Cellular Rejection Post-Cardiac Transplantation: A 13-Year Single Unicentric Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinicopathological Parameters of the Included Cases
3.2. Histopathological Diagnosis
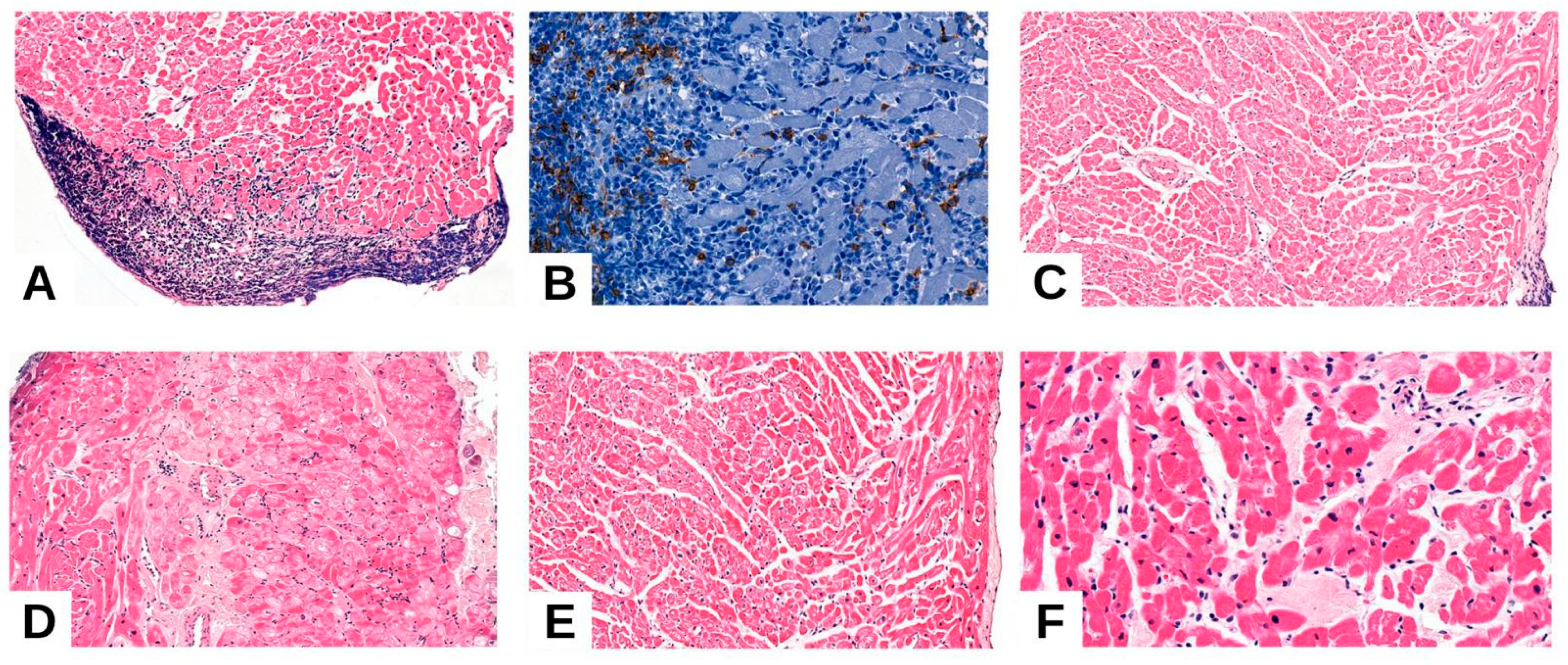
3.2.1. Acute Cellular Rejection (ACR)
3.2.2. Antibody-Mediated Rejection (AMR)
3.2.3. Quilty Effect
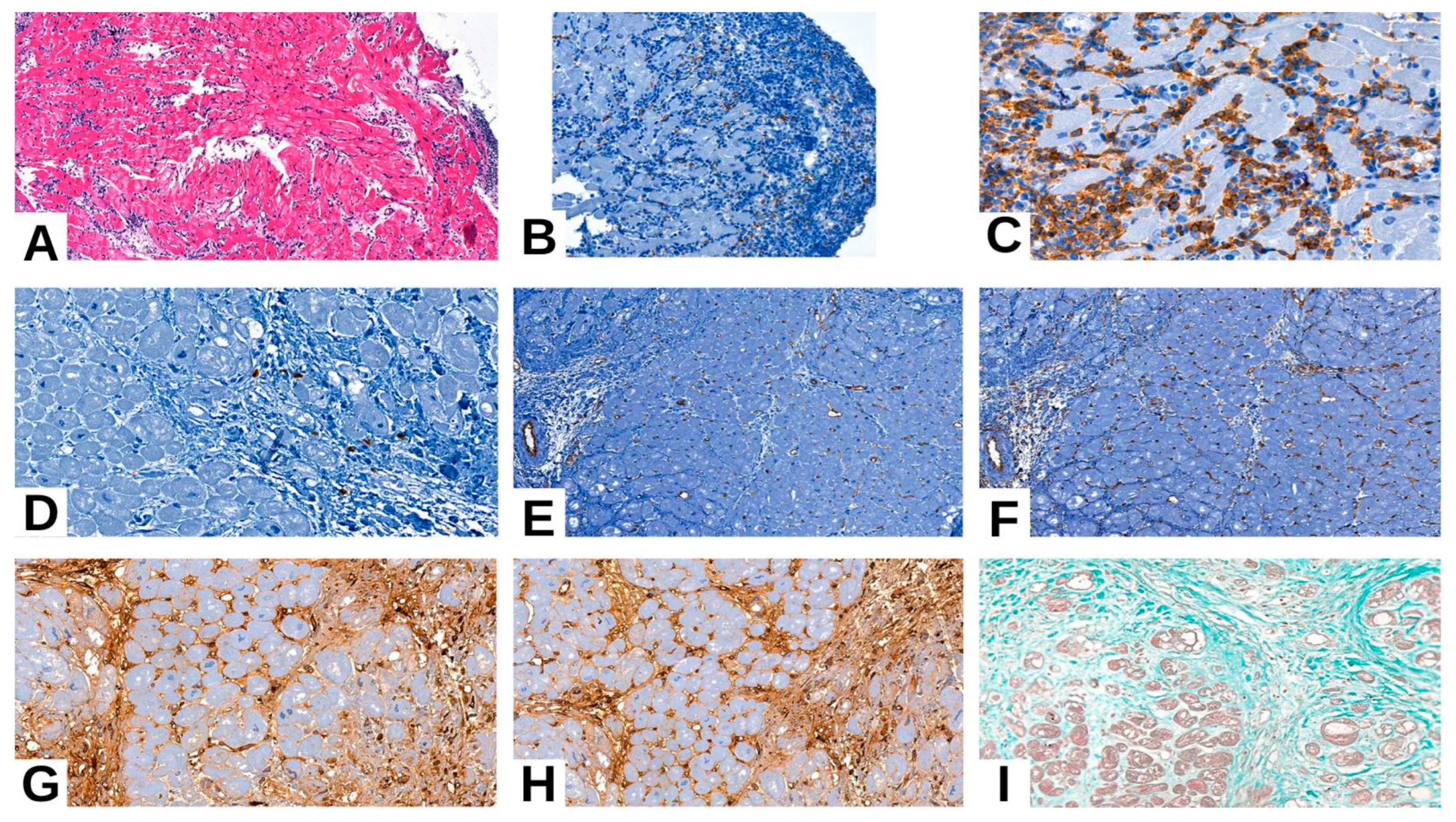
3.3. Clinicopathological Features and Immunohistochemical Markers (Table 5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, M.C. Cardiac transplantation. Heart 2002, 87, 177–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobashigawa, J.; Miller, L.; Renlund, D.; Mentzer, R.; Alderman, E.; Bourge, R.; Costanzo, M.; Eisen, H.; Dureau, G.; Ratkovec, R.; et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation 1998, 66, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Strecker, T.; Rösch, J.; Weyand, M.; Agaimy, A. Endomyocardial biopsy for monitoring heart transplant patients: 11-years-experience at a german heart center. Int. J. Clin. Exp. Pathol. 2013, 6, 55–65. [Google Scholar] [PubMed] [PubMed Central]
- Patel, J.K.; Kobashigawa, J.A. Improving survival during heart transplantation: Diagnosis of antibody-mediated rejection and techniques for the prevention of graft injury. Future Cardiol. 2012, 8, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Ludhwani, D.; Abraham, J.; Sharma, S.; KanmanthareddY, A. Heart Transplantation Rejection; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Joong, A.; Richmond, M.; Addonizio, L. The Quilty effect in pediatric heart transplant recipients. J. Heart Lung Transplant. 2016, 35, S75. [Google Scholar] [CrossRef]
- Costnzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010, 29, 914–956. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Perch, M.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; et al. International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report—2021; Focus on recipient characteristics. J. Heart Lung Transplant. 2021, 40, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colvin, M.M.; Cook, J.L.; Chang, P.; Francis, G.; Hsu, D.T.; Kiernan, M.S.; Kobashigawa, J.A.; Lindenfeld, J.; Masri, S.C.; Miller, D.; et al. Antibody-mediated rejection in cardiac transplantation: Emerging knowledge in diagnosis and management: A scientific statement from the American Heart Association. Circulation 2015, 131, 1608–1639. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Feucht, H.E.; Richter, K.; Reiter, C.; Spes, C.H.; Pongratz, D.; Uberfuhr, P.; Meiser, B.; Theisen, K.; Angermann, C.E. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J. Heart Lung Transplant. 1999, 18, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.F.; Fernández, R.; Fernández, D.; Jaramillo, J.C.; Gil, M.; López García-Asenjo, J.A. C4D immunostaining in surveillance endomyocardial biopsies from well-functioning heart allografts. Transplant. Proc. 2010, 42, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Chantranuwat, C.; Qiao, J.H.; Kobashigawa, J.; Hong, L.; Shintaku, P.; Fishbein, M.C. Immunoperoxidase staining for C4d on paraffin-embedded tissue in cardiac allograft endomyocardial biopsies: Comparison to frozen tissue immunofluorescence. Appl. Immunohistochem. Mol. Morphol. 2004, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Fedrigo, M.; Gambino, A.; Tona, F.; Torregrossa, G.; Poli, F.; Benazzi, E.; Frigo, A.; Feltrin, G.; Toscano, G.; Caforio, A.P.; et al. Can C4d immunostaining on endomyocardial biopsies be considered a prognostic biomarker in heart transplant recipients? Correction in Transplantation 2010, 90, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Fedson, S.E.; Daniel, S.S.; Husain, A.N. Immunohistochemistry staining of C4d to diagnose antibody-mediated rejection in cardiac transplantation. J. Heart Lung Transplant. 2008, 27, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Moseley, E.L.; Atkinson, C.; Sharples, L.D.; Wallwork, J.; Goddard, M.J. Deposition of C4d and C3d in cardiac transplants: A factor in the development of coronary artery vasculopathy. J. Heart Lung Transplant. 2010, 29, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Poelzl, G.; Ullrich, R.; Huber, A.; Ulmer, H.; Antretter, H.; Hoefer, D.; Mairinger, T.; Laufer, G.; Pachinger, O.; Schwarzacher, S. Capillary deposition of the complement fragment C4d in cardiac allograft biopsies is associated with allograft vasculopathy. Transpl. Int. 2005, 18, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Garrett, H.E.; Duvall-Seaman, D.; Helsley, B.; Groshart, K. Treatment of vascular rejection with rituximab in cardiac transplantation. J. Heart Lung Transplant. 