Cardioprotective Effects of Bosentan in Rats Subjected to Lung Ischemia–Reperfusion Injury
Abstract
1. Introduction
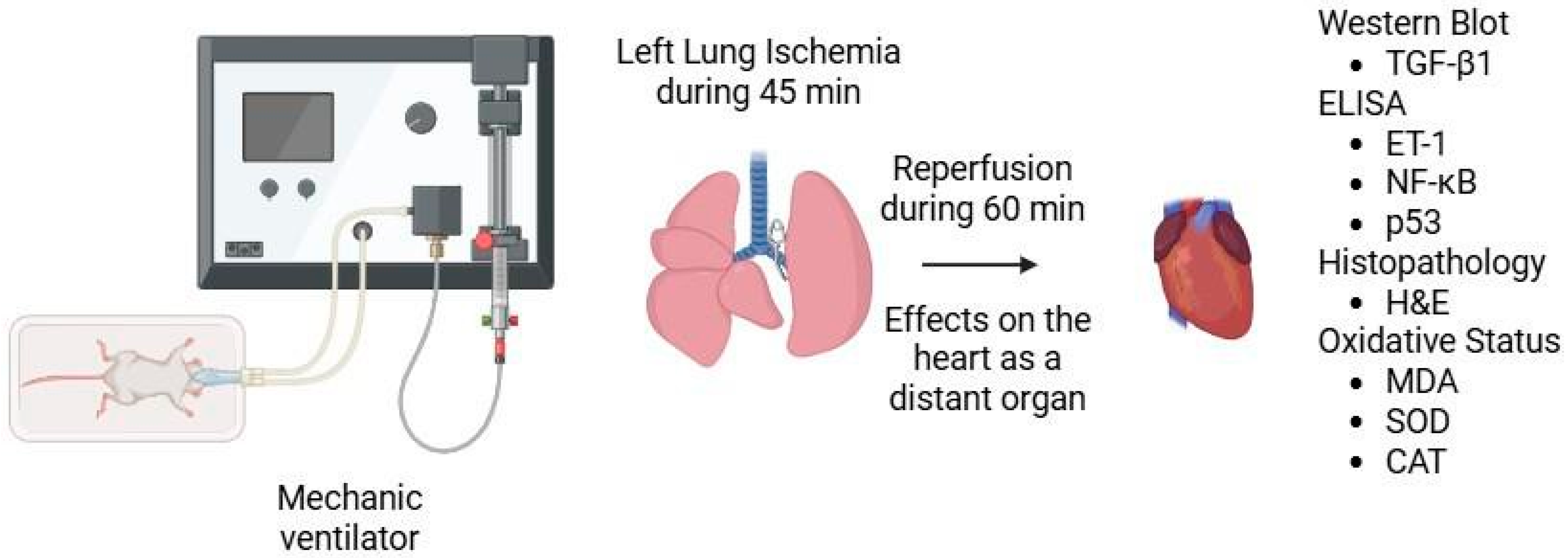
2. Materials and Methods
2.1. Histopathologic Analysis
2.2. Biochemical Analysis
2.3. Statistical Analysis
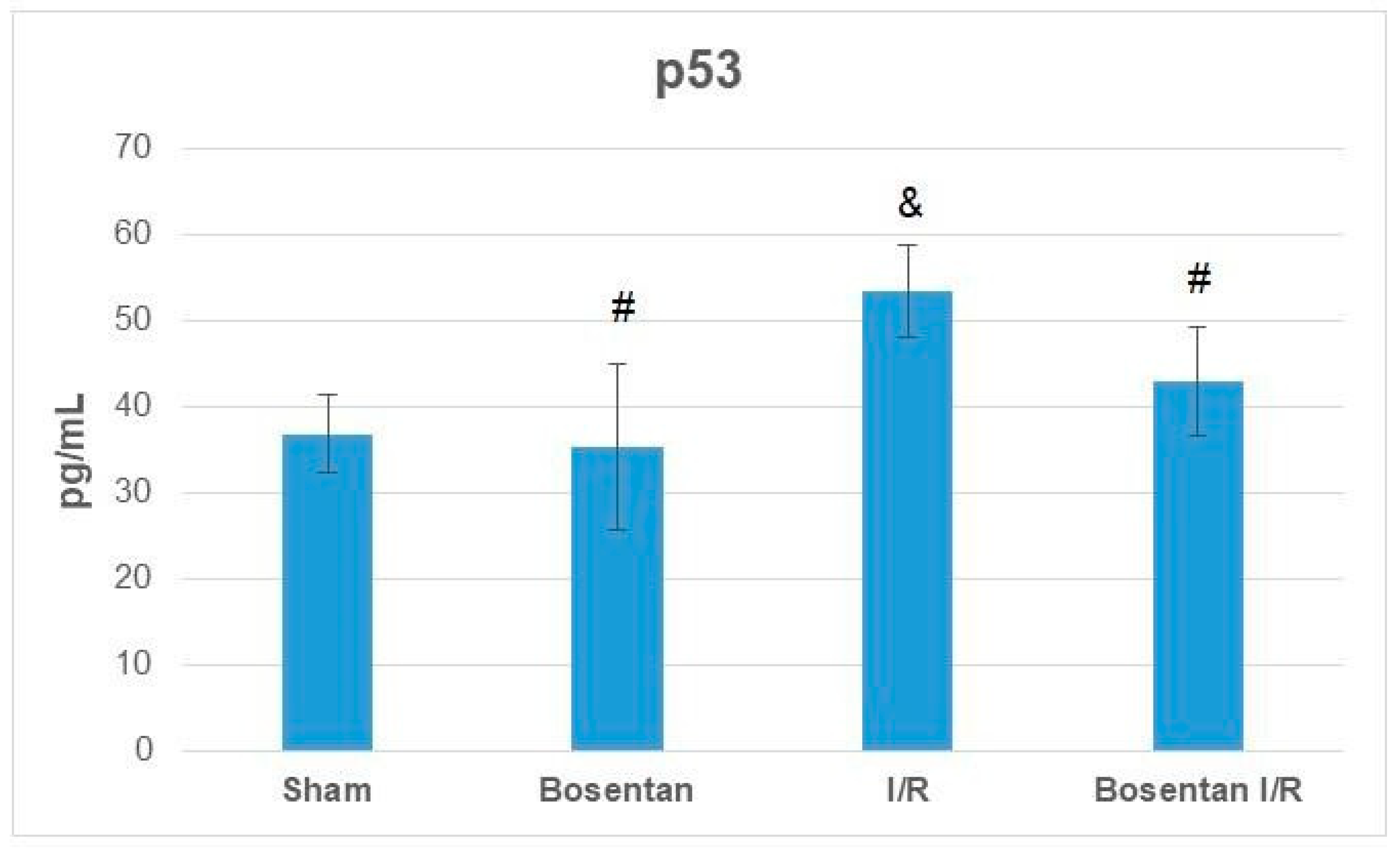
3. Results
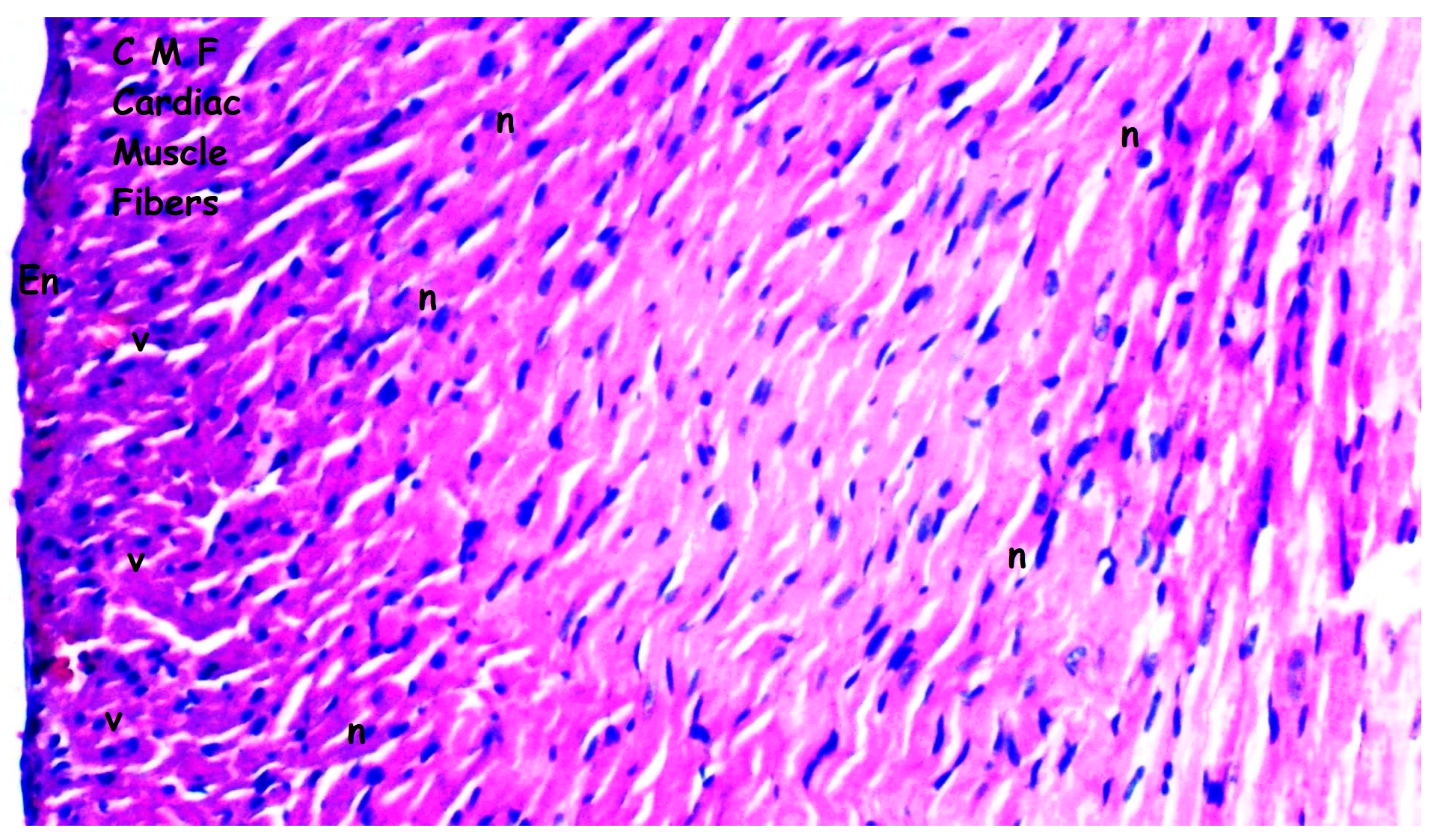
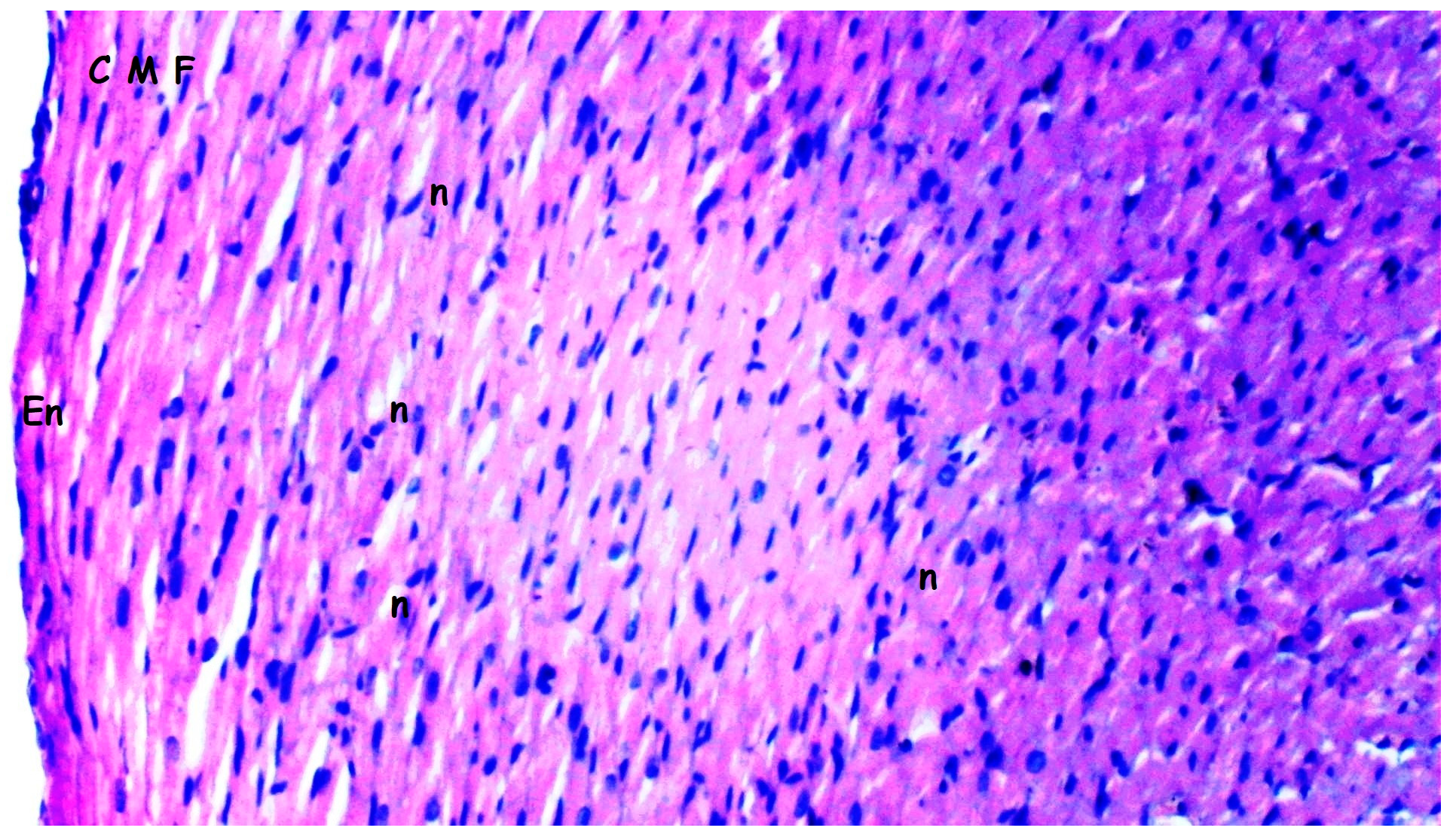
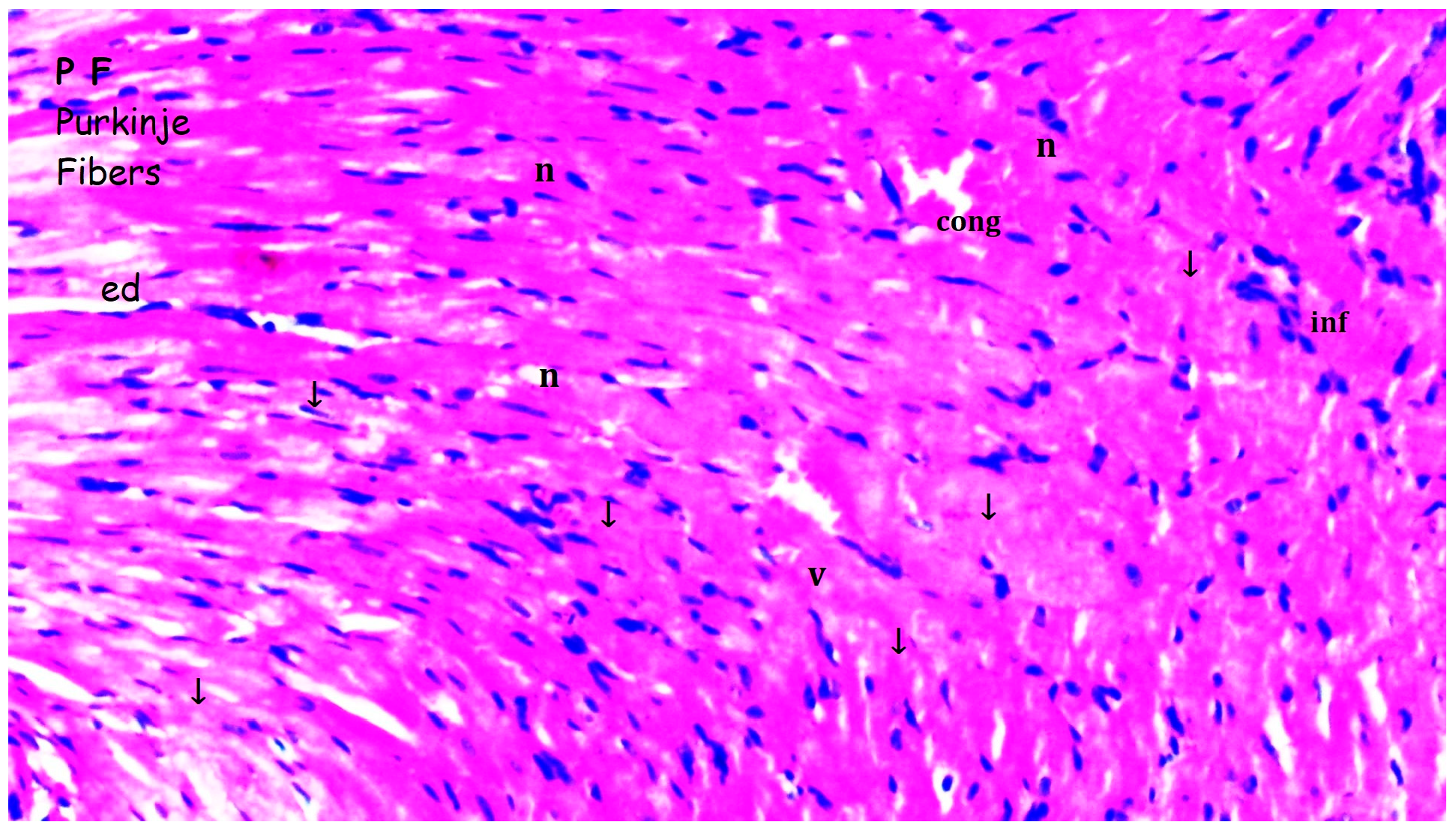
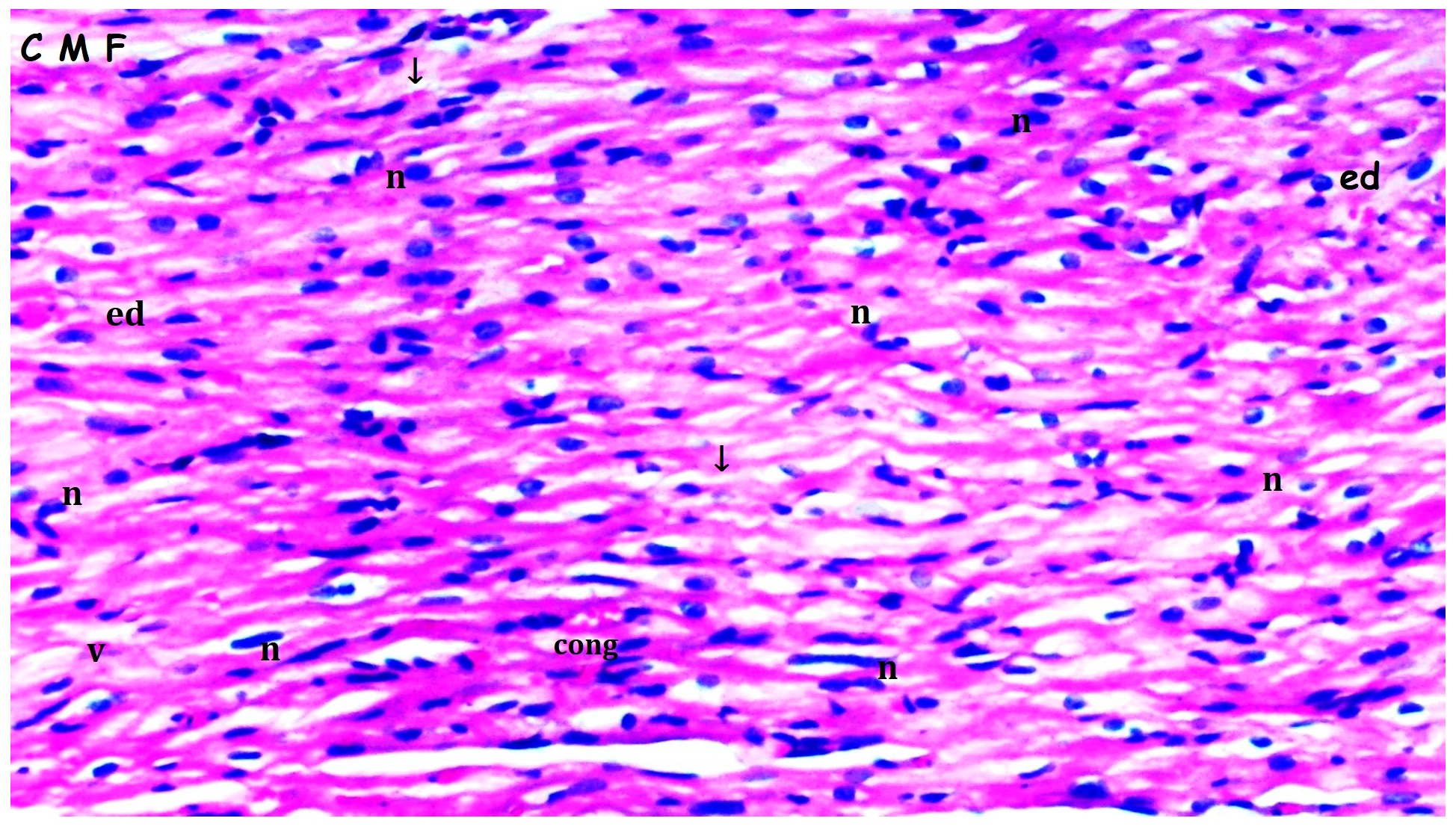
3.1. Histopathologic Findings
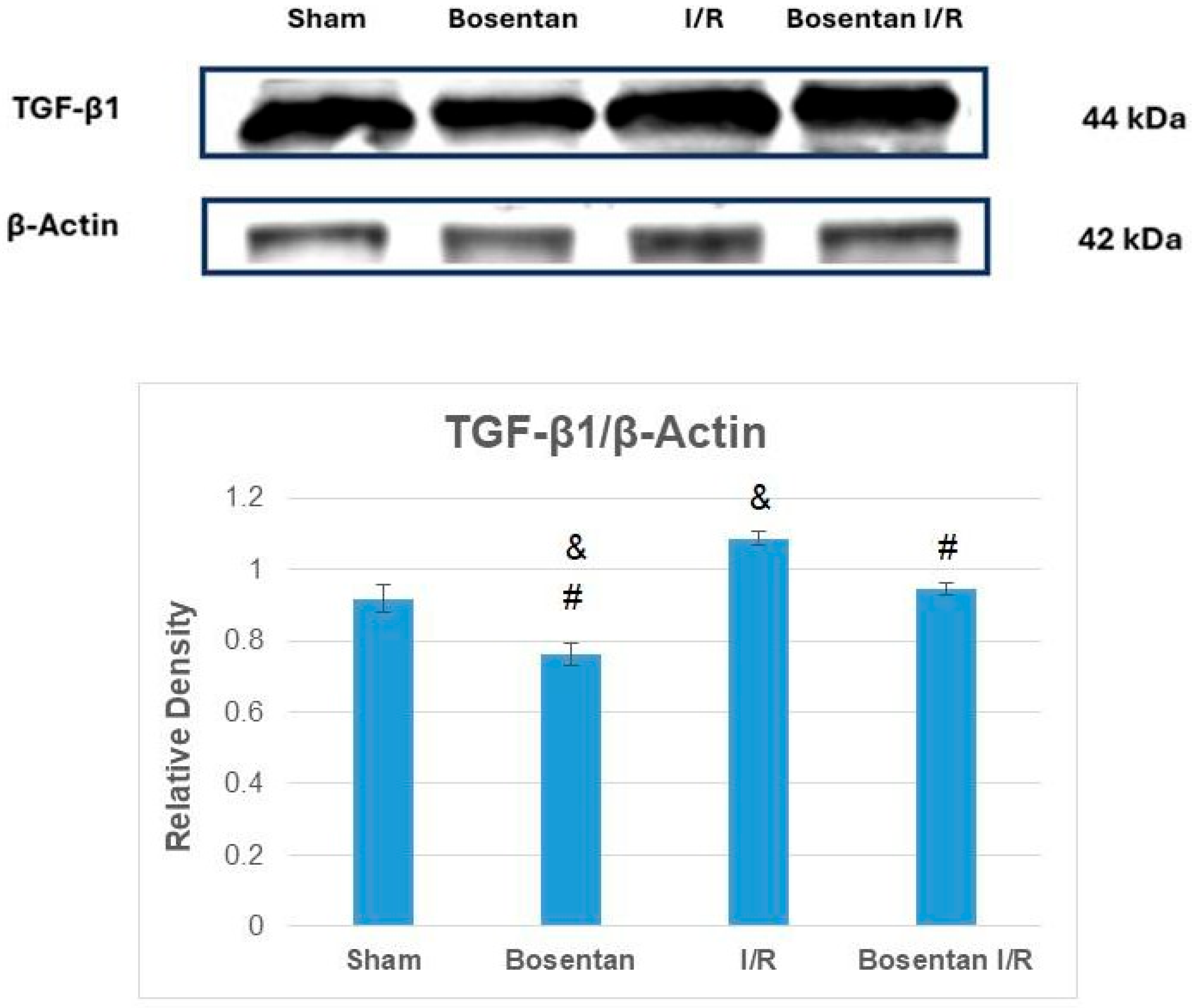
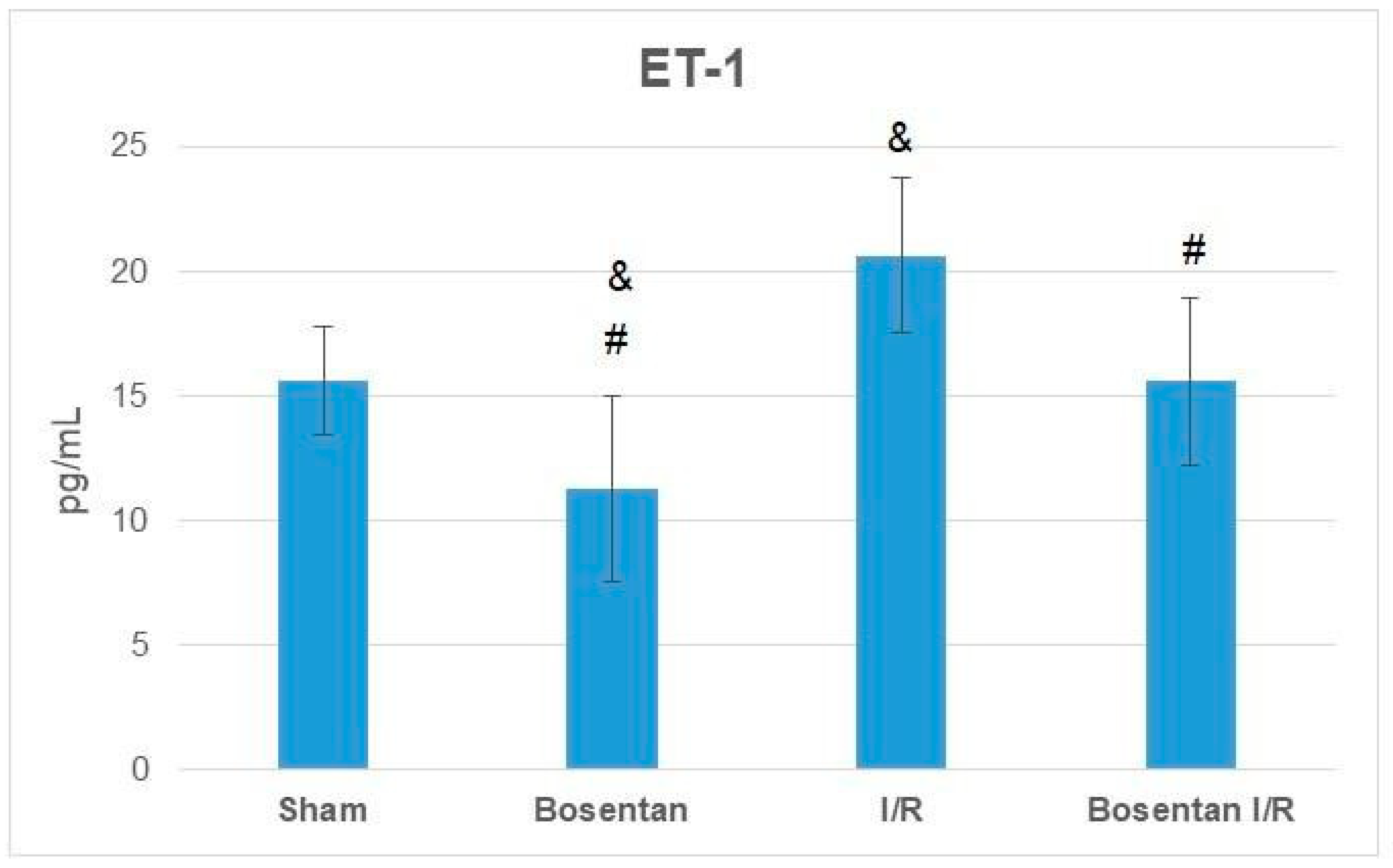
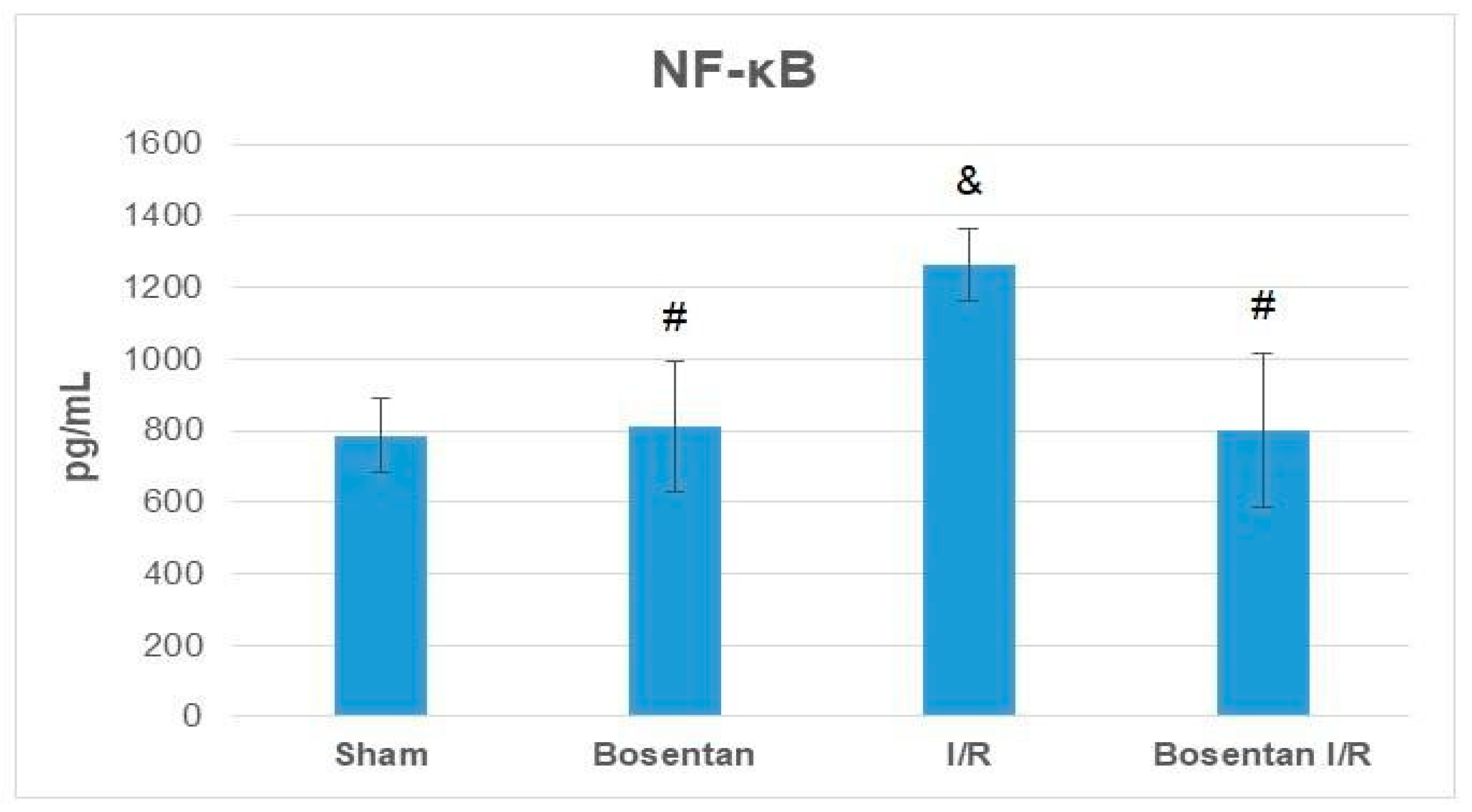
3.2. Biochemical Findings
4. Discussion
4.1. Study Limitations
4.2. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.W.M. Bosentan: A dual endothelin receptor antagonist for the management of pulmonary hypertension. Heart Dis. 2003, 5, 161–169. [Google Scholar] [CrossRef]
- Rubin, L.J.; Roux, S. Bosentan: A dual endothelin receptor antagonist. Expert Opin. Investig. Drugs 2002, 11, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.J.; Badesch, D.B.; Barst, R.J.; Galiè, N.; Black, C.M.; Keogh, A.; Pulido, T.; Frost, A.; Roux, S.; Leconte, I.; et al. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002, 346, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Bruzzone, M.; Di Vittorio, C.C.; Laganà, A.; Peroni, C.; Fusaro, E. Efficacy of bosentan in the treatment of Raynaud’s phenomenon in patients with systemic sclerosis never treated with prostanoids. Reumatismo 2014, 65, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, L.; Dogan, H.T.; Kilicarslan, A.; Aydin, M.E.; Ozer, A.; Demirtas, H.; Kilic, Y.; Iriz, E.; Kucuk, A.; Bayraktar, A.C.; et al. Effect of different doses of pregabalin on skeletal muscle ischaemia-reperfusion injury in rat. Bratisl. Med. J. 2017, 118, 417–422. [Google Scholar] [CrossRef]
- Özer, A.; Demirtaş, H.; Çomu, F.M.; Erer, D.; Kılıç, Y.; Mardin, B.; Arslan, M.; Küçük, A.; Oktar, G.L. Protective effect of erdosteine on erythrocyte deformability in a rat model of lower limb ischemia/reperfusion injury. Turk. J. Med. Sci. 2018, 48, 187–190. [Google Scholar] [CrossRef]
- Demirtas, H.; Özer, A.; Yıldırım, A.K.; Dursun, A.D.; Sezen, Ş.C.; Arslan, M. Protective Effects of BPC157 on Liver, Kidney, and Lung Distant Organ Damagein Ratswith Experimental Lower-Extremity Ischemia–Reperfusion Injury. Medicina 2025, 61, 291. [Google Scholar] [CrossRef]
- Özer, A.; Comu, F.M.; Demirtas, H.; Kılıç, Y.; Mardin, B.; Öztürk, L.; Iriz, E.; Arslan, M.; Küçük, A. Effect of different doses of pregabalin on erythrocyte deformability in rats with lower limb ischemia reperfusion injury. Anaesth. Pain Intensive Care 2017, 21, 350–353. [Google Scholar]
- Özer, A.; Erel, S.; Küçük, A.; Demirtaş, H.; Sezen, Ş.C.; Boyunağa, H.; Oktar, G.L.; Arslan, M. Evaluation of the effect of enriched hydrogen saline solution on distant organ (lung) damage in skeletal muscle ischemia reperfusion in rats. Sci. Prog. 2024, 107, 1–13. [Google Scholar] [CrossRef]
