Association Between ABO or Rh Blood Groups and Chikungunya Virus Infection: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Registered Protocol
2.2. Search Strategy
2.3. Selection of Studies
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Analysis
3. Results
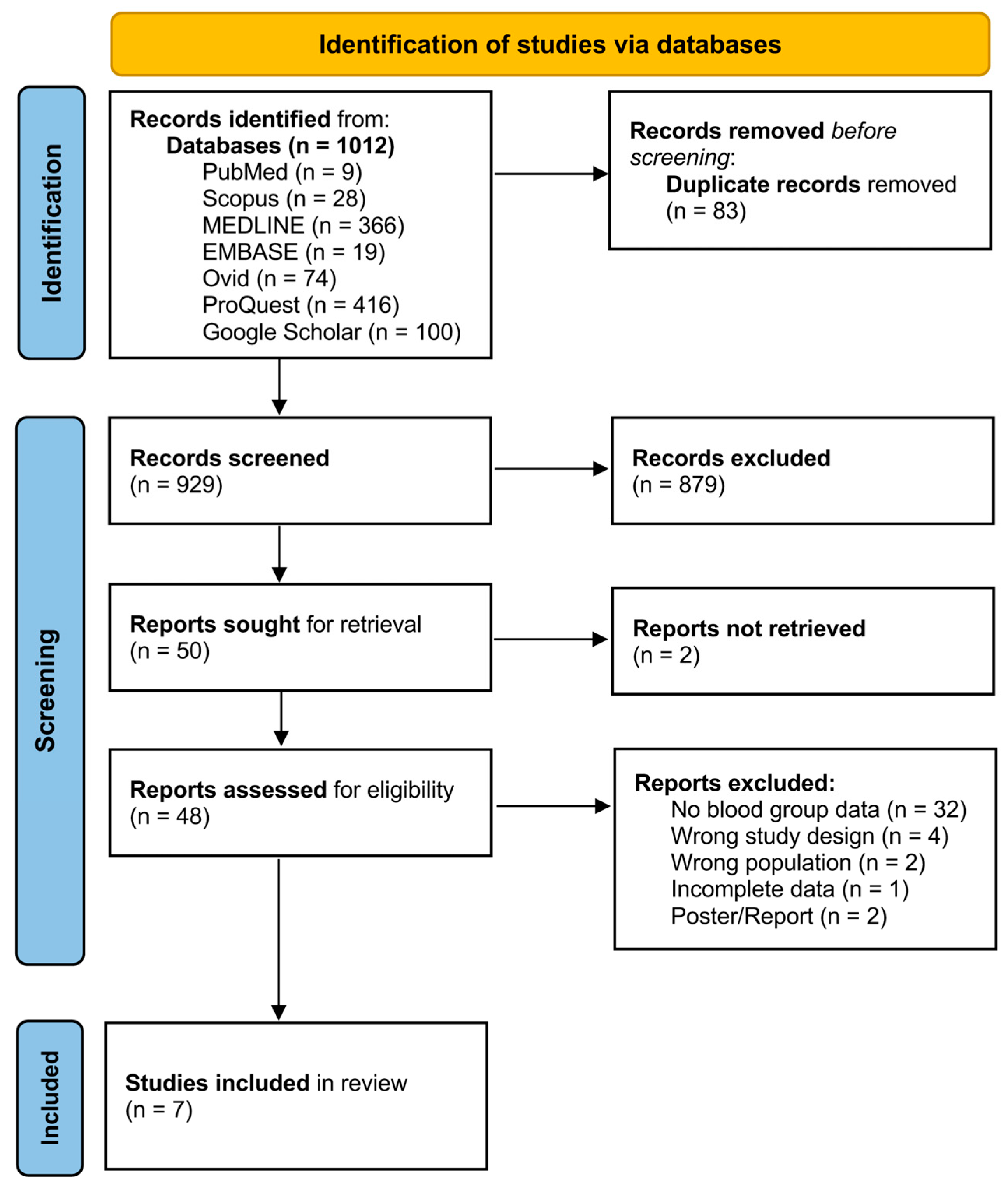
3.1. Search Results
3.2. Characteristics of the Included Studies
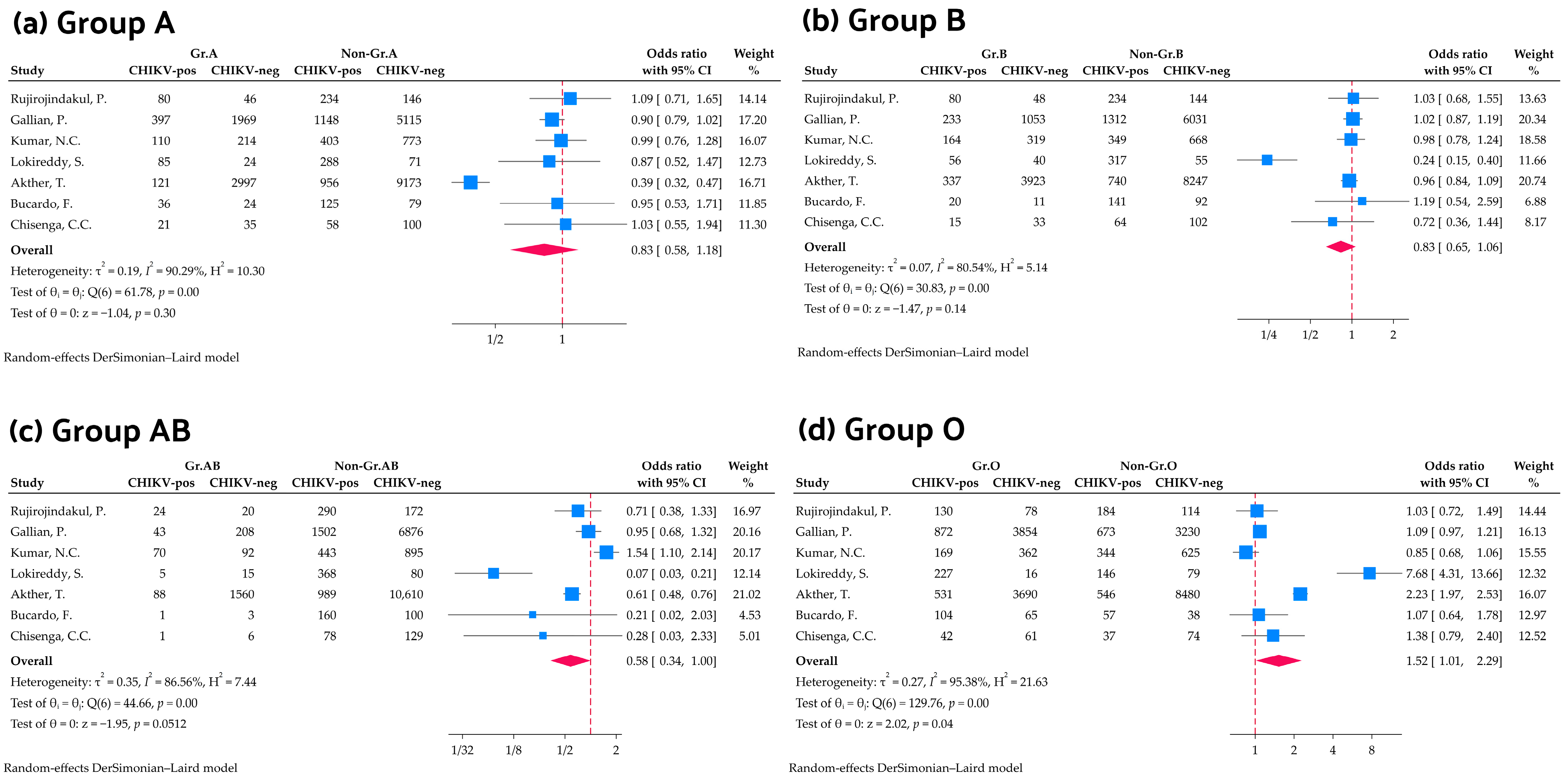
