Visceral Arterial Pseudoaneurysms—A Clinical Review
Abstract
1. Introduction
1.1. Definition
1.2. Epidemiology
1.2.1. Incidence
1.2.2. Demographics
1.2.3. Complications
1.3. Etiology
1.4. Presentation
1.5. Diagnosis
1.5.1. Overview
1.5.2. Computed Tomography (CT) and CTA
1.5.3. DSA
1.5.4. Doppler Ultrasound
1.5.5. MRA
1.5.6. Newer Modalities
1.6. Management
1.6.1. Overview
1.6.2. Endovascular Approach
1.6.3. Percutaneous Approach
1.6.4. Endoscopic Ultrasonography
1.6.5. Open Surgery
1.6.6. Morbidity
Ischemia
Coil Migration
Contrast-Induced Nephropathy (CIN)
Non-Target Embolization
Stent Thrombosis
1.6.7. Literature Search and Review Framework
2. Foregut
2.1. Coeliac Artery
2.1.1. Epidemiology
2.1.2. Etiology
2.1.3. Presentation
2.1.4. Diagnosis
2.1.5. Management
2.2. Left Gastric Artery
2.2.1. Epidemiology
2.2.2. Etiology
2.2.3. Presentation
2.2.4. Diagnosis
2.2.5. Management
2.3. Esophageal Artery
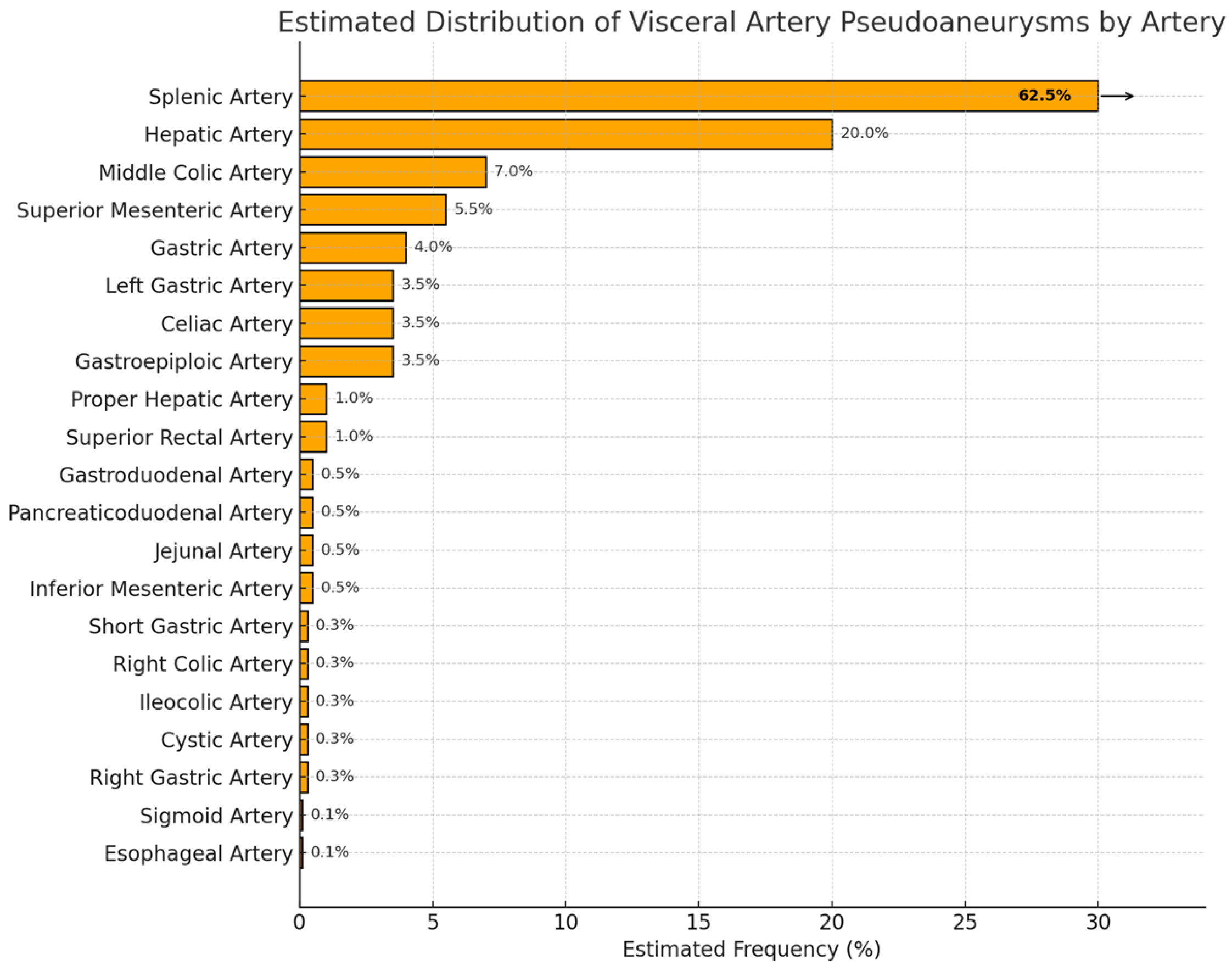
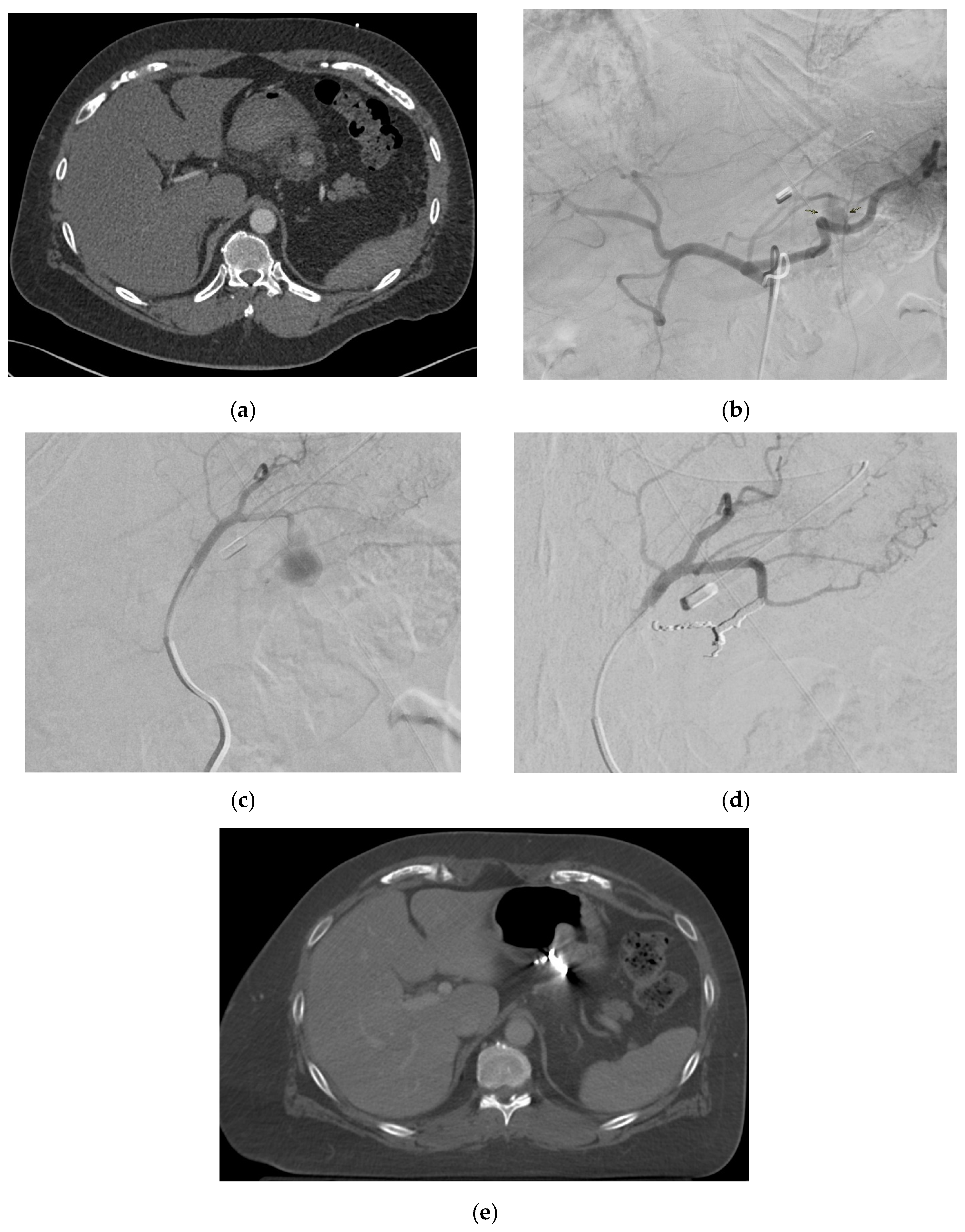
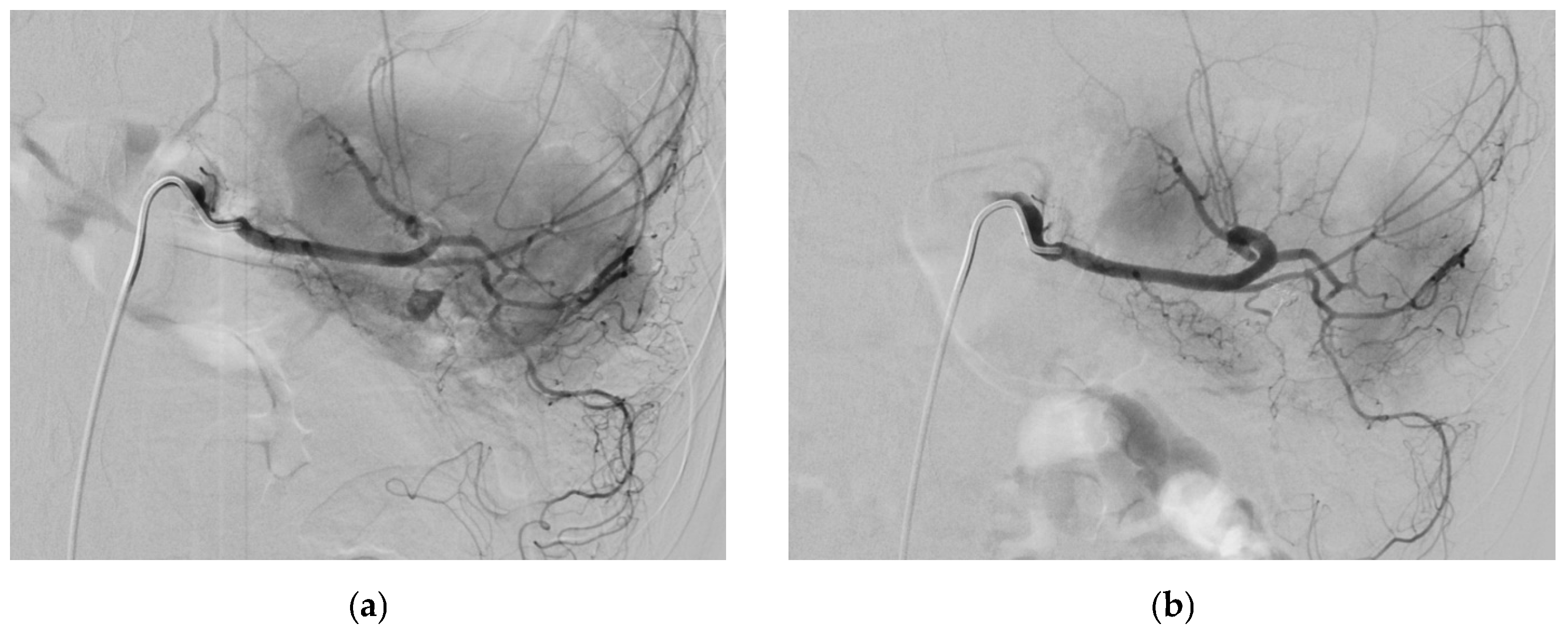
2.4. Splenic Artery
2.4.1. Epidemiology
2.4.2. Etiology
2.4.3. Presentation
2.4.4. Diagnosis
2.4.5. Management
2.5. Left Gastroepiploic Artery
2.6. Short Gastric Artery
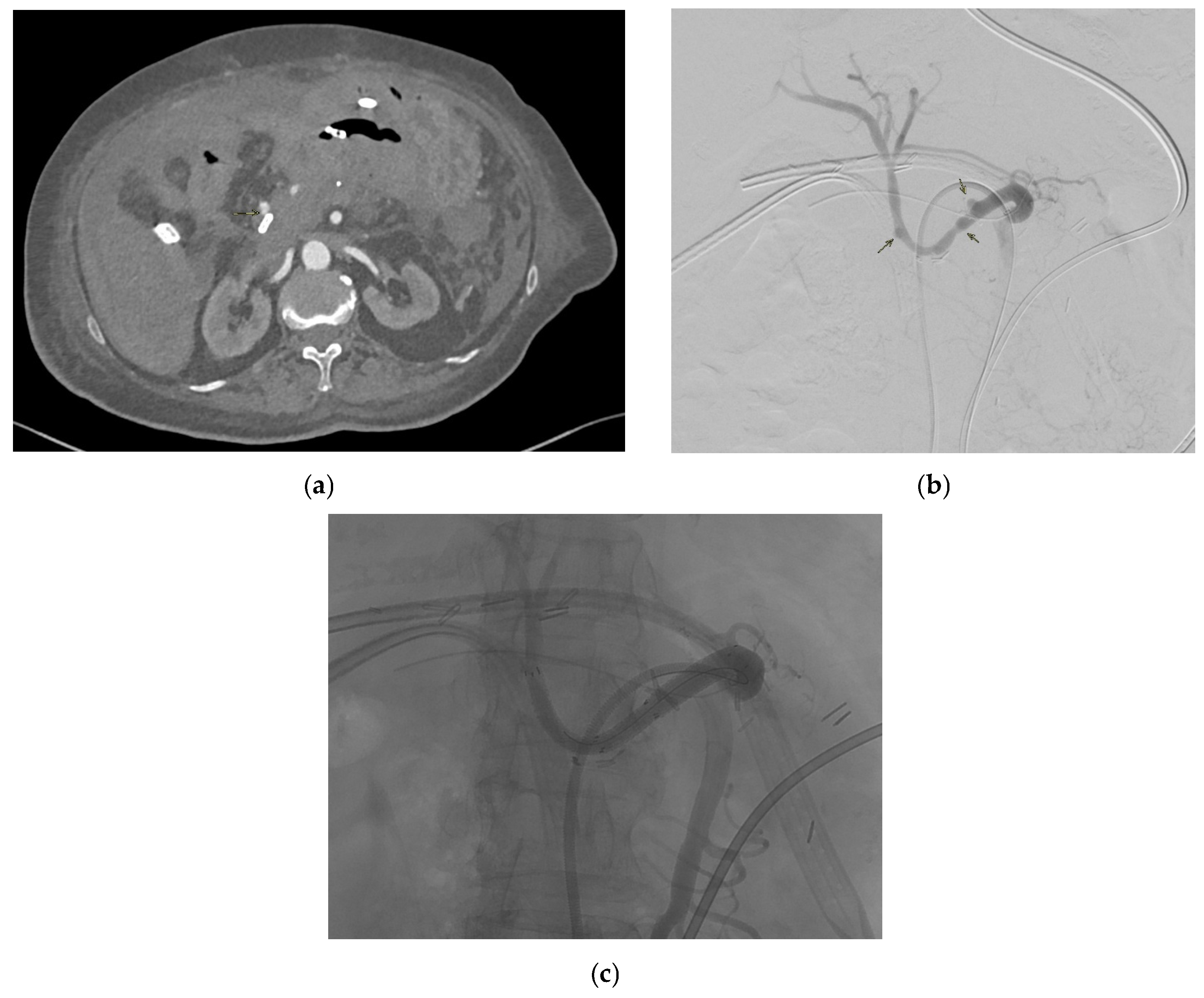
2.7. Common Hepatic Artery
2.7.1. Epidemiology
2.7.2. Etiology
2.7.3. Presentation
2.7.4. Diagnosis
2.7.5. Management
2.8. Proper Hepatic Artery
2.9. Right Gastric Artery
2.10. Right and Left Hepatic Arteries
2.10.1. Epidemiology
2.10.2. Etiology
2.10.3. Presentation
2.10.4. Diagnosis
2.10.5. Management
2.11. Cystic Artery
2.11.1. Epidemiology
2.11.2. Etiology
2.11.3. Presentation
2.11.4. Diagnosis
2.11.5. Management
2.12. Gastroduodenal Artery
2.12.1. Epidemiology
2.12.2. Etiology
2.12.3. Presentation
2.12.4. Diagnosis
2.12.5. Management
2.13. Right Gastroepiploic Artery
2.13.1. Epidemiology
2.13.2. Etiology
2.13.3. Presentation
2.13.4. Diagnosis
2.13.5. Management
2.14. Superior Pancreaticoduodenal Artery
2.14.1. Epidemiology
2.14.2. Etiology
2.14.3. Presentation
2.14.4. Diagnosis
2.14.5. Management
3. Midgut
3.1. Superior Mesenteric Artery
3.1.1. Epidemiology
3.1.2. Etiology
3.1.3. Presentation
3.1.4. Diagnosis
3.1.5. Management
3.2. Inferior Mesenteric Artery
3.2.1. Epidemiology
3.2.2. Etiology
3.2.3. Presentation
3.2.4. Diagnosis
3.2.5. Management
3.3. Jejunal Artery
3.3.1. Epidemiology
3.3.2. Etiology
3.3.3. Presentation
3.3.4. Diagnosis
3.3.5. Management
3.4. Ileal Artery
3.5. Middle Colic Arteries
3.6. Right Colic Arteries
3.7. Superior Mesenteric Artery
3.7.1. Epidemiology
3.7.2. Etiology
3.7.3. Presentation
3.7.4. Diagnosis
3.7.5. Management
4. Hindgut
4.1. Inferior Mesenteric Artery
4.1.1. Epidemiology
4.1.2. Etiology
4.1.3. Presentation
4.1.4. Diagnosis
4.1.5. Management
4.2. Left Colic Artery
4.2.1. Epidemiology
4.2.2. Etiology
4.2.3. Presentation
4.2.4. Diagnosis
4.2.5. Management
4.3. Sigmoid Artery
4.3.1. Epidemiology
4.3.2. Etiology
4.3.3. Presentation
4.3.4. Diagnosis
4.3.5. Management
4.4. Superior Rectal Artery
4.4.1. Epidemiology
4.4.2. Etiology
4.4.3. Presentation
4.4.4. Diagnosis
4.4.5. Management
5. Limitations and Future Perspectives
5.1. Limitations
5.2. Future Perspectives
5.2.1. Investigations
5.2.2. Management
5.2.3. Ethical and Legal Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CAA | celiac artery aneurysm |
| CAP | cystic artery pseudoaneurysm |
| CAPA | celiac artery pseudoaneurysm |
| CEUS | contrast-enhanced ultrasound |
| CIN | contrast induced nephropathy |
| CRP | c-reactive protein |
| CT | computed tomography |
| CTA | computed tomography angiography |
| CTE | computed tomography enterography |
| DECT | dual-energy CT |
| EAP | esophageal artery pseudoaneurysm |
| EUS | endoscopic ultrasonography |
| GAP | gastric artery pseudoaneurysm |
| GADP | gastroduodenal artery pseudoaneurysm |
| GEAA | gastroepiploic artery aneurysm |
| GEAP | gastroepiploic artery pseudoaneurysm |
| GFR | glomerular filtration rate |
| ICG | indocyanine green |
| IMA | inferior mesenteric artery |
| IPAP | inferior pancreaticoduodenal artery pseudoaneurysm |
| LGAP | left gastric artery pseudoaneurysm |
| MALS | median arcuate ligament syndrome |
| MCAP | middle colic artery pseudoaneurysm |
| MRA | magnetic resonance angiography |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| DSA | digital subtraction angiography |
| RCAP | right colic artery pseudoaneurysm |
| RGAP | right gastric artery pseudoaneurysm |
| SAA | splenic artery aneurysm |
| SAM | segmental arterial mediolysis |
| SAPA | splenic artery pseudoaneurysm |
| SMAP | superior mesenteric artery pseudoaneurysm |
| SPAP | superior pancreaticoduodenal artery pseudoaneurysm |
| SRAP | superior rectal artery pseudoaneurysm |
| TAE | transarterial embolization |
| VAPA | visceral arterial pseudoaneurysm |
| WBC | white blood cell |
References
- Hordiychuk, A.; Mehanna, D. Spontaneous rupture of splenic artery pseudoaneurysm. J. Surg. Case Rep. 2022, 2022, rjac604. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.C.; Sumpio, B.E. Visceral Artery Aneurysms and Pseudoaneurysms—Should They All be Managed by Endovascular Techniques? Ann. Vasc. Dis. 2013, 6, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Shanley, C.J.; Shah, N.L.; Messina, L.M. Uncommon splanchnic artery aneurysms: Pancreaticoduodenal, gastroduodenal, superior mesenteric, inferior mesenteric, and colic. Ann. Vasc. Surg. 1996, 10, 506–515. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, M.J.; Raa, S.T.; Hendriks, J.M.; Verhagen, H.J. Visceral aneurysms: Old paradigms, new insights? Best Pract. Res. Clin. Gastroenterol. 2017, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Hassan, S.; Abdou, R.; Torbey, E.; Alkaied, H.; Maniatis, T.; Azab, B.; Chalhoub, M.; Harris, K. Gastroduodenal artery aneurysm, diagnosis, clinical presentation and management: A concise review. Ann. Surg. Innov. Res. 2013, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Chadha, M.; Ahuja, C. Visceral artery aneurysms: Diagnosis and percutaneous management. Semin. Interv. Radiol. 2009, 26, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Saluja, S.S.; Ray, S.; Gulati, M.S.; Pal, S.; Sahni, P.; Chattopadhyay, T.K. Acute cholecystitis with massive upper gastrointestinal bleed: A case report and review of the literature. BMC Gastroenterol. 2007, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.L.; Kan, W.K.; Cheng, S.C. Mycotic cystic artery pseudoaneurysm successfully treated with transcatheter arterial embolisation. Hong Kong Med. J. 2010, 16, 156–157. [Google Scholar] [PubMed]
- Chong, J.J.; O’Connell, T.; Munk, P.L.; Yang, N.; Harris, A.C. Case of the month #176: Pseudoaneurysm of the cystic artery. Can. Assoc. Radiol. J. 2012, 63, 153–155. [Google Scholar] [CrossRef] [PubMed]
- De Molla Neto, O.L.; Ribeiro, M.A.; Saad, W.A. Pseudoaneurysm of cystic artery after laparoscopic cholecystectomy. HPB 2006, 8, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Marusca, G.; Yeddi, A.; Kiwan, W.; Al Masalmeh, N.; Newberger, S.; Kakos, R.; Ehrinpreis, M. A Unique Case of Severe Hematochezia: Ruptured Pseudoaneurysm of the Superior Rectal Artery. ACG Case Rep. J. 2020, 7, e00468. [Google Scholar] [CrossRef] [PubMed]