- Demirtaş, H.; Özer, A.; Gülcan, M.B.; Yığman, Z.; Küçük, A.; Tekin, E.; Ünal, Y.; Dursun, A.; Dağlı, A.; Arslan, M. Protective Effects of Bosentan via Endothelin Receptor Antagonism in Experimental Ischemia-Reperfusion Injury in the Lower Limb of Rats. Drug Des. Devel. Ther. 2025, 19, 1561–1573. [Google Scholar] [CrossRef]
- Gong, S.; Seng, Z.; Wang, W.; Lv, J.; Dong, Q.; Yan, B.; Peng, L.; He, X. Bosentan protects the spinal cord from ischemia reperfusion injury in rats through vascular endothelial growth factor receptors. Spinal Cord 2015, 53, 19–23. [Google Scholar] [CrossRef][Green Version]
- Kazimoglu, H.; Uysal, E.; Dokur, M.; Gurer, A.O.; Batcioglu, K.; Uyumlu, B.A.; Petekkaya, E.; Karadag, M. Comparison of the protective effects of selective endothelin-a receptor antagonist, ambrisentan, and dual endothelin-A/B receptor antagonist, bosentan, in experimental renal ischemia reperfusion injury. Bratisl. Lek. Listy 2020, 121, 547–553. [Google Scholar] [CrossRef]
- Gupta, S.K.; Saxena, A.; Singh, U.; Arya, D.S. Bosentan, the mixed ETA-ETB endothelin receptor antagonist, attenuated oxidative stress after experimental myocardial ischemia and reperfusion. Mol. Cell Biochem. 2005, 275, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Yamashita, C.; Okada, M.; Okada, K. Contribution of endothelin-1 to warm ischemia/reperfusion injury of the rat lung. Am. J. Respir. Crit. Care Med. 1995, 152 Pt 1, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Tamareille, S.; Terwelp, M.; Amirian, J.; Felli, P.; Zhang, X.Q.; Barry, W.H.; Smalling, R.W. Endothelin-1 Release during the Early Phase of Reperfusion Is a Mediator of Myocardial Reperfusion Injury. Cardiology 2013, 125, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Qu, F.; Liu, R.; Xia, Y. Differential expression of miR-142-3p protects cardiomyocytes from myocardial ischemia-reperfusion via TLR4/NFkB axis. J. Cell Biochem. 