1. Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, emerged in late 2019 and rapidly became a global pandemic. The World Health Organization declared it a pandemic on 11 March 2020, as health systems worldwide faced severe disruptions [
1,
2,
3,
4,
5,
6,
7]. With the progression of the pandemic, healthcare infrastructures worldwide experienced critical strain [
8]. Alongside public health interventions such as lockdowns, international travel restrictions, physical distancing, and widespread use of face masks, hospitals adopted radical internal precautions [
9,
10,
11]. Most institutions redirected clinical capacity and human resources to manage COVID-19, leading to a widespread reallocation of health system priorities. As a consequence, the diagnosis and management of non-COVID-19 conditions—including chronic diseases and malignancies—suffered substantial setbacks [
12,
13,
14,
15]. Cancer care was particularly vulnerable to these disruptions [
16]. Delays in diagnostic workups, treatment initiation, and surgical procedures generated concerns about compromised clinical outcomes and increased cancer-related mortality [
17]. These delays affected multiple cancer types, including breast, colorectal, lung, esophageal, and gastric malignancies. Gastric cancer, which constitutes the focus of this study, is of particular concern due to its often asymptomatic course and late-stage presentation [
18,
19,
20,
21,
22,
23,
24,
25]. In an effort to mitigate the potential adverse outcomes of healthcare interruptions, various international and national oncology societies issued consensus guidelines to preserve essential cancer services during the pandemic.
Gastric cancer continues to be a major global health burden, ranking fifth both in cancer incidence and cancer-related deaths. According to the GLOBOCAN 2022 statistics published by the International Agency for Research on Cancer, gastric cancer accounted for approximately 659,853 deaths worldwide in that year alone [
26]. Its risk factors include Helicobacter pylori infection, a positive family history, chronic atrophic gastritis, intestinal metaplasia, tobacco and alcohol use, and prior history of gastric ulcers. In high-risk populations, upper gastrointestinal endoscopy plays a crucial role in early diagnosis and significantly improves survival. Screening programs targeting such individuals can reduce mortality by 30% to 67%, primarily by identifying lesions at curable stages [
27,
28,
29,
30].
The COVID-19 pandemic, which severely disrupted healthcare services globally, also significantly affected the screening, diagnosis, and treatment of patients with gastric cancer. In many countries, elective and even some oncologic surgeries were postponed or canceled, both to reallocate resources toward COVID-19 care and to reduce viral transmission risk [
31,
32]. It is estimated that approximately 38% of oncologic surgeries were delayed or canceled during this period [
33]. As with many other malignancies, surgical intervention remains the cornerstone of curative treatment for gastric cancer. In Turkey, following the first confirmed COVID-19 case, national health authorities implemented similar precautionary strategies, leading to postponement of elective procedures including oncologic surgeries [
21,
34]. In the context of gastric cancer, healthcare disruptions during the COVID-19 pandemic in Turkey led to significant reductions in endoscopy procedures due to concerns over viral transmission, contributing to delayed diagnoses and stage migration. Operating room capacity was constrained by the redeployment of surgical teams and intensive care resources to COVID-19 care, leading to postponed surgeries and interruptions in multidisciplinary cancer management. These specific disruptions highlight the vulnerability of gastric cancer patients during the pandemic.
Although early-phase studies have documented the short-term effects of the pandemic on cancer care, evidence regarding long-term survival outcomes in gastric cancer remains limited [
16,
32,
35,
36]. Studies from various countries have reported significant reductions in early-stage diagnoses and increased rates of advanced-stage presentations, yet few have directly linked surgical delays to survival outcomes in gastric cancer patients. While some meta-analyses suggest that delays up to eight weeks may not significantly affect prognosis, especially in early-stage cases, the impact on more advanced disease is uncertain. This persistent knowledge gap challenges clinicians in making evidence-based decisions about safe delays in surgery or treatment, resource allocation, and patient counseling during health crises like the COVID-19 pandemic. Therefore, the primary aim of this multicenter study is to compare gastric cancer patients operated during the Pre-COVID-19 and COVID-19 Era periods in terms of demographic, clinical, and histopathological characteristics. As a secondary objective, the study also investigates factors associated with postoperative mortality and risk factors influencing survival in gastric cancer patients, incorporating the pandemic period as an independent variable in the analyses. Based on this primary objective, we hypothesized that pandemic-related delays would worsen survival, independent of tumor characteristics.