- Johal, M.; Kalaravy, M.; Ali, F.; Barve, R.; Ahmed, A.; Francis, C.T.; Harky, A. Evolving Diagnostic and Therapeutic Options for Visceral Artery Aneurysms. Ann. Vasc. Surg. 2021, 76, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, P.; Malas, M.B.; Nejim, B.; Haddad, A.; Morrow, A.; Ponce, O.; Hasan, B.; Seisa, M.; Chaer, R.; Murad, M.H. A systematic review and meta-analysis of the management of visceral artery aneurysms. J. Vasc. Surg. 2019, 70, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Jesinger, R.A.; Thoreson, A.A.; Lamba, R. Abdominal and pelvic aneurysms and pseudoaneurysms: Imaging review with clinical, radiologic, and treatment correlation. Radiographics 2013, 33, E71–E96. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Fishman, E.K.; Johnson, P.T. Arterial pseudoaneurysms complicating pancreatitis: Literature review. J. Comput. Assist. Tomogr. 2015, 39, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Regus, S.; Lang, W. Rupture Risk and Etiology of Visceral Artery Aneurysms and Pseudoaneurysms: A Single-Center Experience. Vasc. Endovasc. Surg. 2016, 50, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Giannotti, G.; Harris, E.; Hamblin, M.; Blecha, M. Massive Gastroepiploic Artery Pseudoaneurysm Presenting as Arterial Enteric Fistula. Ann. Vasc. Surg. 2021, 75, e531–e534. [Google Scholar] [CrossRef] [PubMed]
- Nagar, N.; Dubale, N.; Jagadeesh, R.; Nag, P.; Reddy, N.D.; Rao, G. Unusual locations of pseudo aneurysms as a sequel of chronic pancreatitis. J. Interv. Gastroenterol. 2011, 1, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Corvino, F.; Giurazza, F.; Ierardi, A.M.; Lucatelli, P.; Basile, A.; Corvino, A.; Niola, R. Splenic Artery Pseudoaneurysms: The Role of ce-CT for Diagnosis and Treatment Planning. Diagnostics 2022, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, S.A.; Gülcü, A.; Yılmaz, E.; Çiftçi, T.; Göktay, A.Y. Endovascular Stent-Graft Treatment of Giant Celiac Artery Pseudoaneurysm. Pol. J. Radiol. 2015, 80, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Kharel, S.; Shrestha, S.; Gyawali, N.; Maharjan, N.; Thapa, A.; Kandel, B.P.; Lakhey, P.J. Common hepatic artery pseudoaneurysm: A rare complication following total gastrectomy: A case report. Int. J. Surg. Case Rep. 2021, 81, 105816. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Tinelli, G.; Porcu, P.; Aubert, A.; Thony, F.; Magne, J.L. Treatment of visceral artery aneurysms: Description of a retrospective series of 42 aneurysms in 34 patients. Ann. Vasc. Surg. 2004, 18, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Binetti, M.; Lauro, A.; Golfieri, R.; Vaccari, S.; D’Andrea, V.; Marino, I.R.; Cervellera, M.; Renzulli, M.; Tonini, V. False in Name Only-Gastroduodenal Artery Pseudoaneurysm in a Recurrently Bleeding Patient: Case Report and Literature Review. Dig. Dis. Sci. 2019, 64, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, M.; Xu, J.H.; Kaard, A.; Mwipatayi, B.P. Spontaneous Superior Mesenteric Artery Branch Pseudoaneurysm: A Rare Case Report. EJVES Short Rep. 2017, 37, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, N.; Kashyap, V.S.; Greenberg, R.K.; Sarac, T.P.; Clair, D.G.; Pierce, G.; Ouriel, K. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J. Vasc. Surg. 2007, 45, 276–283; discussion 283. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moree, L.H.; Muehlberger, M.J. A Case of Spontaneous Isolated Celiac Artery Dissection with Pseudoaneurysm Formation. Cureus 2017, 9, e1616. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Soave, P.M.; Antonelli, M. Prolonged Stress Causes Depression in Frontline Workers Facing the COVID-19 Pandemic—A Repeated Cross-Sectional Study in a COVID-19 Hub-Hospital in Central Italy. Int. J. Environ. Res. Public Health 2021, 18, 7316. [Google Scholar] [CrossRef] [PubMed]
- Abdelgabar, A.; D’Archambeau, O.; Maes, J.; Brande, F.V.D.; Cools, P.; Rutsaert, R.R. Visceral artery pseudoaneurysms: Two case reports and a review of the literature. J. Med. Case Rep. 2017, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Pitton, M.B.; Dappa, E.; Jungmann, F.; Kloeckner, R.; Schotten, S.; Wirth, G.M.; Mittler, J.; Lang, H.; Mildenberger, P.; Kreitner, K.F.; et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur. Radiol. 2015, 25, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Obara, H.; Kentaro, M.; Inoue, M.; Kitagawa, Y. Current management strategies for visceral artery aneurysms: An overview. Surg. Today 2020, 50, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.E.; Saad, W.E.; Davies, M.G.; Waldman, D.L.; Fultz, P.J.; Rubens, D.J. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics 2005, 25 (Suppl. S1), S173–S189. [Google Scholar] [CrossRef] [PubMed]
- El Aidaoui, K.; Bensaad, A.; Habi, J.; El Yamani, K.; El Kettani, C. Hemorrhagic Shock Revealing Rupture of Splenic Artery Pseudoaneurysm Three Years After Post-Traumatic Pancreatitis. Cureus 2021, 13, e15678. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Weiss, C.; Fishman, E.K.; Johnson, P.T.; Verde, F. Review of visceral aneurysms and pseudoaneurysms. J. Comput. Assist. Tomogr. 2015, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.Z.; Al-Saadi, M.; Abuderman, A.; Alzimami, K.S.; Alkhorayef, M.; Almagli, B.; Sulieman, A. “To-and-fro” waveform in the diagnosis of arterial pseudoaneurysms. World J. Radiol. 2015, 7, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.S.; Venkatesh, H.A.; Gamanagatti, S.; Garg, P.; Srivastava, D.N. Interventional Radiology in the Management of Visceral Artery Pseudoaneurysms: A Review of Techniques and Embolic Materials. Korean J. Radiol. 2016, 17, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.S.; Gamanagatti, S.; Gupta, A.K. Endovascular embolization of pseudoaneurysm of left colic artery developing after renal biopsy. Indian J. Nephrol. 2015, 25, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ohyama, H.; Mikata, R.; Nagashima, H.; Ohno, I.; Takiguchi, Y.; Kato, N. Intraperitoneal bleeding from the right gastroepiploic artery by endoscopic ultrasonography: A case report. J. Rural. Med. 2022, 17, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.A.; Johnson, P.T.; Fishman, E.K. Splenic artery aneurysms and pseudoaneurysms: Clinical distinctions and CT appearances. Am. J. Roentgenol. 2007, 188, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Valluru, B.; Yang, B.; Sharma, K.; Adam, A.A.; Wei, D.; Zhou, Z.; Ali, M.O. Significance of radiology in the diagnosis and management of ruptured left gastric artery aneurysm associated with acute pancreatitis: Case report. Medicine 2019, 98, e14824. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Kumar, A.H.; Pamecha, V.; Gattu, T.; Falari, S.; Sinha, P.K.; Mohapatra, N. Cystic artery pseudoaneurysm-a rare complication of acute cholecystitis: Review of literature. Surg. Endosc. 2022, 36, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Tomimaru, Y.; Hatano, H.; Noguchi, K.; Nagase, H.; Hamabe, A.; Hirota, M.; Oshima, K.; Tanida, T.; Morita, S.; et al. Ruptured Cystic Artery Pseudoaneurysm Successfully Treated with Urgent Cholecystectomy: A Case Report and Literature Review. Am. J. Case Rep. 2018, 19, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Glaysher, M.A.; Cruttenden-Wood, D.; Szentpali, K. A rare cause of upper gastrointestinal haemorrhage: Ruptured cystic artery pseudoaneurysm with concurrent cholecystojejunal fistula. Int. J. Surg. Case Rep. 2014, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- De Martini, I.V.; Pfammatter, T.; Puippe, G.; Clavien, P.A.; Alkadhi, H. Frequency and causes of delayed diagnosis of visceral artery pseudoaneurysms with CT: Lessons learned. Eur. J. Radiol. Open 2020, 7, 100221. [Google Scholar] [CrossRef] [PubMed]
- Bollen, T.L.; Singh, V.K.; Maurer, R.; Repas, K.; van Es, H.W.; Banks, P.A.; Mortele, K.J. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. Am. J. Roentgenol. 2011, 197, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Deek, H.; Newton, P.; Sheerin, N.; Noureddine, S.; Davidson, P.M. Contrast media induced nephropathy: A literature review of the available evidence and recommendations for practice. Aust. Crit. Care 2014, 27, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Benko, A.; Fraser-Hill, M.; Magner, P.; Capusten, B.; Barrett, B.; Myers, A.; Owen, R.J. Canadian Association of Radiologists: Consensus guidelines for the prevention of contrast-induced nephropathy. Can. Assoc. Radiol. J. 2007, 58, 79–87. [Google Scholar] [PubMed]