2020, 121, 3679–3690. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, D.; Zhou, C.; Cheng, K.; Liu, Z.; Chen, L.; Fang, L.; Xie, P. β-Actin as a loading control for plasma-based Western blot analysis of major depressive disorder patients. Anal. Biochem. 2012, 427, 116–120. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Qiu, Z.; Lei, S.Q.; Leng, Y.; Li, W.Y.; Xia, Z.Y. The Role of P53 in Myocardial Ischemia-Reperfusion Injury. Cardiovasc. Drugs Ther. 2025, 39, 195–209. [Google Scholar] [CrossRef]
- Chin, K.; Channick, R. Bosentan . Expert Rev. Cardiovasc. Ther. 2004, 2, 175–182. [Google Scholar] [CrossRef]
- Eriksson, C.; Gustavsson, A.; Kronvall, T.; Tysk, C. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension: A case-report and review of the literature. J. Gastrointestin Liver Dis. 2011, 20, 77–80. [Google Scholar]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Fiser, S.M.; Tribble, C.G.; Long, S.M.; Kaza, A.K.; Cope, J.T.; Laubach, V.E.; Kern, J.A.; Kron, I.L. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J. Thorac. Cardiovasc. Surg. 2001, 121, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, H.; Yildirim, A.K.; Ozer, A.; Dursun, A.D.; Sezen, S.C.; Kucuk, A.; Arslan, M. Protective Effects of Metformin in Non-Diabetic Rats with Experimentally Induced Lower Extremity Ischemia–Reperfusion Injury. Turk. J. Vasc. Surg. 2025, 34, 33–39. [Google Scholar] [CrossRef]
