Efficacy and Safety of Radioligand Therapy with Actinium-225 DOTATATE in Patients with Advanced, Metastatic or Inoperable Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Question
2.2. Search Strategy
2.3. Selection of Studies
- Inclusion criteria for the systematic review were studies or subsets of studies investigating the efficacy and/or safety of radioligand therapy with Actinium-225 DOTATATE in patients with advanced, metastatic or inoperable neuroendocrine neoplasms.
- Exclusion criteria for the systematic review were articles outside the field of interest, preclinical studies, editorials, letters, reviews, comments, conference proceedings, and case reports related to the selected intervention.
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Quality Assessment
3.3. Qualitative Synthesis
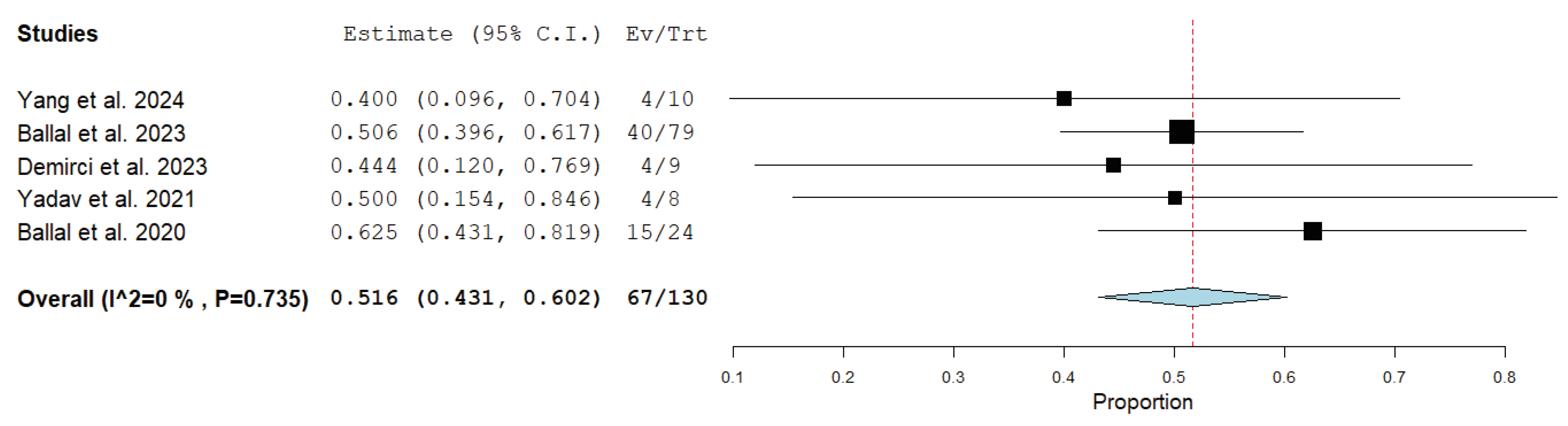
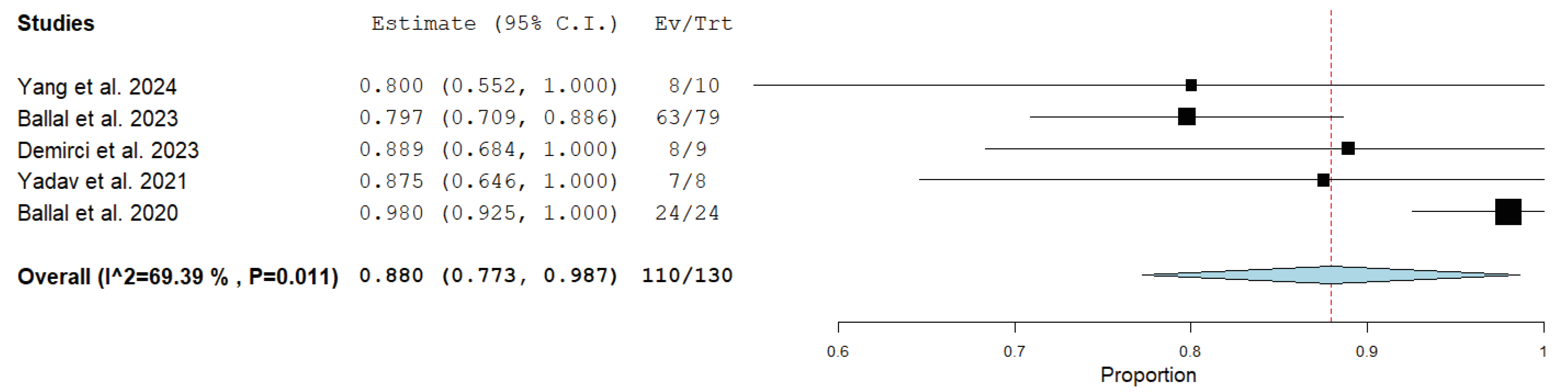
3.4. Quantitative Synthesis (Meta-Analysis)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Di Franco, M.; Zanoni, L.; Fortunati, E.; Fanti, S.; Ambrosini, V. Radionuclide Theranostics in Neuroendocrine Neoplasms: An Update. Curr. Oncol. Rep. 2024, 26, 538–550. [Google Scholar] [CrossRef]
- Hope, T.A.; Allen-Auerbach, M.; Bodei, L.; Calais, J.; Dahlbom, M.; Dunnwald, L.K.; Graham, M.M.; Jacene, H.A.; Heath, C.L.; Mittra, E.S.; et al. SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 204–210. [Google Scholar] [CrossRef]
- Ambrosini, V.; Zanoni, L.; Filice, A.; Lamberti, G.; Argalia, G.; Fortunati, E.; Campana, D.; Versari, A.; Fanti, S. Radiolabeled Somatostatin Analogues for Diagnosis and Treatment of Neuroendocrine Tumors. Cancers 2022, 14, 1055. [Google Scholar] [CrossRef]
- Santo, G.; Di Santo, G.; Virgolini, I. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: Agonist, Antagonist and Alternatives. Semin. Nucl. Med. 2024, 54, 557–569. [Google Scholar] [CrossRef]
- Zoi, V.; Giannakopoulou, M.; Alexiou, G.A.; Bouziotis, P.; Thalasselis, S.; Tzakos, A.G.; Fotopoulos, A.; Papadopoulos, A.N.; Kyritsis, A.P.; Sioka, C. Nuclear Medicine and Cancer Theragnostics: Basic Concepts. Diagnostics 2023, 13, 3064. [Google Scholar] [CrossRef]