- Wong, P.C.; Li, Z.; Guo, J.; Zhang, A. Pathophysiology of contrast-induced nephropathy. Int. J. Cardiol. 2012, 158, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Schoder, M.; Cejna, M.; Längle, F.; Hittmaier, K.; Lammer, J. Glue embolization of a ruptured celiac trunk pseudoaneurysm via the gastroduodenal artery. Eur. Radiol. 2000, 10, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Valle, J.S.; Muñoz-Caicedo, B.; Durán, J.A.M. Multiple Aneurysms or Pseudoaneurysms of the Gastroepiploic Artery: An Anecdotal Cause of Hemoperitoneum. Cureus 2024, 16, e56598. [Google Scholar] [CrossRef] [PubMed]
- Chapa, U.K.; Dutta, S.; Abhinaya, R.; Jain, A.; Kalyanasundaram, A.; Munuswamy, H.; Ramakrishnaiah, V.P.N. Giant Celiac Artery Pseudoaneurysm in a Case of Chronic Pancreatitis: A Rare Case Report With Literature Review. Vasc. Endovasc. Surg. 2021, 55, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Nouhaud, F.X.; Williams, M.; Arnfield, E.; Perera, M.L.; Cho, J.; Esler, R.; Coughlin, G. Is postoperative Doppler ultrasonography useful for the early detection of asymptomatic pseudoaneurysm and prevention of haemorrhagic complications after partial nephrectomy? BJU Int. 2018, 122 (Suppl. S5), 15–21. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.A.; Canders, C.P.; Chiem, A.T. Pseudoaneurysm of the Dorsalis Pedis Artery Diagnosed on Point-of-Care Ultrasound. Pocus J. 2021, 6, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Sonanis, S.; Layton, B.; Nicholson, O.; Subar, D.A. Splenic artery pseudoaneurysm and resultant haematosuccus pancreaticus. BMJ Case Rep. 2021, 14, e239485. [Google Scholar] [CrossRef] [PubMed]
- Pilleul, F.; Beuf, O. Diagnosis of splanchnic artery aneurysms and pseudoaneurysms, with special reference to contrast enhanced 3D magnetic resonance angiography: A review. Acta Radiol. 2004, 45, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, M.; Izzo, A.; Carducci, S.; Giordano, A.V.; Masciocchi, C. Acute-phase endovascular management of an uncommon bleeding peripancreatic pseudoaneurysm. J. Radiol. Case Rep. 2018, 12, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Astore, D.; Guzzo, G.; Frigerio, S.; Tshomba, Y.; Castellano, R.; de Moura, M.R.; Melissano, G. Visceral artery aneurysms. Ann. Vasc. Surg. 2005, 19, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, G.T.; Stone, W.M.; Naidu, S.G.; Oderich, G.S.; Ricotta, J.J.; Bjarnason, H.; Money, S.R. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J. Vasc. Surg. 2011, 53, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Sachdev-Ost, U. Visceral artery aneurysms: Review of current management options. Mt. Sinai J. Med. 2010, 77, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Rao, P.; Ota, S.; De Lin, M.; Kwak, B.K.; Krause, D.; Geschwind, J.F. Packing technique for endovascular coil embolisation of peripheral arterial pseudo-aneurysms with preservation of the parent artery: Safety, efficacy and outcomes. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Khattak, Y.J.; Alam, T.; Shoaib, R.H.; Sayani, R.; Haq, T.U.; Awais, M. Endovascular embolisation of visceral artery pseudoaneurysms. Radiol. Res. Pract. 2014, 2014, 258954. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, S.; Sabharwal, T.; Karnabatidis, D.; Brountzos, E.; Katsanos, K.; Krokidis, M.; Gkoutzios, P.; Siablis, D.; Adam, A. Endovascular treatment of visceral aneurysms and pseudoaneurysms: Long-term outcomes from a multicenter European study. Cardiovasc. Interv. Radiol. 2012, 35, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.K.M.; Othman, M.H.M.; Morgan, R.; Abdallah, A.H.; Seif, H.; Zidan, M.; Khairallah, M.; El-Aleem, R.A. Analysis of outcomes of endovascular embolisation: A cross-sectional two-center study on 46 visceral artery pseudoaneurysms. CVIR Endovasc. 2021, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Song, H.H.; Won, Y.D.; Kim, Y.J. Transcatheter N-butyl cyanoacrylate embolization of pseudoaneurysms. J. Vasc. Interv. Radiol. 2010, 21, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Künzle, S.; Glenck, M.; Puippe, G.; Schadde, E.; Mayer, D.; Pfammatter, T. Stent-graft repairs of visceral and renal artery aneurysms are effective and result in long-term patency. J. Vasc. Interv. Radiol. 2013, 24, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.B.; Moorthy, S.; Pullara, S.K.; Kannan, R.R. Endovascular treatment of aneurysm of splenic artery arising from splenomesentric trunk using stent graft. Korean J. Radiol. 2013, 14, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Piacentino, F.; Coppola, A.; Bettoni, V.; Macchi, E.; Marchi, G.D.; Curti, M.; Ossola, C.; Marra, P.; Palmisano, A.; et al. Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. J. Clin. Med. 2021, 10, 2520. [Google Scholar] [CrossRef] [PubMed]
- Rabuffi, P.; Bruni, A.; Antonuccio, E.G.M.; Ambrogi, C.; Vagnarelli, S. Treatment of visceral artery aneurysms and pseudoaneurysms with the use of cerebral flow diverting stents: Initial experience. CVIR Endovasc. 2020, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Leyon, J.J.; Littlehales, T.; Rangarajan, B.; Hoey, E.T.; Ganeshan, A. Endovascular embolization: Review of currently available embolization agents. Curr. Probl. Diagn. Radiol. 2014, 43, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Keeling, A.N.; McGrath, F.P.; Lee, M.J. Interventional radiology in the diagnosis, management, and follow-up of pseudoaneurysms. Cardiovasc. Interv. Radiol. 2009, 32, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Kasirajan, K.; Greenberg, R.K.; Clair, D.; Ouriel, K. Endovascular management of visceral artery aneurysm. J. Endovasc. Ther. 2001, 8, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.J.; Jones, R.G.; Forde, C.; Marudanayagam, R. Endoscopic ultrasound-guided treatment of visceral artery pseudoaneurysm. HPB 2012, 14, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Gamanagatti, S.; Thingujam, U.; Garg, P.; Nongthombam, S.; Dash, N.R. Endoscopic ultrasound guided thrombin injection of angiographically occult pancreatitis associated visceral artery pseudoaneurysms: Case series. World J. Gastrointest. Endosc. 2015, 7, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, D.; Taglialatela, F.; Mantini, C.; Giammarino, A.; Modestino, F.; Cotroneo, A.R. Endovascular Treatment of Visceral Artery Pseudoaneurysms in Patients with Chronic Pancreatitis: Our Single-Center Experience. Ann. Vasc. Surg. 2017, 45, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Khreisat, A.; Bateman, J.; Kozyk, M.; Strubchevska, K. Spontaneous Celiac Artery Pseudoaneurysm in a Patient With Viral Myocarditis: Coincidence or Consequence? Cureus 2023, 15, e48970. [Google Scholar] [CrossRef] [PubMed]
- Treto, K.; Levitt, A.; Cheatham, M. Endovascular Management of Celiac Artery Pseudoaneurysm after Ballistic Injury. Ann. Vasc. Surg. 2019, 61, 14–15. [Google Scholar] [CrossRef]
- Graham, L.M.; Stanley, J.C.; Whitehouse, W.M., Jr.; Zelenock, G.B.; Wakefield, T.W.; Cronenwett, J.L.; Lindenauer, S.M. Celiac artery aneurysms: Historic (1745–1949) versus contemporary (1950–1984) differences in etiology and clinical importance. J. Vasc. Surg. 1985, 2, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Tamaska, W.; Medlenov, S.; Espinosa, J.; Lucerna, A. Celiac Artery Aneurysm: A Rare Cause of Abdominal Pain. Cureus 2023, 15, e48494. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Iriyama, K.; Azuma, T.; Yamakado, K. Nonoperative treatment for a ruptured pseudoaneurysm of the celiac trunk: Report of a case. Surg. Today 1997, 27, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Jain, A.; Chaudhary, J.; Gautam, M.; Gaur, M.; Grover, S. Concept of mental health and mental well-being, it’s determinants and coping strategies. Indian J. Psychiatry 2024, 66, S231–S244. [Google Scholar] [CrossRef] [PubMed]