- Demirtas, H.; Ozer, A.; Yigit, D.; Dursun, A.D.; Kosa, C.; Kucuk, A.; Simsek, E.; Arslan, M. In-Vivo Antioxidant and Therapeutic Effects of Ellagic Acid on Ischemia–Reperfusion Injury in Skeletal Muscle. Turk. J. Vasc. Surg. 2025, 34, 52–60. [Google Scholar] [CrossRef]
- Iriz, E.; Iriz, A.; Take, G.; Ozgul, H.; Oktar, L.; Demirtas, H.; Helvacioglu, F.; Arslan, M. Iloprost and vitamin C attenuates acute myocardial injury induced by suprarenal aortic ischemia-reperfusion in rabbits. Bratisl. Med. J. 2015, 116, 627–631. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar]
- Durak, I.; Canbolat, O.; Kavutçu, M.; Oztürk, H.S.; Yurtarslani, Z. Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J. Clin. Lab. Anal. 1996, 10, 17–20. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, Netherlands, 1974; pp. 673–884. [Google Scholar]
- Hashimoto, S. A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal. Biochem. 1974, 62, 426–435. [Google Scholar] [CrossRef]
- Kara, H.; Ozer, A.; Arpaci, H.; Demirtas, H.; Comu, F.M.; Oktar, G.L.; Erer, D.; Kucuk, A.; Arslan, M. Effect of alprostadil on erythrocyte deformability in ischemia reperfusion injury. Bratisl. Med. J. 2015, 116, 509–511. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Demirtaş, H.; Yıldırım, A.K.; Özer, A.; Dursun, A.D.; Sezen, Ş.C.; Yığman, Z.; Küçük, A.; Arslan, M. Potential protective effects of boldine in rat with an experimental myocardial ischemia-reperfusion model. J. Updates Cardiovasc. Med. 2025, 13, 41–52. [Google Scholar] [CrossRef]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ. Transplant. 2016, 21, 246–252. [Google Scholar] [CrossRef]
