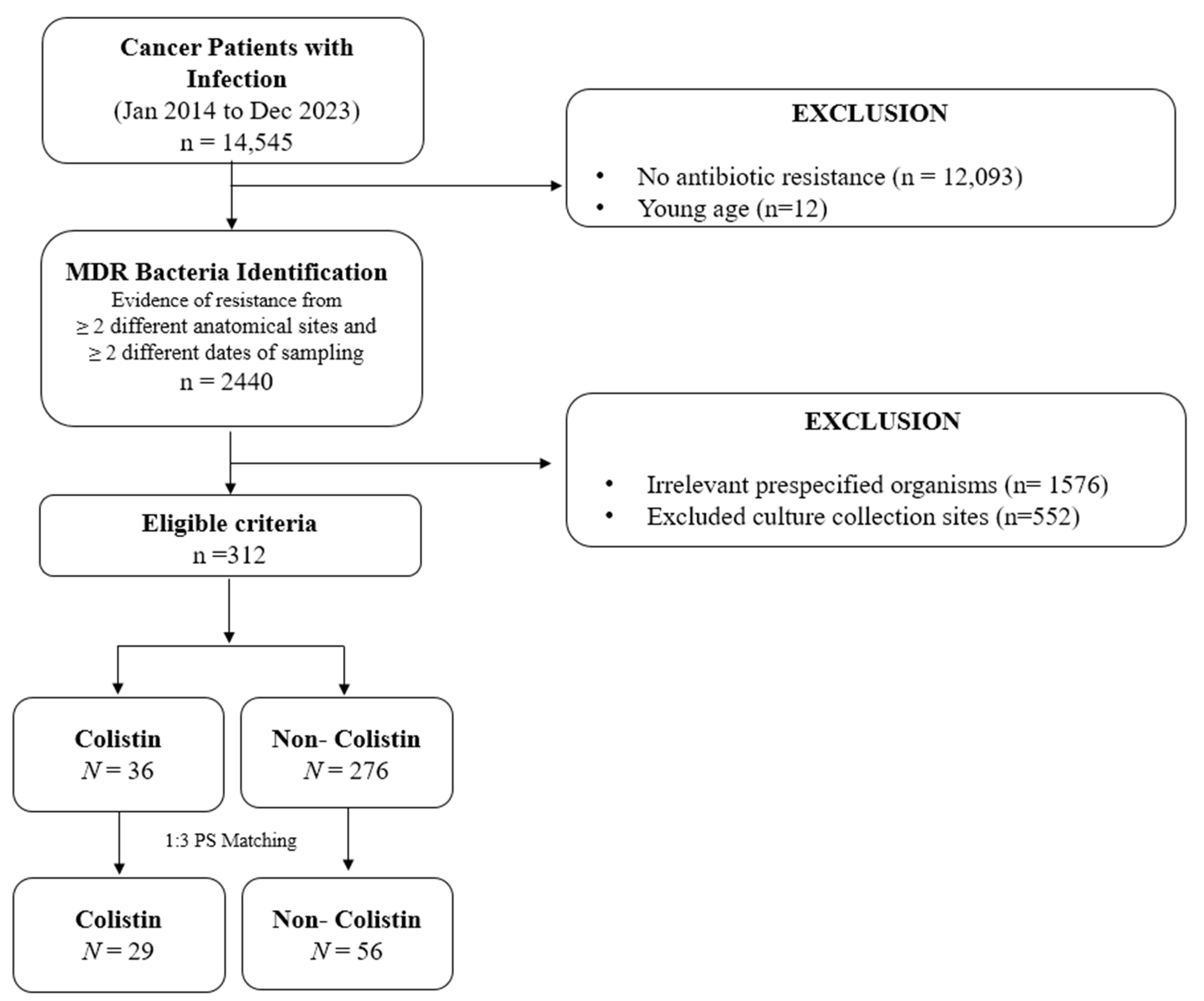
2.1. Study Design and Study Population
2.2. Study Cohort and Study Outcomes
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Patients
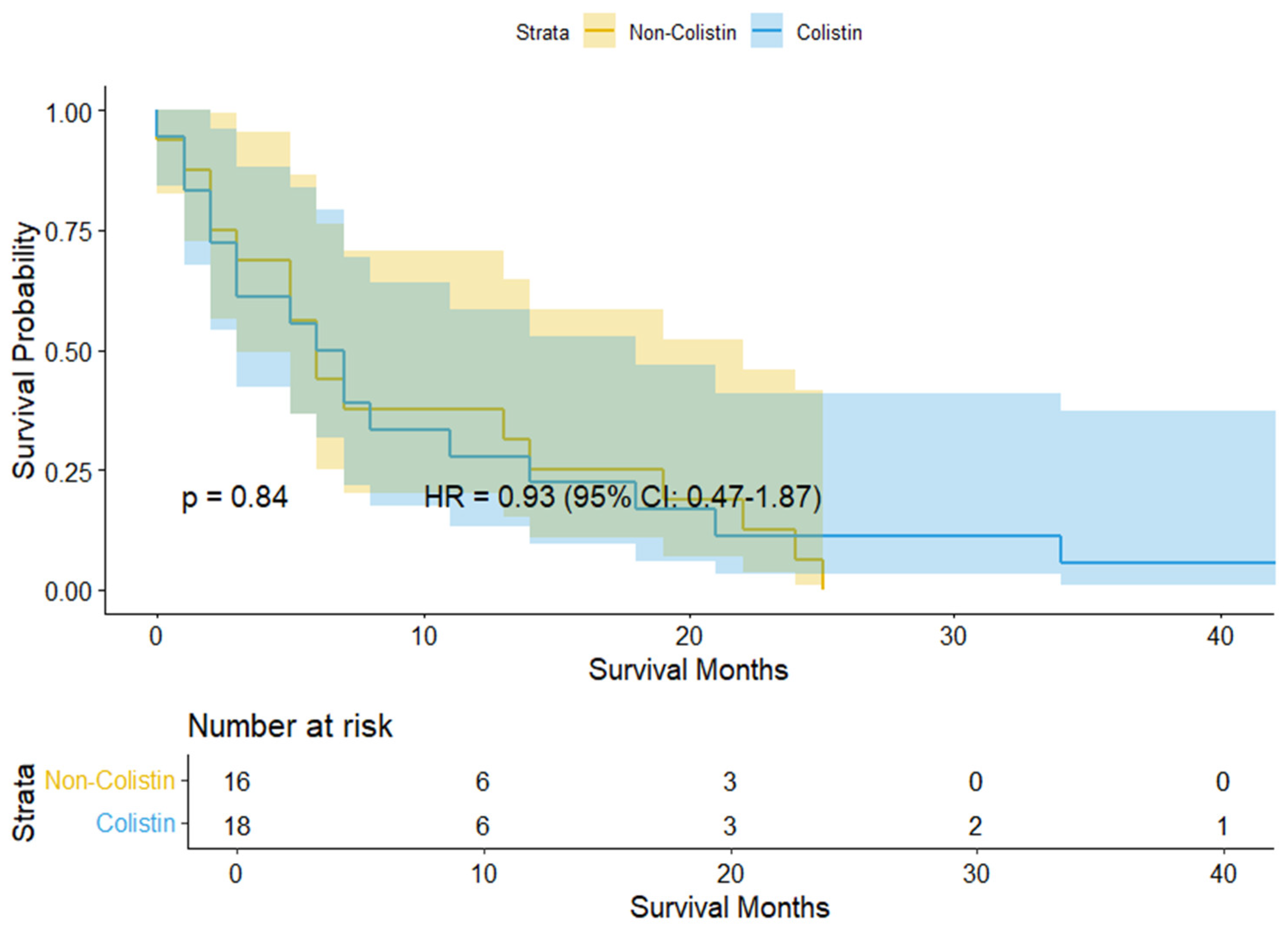
3.2. Study Outcomes
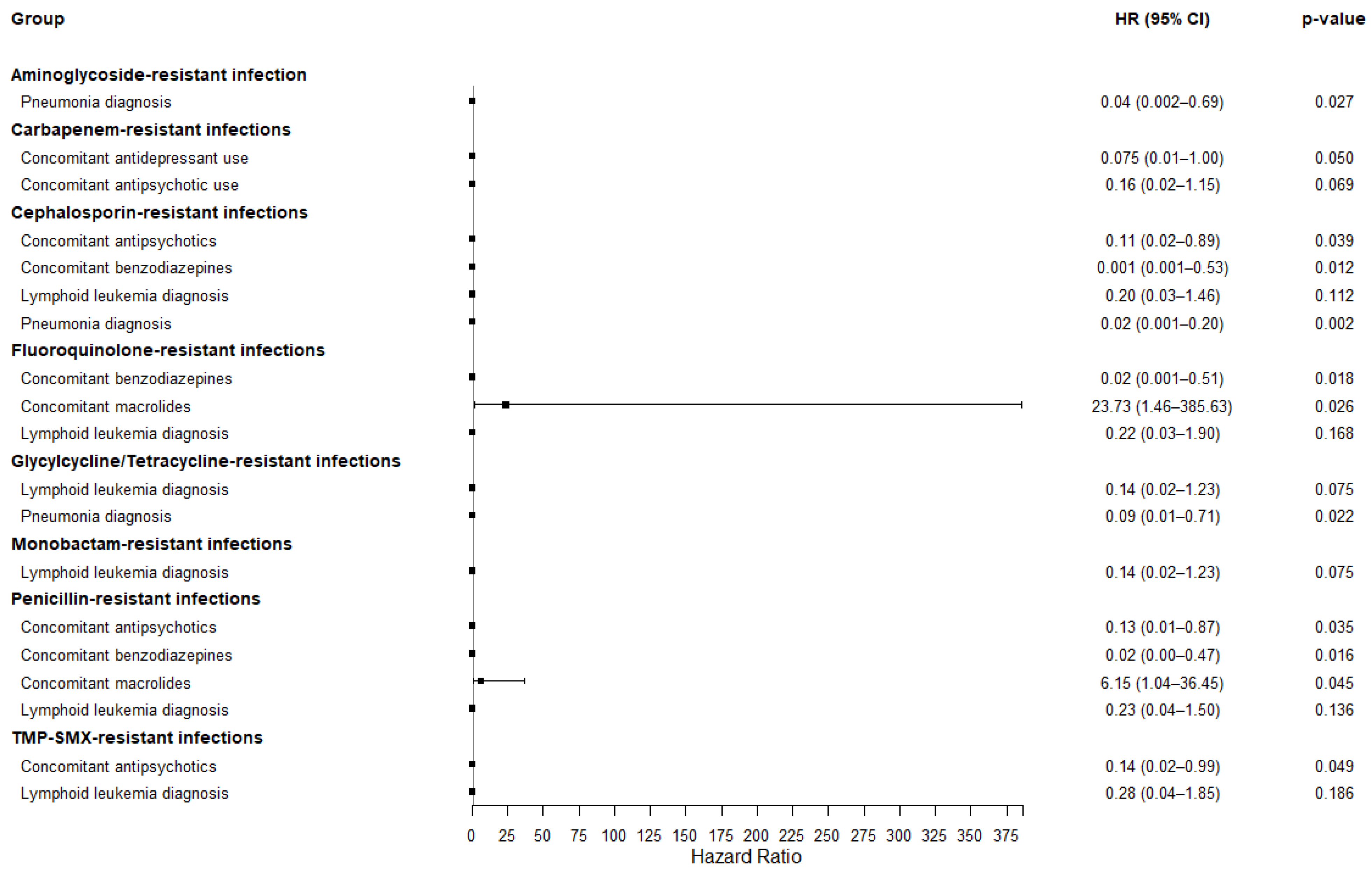
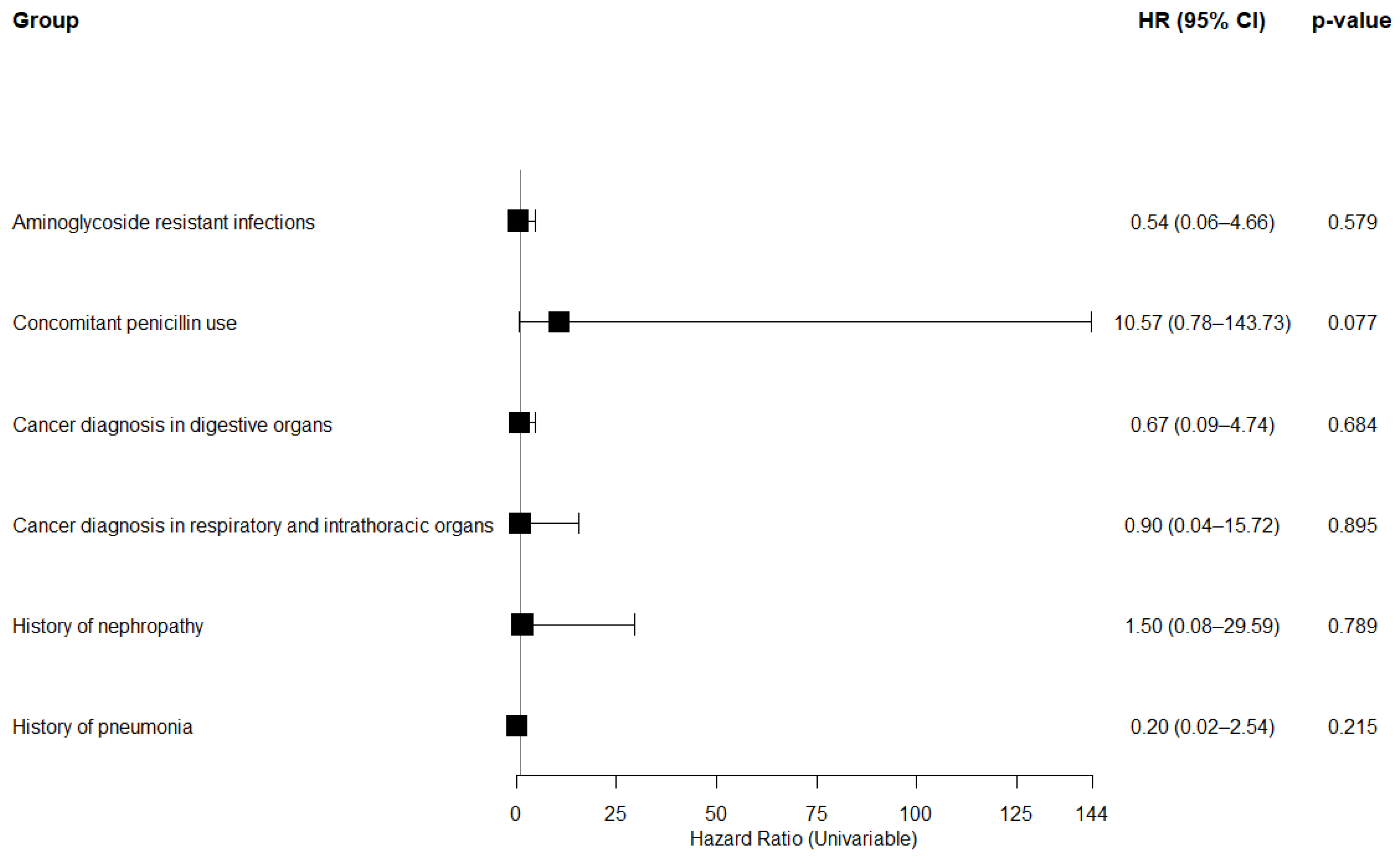
3.3. Subgroup Analysis on 168-Day (6-Month) Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adverse Drug Reactions |
| AR | Antibiotic Resistance |
| CI | Confidence Interval |
| CVC | Central Venous Catheters |
| EMR | Electronic Medical Records |
| GNB | Gram-Negative Bacteria |
| HR | Hazard Ratio |
| ICD | International Classification of Disease |
| MDR | Multidrug Resistant |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| PD | Pharmacodynamics |
| PK | Pharmacokinetics |
| SMD | Standardized Mean Differences |
| TMP_SMX | Trimethoprim-Sulfamethoxazole |
References
- Macesic, N.; Uhlemann, A.C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Simeonova, M.; Leung, V.; Lo, J.; Kan, T.; Raybardhan, S.; Sapin, M.E.; Mponponsuo, K.; Farrell, A.; et al. Antimicrobial resistance in patients with COVID-19: A systematic review and meta-analysis. Lancet Microbe 2023, 4, e179–e191. [Google Scholar] [CrossRef]
- Son, J.Y.; Kim, S.; Porsuk, T.; Shin, S.; Choi, Y.J. Clinical outcomes of colistin methanesulfonate sodium in correlation with pharmacokinetic parameters in critically ill patients with multi-drug resistant bacteria-mediated infection: A systematic review and meta-analysis. J. Infect. Public Health 2024, 17, 843–853. [Google Scholar] [CrossRef]
- Mo, S.H.; Lee, S.H.; Choi, C.-Y.; Sunwoo, Y.; Shin, S.; Choi, Y.J. A Comprehensive 10-Year nationwide pharmacovigilance surveillance on antibacterial agents in Korea: Data mining for signal detection of trends and seriousness of adverse events. Microorganisms 2025, 13, 136. [Google Scholar] [CrossRef]
- Sousa, S.A.; Feliciano, J.R.; Pita, T.; Soeiro, C.F.; Mendes, B.L.; Alves, L.G.; Leitão, J.H. Bacterial nosocomial infections: Multidrug resistance as a trigger for the development of novel antimicrobials. Antibiotics 2021, 10, 942. [Google Scholar] [CrossRef]