3.3. Association Between ABO Blood Group and Chikungunya Virus Infection
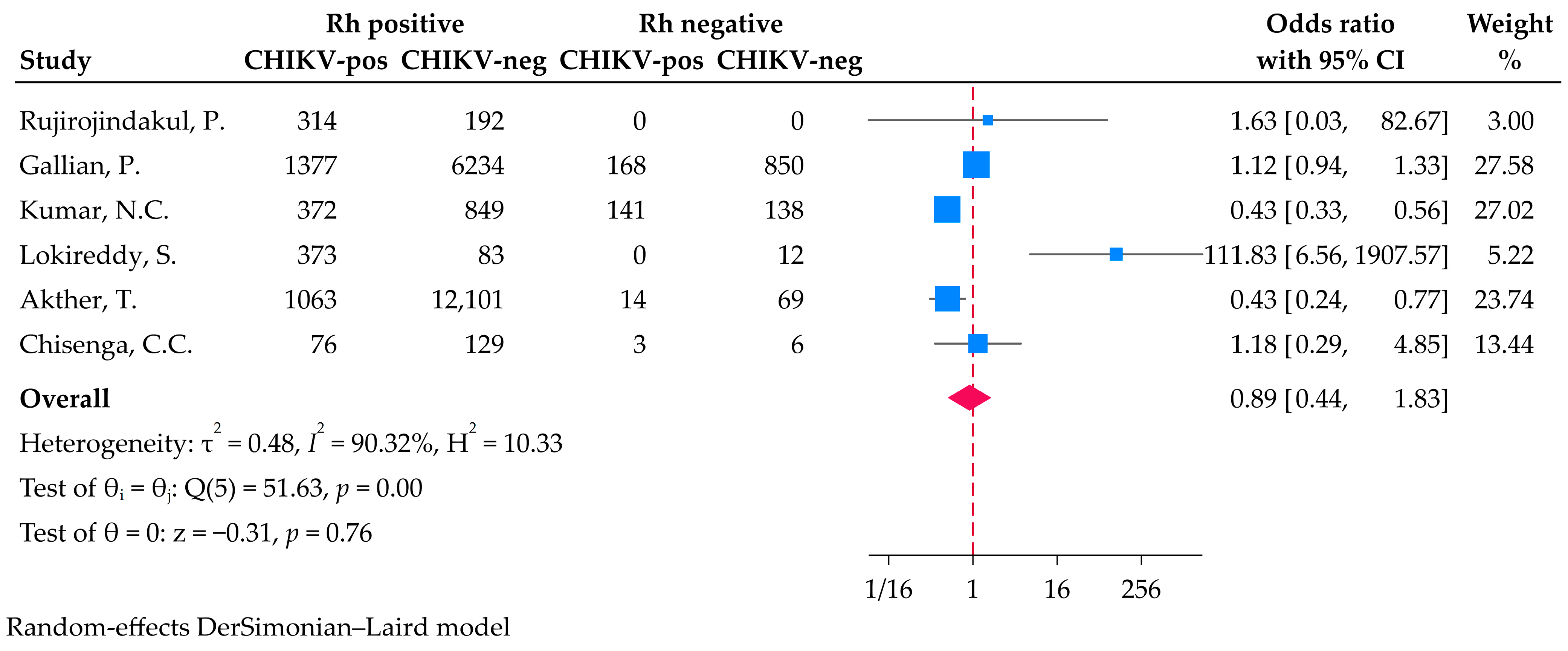
3.4. Association Between Rh Blood Group and Chikungunya Virus Infection
3.5. Subgroup Analysis
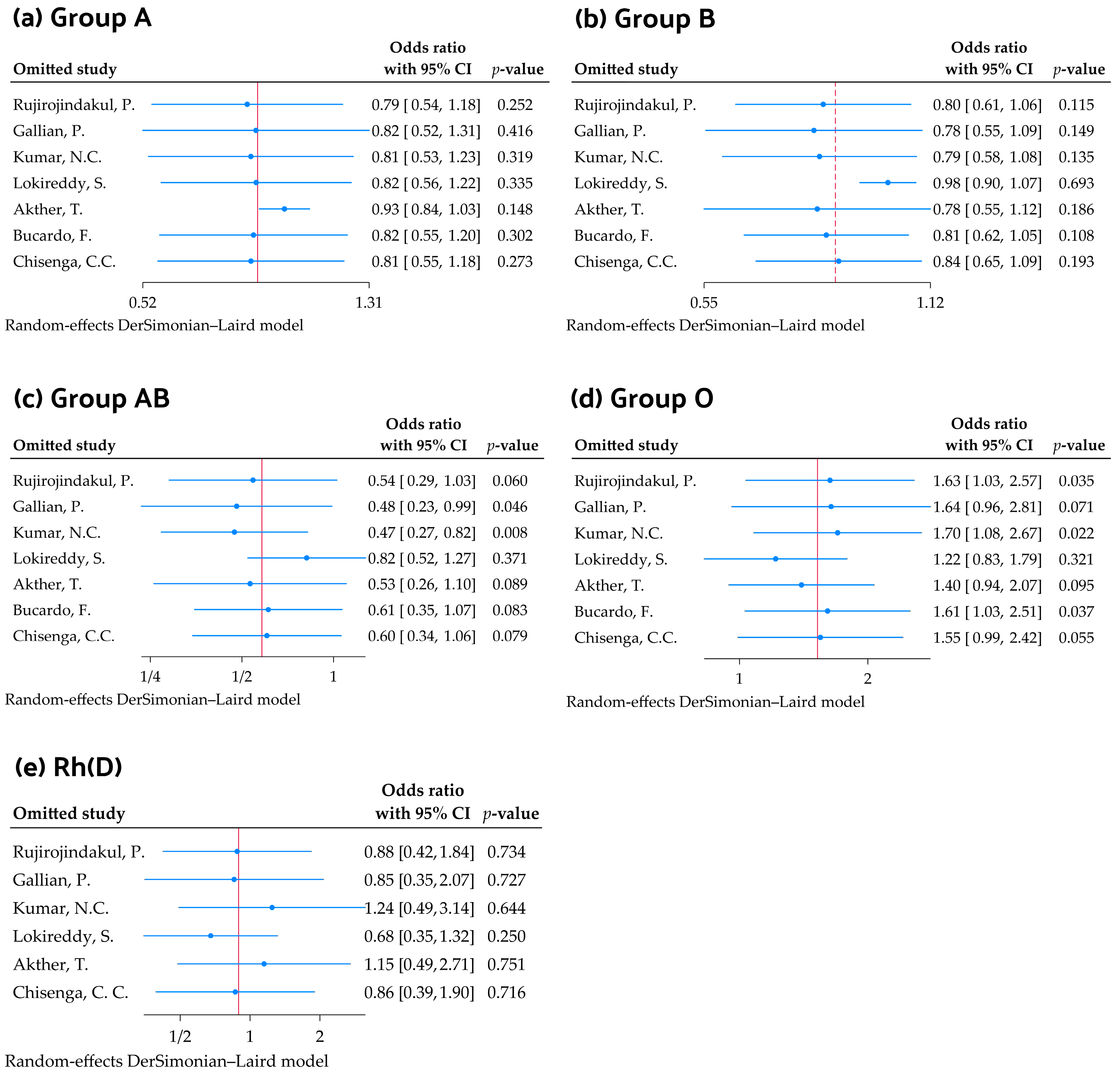
3.6. Sensitivity Analysis
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| CHIKV | Chikungunya virus |
| CI | Confidence interval |
| I2 | Inconsistency index |
| MeSH | Medical Subject Headings |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OR | Odds ratio |
| PEO | Patient, Exposure, and Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Rh | Rhesus |
| SARS-CoV-1 | Severe acute respiratory syndrome coronavirus 1 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Bettis, A.A.; L’Azou Jackson, M.; Yoon, I.K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Neglected Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Thiberville, S.D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; de Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir. Res. 2013, 99, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chikungunya Epidemiology Update–June 2025; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/publications/m/item/chikungunya-epidemiology-update-june-2025 (accessed on 15 July 2025).