2005, 24, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.D.; Sokos, G.G.; Pidwell, D.J.; Smedira, N.G.; Gonzalez-Stawinski, G.V.; Taylor, D.O.; Starling, R.C.; Rodriguez, E.R. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am. J. Transplant. 2009, 9, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, N.B.; McMahon, J.T. Activation of intravascular macrophages within myocardial small vessels is a feature of acute vascular rejection in human heart transplants. J. Heart Lung Transplant. 1995, 14, 338–345. [Google Scholar] [PubMed]
- Holt, D.B.; Liapis, H.; Mohanakumar, T.; Phelan, D.R.; Gandi, S.K.; Huddleston, C.B.; Canter, C.E. Complement fragment C4d and C3d deposition in pediatric heart recipients with a positive crossmatch. J. Heart Lung Transplant. 2008, 27, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Quartel, A.W.; Czer, L.S.; Admon, D.; Chen, G.; Fishbein, M.C.; Schwieger, J.; Steiner, R.W.; Davis, C.; Tyan, D.B. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation 1998, 66, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.C.; Kobashigawa, J. Biopsy-negative cardiac transplant rejection: Etiology, diagnosis, and therapy. Curr. Opin. Cardiol. 2004, 19, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Isobe, M.; Kawauki, M.; Amano, J. Altered expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in acutely rejected myocardium and coronary arteriosclerosis in cardiac allografts of nonhuman primates. Transpl. Int. 2000, 13, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; van Almen, G.C.; Van Aelst, L.N.; Van Cleemput, J.; Droogne, W.; Jin, Y.; Van de Werf, F.; Carmeliet, P.; Vanhaecke, J. Matricellular proteins and matrix metalloproteinases mark the inflammatory and fibrotic response in human cardiac allograft rejection. Eur. Heart J. 2013, 34, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, M.; Ainasoja, O.; Lemström, K.B. Failing Heart Transplants and Rejection-A Cellular Perspective. J. Cardiovasc. Dev. Dis. 2021, 8, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otto, M.E.B.; Martins, A.M.A.; Campos Dall’Orto, A.O.M.; Leite, S.F.; de Queiroz Mauricio Filho, M.A.F.; Martins, N.T.; de Araújo, S.R.; Almeida, S.V.; Paiva, M.U.B.; Atik, F.A. Acute Cellular Rejection in Heart Transplant Patients: Insights of Global Longitudinal Strain, Myocardial Work, and an