- Vink, J.; Edgeworth, J.; Bailey, S.L. Acquisition of MDR-GNB in hospital settings: A systematic review and meta-analysis focusing on ESBL-E. J. Hosp. Infect. 2020, 106, 419–428. [Google Scholar] [CrossRef]
- Rolston, K.V. Infections in cancer patients with solid tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef]
- Li, Y.; Al Maqrashi, Z.; Zhan, L.J.; Siddiqui, Z.; Jin, E.; Ellis, P.M. Use of broad-spectrum antibiotics in adult cancer patients presenting to emergency department with non-neutropenic fever and/or suspected infection. Support Care Cancer 2025, 33, 383. [Google Scholar] [CrossRef]
- Maesaka, F.; Tomioka, A.; Matsushita, C.; Horikawa, N.; Fujimoto, K. Severe neutropenia caused by palliative radiation therapy in a case of metastatic hormone-sensitive prostate cancer. IJU Case Rep. 2024, 7, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Jung, Y.J.; Lee, H.J.; Kim, H.J.; Maeng, C.H.; Baek, S.K.; Han, J.J.; Jeon, W.; Kim, D.Y.; Lee, Y.-M.; et al. Impact of multidrug resistance on outcomes in hematologic cancer patients with bacterial bloodstream infections. Sci. Rep. 2024, 14, 15622. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of infections due to MDR gram-negative bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef]
- Silva, B.; Silva Júnior, M.; Menezes, F.G.; Troster, E.J. Factors associated with multidrug-resistant bacteria in healthcare-associated infections: A pediatric intensive care unit case-control study. Einstein 2022, 20, eAO6704. [Google Scholar] [CrossRef]
- Pallares, R.; Viladrich, P.F.; LiÑAres, J.; Cabellos, C.; Gudiol, F. Impact of antibiotic resistance on chemotherapy for pneumococcal infections. Microb. Drug Resis. 1998, 4, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Kuznicki, M.; Yao, M.; Chichura, A.; Gruner, M.; Reizes, O.; Debernardo, R.; Rose, P.G.; Michener, C.; Vargas, R. Impact of antibiotic treatment during platinum chemotherapy on survival and recurrence in women with advanced epithelial ovarian cancer. Gynecol. Oncol. 2020, 159, 699–705. [Google Scholar] [CrossRef]
- Roger, C. Understanding antimicrobial pharmacokinetics in critically ill patients to optimize antimicrobial therapy: A narrative review. J. Intensive Med. 2024, 4, 287–298. [Google Scholar] [CrossRef]