- Renault, P.; Solet, J.L.; Sissoko, D.; Balleydier, E.; Larrieu, S.; Filleul, L.; Lassalle, C.; Thiria, J.; Rachou, E.; de Valk, H.; et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am. J. Trop. Med. Hyg. 2007, 77, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Ali Mude, A.S.; Nageye, Y.A.; Bello, K.E. Current Epidemiological Status of Chikungunya Virus Infection in East Africa: A Systematic Review and Meta-Analysis. J. Trop. Med. 2024, 2024, 7357911. [Google Scholar] [CrossRef] [PubMed]
- Mowatt, L.; Jackson, S.T. Chikungunya in the Caribbean: An Epidemic in the Making. Infect. Dis. Ther. 2014, 3, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Curlier, E.; Fagour, L.; Herrmann-Storck, C.; Staelen, A.; Vingadassalom, I.; Breurec, S.; Abel, S.; Pierre-François, S.; Jean-Marie, J.; Laouénan, C.; et al. Seroprevalence of chikungunya virus infection among HIV-infected adults in French Caribbean Islands of Martinique and Guadeloupe in 2015: A cross-sectional study. PLoS Neglected Trop. Dis. 2021, 15, e0009267. [Google Scholar] [CrossRef] [PubMed]
- Balmaseda, A.; Gordon, A.; Gresh, L.; Ojeda, S.; Saborio, S.; Tellez, Y.; Sanchez, N.; Kuan, G.; Harris, E. Clinical Attack Rate of Chikungunya in a Cohort of Nicaraguan Children. Am. J. Trop. Med. Hyg. 2016, 94, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. Molecular surveillance of arboviruses circulation and co-infection during a large chikungunya virus outbreak in Thailand, October 2018 to February 2020. Sci. Rep. 2022, 12, 22323. [Google Scholar] [CrossRef] [PubMed]
- Alagarasu, K.; Patil, J.; Jadhav, S.; Chowdhury, D.; Bote, M.; Punekar, M.; More, A.; Kakade, M.; Bachal, R.; Gurav, Y.; et al. Understanding the resurgence of chikungunya virus during 2020–2021 in Pune, India, based on genomic analyses: A seven year study. J. Med. Virol. 2023, 95, e29253. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Taslem Mourosi, J.; Khan, M.F.; Ullah, M.O.; Vanakker, O.M.; Hosen, M.J. Chikungunya outbreak in Bangladesh (2017): Clinical and hematological findings. PLoS Neglected Trop. Dis. 2020, 14, e0007466. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.A.; Parikh, R.; Grandi, S.M.; Ray, J.G.; Cohen, E. ABO and Rh blood groups and risk of infection: Systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 797. [Google Scholar] [CrossRef] [PubMed]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, G.; Chui, C.H.; Lau, F.Y.; Chan, P.K.; Ng, M.H.; Sung, J.J.; Wong, R.S. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005, 293, 1450–1451. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, S.B. Human ABO Blood Groups and Their Associations with Different Diseases. BioMed Res. Int. 2021, 2021, 6629060. [Google Scholar] [CrossRef] [PubMed]
- Aninagyei, E.; Agbenowoshie, P.S.; Akpalu, P.M.; Asiewe, S.B.; Menu, R.Y.; Gbadago, F.; Asmah, R.H. ABO and Rhesus blood group variability and their associations with clinical malaria presentations. Malar. J. 2024, 23, 257. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xue, L.; Gao, J.; Wu, A.; Kou, X. ABO blood group-associated susceptibility to norovirus infection: A systematic review and meta-analysis. Infect. Genet. Evol. 2020, 81, 104245. [Google Scholar] [CrossRef] [PubMed]