- Roma, K.; Sieck, B.; Mikhael, T.; Naik, K.; Wahid, S. A Rare Case of a Gastric Ulcer Causing Left Gastric Artery Pseudoaneurysm. ACG Case Rep. J. 2023, 10, e01010. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.K.; Shaikh, O.; Subramaniyan, S.S.; Reddy, A.; Kumbhar, U. Left Gastric Artery Pseudoaneurysm Due to Pancreatitis. Cureus 2021, 13, e20405. [Google Scholar] [CrossRef] [PubMed]
- Polansky, M.; Yuh, B.; Israel, G. A Rare Left Gastric Artery Pseudoaneurysm Presenting as a Pancreatic Mass. Am. J. Gastroenterol. 2016, 111, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Berjawi, T.; Nasser, H.; Naccour, J.; El-Helou, E.; Kansoun, A. Left gastric artery pseudo-aneurysm post sleeve gastrectomy: A case report. Int. J. Surg. Case Rep. 2020, 76, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Vrazas, J.I. Ruptured left gastric artery aneurysm: Unique presentation with hemothorax and hemomediastinum. Cardiovasc. Interv. Radiol. 2006, 29, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Rebibo, L.; Fuks, D.; Blot, C.; Robert, B.; Boulet, P.O.; Dhahri, A.; Verhaeghe, P.; Regimbeau, J.M. Gastrointestinal bleeding complication of gastric fistula after sleeve gastrectomy: Consider pseudoaneurysms. Surg. Endosc. 2013, 27, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yu, Y.; Shim, K.E.; Kim, J.E.; Koh, J.; Yoon, J.W.; Ahn, C.; Oh, Y.K. Esophageal Artery Pseudoaneurysm and Takayasu Arteritis in a Patient with Autosomal Dominant Polycystic Kidney Disease. Electrolyte Blood Press. 2018, 16, 11–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abbas, M.A.; Stone, W.M.; Fowl, R.J.; Gloviczki, P.; Oldenburg, W.A.; Pairolero, P.C.; Hallett, J.W.; Bower, T.C.; Panneton, J.M.; Cherry, K.J. Splenic artery aneurysms: Two decades experience at Mayo clinic. Ann. Vasc. Surg. 2002, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, Y.; Akbulut, S.; Gumus, S.; Demircan, F. Giant Splenic Artery Pseudoaneurysm: A Case Report and Literature Review. Int. Surg. 2015, 100, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Maatman, T.K.; Heimberger, M.A.; Lewellen, K.A.; Roch, A.M.; Colgate, C.L.; House, M.G.; Nakeeb, A.; Ceppa, E.P.; Schmidt, C.M.; Zyromski, N.J. Visceral artery pseudoaneurysm in necrotizing pancreatitis: Incidence and outcomes. Can. J. Surg. 2020, 63, E272–E277. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Gonçalves, Á.; Passos, P.; Cardoso, M.; Torres, R.; Almeida, T.; Midões, A. Splenic Artery Pseudoaneurysm. J. Gastrointest. Surg. 2018, 22, 1297–1298. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.T.; Comerota, A.J.; Disalle, R.; Kaufman, A.; Pigott, J.P. Endoluminal embolization and revascularization for complicated mesenteric pseudoaneurysms: A report of two cases and a literature review. J. Vasc. Surg. 2007, 45, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.; Pandya, G.J.; Kamalesh, A.; Shelat, V.G. Splenic Artery Pseudoaneurysm Masquerading as a Pancreatic Cyst-A Diagnostic Challenge. Int. Surg. 2015, 100, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Copin, A.; Jenard, S.; Chasse, E. Pseudoaneurysm of the Splenic Artery Following Bariatric Surgery. Obes. Surg. 2021, 31, 2295–2297. [Google Scholar] [CrossRef] [PubMed]
- Tessier, D.J.; Stone, W.M.; Fowl, R.J.; Abbas, M.A.; Andrews, J.C.; Bower, T.C.; Gloviczki, P. Clinical features and management of splenic artery pseudoaneurysm: Case series and cumulative review of literature. J. Vasc. Surg. 2003, 38, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Otan, E. Management of Giant Splenic Artery Aneurysm: Comprehensive Literature Review. Medicine 2015, 94, e1016. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, R.; Carbunaru, A.; Grasu, M.; Toma, M.; Ionescu, M.; Dumitrascu, T. Pseudoaneurysm of the splenic artery—An uncommon cause of delayed hemorrhage after pancreaticoduodenectomy. Ann. Hepatobiliary Pancreat. Surg. 2016, 20, 204–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taslakian, B.; Khalife, M.; Faraj, W.; Mukherji, D.; Haydar, A. Pancreatitis-associated pseudoaneurysm of the splenic artery presenting as lower gastrointestinal bleeding: Treatment with transcatheter embolisation. BMJ Case Rep. 2012, 2012, bcr2012007403. [Google Scholar] [CrossRef] [PubMed]
- Balsarkar, D.J.; Joshi, M.A. Rupture of splenic artery pseudoaneurysm presenting with massive upper gastrointestinal bleed. Am. J. Surg. 2002, 183, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mahmoud, F.; Gaba, W. Splenic artery pseudoaneurysm; a cause or consequence: A case report. J. Med. Case Rep. 2024, 18, 253. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Liu, C.-H.; Lee, C.-Y.; Chiu, N.-C.; Su, C.-W. Splenic artery pseudoaneurysm as a fatal complication of acute pancreatitis. Adv. Dig. Med. 2023, 10, 110–113. [Google Scholar] [CrossRef]
- Chaer, R.A.; Abularrage, C.J.; Coleman, D.M.; Eslami, M.H.; Kashyap, V.S.; Rockman, C.; Murad, M.H. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J. Vasc. Surg. 2020, 72, 3S–39S. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.M.G.; Góes, A.S.; de Albuquerque, P.C.; Palácios, R.M.; Sde, C.A. Endovascular treatment of giant splenic artery aneurysm. Case Rep. Surg. 2012, 2012, 964093. [Google Scholar] [CrossRef] [PubMed]
- Al-Habbal, Y.; Christophi, C.; Muralidharan, V. Aneurysms of the splenic artery—A review. Surgeon 2010, 8, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Zuckerman, D.A.; Matthews, J.B. Occlusion of a giant splenic artery pseudoaneurysm with percutaneous thrombin-collagen injection. J. Vasc. Surg. 2004, 40, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Hemp, J.H.; Sabri, S.S. Endovascular management of visceral arterial aneurysms. Tech. Vasc. Interv. Radiol. 2015, 18, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Shigoka, H.; Maetani, I.; Saito, M. Pseudoaneurysm developed after percutaneous endoscopic gastrostomy: A report of two cases. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Hoshimoto, S.; Aiura, K.; Shito, M.; Kakefuda, T.; Sugiura, H. Successful resolution of a hemorrhagic pancreatic pseudocyst ruptured into the stomach complicating obstructive pancreatitis due to pancreatic cancer: A case report. World J. Surg. Oncol. 2016, 14, 46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishiyama, T.; Yamada, D.; Oba, K.; Kurihara, Y. Left omental artery bleeding in two patients with segmental arterial mediolysis successfully isolated with coil embolization. CVIR Endovasc. 2020, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.; Ng, S.L.; Danaher, L. Gastroepiploic artery pseudoaneurysm: A rare cause of abdominal pain. ANZ J. Surg. 2020, 90, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, A.; Shrivastava, A.; Soni, R.K.; Darbari, A. Pseudo Aneurysm of Aberrant Right Gastroepiploic Artery Mimicking Pseudocyst of Pancreas- A Rare Case Report. J. Clin. Diagn. Res. 2017, 11, PD09–PD10. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Kimura, H.; Kanagaki, M.; Oka, S.; Fukumoto, G.; Otani, T.; Matsubara, N.; Kawabata, K.; Namikawa, M.; Kimura, T. Usefulness of a distal access catheter in embolization of a short gastric artery pseudoaneurysm. Radiol. Case Rep. 2019, 14, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Spezia, L.; Sozzi, C.; Contro, A.; Mansueto, G. Successful Post-Pancreatitis Pseudoaneurysm Coagulation by Percutaneous Computed Tomography (CT)-Guided Thrombin Injection. Pol. J. Radiol. 2017, 82, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Zivari, K.; Niknam, N.; Lapin, S.; Rahmani, R.; Mayer, I. Ruptured Gastric Artery Pseudoaneurysm: A Life-threatening Complication of Percutaneous Endoscopic Gastrostomy (PEG): 1874. Am. J. Gastroenterol. 2017, 112, S1031–S1032. [Google Scholar] [CrossRef]
