Abstract
Psoriasis is a chronic inflammatory autoimmune disease that causes significant deterioration of the quality of life, and due to its multifactorial causes, it is often difficult to manage. Apart from genetic and environmental components, an important part of its pathophysiology comprises an oxidative stress induction that the standard antioxidative mechanisms of the human body cannot compensate for. Moreover, in many psoriatic patients, there is a documented imbalance between antioxidant and pro-oxidative factors. Usually, psoriasis is evaluated using the Psoriasis Area and Severity Index (PASI) score. It has been demonstrated that dietary choices can lead to significant modification of PASI scores. Hypocaloric diets that are rich in antioxidants are highly effective in this regard, especially when focusing on vegetables and restricting consumption of animal-derived protein. Specific dietary regimens, namely the Mediterranean diet and potentially the ketogenic diet, are very beneficial, in the former case owing in large part to the omega-three fatty acids it provides and its ability to alter gut microbiome, a factor which seems to play a notable role in the pathogenesis of the disease. Another option is the topical application of vitamin D and its analogues, combined with corticosteroids, which can ameliorate the manifestations of psoriasis at the level of the skin. Finally, oral vitamin D supplementation has a positive impact on psoriatic arthritis and can mitigate the risk of associated comorbidities.
1. Introduction
In recent years there has been an increased incidence of autoimmune pathologies, and this has led to an heightened awareness of the associated morbidity as well as psychological and mental challenges. One of the most well-known autoimmune diseases is psoriasis, a complex chronic immune-mediated disease presenting with a variety of manifestations, of which the most prominent are usually the cutaneous ones, although other locations and systems may be affected as well [1,2,3,4].
Currently, it is estimated that psoriasis affects over 120 million people at a worldwide level, with incidence and prevalence showing great geographical variations [5]. Notably, it seems that higher-income countries and people of Caucasian ancestry are at higher risk of developing psoriasis [6]. The average prevalence is around 8% in the adult population [6], while, based on recent research results, the actual burden of disease is higher due to underdiagnosis of psoriasis-related morbidities like psoriatic arthritis [7]. The systemic inflammatory nature of the disease means that patients usually exhibit numerous comorbidities [8,9], which in turn decrease their quality of life [10,11,12]. The most common signs of the disease are red and scaly plaques, which usually cause some degree of itching and discomfort [13]; other comorbidities, of varying degrees of severity, are also associated with psoriasis [14].
While there is a genetic component in psoriasis, not all patients carrying the implicated genes will develop the disease. Perhaps the most prominent genetic associations at the moment are genes of the HLA complex [15]. It becomes therefore apparent that since genetic determinism alone cannot justify on its own the pathogenetic model, the onset of the disease must be related to environmental factors as well. It is also well established that the course and severity of the disease are influenced predominantly by environmental rather than intrinsic factors [16,17]. Another important contributing factor, based on recent research, is an increased amount of oxidative stress, which leads to the activation of a number of proinflammatory signalling pathways [18] (Figure 1).
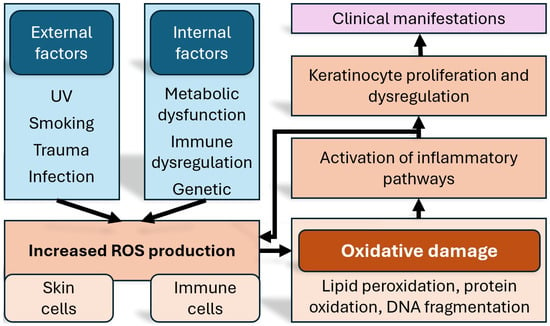
Figure 1.
Schematic representation of the how oxidative stress affects inflammation, and the manifestations of psoriasis. ROS = reactive oxygen species. DNA = deoxyribonucleic acid.
One of the distinguishing features of psoriasis is the exaggerated production of reactive oxygen species (ROS) inside keratinocytes and immune cells [19]. The generated redox imbalance aggravates the inflammation via signalling pathways such as MAPK, STAT3, and NF-κB and contributes not only to local skin lesions but also to systemic comorbidities [20,21]. Cutaneous oxidative stress is generated in response to inflammatory cytokines such as IL-17 and TNF-α, leading to lipid peroxidation, protein oxidation, and DNA fragmentation; in contrast, systemic oxidative stress appears as an extension of the chronic inflammatory process beyond the skin, and the increased pro-inflammatory cytokine levels can negatively affect endothelial cells, leading to dysfunction [22,23].