Exclusive Group of Chagas Disease. Front. Cardiovasc. Med. 2022, 9, 841698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bacal, F.; Marcondes-Braga, F.G.; Rohde, L.E.P.; Xavier, J.L.; Brito, F.D.S.; Moura, L.A.Z.; Colafranceschi, A.S.; Lavagnoli, C.F.R.; Gelape, C.L.; Almeida, D.R. 3a Diretriz Brasileira de Transplante Cardíaco. Arq. Bras. Cardiol. 2018, 111, 230–289. [Google Scholar] [PubMed]
- Söderlund, C.; Rådegran, G. Acute cellular rejection later than one year after heart transplantation: A single-center retrospective study at Skåne University Hospital in Lund 1988-2010. Clin. Transplant. 2017, 31, e12998. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Zerbe, T.; Kaufman, C.; Zeevi, A.; Kormos, R.; Hardesty, R.; Griffith, B.; Duquesnoy, R.J. Propagation of alloreactive lymphocytes from histologically negative endomyocardial biopsies from heart transplant patients. Association with subsequent histological evidence of allograft rejection. Transplantation 1989, 48, 430–435. [Google Scholar] [CrossRef] [PubMed]

| Parameter | n = 146 | |
|---|---|---|
| Mean age (years) | 56 ± 15.8 | |
| Median age | 39 years | |
| Gender | Males | 120 (82.2%) |
| Females | 26 (17.8%) | |
| Age (years) | <40 | 71 (48.6) |
| >40 | 75 (51.4%) | |
| Quilty effect | Absent | 128 (87.7%) |
| Subendocardial | 11 (7.5%) | |
| Endocardial | 7 (4.8%) | |
| Fibrosis | Absent | 31 (21.2%) |
| Mild | 62 (42.5%) | |
| Moderate | 17 (11.6%) | |
| Severe | 36 (24.7%) | |
| Histopathological diagnosis | ACR | 26 (17.8%) |
| Similar injuries unrelated to rejection | 66 (45.2%) | |
| Ischemia and reperfusion injuries | 37 (25.3%) | |
| AMR | 17 (11.7%) | |
| Vasculitis | Present | 39 (26.7%) |
| Absent | 107 (73.3%) | |
| Inflammation | Absent | 13 (8.9%) |
| Mild (1 focus) | 105 (71.9%) | |
| Moderate (2 foci) | 16 (11.0%) | |
| Severe (diffuse) | 12 (8.2%) | |
| Myocite damage | Degenerative lesions | 35 (24.0%) |
| Premiocitolisis | 40 (27.4%) | |
| Myocite necrosis, coagulative necrosis | 71 (48.6%) | |
| Intramyocardial small vessels endothelial damage | Edema | 49 (33.6%) |
| Inflammation | 21 (14.4%) | |
| Distrophic endothelial lesions | 47 (32.2%) | |
| Fibrosis | 29 (19.9%) | |
| AMR | Histologic and immunopathologic studies are both absent (pAMR0) | 129 (88.4%) |
| Histologic findings or immunopathologic findings are present (pAMR1) | 15 (10.3%) | |