- Abbas, M.A.; Fowl, R.J.; Stone, W.M.; Panneton, J.M.; Oldenburg, W.A.; Bower, T.C.; Cherry, K.J.; Gloviczki, P. Hepatic artery aneurysm: Factors that predict complications. J. Vasc. Surg. 2003, 38, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Lu, S.C.; Yan, L.N.; Li, B.; Wen, T.F.; Zeng, Y.; Cheng, N.S.; Wang, J.; Luo, Y.; Pen, Y.L. Incidence and treatment of hepatic artery complications after orthotopic liver transplantation. World J. Gastroenterol. 2003, 9, 2853–2855. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Chen, X.; Zhao, J.; Luo, X.; Guo, Q.; Du, X.; Yuan, D.; Huang, B. Open Repair of Large Hepatic Artery Pseudoaneurysm Without Collateral Circulation: A Case Report. Front. Surg. 2022, 9, 791553. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Mukhopadhyay, S.; Yusuf, J.; Tyagi, S. Emergency hepatic artery embolization in a patient with post-traumatic ruptured hepatic artery pseudoaneurysm. J. Emerg. Trauma. Shock. 2014, 7, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Erben, Y.; De Martino, R.R.; Bjarnason, H.; Duncan, A.A.; Kalra, M.; Oderich, G.S.; Bower, T.C.; Gloviczki, P. Operative management of hepatic artery aneurysms. J. Vasc. Surg. 2015, 62, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Finley, D.S.; Hinojosa, M.W.; Paya, M.; Imagawa, D.K. Hepatic artery pseudoaneurysm: A report of seven cases and a review of the literature. Surg. Today 2005, 35, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Oh, T.Y.C.; Pang, T.C.; Hsu, J.M. Hepatic artery pseudoaneurysm rupture: A case report. Trauma. Case Rep. 2023, 48, 100970. [Google Scholar] [CrossRef] [PubMed]
- Østerballe, L.; Helgstrand, F.; Axelsen, T.; Hillingsø, J.; Svendsen, L.B. Hepatic pseudoaneurysm after traumatic liver injury; is CT follow-up warranted? J. Trauma Manag. Outcomes 2014, 8, 18. [Google Scholar] [CrossRef]
- Razik, R.; Fallah, A.; Sandroussi, C.; Wei, A.C.; McGilvray, I.D. Dissecting pseudoaneurysm of the proper hepatic artery repaired by primary anastomosis: A case report. Case Rep. Surg. 2012, 2012, 804919. [Google Scholar] [CrossRef] [PubMed]
- Shanley, C.J.; Shah, N.L.; Messina, L.M. Common splanchnic artery aneurysms: Splenic, hepatic, and celiac. Ann. Vasc. Surg. 1996, 10, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A.B.; Mattar, S.G.; Allen, R.C.; Bacha, E.A. Hepatic artery aneurysms: The management of 22 patients. J. Surg. Res. 1996, 60, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Heo, J.S.; Choi, S.H.; Choi, D.W. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J. Gastroenterol. 2010, 16, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ota, H.; Matsuura, T.; Seiji, K.; Mugikura, S.; Motoi, F.; Unno, M.; Takase, K. Endovascular Treatment of Hepatic Artery Pseudoaneurysm after Pancreaticoduodenectomy: Risk Factors Associated with Mortality and Complications. J. Vasc. Interv. Radiol. 2017, 28, 50–59.e55. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Wei, B. Proper Hepatic Artery Pseudoaneurysm Treated by Endovascular Stent. Am. J. Roentgenol. 2024, 222, e2330088. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Cho, J.S.; Shepard, A.D.; Nypaver, T.J.; Reddy, D.J. Visceral pseudoaneurysms due to pancreatic pseudocysts: Rare but lethal complications of pancreatitis. J. Vasc. Surg. 2000, 32, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Winick, A.B.; Malloy, P.C.; Lund, G.B. Retained contrast after embolization of a right gastric artery pseudoaneurysm. Cardiovasc. Interv. Radiol. 1996, 19, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Occhionorelli, S.; Morganti, L.; Cappellari, L.; Stano, R.; Andreotti, D.; Vasquez, G. Asymptomatic and early pseudoaneurysm of posterior superior pancreaticoduodenal artery and right gastric artery complicating acute pancreatitis: A case report. Int. J. Surg. Case Rep. 2016, 28, 344–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khalil, A.; Fawzy, F.S.; Zaid, M.H. Management of iatrogenic hepatic artery pseudoaneurysm. Egypt. J. Surg. 2021, 40, 954–958. [Google Scholar] [CrossRef]
- Bulut, T.; Yamaner, S.; Bugra, D.; Akyuz, A.; Acarli, K.; Poyanli, A. False aneurysm of the hepatic artery after laparoscopic cholecystectomy. Acta Chir. Belg. 2002, 102, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.O.; Al-Zadjali, A.; Kakaria, A.K.; Younus, S.; Rahim, M.A.; Al-Sukaiti, R. Hepatic or Cystic Artery Pseudoaneurysms Following a Laparoscopic Cholecystectomy: Literature review of aetiopathogenesis, presentation, diagnosis and management. Sultan Qaboos Univ. Med. J. 2017, 17, e135–e146. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.Y.; Kuo, T.C.; Lai, H.S.; Yang, C.Y.; Tien, Y.W. Obstructive jaundice as a complication of a right hepatic artery pseudoaneurysm after laparoscopic cholecystectomy. J. Minim. Access Surg. 2015, 11, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Traiki, T.A.B.; Madkhali, A.A.; Hassanain, M.M. Hemobilia post laparoscopic cholecystectomy. J. Surg. Case Rep. 2015, 2015, rju159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murugesan, S.D.; Sathyanesan, J.; Lakshmanan, A.; Ramaswami, S.; Perumal, S.; Perumal, S.U.; Ramasamy, R.; Palaniappan, R. Massive hemobilia: A diagnostic and therapeutic challenge. World J. Surg. 2014, 38, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Hylton, J.R.; Pevec, W.C. Successful treatment of an iatrogenic right hepatic artery pseudoaneurysm and stenosis with a stent graft. J. Vasc. Surg. 2010, 51, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.S.; Miraglia, R.; Maruzzelli, L.; Crinò, F.; Cannataci, C.; Gruttadauria, S. Percutaneous Trans-Hepatic Embolization of an Iatrogenic Extra-Hepatic Pseudoaneurysm of the Right Hepatic Artery in a Patient With Previous Occlusion of the Proper Hepatic Artery: An Endovascular Procedure to Avoid a Difficult Surgical Repair. Vasc. Endovasc. Surg. 2021, 55, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Berceli, S.A. Hepatic and splenic artery aneurysms. Semin. Vasc. Surg. 2005, 18, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Helton, W.S. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB 2011, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Genyk, Y.S.; Keller, F.S.; Halpern, N.B. Hepatic artery pseudoaneurysm and hemobilia following laser laparoscopic cholecystectomy. A case report. Surg. Endosc. 1994, 8, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Nezu, Y.; Nakaji, S.; Fujii, H.; Ishii, E.; Hirata, N. Pseudoaneurysm caused by a self-expandable metal stent: A report of three cases. Endoscopy 2014, 46, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Matsubayashi, H.; Ishiwatari, H.; Fujie, S.; Kaneko, J.; Sato, J.; Ito, S.; Kishida, Y.; Imai, K.; Hotta, K.; et al. Rupture of Pseudoaneurysm after Biliary Metallic Stent Placement. Intern. Med. 2019, 58, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sheikh, A.; Partyka, L.; Contractor, S. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin. Imaging 2014, 38, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Boddy, A.; Macanovic, M.; Thompson, J.; Watkinson, A. Use of an endovascular stent graft and percutaneous thrombin injection to treat an iatrogenic hepatic artery pseudoaneurysm. Ann. R. Coll. Surg. Engl. 2010. [Google Scholar]
- Delgadillo, X.; Berney, T.; de Perrot, M.; Didier, D.; Morel, P. Successful treatment of a pseudoaneurysm of the cystic artery with microcoil embolization. J. Vasc. Interv. Radiol. 1999, 10, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Kaman, L.; Kumar, S.; Behera, A.; Katariya, R.N. Pseudoaneurysm of the cystic artery: A rare cause of hemobilia. Am. J. Gastroenterol. 1998, 93, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, L.; Dewaele, T.; Rosseel, F.; Crevits, I.; Aerts, P.; De Man, R. Acute cholecystitis with pseudoaneurysm of the cystic artery. J. Belg. Soc. Radiol. 2012, 95, 136–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gan, Y.Y.; Lim, C.H.; Abdullah, N.A.N.; Soon, K.C. Celiac Artery Aneurysm and Pseudoaneurysm Caused by Median Arcuate Ligament Syndrome (MALS) with Different Presentation: A Case Series. HPB 2021, 23, S395. [Google Scholar] [CrossRef]