4. Discussion
The COVID-19 pandemic has affected millions of people worldwide, delivering significant blows to the healthcare systems of many countries. Due to the limited knowledge about the virus, its high transmissibility, and elevated mortality rates, particularly among patients with comorbidities, both financial and human resources in healthcare were largely redirected to the management of COVID-19 patients, resulting in the neglect of other medical conditions [
41,
42]. The restrictions implemented by many governments, the recommendations to visit hospitals only for emergencies, and the widespread fear of infection in the community led to delays in the diagnosis and treatment of many diseases [
43,
44]. These delays had the most pronounced impact on oncology patients. There is a growing body of literature indicating that cancer patients were neglected during the pandemic and that elective cancer surgeries, particularly in early-stage cases, were frequently postponed [
22]. Moreover, it is known that patients with gastrointestinal system cancers, especially gastric cancer, are at a higher risk of contracting COVID-19 and facing increased mortality compared to non-cancer patients due to their compromised immune systems [
45,
46,
47,
48].
Although one of the most significant causes of diagnostic delay was the implementation of lockdown measures and the fear of hospital visits—particularly among patients with chronic conditions—the most critical factor was the substantial reduction in endoscopic procedures used in gastric cancer screening, due to the aerosol transmission risk associated with COVID-19. The Japan Gastroenterological Endoscopy Society recommended postponing non-urgent gastrointestinal endoscopic procedures [
49]. Studies comparing the pandemic period with Pre-COVID-19 have reported a 42% to 80% decrease in the number of endoscopies performed [
42,
50,
51,
52,
53,
54,
55,
56].
Naturally, the decrease in endoscopy rates was paralleled by a decline in the number of newly diagnosed gastric cancer cases, a reduction in early-stage diagnoses, a rise in advanced-stage disease presentations, and an increase in complicated cases requiring more frequent emergency or palliative surgical interventions [
57,
58,
59]. In fact, a multinational study reported by Herrera-Kok et al. [
59], involving 145 centers from 50 countries, confirmed these global disruptions by reporting a 43.4% decrease in multidisciplinary team meetings, a 54.5% drop in elective gastrectomies, and increases in clinical stage migration (29.0%), metastatic disease rates (33.8%), the need for definitive palliative treatment (26.9%), urgent surgeries (26.9%), and palliative surgeries (16.6%) [
59]. These results highlight the widespread and multifactorial impact of the COVID-19 pandemic on the surgical management and outcomes of gastric cancer patients worldwide. Since the current study focused on the management of patients already diagnosed with gastric cancer, it did not address delays in diagnosis or the decrease in the number of diagnosed patients. Therefore, only general trends from the literature regarding these aspects were presented. In this context, unlike many studies in the literature, the findings of the present study suggest that the pandemic did not significantly impact cancer biology-related parameters (such as tumor stage, pathological grade, or extent of local invasion). However, definitive evidence will require long-term, multicenter studies and systematic analyses.
Several countries reported a decrease in the annual number of surgeries during the pandemic, accompanied by a progression in ASA scores and clinical stages among operated patients [
60]. However, some studies have shown no significant changes in ASA scores or clinical stage distributions during this period [
55,
61]. Meanwhile, there has been a noticeable increase in the use of minimally invasive techniques, diagnostic surgeries, and preoperative chemotherapy. These findings collectively highlight the multifaceted and cumulative impact of the pandemic on cancer care delivery, often contributing to a gradual deterioration in patients’ clinical conditions. In the present study, no significant differences were found between the Pre-COVID-19 and COVID-19 Era groups in terms of basic demographic and clinical features, ASA scores, and tumor characteristics, including TNM staging. Moreover, the rate of neoadjuvant therapy increased from 16.7% in the Pre-COVID-19 group to 24.7% in the COVID-19 Era group (
p = 0.076), indicating a trend approaching statistical significance. Although this increase did not reach conventional significance thresholds, we believe the result is clinically meaningful, as it may reflect evolving treatment strategies during the pandemic aimed at minimizing patient contact, protecting healthcare personnel, and optimizing surgical planning.