- Gielis, J.F.; Boulet, G.A.; Briedé, J.J.; Horemans, T.; Debergh, T.; Kussé, M.; Cos, P.; Van Schil, P.E. Longitudinal quantification of radical bursts during pulmonary ischaemia and reperfusion. Eur. J. Cardiothorac. Surg. 2015, 48, 622–629. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wu, J.L.; Ni, Z.L. Overexpression of microRNA-202-3p protects against myocardial ischemia-reperfusion injury through activation of TGF-β1/Smads signaling pathway by targeting TRPM6. Cell Cycle 2019, 18, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, M.; Sun, Q.; Liu, Z.; Cardounel, A.J.; Swartz, H.M.; He, G. Hyperoxia and transforming growth factor β1 signaling in the post-ischemic mouse heart. Life Sci. 2013, 92, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Azad, A.; Schnitt, R.; He, G.; Weigert, C.; Ichijo, H.; Sen, C.K. Fra-2 mediates oxygen-sensitive induction of transforming growth factor beta in cardiac fibroblasts. Cardiovasc. Res. 2010, 87, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Redondo, S.; Santos-Gallego, C.G.; Tejerina, T. TGF-beta1: A novel target for cardiovascular pharmacology. Cytokine Growth Factor Rev. 2007, 18, 279–286. [Google Scholar] [CrossRef]
- Fu, X.B.; Yang, Y.H.; Sun, T.Z.; Gu, X.-M.; Jiang, L.-X.; Sun, X.-Q.; Sheng, Z.-Y. Effect of intestinal ischemia-reperfusion on expressions of endogenous basic fibroblast growth factor and transforming growth factor betain lung and its relation with lung repair. World J. Gastroenterol. 2000, 6, 353–355. [Google Scholar] [CrossRef]