- Alam, A.S.; Elkhawaga, M.; Yashi, K. Gastroduodenal Artery Pseudoaneurysm: A Rare Cause of Upper Gastrointestinal Bleeding and Pancreatic Duct Compression. Cureus 2022, 14, e29971. [Google Scholar] [CrossRef] [PubMed]
- Bergert, H.; Hinterseher, I.; Kersting, S.; Leonhardt, J.; Bloomenthal, A.; Saeger, H.D. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 2005, 137, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.M.; Bolton, J.S.; Gioe, S.M.; Conway, W.C. Gastroduodenal Artery Pseudoaneurysm Causing Obstructive Jaundice. Ochsner J. 2021, 21, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Awada, Z.; Al Moussawi, H.; Alsheikh, M. Gastroduodenal Artery Pseudoaneurysm Rupture Post-Billroth II Surgery: Case Report. Cureus 2019, 11, e3833. [Google Scholar] [CrossRef] [PubMed]
- Koyazounda, A.; Jaillot, P.; Persico, J.; Thouret, J.M.; Grand, A. Aneurysm of the gastroduodenal artery ruptured into the peritoneum. Treatment by embolization. Presse Med. 1994, 23, 661–664. [Google Scholar] [PubMed]
- Sethi, H.; Peddu, P.; Prachalias, A.; Kane, P.; Karani, J.; Rela, M.; Heaton, N. Selective embolization for bleeding visceral artery pseudoaneurysms in patients with pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2010, 9, 634–638. [Google Scholar] [PubMed]
- Antunes, A.G.; Peixe, B.; Guerreiro, H. Spontaneous thrombosis of a gastroduodenal artery pseudoaneurysm manifesting as a subepithelial lesion. Rev. Gastroenterol. De Mex 2018, 83, 354–356. [Google Scholar] [CrossRef]
- Reis, J. Gastroduodenal artery pseudoaneurysm. Ultrasound Q. 2012, 28, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Germanos, S.; Soonawalla, Z.; Stratopoulos, C.; Friend, P.J. Pseudoaneurysm of the gastroduodenal artery in chronic pancreatitis. J. Am. Coll. Surg. 2009, 208, 316. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Thompson, N.W.; Fry, W.J. Splanchnic artery aneurysms. Arch. Surg. 1970, 101, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Shimohira, M.; Ogino, H.; Kitase, M.; Sasaki, S.; Ishii, M.; Shibamoto, Y. Embolization of a right gastroepiploic artery pseudoaneurysm associated with Churg-Strauss syndrome. J. Vasc. Interv. Radiol. 2008, 19, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Shimada, K.; Kobayashi, N.; Kakita, A. Migration of steel-wire coils into the stomach after transcatheter arterial embolization for a bleeding splenic artery pseudoaneurysm: Report of a case. Surg. Today 2001, 31, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Sinnadurai, S.; Sowa, P.; Jankowski, P.; Gasior, Z.; Kosior, D.A.; Haberka, M.; Czarnecka, D.; Pajak, A.; Setny, M.; Jamiolkowski, J.; et al. Effects of cardiac rehabilitation on risk factor management and quality of life in patients with ischemic heart disease: A multicenter cross-sectional study. Pol. Arch. Intern. Med. 2021, 131, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Sato, J.; Tateishi, K. Posterior Superior Pancreaticoduodenal Artery Pseudoaneurysm Caused by a Large-bore Biliary Metal Stent. J. Gastrointest. Liver Dis. 2022, 31, 382. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Morita, Y.; Muraki, R.; Furuhashi, S.; Takeda, M.; Kikuchi, H.; Hiramatsu, Y.; Tanahashi, Y.; Goshima, S.; Takeuchi, H. Anterior superior pancreaticoduodenal artery pseudoaneurysm after distal pancreatectomy with en bloc celiac axis resection successfully treated with balloon-assisted coil embolization. Clin. J. Gastroenterol. 2022, 15, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Kaman, L.; Sanyal, S.; Menakuru, S.R.; Singh, R. Pseudoaneurysm of the superior pancreaticoduodenal artery, a rare cause of hemosuccus pancreaticus: Report of a case. Surg. Today 2004, 34, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.C.; Vargo, J.J.; Chahal, P. Superior pancreaticoduodenal artery pseudoaneurysm mimicking a cystic neoplasm with worrisome features. VideoGIE 2017, 2, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Kochar, S.; Sharparis, Y.; Banerjee, B.; Karasek, M.; Brennan, S. Endovascular Coil Embolization of Superior Mesenteric Artery Branch Pseudoaneurysm. Cureus 2021, 13, e18014. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.G.; Thompson, E.; Wartman, S.; Woo, K. Infective endocarditis associated superior mesenteric artery pseudoaneurysm. Ann. Vasc. Surg. 2014, 28, e1561–e1565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pu, X.; Li, Z.; Zeng, Q.; He, Y.; He, Y.; Xiang, Y.; Wu, Z.; Li, D.; Lu, T.; et al. Endovascular Treatment of Superior Mesenteric Artery Pseudoaneurysm Due to Infective Endocarditis With Stent-Graft. J. Endovasc. Ther. 2023, 30, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.M.; Wu, T.L.; Cheong, C.U.; Chang, Y.K.; Lai, C.C. Superior mesenteric artery pseudoaneurysm. J. Emerg. Med. 2013, 45, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Al Harthy, A.; Belot, A.; Feugier, P. Superior Mesenteric Artery Pseudoaneurysm Induced by Accidental Ingestion of a Foreign Body: Case Report. EJVES Vasc. Forum 2022, 54, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Burli, P.; Winterbottom, A.P.; Cousins, C.; Appleton, D.S.; See, T.C. Imaging appearances and endovascular management of uncommon pseudoaneurysms. Clin. Radiol. 2008, 63, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Saboo, S.S.; Khurana, B.; Juan, Y.H.; Sodickson, A.; Gates, J. Mesenteric mycotic pseudoaneurysm: A hidden catastrophe. Emerg. Radiol. 2014, 21, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, H.; Date, Y.; Takagi, T.; Kawase, Y. Spontaneous complete resolution of mycotic superior mesenteric artery pseudoaneurysm. J. Vasc. Surg. 2018, 68, 1914–1915. [Google Scholar] [CrossRef] [PubMed]
- Aymonnier, K.; Amsler, J.; Lamprecht, P.; Salama, A.; Witko-Sarsat, V. The neutrophil: A key resourceful agent in immune-mediated vasculitis. Immunol. Rev. 2023, 314, 326–356. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.H.; Ren, J.Z.; Zhou, G.F.; Zheng, C.S.; Liang, H.M.; Dong, X.J.; Feng, G.S. Clinical features and endovascular treatment of visceral artery pseudoaneurysms. Ann. Vasc. Surg. 2015, 29, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Cumbie, T.A.; Kedora, J.C.; Pearl, G.J.; Shutze, W.P. A hybrid repair of a superior mesenteric artery pseudoaneurysm using open mesenteric bypass and endovascular exclusion. Bayl. Univ. Med Cent. Proc. 2015, 28, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.Y.; Mohan, S.; Balasubramaniam, S.; Ahmed, S.; Siew, C.C.H.; Shelat, V.G. Indocyanine green dye and its application in gastrointestinal surgery: The future is bright green. World J. Gastrointest. Surg. 2023, 15, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Abu-Zidan, F.M.; Ansaloni, L.; Bala, M.; Beltrán, M.A.; Biffl, W.L.; Catena, F.; Chiara, O.; Coccolini, F.; Coimbra, R.; et al. The role of the open abdomen procedure in managing severe abdominal sepsis: WSES position paper. World J. Emerg. Surg. 2015, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Dallara, H.; Habboushe, J. Spontaneous inferior pancreaticoduodenal artery pseudoaneurysm rupture. Intern. Emerg. Med. 2017, 12, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Muscara, F.; Farghal, A.; Shaikh, I. Haematochezia from a Splenic Artery Pseudoaneurysm Communicating with Transverse Colon: A Case Report and Literature Review. Case Rep. Vasc. Med. 2016, 2016, 8461501. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.W.K.; Shelat, V.G.; Monteiro, A.Y.; Low, J.K. Spontaneous retroperitoneal haemorrhage from pancreatoduodenal artery (PDA) rupture and associated complications. BMJ Case Rep. 2022, 15, e250383. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.M.; Cook, J.L.; Jackson, J.E.; Hocken, D.B. Infective aneurysm of the inferior pancreaticoduodenal artery. Ann. R. Coll. Surg. Engl. 2011, 93, e87–e88. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Dugalic, V.; Kovac, J.; Tadic, B.; Milosevic, S.; Lukic, B.; Lekic, N.; Cvetic, V. Successful Embolization of Posterior Inferior Pancreaticoduodenal Artery Pseudoaneurysm on the Grounds of Chronic Pancreatitis-Case Report and Literature Review. Medicina 2020, 56, 617. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.M.; Tadic, B.; Jankovic, A.; Stosic, K.; Lukic, B.; Cvetic, V.; Knezevic, D. Endovascular treatment of a pseudoaneurysm of the posterior inferior pancreaticoduodenal artery as a complication of chronic pancreatitis: A case report. J. Int. Med. Res. 2022, 50, 3000605221083441. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Besant, S.; Cleveland, T.; Al-Mukhtar, A. Pseudoaneurysm of the Inferior Pancreaticoduodenal Artery Due to Cholecystitis. Reports 2019, 2, 11. [Google Scholar] [CrossRef]