- Marini, I.; Sansovini, M.; Bongiovanni, A.; Nicolini, S.; Grassi, I.; Ranallo, N.; Monti, M.; DI Iorio, V.; Germanò, L.; Caroli, P.; et al. Theragnostic in neuroendocrine tumors. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 342–352. [Google Scholar] [CrossRef]
- Rodrigues, M.; Svirydenka, H.; Virgolini, I. Theragnostics in Neuroendocrine Tumors. PET Clin. 2021, 16, 365–373. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Giovinazzo, F.; Galiandro, F.; Annunziata, S.; Muoio, B.; Kroiss, A.S. PET with Different Radiopharmaceuticals in Neuroendocrine Neoplasms: An Umbrella Review of Published Meta-Analyses. Cancers 2021, 13, 5172. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Ciuciulkaite, I.; Herrmann, K.; Lahner, H. Importance of peptide receptor radionuclide therapy for the management of neuroendocrine tumours. Radiologie 2025, 65, 371–380. [Google Scholar] [CrossRef]
- Dadgar, H.; Pashazadeh, A.; Norouzbeigi, N.; Assadi, M.; Al-Balooshi, B.; Baum, R.P.; Al-Ibraheem, A.; Haidar, M.; Beheshti, M.; Geramifar, P.; et al. Targeted radioligand therapy: Physics and biology, internal dosimetry and other practical aspects during 177Lu/225Ac treatment in neuroendocrine tumors and metastatic prostate cancer. Theranostics 2025, 15, 4368–4397. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Q.; Cai, L.; Xie, Y.; Chen, Y. The efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 1533–1543. [Google Scholar] [CrossRef]
- Wang, L.F.; Lin, L.; Wang, M.J.; Li, Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine 2020, 99, e19304. [Google Scholar] [CrossRef]
- Saravana-Bawan, B.; Bajwa, A.; Paterson, J.; McEwan, A.J.B.; McMullen, T.P.W. Efficacy of 177Lu Peptide Receptor Radionuclide Therapy for the Treatment of Neuroendocrine Tumors: A Meta-analysis. Clin. Nucl. Med. 2019, 44, 719–727. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yoshino, M.; Shirasaki, K.; Nakanishi, K.; Kamada, K.; Yoshikawa, A.; Kataoka, J. Real-time trajectory imaging of alpha particles emitted from actinium-225 and its daughter radionuclides. Sci. Rep. 2025, 15, 2534. [Google Scholar] [CrossRef]