- Mourad, D.F.; Radwan, S.; Hamdy, R.; Elkhashab, D.M.; Kamel, M.M.; Abdel-Moneim, A.S.; Kadry, D.Y. Identification of lower respiratory tract pathogens in cancer patients: Insights into fatal outcomes. Microorganisms 2024, 12, 1686. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Hachem Ray, Y.; Chemaly Roy, F.; Ahmar Corine, A.; Jiang, Y.; Boktour Maha, R.; Rjaili Georges, A.; Bodey Gerald, P.; Raad Issam, I. Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob. Agents Chemother. 2007, 51, 1905–1911. [Google Scholar] [CrossRef]
- Salman, A.; Ghannam, A.; Kittaneh, A.a.; Abu-zant, A.; Sahoury, Y.; Sahouri, E.; Abuamsha, R.; Salman, M. Effectiveness and safety of colistimethate sodium used in the treatment of neutropenic blood cancer patients infected with multidrug-resistant Pseudomonas aeruginosa. Arab. J. Sci. Eng. 2024, 49, 9111–9120. [Google Scholar] [CrossRef]
- Jo, H.G.; Jeong, K.; Ryu, J.Y.; Park, S.; Choi, Y.S.; Kwack, W.G.; Choi, Y.J.; Chung, E.K. Fatal events associated with adverse drug reactions in the Korean national pharmacovigilance database. J. Pers. Med. 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.G.; Clark, R.B. Discovery of macrolide antibiotics effective against multi-drug resistant gram-negative pathogens. Acc. Chem. Res. 2021, 54, 1635–1645. [Google Scholar] [CrossRef]
- Wei, J.; Walker, A.S.; Eyre, D.W. Addition of macrolide antibiotics for hospital treatment of community-acquired pneumonia. J. Infect. Dis. 2025, 231, e713–e722. [Google Scholar] [CrossRef] [PubMed]
- Sandherr, M.; Stemler, J.; Schalk, E.; Hattenhauer, T.; Hentrich, M.; Hertenstein, B.; Hohmann, C.; Mellinghoff, S.C.; Mispelbaum, R.; Rieger, C.; et al. 2024 update of the AGIHO guideline on diagnosis and empirical treatment of fever of unknown origin (FUO) in adult neutropenic patients with solid tumours and hematological malignancies. Lancet Reg. Health Eur. 2025, 51, 101214. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27 (Suppl. S5), v111–v118. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Tomida, J.; Kawamura, Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front. Microbiol. 2014, 4, 422. [Google Scholar] [CrossRef]