Early diagnosis and prompt initiation of treatment are critically important for survival in gastric cancer [
62]. The survival rate exceeds 90% for stage I, drops to approximately 45% for stage III, and falls to 9% for stage IV patients [
63]. During the pandemic, it has been reported that the time from diagnosis to surgery was prolonged and there were delays in initiating chemotherapy due to COVID-19-related factors [
64]. However, the literature presents conflicting findings regarding these time intervals. Some studies have shown that the interval between diagnosis and treatment does not significantly affect prognosis in gastric cancer [
65,
66,
67,
68]. Similarly, various studies have demonstrated that surgical delays of 2, 4, 6, or even 8 weeks do not negatively impact prognosis, especially in stage I and II patients [
19,
43,
69,
70,
71]. Another meta-analysis also suggested that delaying oncological surgery by less than 8 weeks may not adversely affect overall survival in gastric cancer [
48].
If left untreated, early-stage gastric cancer has been reported to progress to advanced stages within 34–44 months [
72]. In another study, the progression from stage I to stage II occurred within 34 months, whereas progression from stage III to IV was reported to occur in as little as 1.8 months [
19]. To date, there is no specific guideline regarding the optimal timing of surgery in gastric cancer [
73]. In a multicenter survey involving 36 hospitals in Italy, the number of surgical procedures significantly decreased during the pandemic, and the interval between patient assessment and surgery nearly doubled [
74].
Studies conducted during the COVID-19 pandemic have primarily focused on how delays in diagnosis, neoadjuvant therapy, surgery, and the initiation of adjuvant therapy influence patient outcomes. In the present study, although the time from diagnosis to surgery was similar between the groups, the turnaround time for pathology reporting was slightly longer during the COVID-19 Era, which could have potentially led to modest delays in planning adjuvant therapy. However, a review of patient records indicated that the vast majority of pathology reports were finalized within 30 days, generally falling within the usual postoperative recovery timeframe before initiating adjuvant treatment. Moreover, in the present study, no association was observed between the timing of adjuvant therapy and mortality or survival outcomes. Similar findings have been reported in other malignancies, such as biliary cancers, where delays of up to three or four months in initiating adjuvant therapy did not significantly impact overall survival [
75]. To our knowledge, no previous studies have specifically compared the interval between surgery and the completion of pathology reports. In our prior publication on breast cancer, we explored this issue and found no significant differences [
21]. These observations suggest that moderate delays in postoperative management might not universally translate into worse outcomes, including in gastric cancer. The discrepancy noted in our study may be related to organizational factors at university hospitals, where pathologists are subspecialized by organ, potentially contributing to variations in reporting times.
During the COVID-19 pandemic, numerous studies have reported a significant decline in the number of surgical procedures performed for gastric cancer, although some contrary findings have also been reported in the literature. Feier et al. [
22] reported a 44% reduction in such surgeries during the pandemic. Similarly, hospitals across India observed reductions ranging between 17% and 63%, a study from Tokyo reported a 50% decrease, and Italy experienced a 30% decline [
41,
69,
70,
76,
77,
78,
79]. However, Sun et al. [
15] reported a 21.4% increase in the number of patients undergoing surgery for gastric cancer during the pandemic period. These disruptions likely contributed to delays in diagnosis and treatment, negatively affecting prognosis and increasing the risk of complications. In the present study, only a slight decrease in patient volume was observed between the Pre-COVID-19 and COVID-19 Era periods within the evaluated timeframe. Notably, consistent with the findings of Arneiro et al. [
41], no significant differences were identified in tumor characteristics, morbidity, or mortality between the two periods.