- Göring, L.; Schumann, S.; Müller, J.; Buck, A.K.; Port, M.; Lassmann, M.; Scherthan, H.; Eberlein, U. Repair of α-particle-induced DNA damage in peripheral blood mononuclear cells after internal ex vivo irradiation with 223Ra. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3981–3988. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 May 2025).
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Li, H.; Zhang, Y.; Feng, Y.; Yang, X.; Chen, Y. Efficacy and Safety of 225 Ac-DOTATATE in the Treatment of Neuroendocrine Neoplasms With High SSTR Expression. Clin. Nucl. Med. 2024, 49, 505–512. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Tripathi, M.; Sahoo, R.K.; Bal, C. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients receiving Concomitant 225Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-world Scenario Management Based Long-term Outcome Study. J. Nucl. Med. 2023, 64, 211–218. [Google Scholar] [CrossRef]
- Demirci, E.; Alan Selçuk, N.; Beydağı, G.; Ocak, M.; Toklu, T.; Akçay, K.; Kabasakal, L. Initial Findings on the Use of [225Ac]Ac-DOTATATE Therapy as a Theranostic Application in Patients with Neuroendocrine Tumors. Mol. Imaging Radionucl. Ther. 2023, 32, 226–232. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Giammarile, F.; Carrara, M.; Paez, D.; Hricak, H.; Ayati, N.; Li, J.J.; Mueller, M.; Aggarwal, A.; Al-Ibraheem, A.; et al. Radiotherapy and theranostics: A Lancet Oncology Commission. Lancet Oncol. 2024, 25, e545–e580. [Google Scholar] [CrossRef]
- Grewal, U.S.; Gbolahan, O.B.; Takalkar, A.M.; Halperin, D.M. Alpha radioligand therapy in neuroendocrine neoplasms: Current landscape and spotlight on RYZ101. Future Oncol. 2025, 21, 1357–1363. [Google Scholar] [CrossRef]
- Zuo, D.; Wang, H.; Yu, B.; Li, Q.; Gan, L.; Chen, W. Astatine-211 and actinium-225: Two promising nuclides in targeted alpha therapy. Acta Biochim. Biophys. Sin. 2024, 57, 327–343. [Google Scholar] [CrossRef]
- Song, H.; Sgouros, G. Alpha and Beta Radiation for Theragnostics. PET Clin. 2024, 19, 307–323. [Google Scholar] [CrossRef]
- Morgan, K.A.; Rudd, S.E.; Noor, A.; Donnelly, P.S. Theranostic Nuclear Medicine with Gallium-68, Lutetium-177, Copper-64/67, Actinium-225, and Lead-212/203 Radionuclides. Chem. Rev. 2023, 123, 12004–12035. [Google Scholar] [CrossRef]