- Nor Amdan, N.A.; Shahrulzamri, N.A.; Hashim, R.; Mohamad Jamil, N. Understanding the evolution of macrolides resistance: A mini review. J. Glob. Antimicrob. Resist. 2024, 38, 368–375. [Google Scholar] [CrossRef]
- McDonagh, F.; Murray, E.K.; Hallahan, B.; Miliotis, G. Systematic examination of off-target effects of antipsychotic medications associated with microbiome disruption and heightened bacterial infection risks. J. Psychiatr. Res. 2025, 189, 171–183. [Google Scholar] [CrossRef]
- Nersesjan, V.; Christensen, R.H.B.; Andersen, E.W.; Kondziella, D.; Benros, M.E. Antipsychotic exposure and infection risk in people with schizophrenia spectrum disorders during the COVID-19 pandemic: A Danish nationwide registry study. Lancet Psychiatry 2024, 11, 796–806. [Google Scholar] [CrossRef]
- Wang, M.T.; Lin, C.W.; Tsai, C.L.; Wang, Y.H.; Lai, J.H.; Yeh, C.B.; Huang, Y.L.; Hsu, Y.J. Use of antipsychotics and the risk of acute respiratory failure among adults: A disease risk score-matched nested case-control study. Br. J. Clin. Pharmacol. 2020, 86, 2204–2216. [Google Scholar] [CrossRef]
- Wu, M.; Li, Z.; Zheng, W.; Zhuang, J.; Wu, S.; Zhou, Q.; Cai, J.; Zheng, H.; Zeng, G.; Zhang, W.; et al. Association between anticholinergic medication uses and the risk of pneumonia in elderly adults: A meta-analysis and systematic review. Ann. Med. 2023, 55, 2209736. [Google Scholar] [CrossRef]
- Korkatti-Puoskari, N.; Tiihonen, M.; Caballero-Mora, M.A.; Topinkova, E.; Szczerbińska, K.; Hartikainen, S. Therapeutic dilemma’s: Antipsychotics use for neuropsychiatric symptoms of dementia, delirium and insomnia and risk of falling in older adults, a clinical review. Eur. Geriatr. Med. 2023, 14, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Bressi, J.; Lê, M.-L.; Neal, D.; Cadogan, C.; Witt-Doerring, J.; Witt-Doerring, M.; Wright, S. Prescribing and deprescribing guidance for benzodiazepine and benzodiazepine receptor agonist use in adults with depression, anxiety, and insomnia: An international scoping review. eClinicalMedicine 2024, 70, 102507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Isomura, K.; Lichtenstein, P.; Kuja-Halkola, R.; D’Onofrio, B.M.; Brikell, I.; Quinn, P.D.; Zhu, N.; Jayaram-Lindström, N.; Chang, Z.; et al. Incident benzodiazepine and Z-drug use and subsequent risk of serious infections. CNS Drugs 2024, 38, 827–838. [Google Scholar] [CrossRef]