The strict public health measures implemented during the pandemic, in addition to causing diagnostic delays, influenced both the prioritization and modality of cancer treatment. During periods of tightened restrictions, surgical treatment options were often suspended, and patients were redirected toward non-surgical modalities such as chemotherapy whenever possible [
64]. One major contributing factor was the reallocation of healthcare personnel to pandemic response, which resulted in a reduced number of available operating rooms for elective procedures [
80]. In response to these challenges, several clinical guidelines were issued. The Italian Society of Surgical Oncology recommended neoadjuvant chemotherapy for all patients with cT2N0 gastric cancer and advised continuing medical treatment for those who could tolerate it [
81]. In another publication, Kang et al. [
19] proposed deferring elective surgery for early-stage gastric cancer patients and utilizing neoadjuvant chemotherapy for locally advanced cases, with surgery planned four weeks after the completion of treatment. Moreover, studies on neoadjuvant chemotherapy in gastric surgery have suggested that extending the waiting period beyond six weeks before surgery may improve complete response rates, without adversely impacting prognosis [
82,
83]. A study from Turkey also reported an increased use of neoadjuvant therapy in gastric cancer patients treated during the post-pandemic period [
61]. As previously discussed, although not statistically significant, the present study demonstrated a modest increase in the use of neoadjuvant therapy, which may reflect evolving treatment strategies during the pandemic.
COVID-19 is primarily transmitted through airborne particles and droplets, raising significant concerns in surgical environments. Early in the pandemic, several studies suggested that laparoscopic procedures might lead to aerosol generation due to carbon dioxide insufflation and surgical smoke escaping through trocars, potentially facilitating aerosol transmission of the virus within the operating room [
84,
85]. As a result, initial guidance from organizations such as the Intercollegiate General Surgery Guidance on COVID-19 and the Society of Gastrointestinal and Endoscopic Surgeons (SAGES) advised caution or avoidance of laparoscopy; however, later updates acknowledged the absence of definitive evidence supporting this risk [
86,
87]. Techniques like closed vacuum systems with filtration, as described by Li et al. [
77], were introduced to reduce the potential for aerosol dispersion and subsequent viral transmission. In the present study, we observed a statistically significant decline in the use of laparoscopic surgery during the pandemic, likely reflecting both early recommendations discouraging laparoscopy and surgeons’ efforts to minimize operative time and exposure risks.
Although there are studies with opposing findings, several publications in the literature have shown an increase in the proportion of stage III and IV gastric cancers during the pandemic. Solaini et al. [
64] reported a higher number of T4- and M1-stage patients during the pandemic compared to the same period before. Arneiro et al. [
60] also showed a higher incidence of T3/T4 gastric cancer cases during the pandemic. Seker et al. [
55] found higher clinical T, pathological T, and clinical N stages in patients diagnosed during the pandemic. In a multicenter retrospective study from Japan, a 32.9% decrease in stage I and an 11.4% increase in stage IV cases were reported [
58]. Shigenobu et al. [
88] similarly demonstrated a decline in early-stage and an increase in advanced-stage gastric cancer cases. Feier et al. [
22] reported an increase in stage III and IV patients compared to the pre-pandemic period. Other studies in the literature also support these findings, showing a decrease in early-stage cancers and a concurrent increase in advanced-stage disease [
41,
70,
71]. Cınkıl et al. [
61] reported an increase in stage IIIB patients treated during the pandemic, although overall TNM stage distributions varied. Conversely, Solaini et al. [
64] found no significant impact of the pandemic on TNM staging in their large patient series. Similarly, Sun et al. [
15] reported that the pandemic had no significant effect on TNM stage distribution. In the present study, no statistically significant differences were found between the Pre-COVID-19 and COVID-19 Era periods in terms of pathological T, pathological N, clinical M, TNM stage, or detailed histopathological tumor characteristics.
There are only a limited number of studies investigating the direct and indirect impact of the COVID-19 pandemic on mortality and long-term survival in patients with gastric cancer, likely due to the recent onset of the pandemic. Ma et al. [
48] conducted a meta-analysis including eight studies and a total of 4052 gastric cancer patients. This study demonstrated that delaying surgery for less than 8 weeks does not adversely affect overall survival or disease-free survival. Moreover, postponing surgery by 4, 6, or even 8 weeks was not significantly associated with reduced overall survival.