- Hassan, M.; Bokhari, T.H.; Lodhi, N.A.; Khosa, M.K.; Usman, M. A review of recent advancements in Actinium-225 labeled compounds and biomolecules for therapeutic purposes. Chem. Biol. Drug. Des. 2023, 102, 1276–1292. [Google Scholar] [CrossRef]
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef]
- Rubira, L.; Deshayes, E.; Santoro, L.; Kotzki, P.O.; Fersing, C. 225Ac-Labeled Somatostatin Analogs in the Management of Neuroendocrine Tumors: From Radiochemistry to Clinic. Pharmaceutics 2023, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Kratochwil, C.; Ahmadzadehfar, H.; Morgenstern, A.; Eiber, M.; Herrmann, K.; Pomykala, K.L. Clinical Translation of Targeted α-Therapy: An Evolution or a Revolution? J. Nucl. Med. 2023, 64, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Chandekar, K.R.; Bal, C. Gastro-Enteric-Pancreatic Neuroendocrine Tumor Treatment: Actinium-225-DOTATATE and Combined Therapies. PET Clin. 2023, 18, 215–221. [Google Scholar] [CrossRef]
- Zhang, X.; Wakabayashi, H.; Hiromasa, T.; Kayano, D.; Kinuya, S. Recent Advances in Radiopharmaceutical Theranostics of Pheochromocytoma and Paraganglioma. Semin. Nucl. Med. 2023, 53, 503–516. [Google Scholar] [CrossRef]
- Shi, M.; Jakobsson, V.; Greifenstein, L.; Khong, P.L.; Chen, X.; Baum, R.P.; Zhang, J. Alpha-peptide receptor radionuclide therapy using actinium-225 labeled somatostatin receptor agonists and antagonists. Front. Med. 2022, 9, 1034315. [Google Scholar] [CrossRef]
- Harris, P.E.; Zhernosekov, K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front. Endocrinol. 2022, 13, 941832. [Google Scholar] [CrossRef]
- Dhiman, D.; Vatsa, R.; Sood, A. Challenges and opportunities in developing Actinium-225 radiopharmaceuticals. Nucl. Med. Commun. 2022, 43, 970–977. [Google Scholar] [CrossRef]
- Michler, E.; Kästner, D.; Pretze, M.; Hartmann, H.; Freudenberg, R.; Schultz, M.K.; Bundschuh, R.A.; Kotzerke, J.; Brogsitter, C. [203/212Pb]Pb-VMT-α-NET as a novel theranostic agent for targeted alpha radiotherapy-first clinical experience. Eur. J. Nucl. Med. Mol. Imaging 2025. [CrossRef]
- Chapeau, D.; Koustoulidou, S.; Handula, M.; Beekman, S.; de Ridder, C.; Stuurman, D.; de Blois, E.; Buchatskaya, Y.; van der Schilden, K.; de Jong, M.; et al. [212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors. Pharmaceuticals 2023, 16, 985. [Google Scholar] [CrossRef]