- Park, H.Y.; Kwon, J.; An, S.K.; Park, E.-C. A nationwide cohort study of the association of benzodiazepines with SARS-CoV-2 infection and clinical outcomes. Sci. Rep. 2022, 12, 15947. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.E., 3rd; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner. J. 2013, 13, 214–223. [Google Scholar]
- Cornwell, A.C.; Tisdale, A.A.; Venkat, S.; Maraszek, K.E.; Alahmari, A.A.; George, A.; Attwood, K.; George, M.; Rempinski, D.; Franco-Barraza, J.; et al. Lorazepam stimulates IL6 production and is associated with poor survival outcomes in pancreatic cancer. Clin. Cancer Res. 2023, 29, 3793–3812. [Google Scholar] [CrossRef]
- Nates, J.L.; Pene, F.; Darmon, M.; Mokart, D.; Castro, P.; David, S.; Povoa, P.; Russell, L.; Nielsen, N.D.; Gorecki, G.P.; et al. Septic shock in immunocompromised cancer patient: A narrative review. Crit. Care 2024, 28, 285. [Google Scholar] [CrossRef]
| Characteristics | No. of Cases (% Relative Frequency) or Median (IQR) | |||
|---|---|---|---|---|
| Total (n = 85) | Non-Colistin (n = 56) | Colistin (n = 29) | p-Values | |
| Age (years) | 65.49 ± 11.49 | 65.34 ± 12.23 | 64.64 ± 12.81 | >0.05 |
| 30~39 | 3 (3.53%) | 1 (1.79%) | 2 (6.90%) | |
| 40~49 | 7 (8.24%) | 5 (8.93%) | 2 (6.90%) | |
| 50~59 | 14 (16.47%) | 9 (16.07%) | 5 (17.24%) | |
| 60~69 | 24 (28.24%) | 16 (28.57%) | 8 (27.59%) | |
| 70~79 | 30 (35.29%) | 21 (37.50%) | 9 (31.03%) | |
| 80~89 | 7 (8.24%) | 4 (7.14%) | 3 (10.34%) | |
| Sex | ||||
| Men | 62 (72.94%) | 40 (71.43%) | 22 (75.86%) | >0.05 |
| Women | 23 (27.06%) | 16 (28.57%) | 7 (24.14%) | |
| Culture | ||||
| Blood/Fluid | 12 (14.12%) | 8 (14.29%) | 4 (13.79%) | >0.05 |
| Genital/Urinary | 8 (9.41%) | 8 (14.29%) | 0 (0.00%) | |
| Respiratory | 36 (42.35%) | 25 (44.64%) | 11 (37.93%) | |
| Duplicate | 29 (34.12%) | 15 (26.79%) | 14 (48.28%) | |
| Microorganism | ||||