- Rim, D.; Yu, Q.; Cieslak, J.; Wang, W. Bleeding Pseudoaneurysm of the Inferior Pancreaticoduodenal Artery as an Endoscopic Retrograde Cholangiopancreatography Complication. ACG Case Rep. J. 2021, 8, e00695. [Google Scholar] [CrossRef] [PubMed]
- Colak, M.C.; Kocaturk, H.; Bayram, E.; Karaca, L. Inferior pancreaticoduodenal artery false aneurysm: A rare cause of gastrointestinal bleeding diagnosed by three-dimensional computed tomography. Singap. Med. J. 2009, 50, e346–e349. [Google Scholar]
- Al-Jeroudi, A.; Belli, A.M.; Shorvon, P.J. False aneurysm of the pancreaticoduodenal artery complicating therapeutic endoscopic retrograde cholangiopancreatography. Br. J. Radiol. 2001, 74, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.T.; Yeh, C.N.; Hung, C.F.; Chen, H.M.; Hwang, T.L.; Jan, Y.Y.; Chen, M.F. Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol. 2006, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Kiviluoto, T.; Kivisaari, L.; Kivilaakso, E.; Lempinen, M. Pseudocysts in chronic pancreatitis. Surgical results in 102 consecutive patients. Arch. Surg. 1989, 124, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kumar, S.; Kumar, P.; Chandra, A. Giant pseudoaneurysm of the splenic artery. Jop 2011, 12, 190–193. [Google Scholar] [PubMed]
- Balachandra, S.; Siriwardena, A.K. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am. J. Surg. 2005, 190, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, S.S.; Sharma, D.; Sapra, B.; Nijhawan, S. Endoscopic ultrasound-guided thrombin injection, a management approach for visceral artery pseudoaneurysms. Endosc. Int. Open 2020, 8, e407–e412. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Solomon, J.; Carpenter, J.P. Stent graft repair of visceral artery aneurysms. J. Vasc. Surg. 2002, 36, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Bavunoglu, I.; Ayan, F.; Karabicak, I.; Ledamo, Y.; Sayilgan, C.; Numan, F.; Sirin, F. Selective jejunal artery pseudoaneurysm embolization in a patient with massive gastrointestinal bleeding due to intestinal tuberculosis. J. Emerg. Med. 2006, 31, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Breguet, R.; Pupulim, L.F.; Terraz, S. Embolization of a Jejunal Artery Pseudoaneurysm via Collateral Vessels. Case Rep. Surg. 2015, 2015, 465143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Provost, H.; Drudi, L.M.; Schwenter, F.; Elkouri, S.; Blair, J.F.; Charbonneau, P. A mycotic aneurysm of a jejunal branch treated by aneurysmectomy without bowel resection. J. Vasc. Surg. Cases Innov. Tech. 2024, 10, 101364. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Shih, M.C.; Chao, M.F.; Lin, Y.F.; Lu, C.H.; Wu, D.K. Gigantic Jejunal Artery Pseudoaneurysm:A Very Rare Case Report, its Management with Endovascular Coiling and Review of the Literature. J. Rare Disord. Diagn. Ther. 2018, 4, 18. [Google Scholar] [CrossRef]
- Ismaili, M.; Mvumbi, F.; Akach, I.; Alami, B.; Boubbou, M.; Lahmidani, N.; El Youssfi, M.; Maâroufi, M.; Lamrani, Y.A. A jejunal artery pseudoaneurysm ruptured in the jejunal lumen: A rare vascular complication of severe pancreatitis. J. Med. Vasc. 2019, 44, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, P.; Gaudenzi, D.; Mulazzani, L.; Rebonato, A.; Patriti, A. Crohn’s Disease and Jejunal Artery Aneurysms: A Report of the First Case and a Review of the Literature. Medicina 2022, 58, 1344. [Google Scholar] [CrossRef] [PubMed]
- Minaya-Bravo, A.M.; Vera-Mansilla, C.; Ruiz-Grande, F. Presentation of a large jejunal artery aneurysm: Management and review of the literature. Int. J. Surg. Case Rep. 2018, 48, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, M.J.; Anderson, S.E.; Lourens, S.; Triller, J. A rare case of jejunal arterio-venous fistula: Treatment with superselective catheter embolization with a tracker-18 catheter and microcoils. Cardiovasc. Interv. Radiol. 2004, 27, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Shimohira, M.; Ogino, H.; Kitase, M.; Takeuchi, M.; Shibamoto, Y. Embolization for asymptomatic aneurysms of the first jejunal artery. Vasa 2006, 35, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.C.; Wakefield, T.W.; Graham, L.M.; Whitehouse, W.M., Jr.; Zelenock, G.B.; Lindenauer, S.M. Clinical importance and management of splanchnic artery aneurysms. J. Vasc. Surg. 1986, 3, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.G.; Pogo, G.J.; Moccio, C.G. Mycotic aneurysm of the superior mesenteric artery. J. Vasc. Surg. 1987, 6, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Takata, N.; Watanabe, A.; Fukai, S.; Yoshikawa, K.; Sasamatsu, S.; Lefor, A.K.; Sakamoto, T.; Mizokami, K.; Kanzaki, M.; et al. Pseudoaneurysm of an ileal mesenteric artery after a stapled anastomosis. Surgery 2018, 163, 968–969. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, F.; Brisbois, D. Post-traumatic ileal branch pseudoaneurysm. Rev. Med. Liege 2022, 77, 199–201. [Google Scholar] [PubMed]
- Kassamali, F.; Sharma, A.R. Ileal artery pseudoaneurysm: A rare cause of gastrointestinal bleed. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cha, J.G.; Park, J. Endovascular treatment of traumatic pseudoaneurysm of the ileal branch of the superior mesenteric artery in a 9-year-old girl: Case report and literature review. Medicine 2024, 103, e37978. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J., Jr.; Mineau, D.E. Transcatheter arterial embolization--major complications and their prevention. Cardiovasc. Interv. Radiol. 1983, 6, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Borzelli, A.; Cangiano, G.; Silvestre, M.; Corvino, F.; Corvino, A.; Magistris, G.; Niola, R. Successful Endovascular Embolisation of an Unusual Giant Pseudoaneurysm of the Middle Colic Artery. J. Endovasc. Resusc. Trauma. Manag. 2022, 6, 84–90. [Google Scholar] [CrossRef]
- Toyonaga, T.; Nagaoka, S.; Ouchida, K.; Nagata, M.; Shirota, T.; Ogawa, T.; Yoshida, J.; Sinohara, M.; Matuo, K.; Sumitomo, K.; et al. Case of a bleeding pseudoaneurysm of the middle colic artery complicating acute pancreatitis. Hepatogastroenterology 2002, 49, 1141–1143. [Google Scholar] [PubMed]
- Wang, A.Y.; Lin, T.H.; Liu, K.L.; Wang, H.P.; Lien, W.C. Rupture of middle colic artery pseudoaneurysm. Am. J. Emerg. Med. 2013, 31, 454.e5–454.e7. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, P.B.; Maheshwari, G.; Haynes, M.; Amaechi, I.; Dickson-Lowe, R. Intraperitoneal Haemorrhage Secondary to Rupture of Right Colic Artery Pseudoaneurysm: A Case Report and Literature Review. Cureus 2023, 15, e44634. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, Y.M. A Rare Case of Coil Migration into the Duodenum after Embolization of a Right Colic Artery Pseudoaneurysm. Clin. Endosc. 2021, 54, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, C.V.; Kuhl, F.G.; Leite, A.M.; Raymundo, S.R.d.O.; Miquelin, A.R.; Acar, V.; Fachini, V.B.; Canuti, M.R. Ruptured ileocolic artery pseudoaneurysm: Case report. J. Vasc. Bras. 2021, 20, e20210163. [Google Scholar] [CrossRef] [PubMed]