- Delgado Bolton, R.C.; Calapaquí Terán, A.K.; Fanti, S.; Giammarile, F. New Biomarkers with Prognostic Impact Based on Multitracer PET/CT Imaging in Neuroendocrine Neoplasms: The Light Leading Out of the Darkness in Challenging Tumors. Clin. Nucl. Med. 2022, 47, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Holzgreve, A.; Unterrainer, L.M.; Tiling, M.; Mansour, N.; Spitzweg, C.; Brendel, M.; Ricke, J.; Unterrainer, M.; Kunz, W.G.; Mehrens, D. Cost-Effectiveness of [177Lu]Lu-DOTATATE for the Treatment of Newly Diagnosed Advanced Gastroenteropancreatic Neuroendocrine Tumors: An Analysis Based on Results of the NETTER-2 Trial. J. Nucl. Med. 2025, 66, 1075–1081. [Google Scholar] [CrossRef]
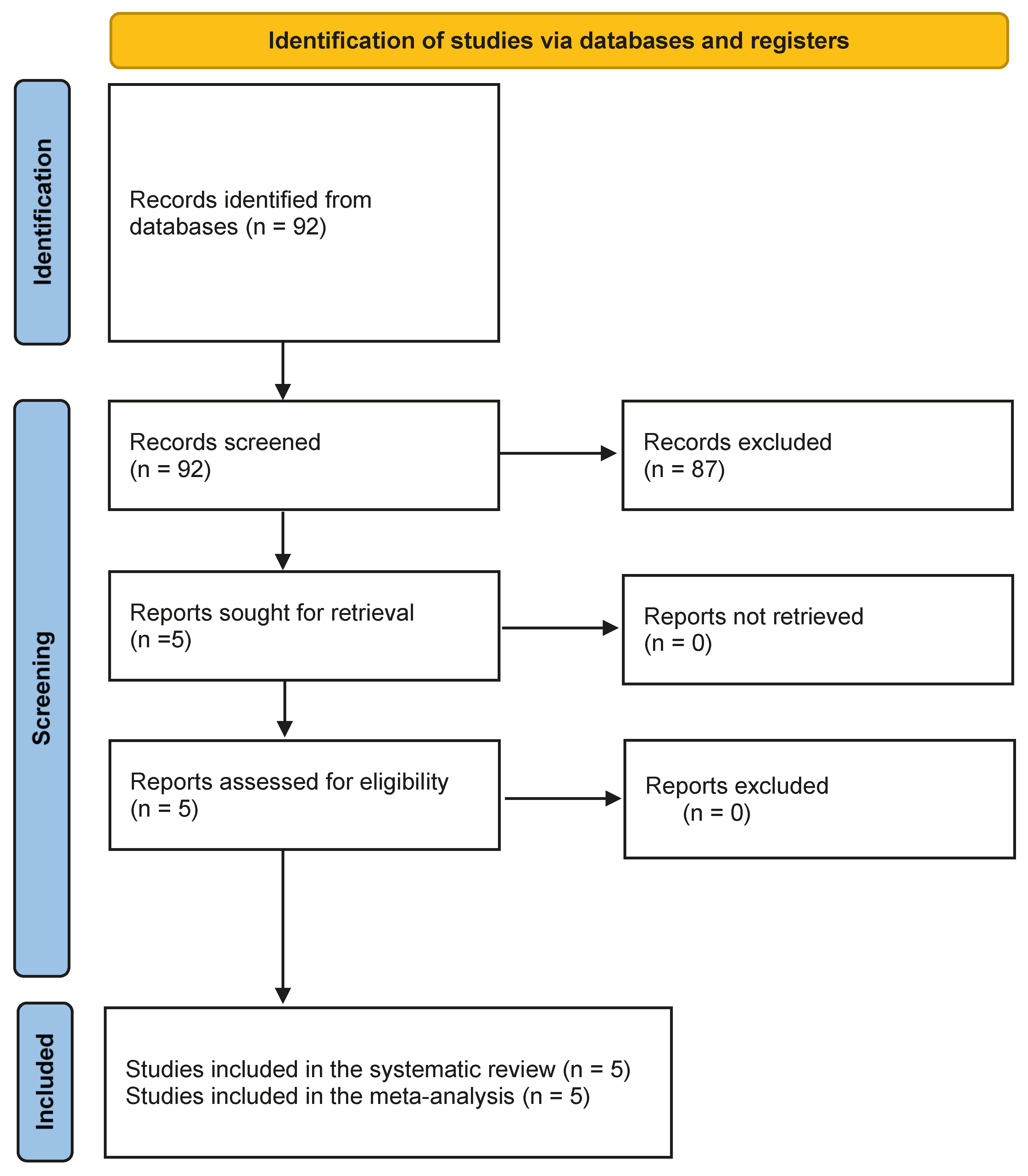
| Authors | Year | Country | Study Design | No. of Cases | Mean or Median Age | Sex Ratio | Types of Tumor | Tumor Grade (Based on Ki-67%) |
|---|---|---|---|---|---|---|---|---|
| Yang et al. [21] | 2024 | China | Prospective | 10 | 47.5 y | 3 F/7 M | Various metastatic NENs | I = 10%, II = 80%; NA = 10% |
| Ballal et al. [22] | 2023 | India | Prospective | 91 | 54 y | 37 F/54 M | Metastatic GEP-NENs | I = 36%, II = 53%; III = 8%; NA = 3% |
| Demirci et al. [23] | 2023 | Turkey | Retrospective | 11 | 59 y | 3 F/8 M | Various metastatic NENs | I = 20%, II = 70%; NA = 10% |
| Yadav et al. [24] | 2022 | India | Prospective | 9 | 41 y | 3 F/6 M | Advanced PGLs | I = 33%, II = 33%; III = 11%; NA = 22% |
| Ballal et al. [25] | 2020 | India | Prospective | 32 | 52 y | 17 F/15 M | Metastatic GEP-NENs | I = 34%, II = 50%; III = 9%; NA = 6% |
| Authors | Radiopharmaceutical | Median Number of Cycles (Range) | Dose per Cycle and Interval | Median/Mean Cumulative Activity (Range) | Treatment Response Criteria | Median Follow-Up Time (Range) | DCR | DRR | Serious Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [21] | 225Ac-DOTATATE | 3 (2–6) | 100 kBq/kg; 8-week interval | 22.9 MBq (14.8–44.4) | PERCIST 1.0 | 14 months (7–22) | 8/10 (80%) | 4/10 (40%) | None |