| Acinetobacter baumannii | 23 (27.06%) | 13 (23.21%) | 10 (34.48%) | >0.05 |
| Klebsiella pneumoniae | 20 (23.53%) | 15 (26.79%) | 5 (17.24%) | |
| Pseudomonas aeruginosa | 15 (17.65%) | 10 (17.86%) | 5 (17.24%) | |
| ≥2 cultures | 27 (31.76%) | 18 (32.14%) | 9 (31.03%) | |
| Antibiotic resistance | ||||
| Aminoglycoside | 61 (10.93%) | 40 (10.96%) | 21 (10.88%) | >0.05 |
| Carbapenems | 65 (11.65%) | 42 (11.51%) | 23 (11.92%) | |
| Cephalosporin | 71 (12.72%) | 46 (12.60%) | 25 (12.95%) | |
| Colistin | 2 (0.36%) | 1 (0.27%) | 1 (0.52%) | |
| Fluoroquinolone | 73 (13.08%) | 47 (12.88%) | 26 (13.47%) | |
| Glycylcycline | 48 (8.60%) | 32 (8.77%) | 16 (8.29%) | |
| Monobactam | 64 (11.47%) | 42 (11.51%) | 22 (11.40%) | |
| Penicillin | 76 (13.62%) | 51 (13.97%) | 25 (12.95%) | |
| Tetracycline | 18 (3.23%) | 11 (3.01%) | 7 (3.63%) | |
| TMP_SMX | 80 (14.34%) | 53 (14.52%) | 27 (13.99%) | |
| Concomitant antibiotics | ||||
| Aminoglycoside | 44 (5.48%) | 30 (5.78%) | 14 (4.93%) | >0.05 |
| Carbapenem | 68 (8.47%) | 43 (8.29%) | 25 (8.80%) | |
| Cephalosporin | 78 (9.71%) | 51 (9.83%) | 27 (9.51%) | |
| Fluoroquinolones | 57 (7.10%) | 38 (7.32%) | 19 (6.69%) | |
| Glycopeptide | 76 (9.46%) | 49 (9.44%) | 27 (9.51%) | |
| Lincosamides | 13 (1.62%) | 8 (1.54%) | 5 (9.51%) | |
| Macrolide | 17 (2.12%) | 11 (2.12%) | 6 (0.70%) | |
| Monobactam | 1 (0.12%) | 1 (0.19%) | 0 (0.00%) | |
| Oxazolidinone | 8 (1.00%) | 6 (1.16%) | 2 (0.70%) | |
| Penicillin | 73 (9.09%) | 47 (9.06%) | 26 (9.15%) | |
| Rifamycin | 8 (1.00%) | 5 (0.96%) | 3 (1.06%) | |
| Tetracycline | 6 (0.75%) | 2 (0.39%) | 4 (1.41%) | |
| TMP_SMX | 11 (1.37%) | 5 (0.96%) | 6 (2.11%) | |
| Comedications | ||||
| Anesthetics | 64 (7.97%) | 42 (8.09%) | 22 (7.75%) | >0.05 |
| Antidepressants | 20 (2.49%) | 15 (2.89%) | 5 (1.76%) | |
| Antipsychotic | 50 (6.23%) | 32 (6.17%) | 18 (6.34%) | |
| Anxiolytics | 6 (0.75%) | 3 (0.58%) | 3 (1.06%) | |
| Benzodiazepine | 71 (8.84%) | 46 (8.86%) | 25 (8.80%) | |
| Buprenorphine | 1 (0.12%) | 1 (0.19%) | 0 (0.00%) | |
| Hypnotics and sedatives | 18 (2.24%) | 11 (2.12%) | 7 (2.46%) | |
| Immunosuppressants | 8 (1.00%) | 3 (0.58%) | 5 (1.76%) | |