- Oladeinde, B.H.; Olaniyan, M.F.; Muhibi, M.A.; Uwaifo, F.; Richard, O.; Omabe, N.O.; Daud, A.; Ozolua, O.P. Association between ABO and RH blood groups and Hepatitis B virus infection among young Nigerian adults. J. Prev. Med. Hyg. 2022, 63, E109–E114. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Schull, M.J.; Vermeulen, M.J.; Park, A.L. Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness: A Population-Based Cohort Study. Ann. Intern. Med. 2021, 174, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.C.V.M.; Nadimpalli, M.; Vardhan, V.R.; Gopal, S.D.V.R. Association of ABO blood groups with Chikungunya virus. Virol. J. 2010, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Akther, T.; Karim, M.N.; Akther, T.; Munshi, S.U. Investigating the Association Between ABO Blood Groups and Rhesus Factors with Dengue and Chikungunya Virus Infections During the 2017 Outbreak in Bangladesh. Vector Borne Zoonotic Dis. 2024, 24, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Rujirojindakul, P.; Chongsuvivatwong, V.; Limprasert, P. Association of ABO Blood Group Phenotype and Allele Frequency with Chikungunya Fever. Adv. Hematol. 2015, 2015, 543027. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid. Based Healthc. 2015, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Gallian, P.; Leparc-Goffart, I.; Richard, P.; Maire, F.; Flusin, O.; Djoudi, R.; Chiaroni, J.; Charrel, R.; Tiberghien, P.; de Lamballerie, X. Epidemiology of Chikungunya Virus Outbreaks in Guadeloupe and Martinique, 2014: An Observational Study in Volunteer Blood Donors. PLoS Neglected Trop. Dis. 2017, 11, e0005254. [Google Scholar] [CrossRef] [PubMed]
- Lokireddy, S.; Sarojamma, V.; Ramakrishna, V. Genetic predisposition to Chikungunya—A blood group study in Chikungunya affected families. Virol. J. 2009, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Bucardo, F.; Reyes, Y.; Morales, M.; Briceño, R.; González, F.; Lundkvist, Å.; Svensson, L.; Nordgren, J. Association of Genetic Polymorphisms in DC-SIGN, Toll-Like Receptor 3, and Tumor Necrosis Factor α Genes and the Lewis-Negative Phenotype with Chikungunya Infection and Disease in Nicaragua. J. Infect. Dis. 2021, 223, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Chisenga, C.C.; Bosomprah, S.; Musukuma, K.; Mubanga, C.; Chilyabanyama, O.N.; Velu, R.M.; Kim, Y.C.; Reyes-Sandoval, A.; Chilengi, R. Sero-prevalence of arthropod-borne viral infections among Lukanga swamp residents in Zambia. PLoS ONE 2020, 15, e0235322. [Google Scholar] [CrossRef] [PubMed]
- Zerihun, T.; Degarege, A.; Erko, B. Association of ABO blood group and Plasmodium falciparum malaria in Dore Bafeno Area, Southern Ethiopia. Asian Pac. J. Trop. Biomed. 2011, 1, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Cruciani, M.; Mengoli, C.; Marano, G.; Candura, F.; Lopez, N.; Pati, I.; Pupella, S.; De Angelis, V. ABO blood group and COVID-19: An updated systematic literature review and meta-analysis. Blood Transfus. 2021, 19, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Ramya, S.R.; Kanungo, R. Association of ABO blood groups with dengue fever and its complications in a tertiary care hospital. J. Lab. Physicians 2019, 11, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Shanthamurthy, C.D.; Jain, P.; Yehuda, S.; Monteiro, J.T.; Leviatan Ben-Arye, S.; Subramani, B.; Lepenies, B.; Padler-Karavani, V.; Kikkeri, R. ABO Antigens Active Tri- and Disaccharides Microarray to Evaluate C-type Lectin Receptor Binding Preferences. Sci. Rep. 2018, 8, 6603. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Capra, F.; Targher, G.; Montagnana, M.; Lippi, G. Relationship between ABO blood group and von Willebrand factor levels: From biology to clinical implications. Thromb. J. 2007, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Sticchi Damiani, A.; Zizza, A.; Banchelli, F.; Gigante, M.; De Feo, M.L.; Ostuni, A.; Marinelli, V.; Quagnano, S.; Negro, P.; Di Renzo, N.; et al. Association between ABO blood groups and SARS-CoV-2 infection in blood donors of Puglia region. Ann. Hematol. 2023, 102, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Farkas, T.; Zhong, W.; Tan, M.; Thornton, S.; Morrow, A.L.; Jiang, X. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 2005, 79, 6714–6722. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Y.; Tan, M. Histo-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategy. Emerg. Microbes Infect. 2017, 6, e22. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.C.; Brewer-Jensen, P.D.; Mallory, M.L.; Jensen, K.; Yount, B.L.; Costantini, V.; Collins, M.H.; Edwards, C.E.; Sheahan, T.P.; Vinjé, J.; et al. Virus-Host Interactions Between Nonsecretors and Human Norovirus. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Alkout, A.M.; Blackwell, C.C.; Weir, D.M. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J. Infect. Dis. 2000, 181, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.; Fernandes, Â.; Santos-Pereira, B.; Azevedo, C.M.; Pinho, S.S. Glycans as a key factor in self and nonself discrimination: Impact on the breach of immune tolerance. FEBS Lett. 2022, 596, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Cruse, J.M.; Lewis, R.E.; Wang, H.; Schreuder, G.M.T.; Marsh, S.G.E.; Kennedy, L.J. Immunohematology and Transfusion Medicine. In Immunology Guidebook; Cruse, J.M., Lewis, R.E., Wang, H., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 431–443. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Garner, P.; Donegan, S. Interpretation of subgroup analyses in systematic reviews: A tutorial. Clin. Epidemiol. Glob. Health 2018, 7, 192–198. [Google Scholar] [CrossRef]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.J.; Mentzer, A.; Knight, J.C. Host genetics and infectious disease: New tools, insights and translational opportunities. Nat. Rev. Genet. 2021, 22, 137–153. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattanapan, Y.; Chulrik, W.; Rasaratnam, K.; Duangchan, T. Association Between ABO or Rh Blood Groups and Chikungunya Virus Infection: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1316. https://doi.org/10.3390/medicina61081316
Rattanapan Y, Chulrik W, Rasaratnam K, Duangchan T. Association Between ABO or Rh Blood Groups and Chikungunya Virus Infection: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(8):1316. https://doi.org/10.3390/medicina61081316
Chicago/Turabian StyleRattanapan, Yanisa, Wanatsanan Chulrik, Karunaithas Rasaratnam, and Thitinat Duangchan. 2025. "Association Between ABO or Rh Blood Groups and Chikungunya Virus Infection: A Systematic Review and Meta-Analysis" Medicina 61, no. 8: 1316. https://doi.org/10.3390/medicina61081316
APA StyleRattanapan, Y., Chulrik, W., Rasaratnam, K., & Duangchan, T. (2025). Association Between ABO or Rh Blood Groups and Chikungunya Virus Infection: A Systematic Review and Meta-Analysis. Medicina, 61(8), 1316. https://doi.org/10.3390/medicina61081316