| Histologic and immunopathologic findings are both present (pAMR2) | 0 (0.0%) | |
| Severe pathologic AMR (pAMR3) (interstitial hemorrhage, capillary fragmentation, mixed inflammatory infiltrates, pyknosis, karyorrhexis, edema | 2 (1.4%) | |
| ACR | Absent (0) | 120 (82.2%) |
| Mild (1R) | 19 (13.0%) | |
| Moderate (2R) | 6 (4.1%) | |
| Severe (3R) | 1 (0.7%) | |
| Evolution | Resolved ACR | 136 (93.2%) |
| Remissive ACR | 9 (6.2%) | |
| Continuous ACR | 1 (0.6%) | |
| CMV infection | Absent | 140 (95.9%) |
| Present | 6 (4.1%) |
| Parameter | ACR Absent | ACR Present | OR | CI | p-Value | |
|---|---|---|---|---|---|---|
| Sex | Male | 83.33% (100) | 16.67% (20) | 0.67 | 0.24–1.87 | 0.411 |
| Female | 76.92% (20) | 23.08% (6) | ||||
| Age | <40 | 84.50% (60) | 15.5% (11) | 0.73 | 0.31–1.73 | 0.522 |
| >40 | 80% (60) | 20% (15) | ||||
| Environment | Rural | 81.25% (65) | 18.75% (15) | 1.15 | 0.49–2.72 | 0.830 |
| Urban | 83.33% (55) | 16.67% (11) | ||||
| Diagnosis | Dilatative Cardiomyopathy | 87.09% (81) | 12.91% (12) | 0.41 | 0.17–0.98 | 0.046 |
| Restrictive Cardiomyopathy | 73.58% (39) | 26.42% (14) | ||||
| CMV infection | Male | 66.67% (4) | 33.33% (2) | 2.42 | 0.42–13.95 | 0.290 |
| Female | 82.85% (116) | 17.15% (24) | ||||
| Parameter | AMR Absent | AMR Present | OR | CI | p-Value | |
|---|---|---|---|---|---|---|
| Sex | Male | 90.83% (109) | 9.17% (11) | 0.34 | 0.11–1.01 | 0.083 |
| Female | 76.92% (20) | 23.08% (6) | ||||
| Age | <40 | 81.69% (58) | 18.31% (13) | 3.98 | 1.23–12.86 | 0.019 |
| >40 | 94.67% (71) | 5.33% (4) | ||||
| Environment | Rural | 85% (68) | 15% (12) | 2.15 | 0.72–6.46 | 0.200 |
| Urban | 92.42% (61) | 7.58% (5) | ||||
| Diagnosis | Dilatative Cardiomyopathy | 89.24% (83) | 10.76% (10) | 0.79 | 0.28–2.22 | 0.789 |
| Restrictive Cardiomyopathy | 86.79% (46) | 13.21% (7) | ||||
| CMV infection | Male | 50% (3) | 50% (3) | 9.0 | 1.66–48.92 | 0.021 |
| Female | 90% (126) | 10% (14) | ||||
| (a) | ||||||
| Parameter | Quilty Effect Absent | Quilty Effect Present | OR | CI | p-Value | |
| Sex | Male | 90% (108) | 10% (12) | 0.37 | 0.12–1.10 | 0.094 |
| Female | 76.92% (20) | 23.08% (6) | ||||
| Age | <40 | 83.09% (59) | 16.91% (12) | 2.34 | 0.83–6.62 | 0.132 |
| >40 | 92% (69) | 8% (6) | ||||
| Environment | Rural | 90% (72) | 10% (8) | 0.62 | 0.23–1.68 | 0.449 |