| NSAIDs | 21 (2.62%) | 16 (3.08%) | 5 (1.76%) | |
| Opioid | 78 (9.71%) | 51 (9.83%) | 27 (9.51%) | |
| Cancer types | ||||
| Digestive organs | 41 (41.41%) | 26 (37.68%) | 15 (50.00%) | >0.05 |
| Respiratory and intrathoracic organs | 15 (15.15%) | 10 (14.49%) | 5 (16.67%) | |
| Mesothelial | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Renal and urinary tract | 2 (2.02%) | 2 (2.90%) | 0 (0.00%) | |
| Thyroid and other endocrine glands | 1 (1.01%) | 0 (0.00%) | 1 (3.33%) | |
| Lymphoid leukemia | 16 (16.16%) | 11(15.94%) | 5 (16.67%) | |
| Malignant neoplasm of the breast | 1 (1.01%) | 1 (1.45%) | 0 (0.00%) | |
| Renal cell carcinoma | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Malignant neoplasm of meninges | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Malignant neoplasm of spinal cord, cranial nerves, and other parts of central nervous system | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Malignant neoplasm of peripheral nerves and autonomic nervous system | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Malignant neoplasm brain | 2 (2.02%) | 1 (1.45%) | 1 (3.33%) | |
| Malignant neoplasms | 12 (12.12%) | 10 (14.49%) | 2 (6.67%) | |
| Comorbidities | ||||
| Cardiovascular disease | 10 (22.22%) | 7 (25.00%) | 3 (17.65%) | >0.05 |
| Diabetes mellitus | 4 (8.89%) | 2 (7.14%) | 2 (11.76%) | |
| Hypertensive | 2 (4.44%) | 1 (3.57%) | 1 (5.88%) | |
| Liver disease | 3 (6.67%) | 2 (7.14%) | 1 (5.88%) | |
| Nephropathy | 5 (11.11%) | 3 (10.71%) | 2 (11.76%) | |
| Pneumonia | 17 (37.78%) | 10 (35.71%) | 7 (41.18%) | |
| Cerebrovascular disease | 4 (8.89%) | 3 (10.71%) | 1 (5.88%) | |
| Characteristic | Multivariable Cox Analysis | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Cardiovascular disease | 1.72 (0.29–10.25) | 0.552 |
| Diabetes mellitus | 1.03 (0.22–4.86) | 0.973 |
| Hypertension | 0.26 (0.01–4.52) | 0.355 |
| Liver diseases | 1.08 (0.22–5.27) | 0.928 |
| Nephropathy | 0.97 (0.26–3.68) | 0.963 |
| Cerebrovascular disease | 4.34 (0.51–37.08) | 0.180 |
| Sepsis | 0.66 (0.16–2.72) | 0.565 |
| Septic shock | 1.27 (0.62–3.13) | 0.957 |
| Characteristic | Univariable Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Based on Colistin use status | ||||