Aside from medical interventions, the environmental factors implicated are psychological stress, substance and tobacco use and abuse, and diet. By themselves, these factors have been studied for decades by numerous researchers trying to establish causal relationships between diseases and risk factors. Indeed, physiological and psychological stress is now seen as being associated with both mortality and morbidity [24,25,26]. Smoking and excessive alcohol consumption have a host of research data demonstrating their importance as risk factors [27,28,29,30,31,32], while the role of diet in health maintenance and disease prevention has come into focus in the 21st century [33,34,35,36,37,38].
In the specific case of psoriasis, it seems that the principal associated comorbidity is obesity [39,40,41,42,43]. Obesity increases the risk of psoriasis incidence, while the cytokine profile in patients with metabolic syndrome and obesity and psoriatic patients is similar [44,45]. Obesity is more likely to develop in people following a sedentary lifestyle [46,47] and with high-fat diets [48,49]; dietary factors, both in the context of obesity and on their own, can modify disease progression and severity in the case of psoriasis.
In the last decades medical innovations have led to the development of novel therapeutic strategies [50]. Given the recent focus on the influence of nutrition and oxidative stress in psoriatic patients, the focus of this review will be the presentation of how diet, both from a quantitative and a qualitative perspective, influences the onset and severity of psoriasis, and via which mechanisms such influences are exerted.
2. Materials and Methods
In endeavoring to gather a complete and representative sample of research on psoriasis and nutrition, we have performed a thorough search in the most complete and widely used medical databases, namely PubMed, Scopus, Science Direct, and Medline. The search strategy relied on the terms “psoriasis”, “diet”, “obesity”, “oxidative”, “oxidants”, “antioxidants”, and “inflammation” in different combinations so as to cover as much probable relevant research material as possible, using the appropriate Boolean operators. For a paper to be considered as appropriate, the search terms should have to be included either in the title or abstract, preferably both. We tried different combinations of these keywords to ensure, as much as possible, the inclusion of all possible relevant papers, and we subsequently removed from our selection papers that did not exclusively deal with psoriasis but with oxidative stress in the context of autoimmune diseases. We have further excluded papers published in languages other than English, manuscripts not published in peer-reviewed journals, and those with a research question or patient population that were not relevant for our study.
Even though psoriasis was first described in modern medical terminology in the 18th century [51], the role of diet in its evolution and management began to be assessed after the 1950s, and research has only specifically focused on this aspect after 2010. Therefore, we have elected to confine our search to papers published between 2014 and 2024; the starting year has been selected specifically due to the relevant WHO resolution [52], which raised awareness of psoriasis and served as an impetus for intensifying research efforts. We have chosen to include, wherever possible, reviews that delve into mechanisms and associations, but where original papers reported essential or novel information, we have included them as well.
3. The Interplay Between Psoriasis and Nutrition
The profound influence of diet on human health and disease [53] has begun to be understood in the last decades [38,54]. At the same time, a notable majority of adults in the Western world suffer from at least one chronic disease, whose evolution and even risk of incidence may be associated with dietary factors [55,56].
At the same time, several different diet patterns have been implicated in the management of different diseases. For example, ketogenic diets may be useful in the management of neurological conditions [57]. Furthermore, nutritional supplementation may have potential in inflammatory biomarker control [58], and certain nutritional products may be useful in managing macular degeneration [59,60]. Other authors have focused on the role of gut microbiota in disease [61,62]. All these and other factors have been implicated in the incidence and management of psoriasis. As such, in this section, we will explore the role of oxidative stress, of diet and nutritional choices, of other supplements in general, and of vitamin D supplementation in particular.
3.1. The Role of Oxidative Stress in the Pathogenesis of Psoriasis
Oxidative stress can be defined as an imbalance manifesting as a temporary or chronic increase in the levels of free oxygen/nitrogen radicals resulting either from an increased production or a decrease in the ability of antioxidant systems to eliminate them [63], contributing to the pathogenesis of psoriasis.