| Urban | 84.84% (56) | 15.16% (10) | ||||
| Diagnosis | Dilatative Cardiomyopathy | 94.16% (113) | 5.84% (7) | 0.08 | 0.03–0.25 | <0.001 |
| Restrictive Cardiomyopathy | 57.69% (15) | 42.31% (11) | ||||
| CMV infection | Male | 16.67% (1) | 83.33% (5) | 0.83 | 0.09–7.51 | <0.001 |
| Female | 14.28% (20) | 85.72% (120) | ||||
| (b) | ||||||
| Parameter | Quilty Effect Absent | Quilty Effect Present | OR | CI | p-Value | |
| Vasculitis | Absent | 94.39% (101) | 5.61% (6) | 0.13 | 0.045–0.38 | <0.001 |
| Present | 69.23% (27) | 30.77% (12) | ||||
| Inflammation | Absent | 80.48% (33) | 19.52% (8) | 2.3 | 0.84–6.32 | 0.158 |
| Present | 90.47% (95) | 9.53% (10) | ||||
| Myocyte damage | Absent | 85.71% (30) | 14.29% (5) | 1.26 | 0.42–3.82 | 0.769 |
| Present | 88.28% (98) | 11.72% (13) | ||||
| Intramyocardial small vessel endothelial damage | Absent | 87.75% (43) | 12.25% (6) | 0.99 | 0.35–2.82 | 1.000 |
| Present | 87.62% (85) | 12.38% (12) | ||||
| Parameter | CD20 Positive | CD20 Negative | OR | CI | p-Value | |
| Sex | Male | 10% (12) | 90% (108) | 0.37 | 0.12–1.10 | 0.094 |
| Female | 23.07% (6) | 76.93% (20) | ||||
| Age | <40 | 16.90% (12) | 83.1% (59) | 2.34 | 0.83–6.62 | 0.132 |
| >40 | 8% (6) | 92% (69) | ||||
| Parameter | CD4 Positive | CD4 Negative | OR | CI | p-Value | |
| Sex | Male | 16.67% (20) | 83.33% (100) | 0.45 | 0.17–1.18 | 0.106 |
| Female | 30.76% (8) | 69.24% (18) | ||||
| Age | <40 | 22.53% (16) | 77.47% (55) | 1.53 | 0.67–3.51 | 0.401 |
| >40 | 16% (12) | 84% (63) | ||||
| Parameter | CD8 Positive | CD8 Negative | OR | CI | p-Value | |
| Sex | Male | 16.67% (20) | 83.33% (100) | 0.45 | 0.17–1.18 | 0.106 |
| Female | 30.76% (8) | 69.24% (18) | ||||
| Age | <40 | 22.53% (16) | 77.47% (55) | 1.53 | 0.67–3.51 | 0.401 |
| >40 | 16% (12) | 84% (63) | ||||
| Parameter | CD68 Positive | CD68 Negative | OR | CI | p-Value | |
| Sex | Male | 22.5% (27) | 77.5% (93) | 0.79 | 0.30–2.08 | 0.616 |
| Female | 26.92% (7) | 73.08% (19) | ||||
| Age | <40 | 28.16% (20) | 71.84% (51) | 1.71 | 0.79–3.72 | 0.240 |
| >40 | 18.67% (14) | 81.33% (61) | ||||
| Parameter | CD31 Positive | CD31 Negative | OR | CI | p-Value | |
| Sex | Male | 16.67% (20) | 83.33% (100) | 0.54 | 0.20–1.45 | 0.265 |
| Female | 26.92% (7) | 73.08% (19) | ||||
| Age | <40 | 22.53% (16) | 77.47% (55) | 1.69 | 0.72–3.95 | 0.287 |