- Grünenfelder, J.; Miniati, D.N.; Murata, S.; Falk, V.; Hoyt, E.G.; Robbins, R.C. Up-regulation of Bcl-2 through hyperbaric pressure transfection of TGF-beta1 ameliorates ischemia-reperfusion injury in rat cardiac allografts. J. Heart Lung Transplant. 2002, 21, 244–250. [Google Scholar] [CrossRef]
- Daddi, N.; Suda, T.; D’Ovidio, F.; Kanaan, S.A.; Tagawa, T.; Grapperhaus, K.; Kozower, B.D.; Ritter, J.H.; Yew, N.S.; Mohanakumar, T.; et al. Recipient intramuscular cotransfection of naked plasmid transforming growth factor beta1 and interleukin 10 ameliorates lung graft ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2002, 124, 259–269. [Google Scholar] [CrossRef]
| Group Sham (n = 6) | Group Bosentan (n = 6) | Group I/R (n = 8) | Group Bosentan + I/R (n = 8) | p Value (ANOVA) | |
|---|---|---|---|---|---|
| [23,33] Myocardial disorganization | 0.50 ± 0.22 | 0.50 ± 0.22 | 1.25 ± 0.16 *,& | 0.63 ± 0.18+ | 0.028 |
| Inflammation and neutrophil infiltration | 0.33 ± 0.21 | 0.50 ± 0.22 | 1.25 ± 0.16 *,& | 0.63 ± 0.18+ | 0.012 |
| Interstitial fibrosis | 0.33 ± 0.21 | 0.50 ± 0.22 | 1.25 ± 0.16 *,& | 0.75 ± 0.16 | 0.010 |
| Interstitial edema | 0.33 ± 0.21 | 0.50 ± 0.22 | 1.25 ± 0.16 *,& | 0.63 ± 0.18 + | 0.012 |
| Swelling of myocardial cells | 0.33 ± 0.21 | 0.33 ± 0.21 | 1.13 ± 0.13 *,& | 0.63 ± 0.18 + | 0.013 |
| Myocardial necrosis | 0.17 ± 0.17 | 0.33 ± 0.21 | 0.75 ± 0.16 | 0.63 ± 0.18 | 0.126 |
| Group Sham (n = 6) | Group Bosentan (n = 6) | Group I/R (n = 8) | Group Bosentan + I/R (n = 8) | p Value (ANOVA) | |