Oxidative stress levels are measured by markers such as malondialdehyde (MDA), total oxidative stress (TOS), oxidative stress index (OSI), catalase (CAT), myeloperoxidase (MPO), ferroxidase (FOX), ischemia-modified albumin (IMA), paraoxonase-1 (PON-1), total antioxidant status (TAS), and 8-hydroxy 2′-deoxyguanosine (8H2D) [18,64]. Other useful markers include adipokines, namely adiponectin, leptin, visfatin, and resistin, due to their immunomodulating action [63,65], as well as urinary biopyrins [63,66]. There exist correlations between these markers and the severity of psoriasis, as measured by the Psoriasis Area and Severity Index (PASI) [18,63,66]; higher oxidative stress levels were associated with increased PASI scores. Out of all of them, the strongest correlation was found to exist between MDA levels and PASI, highlighting its potential as a biomarker for assessing psoriasis severity [67]. Thus, these findings support the theory that oxidative stress plays a crucial role in the development and complications of psoriasis.
Antioxidant enzymes, such as superoxide dismutase (SOD) and CAT, play a crucial role in reducing ROS levels, which are implicated in the pathogenesis of psoriasis [68,69,70]. A deficiency in these enzymes can lead to oxidative stress, contributing to inflammation and hyperproliferation of keratinocytes [43]. Therefore, maintaining or restoring the activity of these enzymes may offer therapeutic benefits in managing psoriasis symptoms [18].
In patients with active psoriasis, the levels of antioxidant enzyme levels, particularly SOD and CAT, are often reduced, indicating an impaired antioxidant defense system [18]. This deficiency can lead to increased oxidative stress, exacerbating inflammation and skin lesions associated with psoriasis. Consequently, monitoring and potentially restoring antioxidant enzyme levels may have therapeutic implications, offering a strategy to mitigate oxidative damage and improve disease management [18].
Another study emphasizes systemic inflammation through redox mechanisms, showing that oxidative stress associated with the development of psoriasis leads to oxidative protein changes, including a wide range of lipid peroxidation products [71]; the authors demonstrate that psoriasis-associated diseases can be effectively treated by inhibiting the formation of lipid peroxidation product–protein adducts and adjusting their concentrations during psoriasis therapy [71].
3.2. The Role of Diet in the Management of Psoriasis
Most studies agree that dietary patterns play a significant role in the management of psoriasis [40,70,72,73,74,75,76,77,78,79,80], as various skin diseases and conditions can be prevented or improved through changes in diet. Various dietary interventions, as well as single nutrients, can positively impact the clinical presentation, severity, and course of the disease [81]. Hypocaloric diets as well as antioxidant-rich diets can promote weight loss, reduce oxidative stress, and improve both the severity and the response of the condition to systemic treatments [40,73,76,82].
Since reducing caloric intake was found to alleviate symptoms [80,81], some forms of fasting, such as intermittent circadian fasting [74,83,84] and TRE (time-restricted eating) [78], may be promising in terms of their potential for symptom amelioration. This is in line with findings that suggest that intermittent fasting is associated with beneficial effects on autoimmune pathologies [74,83,84].
Generally, specialized diets, such as protein-restricted and vegetarian diets, may suppress systemic inflammation and inhibit angiogenesis [73], creating a less favorable environment for psoriasis. Diets rich in vegetables, with low or even zero animal protein intake, when combined with appropriate nutritional supplementation, seem to be associated with symptom amelioration in some patients, presumably due to their high fiber content and low saturated fat intake [73]. Although they may be wrongly characterized as risky for skin health due to nutritional deficiencies, such as lack of riboflavin (B2) and vitamin A, well-devised vegan diets can nevertheless meet nutritional needs and benefit inflammatory skin conditions like psoriasis, acne, and atopic dermatitis [85].