| >40 | 14.67% (11) | 85.33% (64) | ||||
| Parameter | CD34 Positive | CD34 Negative | OR | CI | p-Value | |
| Sex | Male | 18.33% (22) | 81.67% (98) | 0.61 | 0.23–1.63 | 0.415 |
| Female | 26.92% (7) | 73.08% (19) | ||||
| Age | <40 | 22.54% (16) | 77.46% (55) | 1.53 | 0.67–3.51 | 0.401 |
| >40 | 16% (12) | 84% (63) | ||||
| Parameter | Ig A Positive | Ig A Negative | OR | CI | p-Value | |
| Sex | Male | 16.67% (20) | 83.33% (100) | 0.54 | 0.20–1.45 | 0.265 |
| Female | 26.92% (7) | 73.08% (19) | ||||
| Age | <40 | 21.12% (15) | 78.88% (56) | 1.41 | 0.61–3.27 | 0.523 |
| >40 | 16% (12) | 84% (63) | ||||
| Parameter | Ig M Positive | Ig M Negative | OR | CI | p-Value | |
| Sex | Male | 15.83% (19) | 84.17% (101) | 0.51 | 0.19–1.38 | 0.255 |
| Female | 26.92% (7) | 73.08% (19) | ||||
| Age | <40 | 26.78% (15) | 73.22% (56) | 1.56 | 0.66–3.67 | 0.388 |
| >40 | 14.67% (11) | 85.33% (64) | ||||
| Parameter | EGFR Positive | EGFR Negative | OR | CI | p-Value | |
| Sex | Male | 14.17% (17) | 85.83% (103) | 0.91 | 0.28–2.97 | 1.000 |
| Female | 15.38% (4) | 84.62% (22) | ||||
| Age | <40 | 15.49% (11) | 84.51% (60) | 1.19 | 0.47–3.00 | 0.815 |
| >40 | 13.33% (10) | 86.67% (65) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrichi, G.; Satala, C.-B.; Patrichi, A.I.; Voidăzan, T.S.; Tomuț, A.-N.; Mihalache, D.; Sin, A.I. Cellular Rejection Post-Cardiac Transplantation: A 13-Year Single Unicentric Study. Medicina 2025, 61, 1317. https://doi.org/10.3390/medicina61081317
Patrichi G, Satala C-B, Patrichi AI, Voidăzan TS, Tomuț A-N, Mihalache D, Sin AI. Cellular Rejection Post-Cardiac Transplantation: A 13-Year Single Unicentric Study. Medicina. 2025; 61(8):1317. https://doi.org/10.3390/medicina61081317
Chicago/Turabian StylePatrichi, Gabriela, Catalin-Bogdan Satala, Andrei Ionut Patrichi, Toader Septimiu Voidăzan, Alexandru-Nicușor Tomuț, Daniela Mihalache, and Anca Ileana Sin. 2025. "Cellular Rejection Post-Cardiac Transplantation: A 13-Year Single Unicentric Study" Medicina 61, no. 8: 1317. https://doi.org/10.3390/medicina61081317
APA StylePatrichi, G., Satala, C.-B., Patrichi, A. I., Voidăzan, T. S., Tomuț, A.-N., Mihalache, D., & Sin, A. I. (2025). Cellular Rejection Post-Cardiac Transplantation: A 13-Year Single Unicentric Study. Medicina, 61(8), 1317. https://doi.org/10.3390/medicina61081317