- AbuDalu, M.; Munz, Y.; Ohana, G. Symptomatic ileocolic pseudoaneurysm following laparoscopic ileocecectomy for Crohn’s disease: A case report. Int. J. Surg. Case Rep. 2022, 94, 107059. [Google Scholar] [CrossRef] [PubMed]
- Edden, Y.; Shussman, N.; Cohen, M.J.; Verstandig, A.; Pikarsky, A.J. Endovascular treatment of ileocolic pseudoaneurysm after a laparoscopic-assisted bowel resection for Crohn disease. Vasc. Endovasc. Surg. 2008, 42, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Takashima, J.; Taniguchi, K.; Koizumi, A.; Shigehara, F.; Yamazaki, K.; Fujimoto, D.; Miura, F.; Kobayashi, H. Ruptured ileocolic artery pseudoaneurysm after laparoscopic appendectomy for acute appendicitis. Surg. Case Rep. 2022, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Roche-Nagle, G.; O’Donnell, D.; O’Hanrahan, T. Visceral artery aneurysms: A symptomatic aneurysm of the ileocolic artery. Vascular 2007, 15, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Elango, S.; Ishita, L.; Shanmugam, D. Traumatic Pseudoaneurysm of the Inferior Mesenteric Artery Branch: A Rare Cause of Lower GI Bleeding and Treatment with Selective Coil Embolization. Cardiovasc. Interv. Radiol. 2016, 39, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Khatri, S.; Parikh, R.; Smith, M.; Friedman, J. Endovascular management of colic artery pseudoaneurysm: A case report of successful intervention in a 59-year-old male with abdominal hematoma. Radiol. Case Rep. 2024, 19, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Kaman, L.; Parkash, M. Traumatic Pseudoaneurysm of Superior Rectal Artery—An Unusual Cause of Massive Lower Gastrointestinal Bleed: A Case Report. Gastroenterol. Res. 2011, 4, 36–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marichal, D.A.; Savage, C.; Meler, J.D.; Kirsch, D.; Rees, C.R. Left colic artery pseudoaneurysm from pancreatitis presenting as upper gastrointestinal hemorrhage. J. Vasc. Interv. Radiol. 2009, 20, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Callebaut, G.; Laureys, M.; De Quin, I.; Goffin, C.; Dernier, Y.; Bellens, B. Inferior Mesenteric Artery Aneurysm: Report of a Case. Eur. J. Vasc. Endovasc. Surg. 2009, 17, 1–3. [Google Scholar] [CrossRef]
- Carriero, S.; Lanza, C.; Biondetti, P.; Renzulli, M.; Bonelli, C.; Piacentino, F.; Fontana, F.; Venturini, M.; Carrafiello, G.; Ierardi, A.M. Imaging-Guided Percutaneous Puncture and Embolization of Visceral Pseudoaneurysms: Feasibility and Outcomes. J. Clin. Med. 2022, 11, 2952. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Baliss, M.; Tayyem, O.; Merwat, S. Lower Gastrointestinal Hemorrhage Caused by Superior Rectal Artery Pseudoaneurysm. ACG Case Rep. J. 2020, 7, e00387. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.; Schwarz, F.; Sommer, F.; Ayoub, M.; Messmann, H. Hemorrhagic Shock After Endoscopic Biopsy of Sigmoid Cancer: Pseudoaneurysm of the Rectal Superior Artery. ACG Case Rep. J. 2023, 10, e01074. [Google Scholar] [CrossRef] [PubMed]
- Curfman, K.R.; Shuman, M.P.; Gorman, K.M.; Schrock, W.B.; Meade, P.G. Post-Traumatic Retroperitoneal Hematoma Caused by Superior Rectal Artery Pseudoaneurysm. Am. J. Case Rep. 2020, 21, e924529. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.K.; Lewis, M.; Stebbing, J.F.; Marks, C.G. Multiple microaneurysms of the superior hemorrhoidal artery: Unusual recurrent massive rectal bleeding: Report of a case. Dis. Colon. Rectum 2003, 46, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Bandi, R.; Shetty, P.C.; Sharma, R.P.; Burke, T.H.; Burke, M.W.; Kastan, D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J. Vasc. Interv. Radiol. 2001, 12, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.W.; Karthikesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. NPJ Digit. Med. 2021, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Scallan, O.H.; Duncan, A.A. Current Approaches for Mesenteric Ischemia and Visceral Aneurysms. Surg. Clin. N. Am. 2023, 103, 703–731. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Miura, Y.; Edo, N.; Mitsuishi, A.; Matsumoto, T.; Kitagawa, H. A Case of Successful Hybrid Treatment of Dual Arterial Bypass Using Indocyanine Green Fluorescence Angiography and Endovascular Treatment for Recurrent Superior Mesenteric Artery Aneurysm. Ann. Vasc. Dis. 2024, 17, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Forgione, A.; Barberio, M.; Agnus, V.; Swanström, L.; Marescaux, J.; Diana, M.; Gallix, B. Precision image-guided colonic surgery: Proof of concept for enhanced preoperative and intraoperative vascular imaging. Surg. Endosc. 2021, 35, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Treskes, N.; Persoon, A.M.; van Zanten, A.R.H. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: A systematic review and meta-analysis. Intern. Emerg. Med. 2017, 12, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Yaddanapudi, S.; Yaddanapudi, L. Indications for blood and blood product transfusion. Indian J. Anaesth. 2014, 58, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Casciani, F.; Trudeau, M.T.; Vollmer, C.M., Jr. Perioperative Immunization for Splenectomy and the Surgeon’s Responsibility: A Review. JAMA Surg. 2020, 155, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, C.; Esposito, D.; Martini, R.; Novali, C.; Zaninelli, A.; Annese, A.L.; Baggi, P.; Bellosta, R.; Massoni, C.B.; Bonardelli, S.; et al. Guidelines on the diagnosis, treatment and management of visceral and renal artery aneurysms: A joint assessment by the Italian Societies of Vascular and Endovascular Surgery (SICVE) and Medical and Interventional Radiology (SIRM). J. Cardiovasc. Surg. 2024, 65, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Kassir, R.; Lointier, P.; Papastathi-Boureau, C.; Fiquet, J.M.; Blanc, P. Medicolegal Analysis of 81 Malpractice Claims Against Bariatric Surgeons in France. Obes. Surg. 2020, 30, 1726–1735. [Google Scholar] [CrossRef] [PubMed]


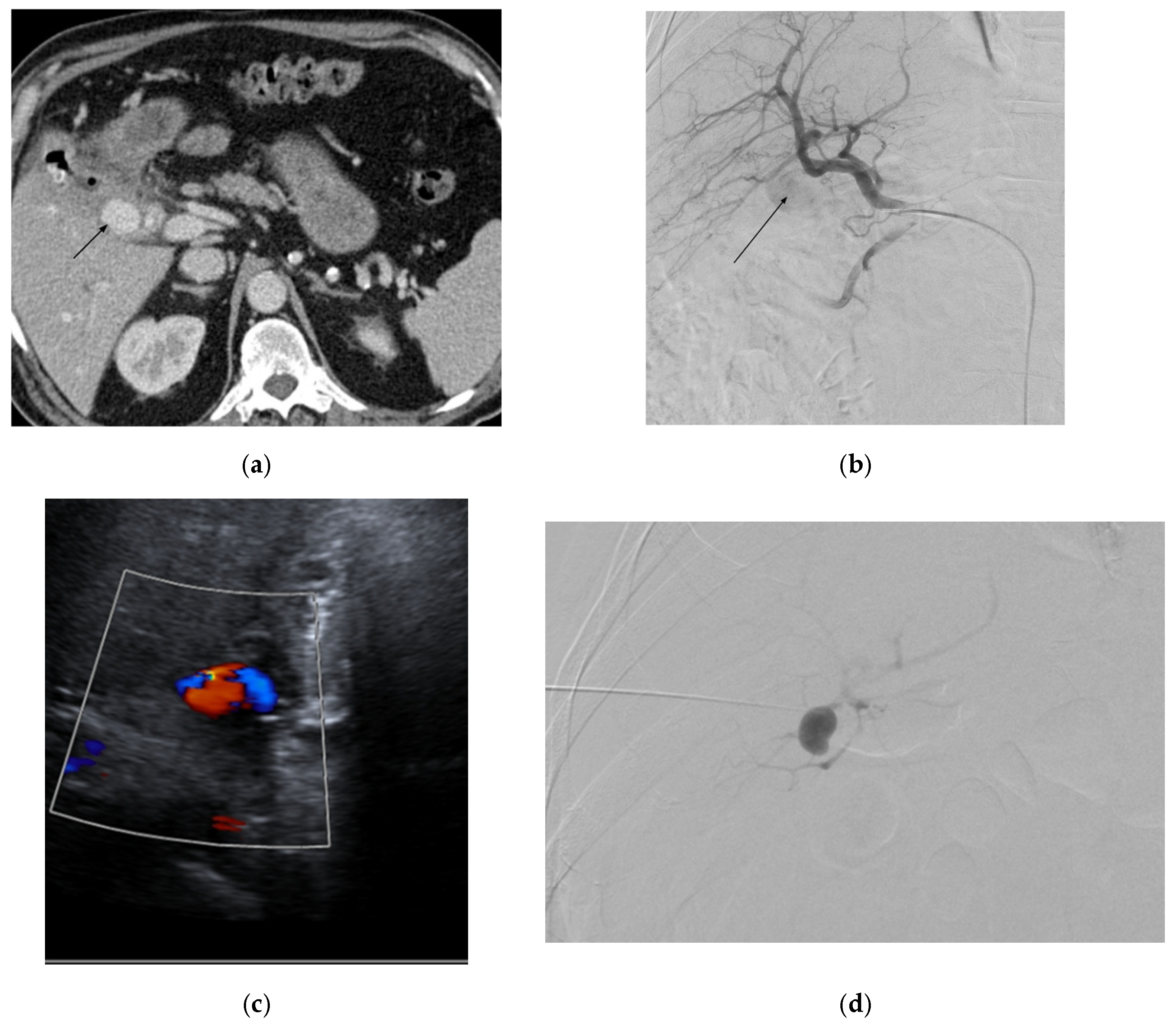

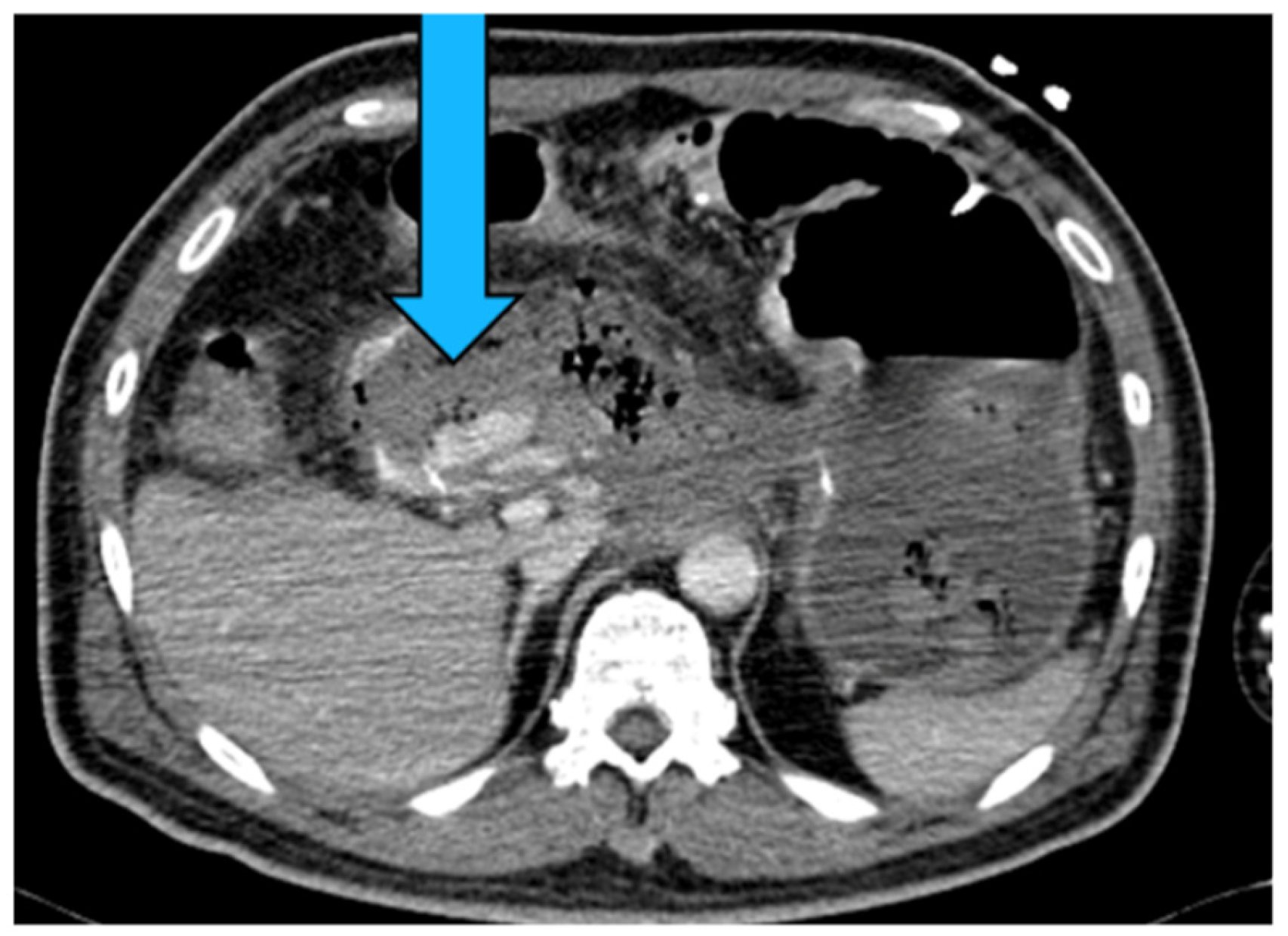
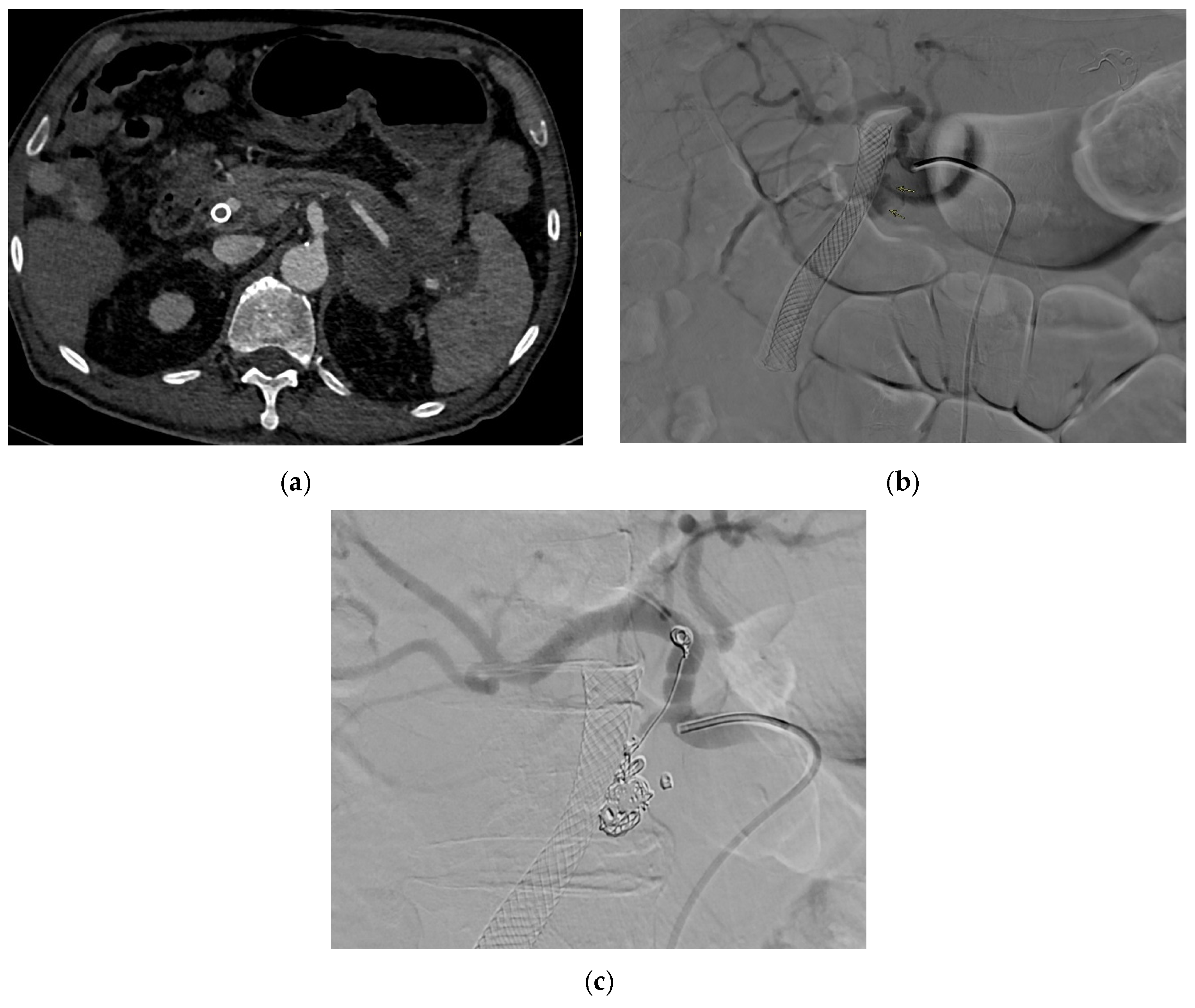
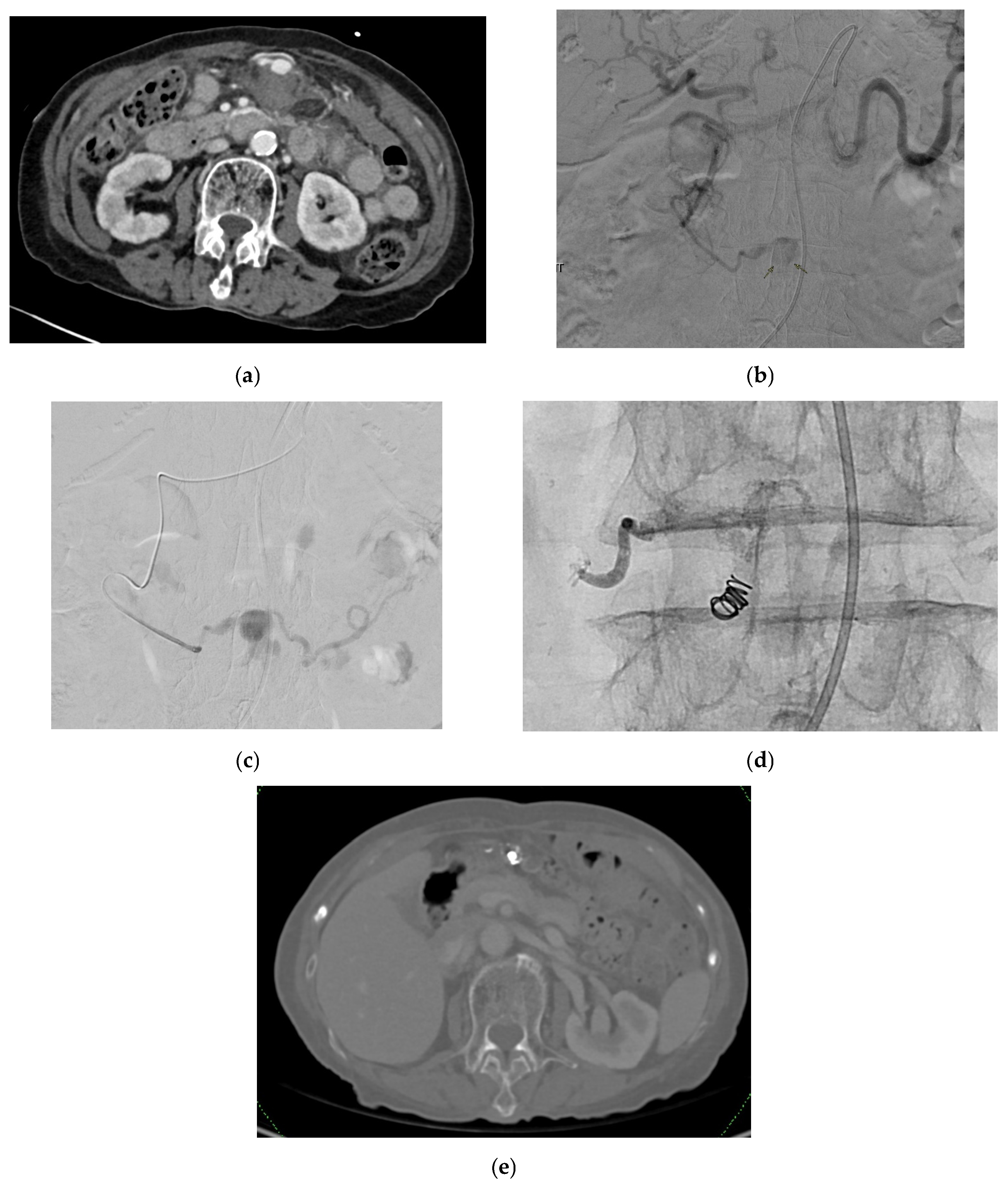
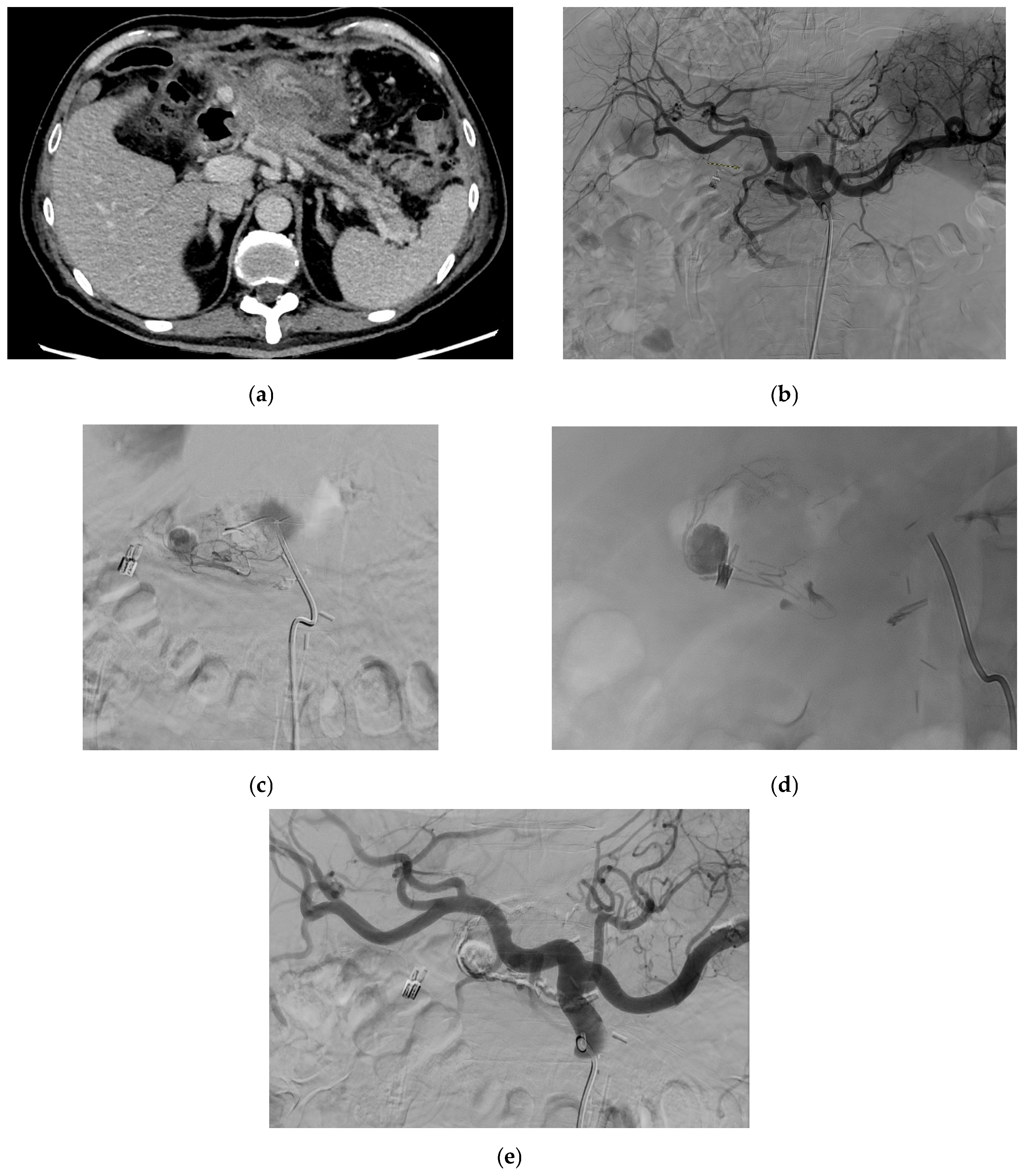
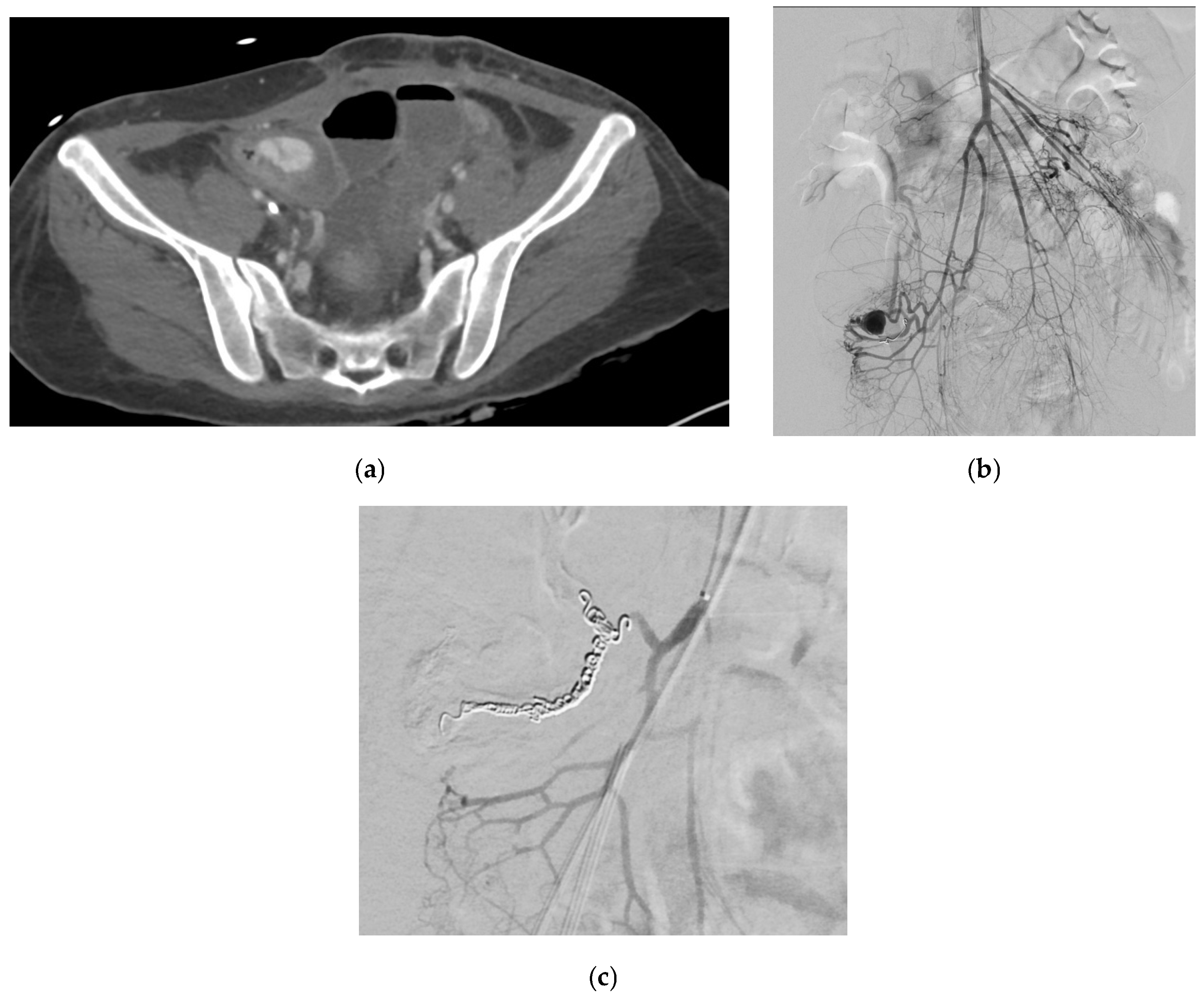
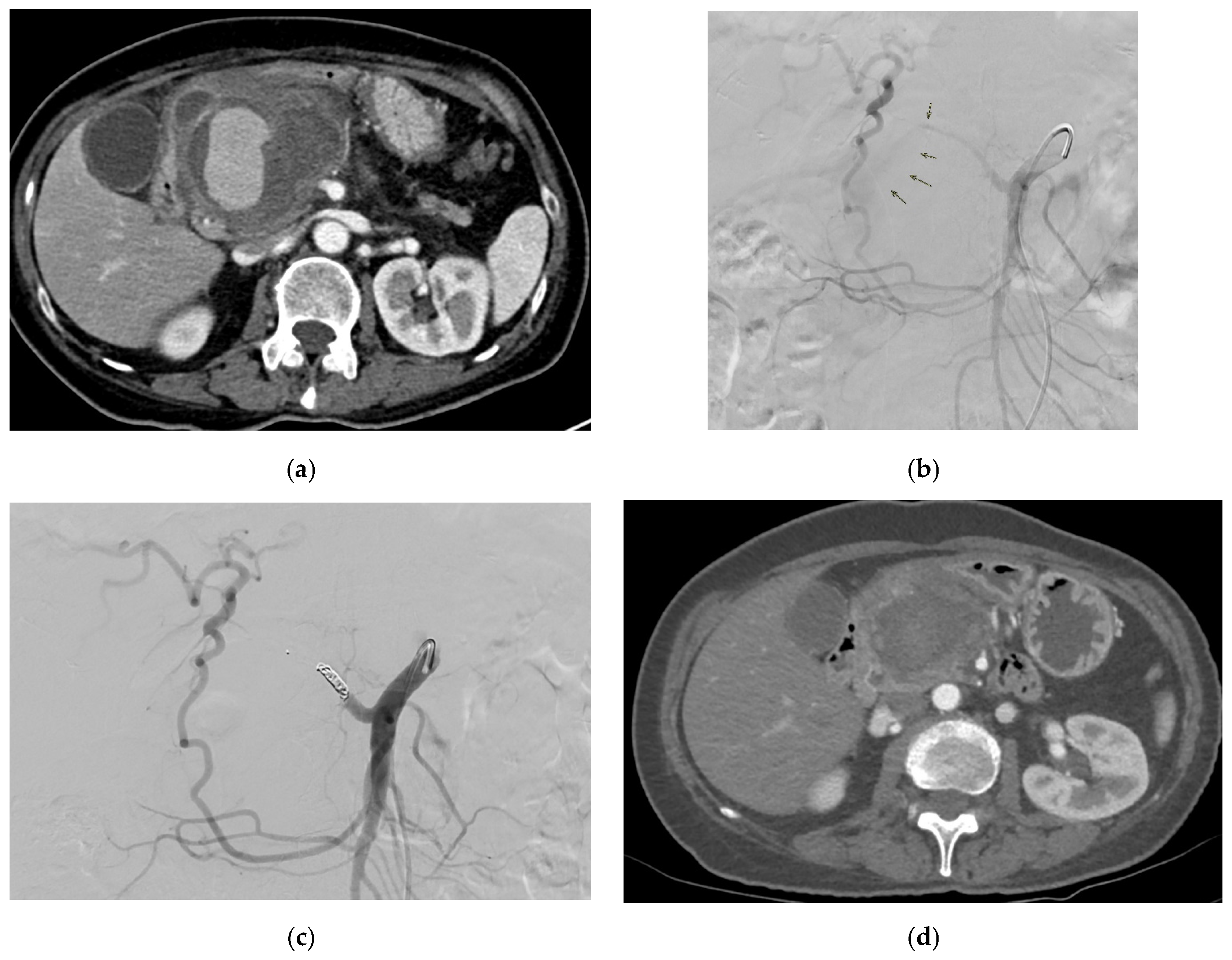
| Aneurysm Morphology | Common Locations | Embolization Options | Technical Notes |
|---|---|---|---|
| Saccular | Splenic, GDA, SMA | Coil, vascular plug | Suitable for super-selective embolization; easy to isolate neck |
| Fusiform | Hepatic, Celiac | Stent graft, parent artery sacrifice | Preservation of flow preferred; stent placement may be challenging in tortuous vessels |
| Wide-necked | Pancreaticoduodenal, Hepatic | Balloon-assisted coiling, stent + coil | Risk of coil migration; adjunctive techniques required |
| Mycotic or infected | Mesenteric, colic branches | Selective embolization, followed by surgery | Definitive treatment often surgical; embolization may temporize bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sule, A.A.; Sah, S.; Kwan, J.; Punamiya, S.; Shelat, V.G. Visceral Arterial Pseudoaneurysms—A Clinical Review. Medicina 2025, 61, 1312. https://doi.org/10.3390/medicina61071312
Sule AA, Sah S, Kwan J, Punamiya S, Shelat VG. Visceral Arterial Pseudoaneurysms—A Clinical Review. Medicina. 2025; 61(7):1312. https://doi.org/10.3390/medicina61071312
Chicago/Turabian StyleSule, Ashita Ashish, Shreya Sah, Justin Kwan, Sundeep Punamiya, and Vishal G. Shelat. 2025. "Visceral Arterial Pseudoaneurysms—A Clinical Review" Medicina 61, no. 7: 1312. https://doi.org/10.3390/medicina61071312
APA StyleSule, A. A., Sah, S., Kwan, J., Punamiya, S., & Shelat, V. G. (2025). Visceral Arterial Pseudoaneurysms—A Clinical Review. Medicina, 61(7), 1312. https://doi.org/10.3390/medicina61071312