A study from Portugal reported higher mortality during the early phase of the pandemic, attributing this increase to indirect effects of the pandemic [
89]. Another study also found increased mortality during the pandemic, which was associated with increased comorbidity indices, more advanced T staging, and a higher rate of total gastrectomy [
22]. A study from Japan reported an increased risk of mortality in gastric cancer patients during the pandemic, with a mean follow-up of 157 days [
88]. In contrast, a study from Germany reported no difference in perioperative morbidity and mortality [
90], while a Brazilian study found no significant differences in 30- and 90-day mortality rates [
60]. A multicenter study from India reported a slight increase in mortality during the pandemic in 6 out of 16 centers [
78].
Across these studies, various contributing factors have been emphasized, including delayed hospital admissions, patients waiting until symptoms worsened, prior COVID-19 infection, diagnostic delays, increased tumor burden, poorer clinical and psychological status of patients, and an increase in complications.
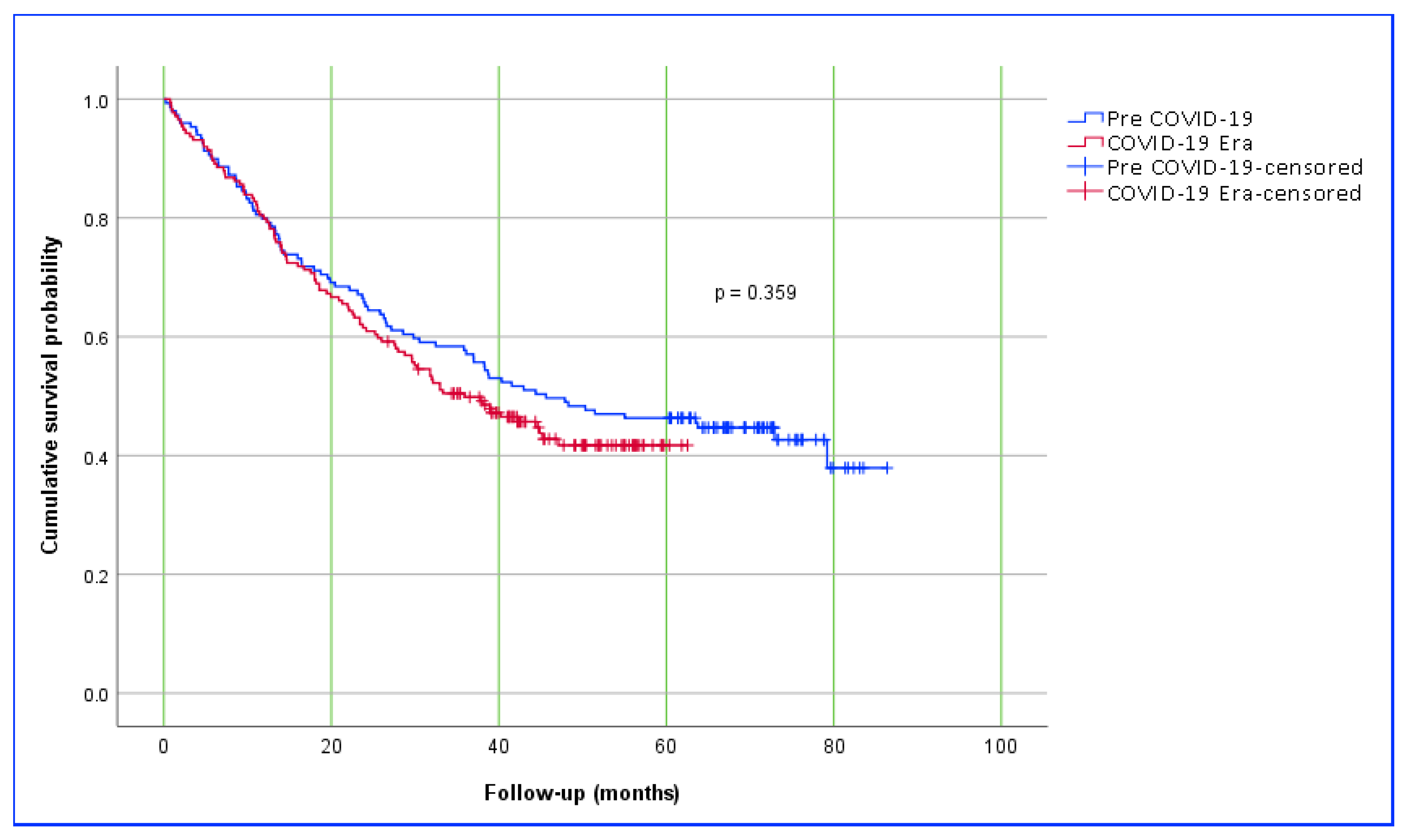
Interestingly, a study from Romania reported better survival in gastric cancer patients operated on during the pandemic period; however, the authors could not explain this finding [
91]. In our study, no difference in mortality was observed between patients who underwent cancer surgery before and during the pandemic throughout the follow-up period. Similarly, survival analysis showed comparable results. The 1-, 3-, and 5-year survival rates in the pre-pandemic group were 80.7%, 50.7%, and 46.7%, respectively, while these rates in the pandemic group were 79.9%, 49.8%, and 41.7%, respectively. Nevertheless, it is important to note that some of the patients included in this study have not yet completed the full 5-year follow-up period. In summary, due to the naturally shorter follow-up time in patients treated during the pandemic compared to those in the pre-pandemic period, statistically significant results related to follow-up may be misleading. On the other hand, most gastric cancers are diagnosed at an advanced stage and are inherently associated with high mortality and poor survival. Therefore, more definitive conclusions regarding follow-up outcomes will require long-term data.
Numerous studies in the literature have identified key prognostic factors associated with mortality and long-term survival in patients undergoing surgery for gastric cancer. Among the most consistently reported predictors are advanced tumor size (T4), extensive nodal involvement (N2–N3), lymphovascular and perineural invasion, poor tumor differentiation, and the presence of distant metastases. Additionally, factors such as patient age, comorbidities, and ASA score have also been shown to influence surgical outcomes [
92,
93,