| Colistin use | 1.18 (0.45–3.06) | 0.738 | 4.66 (0.74–29.45) | 0.102 |
| Sex | ||||
| SEX (female) | 1.05 (0.29–3.73) | 0.944 | 0.15 (0.01–1.54) | 0.110 |
| Antibiotic susceptibility testing | ||||
| Aminoglycoside resistance | 1.66 (0.61–4.53) | 0.323 | 54.50 (3.30–900.20) | 0.005 |
| Concomitant Antibiotics | ||||
| Penicillin | 0.96 (0.33–2.77) | 0.936 | 51.91 (2.83–950.60) | 0.008 |
| Cancer Types | ||||
| Digestive organs | 0.69 (0.24–1.95) | 0.483 | 0.02 (0.00–0.20) | 0.002 |
| Respiratory and intrathoracic organs | 0.54 (0.12–2.45) | 0.425 | 2.45 × 10−4 (1.49 × 10−6–4.02 × 10−2) | 0.001 |
| Comorbidities | ||||
| Nephropathy | 0.72 (0.16–3.22) | 0.667 | 0.021 (3.11 × 10−4–1.42) | 0.072 |
| Pneumonia | 1.23 (0.44–3.81) | 0.638 | 0.06 (1.94 × 10−3–1.85) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Choi, Y.; Choi, C.-Y.; Choi, Y.J.; Shin, S. Mortality Risk of Colistin vs. Non-Colistin Use in Cancer Patients with Multidrug-Resistant Gram-Negative Bacterial Infections: Stratified by Resistance Profile and Concomitant Medications. Medicina 2025, 61, 1361. https://doi.org/10.3390/medicina61081361
Lee SH, Choi Y, Choi C-Y, Choi YJ, Shin S. Mortality Risk of Colistin vs. Non-Colistin Use in Cancer Patients with Multidrug-Resistant Gram-Negative Bacterial Infections: Stratified by Resistance Profile and Concomitant Medications. Medicina. 2025; 61(8):1361. https://doi.org/10.3390/medicina61081361
Chicago/Turabian StyleLee, Soo Hyeon, Yongwon Choi, Chang-Young Choi, Yeo Jin Choi, and Sooyoung Shin. 2025. "Mortality Risk of Colistin vs. Non-Colistin Use in Cancer Patients with Multidrug-Resistant Gram-Negative Bacterial Infections: Stratified by Resistance Profile and Concomitant Medications" Medicina 61, no. 8: 1361. https://doi.org/10.3390/medicina61081361
APA StyleLee, S. H., Choi, Y., Choi, C.-Y., Choi, Y. J., & Shin, S. (2025). Mortality Risk of Colistin vs. Non-Colistin Use in Cancer Patients with Multidrug-Resistant Gram-Negative Bacterial Infections: Stratified by Resistance Profile and Concomitant Medications. Medicina, 61(8), 1361. https://doi.org/10.3390/medicina61081361