|---|---|---|---|---|---|
| MDA (nmol/g protein) | 111.22 ± 2.94 | 112.39 ± 3.41 | 136.88 ± 5.02 *,& | 120.18 ± 2.67 + | <0.001 |
| SOD (IU/mg protein) | 34.18 ± 2.35 | 28.73 ± 2.85 | 18.22 ± 1.66 *,& | 26.17 ± 1.73 + | <0.001 |
| CAT (IU/mg protein) | 52.21 ± 2.36 | 51.24 ± 1.17 | 38.16 ± 1.59 *,& | 50.40 ± 2.32+ | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demiröz, Ş.M.; Küçük, A.; Tekin, E.; Söylemez, S.; Yılmaz, H.; Sezen, Ş.C.; Atlı, M.; Demirtaş, H.; Özer, A.; Ünal, Y.; et al. Cardioprotective Effects of Bosentan in Rats Subjected to Lung Ischemia–Reperfusion Injury. Medicina 2025, 61, 1298. https://doi.org/10.3390/medicina61071298
Demiröz ŞM, Küçük A, Tekin E, Söylemez S, Yılmaz H, Sezen ŞC, Atlı M, Demirtaş H, Özer A, Ünal Y, et al. Cardioprotective Effects of Bosentan in Rats Subjected to Lung Ischemia–Reperfusion Injury. Medicina. 2025; 61(7):1298. https://doi.org/10.3390/medicina61071298
Chicago/Turabian StyleDemiröz, Şevki Mustafa, Ayşegül Küçük, Esra Tekin, Sibel Söylemez, Hanife Yılmaz, Şaban Cem Sezen, Muharrem Atlı, Hüseyin Demirtaş, Abdullah Özer, Yusuf Ünal, and et al. 2025. "Cardioprotective Effects of Bosentan in Rats Subjected to Lung Ischemia–Reperfusion Injury" Medicina 61, no. 7: 1298. https://doi.org/10.3390/medicina61071298
APA StyleDemiröz, Ş. M., Küçük, A., Tekin, E., Söylemez, S., Yılmaz, H., Sezen, Ş. C., Atlı, M., Demirtaş, H., Özer, A., Ünal, Y., & Arslan, M. (2025). Cardioprotective Effects of Bosentan in Rats Subjected to Lung Ischemia–Reperfusion Injury. Medicina, 61(7), 1298. https://doi.org/10.3390/medicina61071298