Elimination diets (e.g., gluten-free diet) may work for some patients, who report improvement by eliminating specific foods that may trigger flare-ups [82,86,87,88]. Common triggers include gluten-containing grains for those with gluten sensitivity or intolerance, dairy products, refined carbohydrates, nightshade vegetables (like tomatoes and potatoes), high-sugar foods, and processed foods [88]. However, these effects are individualistic; what triggers one person’s condition may not affect another. Consequently, when it comes to the totality of psoriatic patients, the benefits of a gluten-free diet are indeterminate [81].
The Mediterranean diet, which is rich in fruits and vegetables, provides polyphenols and antioxidants that help combat oxidative stress. Antioxidants such as flavonoids, vitamins A, C, and E, and β-carotene, along with oligo-elements like copper, manganese, zinc, and selenium, may offer protective effects against intrinsic skin damage [89]. Interestingly, it has been suggested that this diet’s beneficial effects can also be tied to changes in the gut microbiota [90]. Overall, greater adherence to the Mediterranean diet is linked to less severe forms of psoriasis and improved quality of life for patients [73,76,78,82,89,91,92]. The Mediterranean-like model tested by Castaldo et al. [93] on obese, drug-naïve patients in particular, consisting of a protein-sparing, very-low-calorie ketogenic diet, resulted in notable alterations of PASI scores. While the Mediterranean diet sensu stricto may be considered culturally specific to the Mediterranean region, its basic tenets and principles may be applicable in a wide range of populations, irrespective of their nationality or residential region. The effectiveness of the Mediterranean diet, but also of the ketogenic diet, in affecting psoriatic activity and the associated inflammatory markers is also corroborated by Lambadiari et al. [94] and Katsimbri et al. [95]. It is worth mentioning, however, that the ketogenic diet has been reported to have both ameliorating and exacerbating effects on psoriasis [96]. Apart from the presence of ambiguous data on this matter, it should also be mentioned that ketogenic diets have a number of potential adverse effects, including nutrient deficiency, fatigue, increased LDL cholesterol, or other chronic diseases [97,98]; therefore, a careful risk-benefit assessment should be undertaken on a patient-by-patient basis.
At the opposite end of the spectrum, there are the high-fat diets, like the Western diet, which may exacerbate the cutaneous manifestations, particularly through the action of saturated fatty acids (SFAs), such as palmitic acid, due to enhancement of skin inflammation via immune activation, independent of obesity [78,99].
Thus, this kind of fatty acid should be avoided in order to reduce psoriasis-related inflammatory lesions at the level of the skin [72]. The opposite is true of unsaturated fatty acids, namely monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids, which have been found to be able to decrease the risk of immunometabolic diseases. MUFAs, such as oleic acid, which are found in extra-virgin olive oil, safeguard lipoproteins and cell membranes from detrimental oxidative damage. PUFAs are divided into omega-3 and omega-6 fatty acids. Patients should be encouraged to increase their intake of omega-3 fatty acids (found in fish, flaxseed, and walnuts) since they can reduce inflammation [67,76,89]. Conversely, omega-6 fatty acids (commonly found in processed foods and vegetable oils) are promoters of inflammation, and consumption of the associated food sources should be restricted [43]. A cross-sectional study focusing on patterns of food consumption in psoriasis patients illustrates this point by assessing Pattern 1, consisting mostly of processed foods, and Pattern 2, being made up predominantly of fresh foods. Compliance with Pattern 2 was linked to normal serum lipids and blood pressure, a lower waist-to-hip ratio, and decreased psoriasis (skin) activity [100]. In the same vein, Clark et al. [101] conclude that the supplementation of omega-3 fatty acids leads to improvement of PASI score, as well as of the erythema and scaling, though it remains uncertain whether they can help with other signs and symptoms, like desquamation and itching. The beneficial nature of omega-3 fatty acids is also supported by the fact that the Mediterranean diet, the effectiveness of which was described previously, has a favorable omega-3 to omega-6 ratio [40].
As far as specific dietary instructions are concerned, according to the United States National Institutes of Health Panel [102], the ideal ratio of omega-3 to omega-6 fatty acids should be approximately 1:1.80, though other reports place it at a range of 1:30 to 1:50 [103,104,105].