94]. Some studies have shown that TNM staging serves as an independent prognostic factor for survival, while biological markers of tumor aggressiveness such as grading, lymphovascular invasion, and perineural invasion were not found to be significant risk factors [
95]. In the current study, multivariable analyses were conducted to evaluate the effect of independent variables on mortality and survival in patients who underwent gastric cancer surgery either the Pre-COVID-19 or COVID-19 Era cohorts. In both the logistic and Cox regression models, the period of surgery (Pre-COVID-19 vs. COVID-19 Era) was included as an independent variable but did not demonstrate a statistically significant association with either mortality or overall survival. In the logistic regression model, total gastrectomy (OR: 2.14), advanced tumor stage T4 (OR: 2.93), extensive nodal involvement N3, and the presence of lymphovascular invasion (OR: 2.87) were associated with increased mortality. Similarly, in the Cox proportional hazards model, the variables were identified as independent risk factors significantly associated with decreased survival, including combined tumor location (HR: 1.73), total gastrectomy (HR: 1.56), lymphovascular invasion, T4 stage (HR: 1.93), N3 nodal disease (HR: 1.71), and the presence of distant metastasis (HR: 1.74). These results suggest that classical oncologic prognostic factors were the main determinants of mortality and survival in this cohort, regardless of the timing of surgery relative to the pandemic.
Limitations
This study has several limitations. First, the retrospective design inherently limits causal inference and may be subject to selection and information biases. Although the multicenter nature of the study enhances its scope, differences in diagnostic protocols, surgical indications, and postoperative management across the participating centers could have introduced variability. Moreover, precise data on key diagnostic intervals—such as the time from symptom onset to endoscopy or from diagnosis to surgery—were not systematically recorded, preventing detailed analysis of diagnostic delays. Another important limitation is the significantly shorter follow-up duration in the COVID-19-era cohort compared to the Pre-COVID-19 group. This disparity limits our ability to evaluate 5- and 10-year survival outcomes and may underestimate the potential late effects of pandemic-related disruptions. Additionally, although pathology report turnaround times were longer during the COVID-19 Era, determining their true impact on survival and mortality will require highly coordinated, multicenter, and large-scale studies to provide reliable evidence. Finally, the unique healthcare structure and pandemic management strategies in Turkey may limit the generalizability of our findings to other countries with different healthcare systems and COVID-19 protocols.