| Ballal et al. [22] | 225Ac-DOTATATE | 4 (1–10) | 100–120 kBq/kg; 8-week interval | 35.5 MBq (21.6–59.5) | RECIST 1.1 | 24 months (5–41) | 63/79 (80%) | 40/79 (51%) | Grade III thrombocytopenia (1/91) |
| Demirci et al. [23] | 225Ac-DOTATATE | 1 (1–3) | 100–120 kBq/kg; interval NR | 8.2 MBq (7.5–10) | RECIST 1.1 | NR | 8/9 (89%) | 4/9 (44%) | None |
| Yadav et al. [24] | 225Ac-DOTATATE | 3 (2–9) | 100 kBq/kg; 8-week interval | 42.4 MBq (15.5–86.6) | RECIST 1.1 | 22.5 months (8–30) | 7/8 (87.5%) | 4/8 (50%) | None |
| Ballal et al. [25] | 225Ac-DOTATATE | 3 (1–5) | 100 kBq/kg; 8-week interval | 22.5 MBq (7.8–44.4) | RECIST 1.1 | 8 months (2–13) | 24/24 (100%) | 15/24 (62.5%) | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, A.; Imperiale, A.; Annunziata, S.; Delgado Bolton, R.C.; Albano, D.; Fiz, F.; Piccardo, A.; Cuzzocrea, M.; Paone, G.; Treglia, G. Efficacy and Safety of Radioligand Therapy with Actinium-225 DOTATATE in Patients with Advanced, Metastatic or Inoperable Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1341. https://doi.org/10.3390/medicina61081341
Rizzo A, Imperiale A, Annunziata S, Delgado Bolton RC, Albano D, Fiz F, Piccardo A, Cuzzocrea M, Paone G, Treglia G. Efficacy and Safety of Radioligand Therapy with Actinium-225 DOTATATE in Patients with Advanced, Metastatic or Inoperable Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(8):1341. https://doi.org/10.3390/medicina61081341
Chicago/Turabian StyleRizzo, Alessio, Alessio Imperiale, Salvatore Annunziata, Roberto C. Delgado Bolton, Domenico Albano, Francesco Fiz, Arnoldo Piccardo, Marco Cuzzocrea, Gaetano Paone, and Giorgio Treglia. 2025. "Efficacy and Safety of Radioligand Therapy with Actinium-225 DOTATATE in Patients with Advanced, Metastatic or Inoperable Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis" Medicina 61, no. 8: 1341. https://doi.org/10.3390/medicina61081341
APA StyleRizzo, A., Imperiale, A., Annunziata, S., Delgado Bolton, R. C., Albano, D., Fiz, F., Piccardo, A., Cuzzocrea, M., Paone, G., & Treglia, G. (2025). Efficacy and Safety of Radioligand Therapy with Actinium-225 DOTATATE in Patients with Advanced, Metastatic or Inoperable Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Medicina, 61(8), 1341. https://doi.org/10.3390/medicina61081341