Natural antioxidants can be used to treat inflammatory diseases, including psoriasis. Because of its anti-inflammatory and antioxidant properties, it has been discovered that the natural phytocannabinoid oil has been suggested as a treatment for psoriasis [106].
Similarly, it has been discovered that cannabidiol (CBD) can provide considerable protection from UV-induced oxidative stress to skin cells [107,108], as, after applying CBD to psoriatic skin, there was a decrease in proinflammatory mediators and relevant proteins [107], and it induces metabolic alterations in keratinocytes [109]; other authors report a decrease in keratinocyte proliferation [103,108]. Furthermore, psoriatic arthritis patients report less pain when using CBD [75,108]. Although there is insufficient evidence to confirm this, other antioxidant and anti-inflammatory substances, such as the lipid extract of microalgae [69], are proposed as possible anti-psoriatic factors, their actions being based on preventing lipid peroxidation products from interacting with proteins. Regarding CBD, it must be stressed that this is not a risk-free substance, and a host of adverse effects have been reported [110]; at the same time, more clinical trials on its use in psoriasis patients must be designed, with the aim of elucidating the full spectrum of its therapeutical value and its benefit-to-adverse-effect ratio. We must note that the aforementioned CBD applications are mostly researched at a cellular level in vitro; however, based on these encouraging results, several authors have explored its possible use as a supplement [111,112].
Anti-inflammatory effects can be exerted by adhering to diets that include foods such as fruits, vegetables, whole grains, fatty fish (rich in omega-3 fatty acids), nuts, and seeds [85,87,89]. This is made possible due to their ability to assist in lowering oxidative stress and modulating immune responses, having been associated with lower levels of inflammation markers, such as C-reactive protein (CRP) [87].
As mentioned before, obesity is a known risk factor for psoriasis. This is because adipose tissue produces adipokines, the proinflammatory action of which is implicated in the pathophysiology of the condition, being able to bring about exacerbations [79]; hence, this is why they are useful as markers [63,65]. As such, it can be said that adipokines play a crucial role in the relationship between obesity, psoriasis, and nonalcoholic fatty liver disease (NAFLD); adiponectin, a hormone that promotes insulin sensitivity and fatty acid oxidation, is closely associated with both psoriasis and NAFLD [113]. Therefore, weight loss or weight management through any of the aforementioned balanced diet patterns can lead to a decrease in blood serum inflammatory factors and consequently to significant improvements in psoriatic symptoms [40,42,70,75,78,87]. Since high alcohol consumption and smoking are associated with increased severity of psoriasis [105,114], studies also show that reducing or excluding these substances can lead to improvements in skin health and overall well-being [80,87].
3.3. The Role of Vitamin D in the Management of Psoriasis
Vitamins play a significant role in the treatment of mild to moderate psoriasis. Currently, various vitamins and their analogues are employed to manage these forms of psoriasis, either administered individually or in combination with other medications [68]. Vitamins are essential in psoriasis treatment, with two primary therapeutic vitamins and their derivatives being vitamin A and vitamin D [68].
Vitamin D, also called the sunshine vitamin, is mainly produced by the skin upon exposure to UVB radiation [115]. It is then hydroxylated in the liver to form 25-hydroxyvitamin D, or 25(OH)D3, an inactive form of the vitamin, and undergoes further hydroxylation in the kidneys to produce the biologically active form of vitamin D, calcitriol, or 1,25(OH)D3. In order to ensure the required amount of vitamin D, it is necessary to expose the forearm for 15–20 min to UV sunrays. A major problem is advanced technology, which induces people to spend more and more time indoors, and this leads to a significant decrease in vitamin D levels [116,117].
There are two main forms of this vitamin, ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3); only vitamin D3 is the bioactive form [118]. Few foods naturally contain vitamin D, and they are primarily of animal origin [118]. Vitamin D2 is produced by plants, but fruits and vegetables in the human diet contain only minimal amounts. The best sources of vitamin D2 are nuts, whole cereals, and vegetables. Mushrooms provide variable amounts of vitamin D2, which can be enhanced through ultraviolet light exposure under controlled conditions [119]. In contrast, vitamin D3 is naturally found in certain animal foods, particularly meat, egg yolk, butter, pork liver, and fatty fish such as salmon, herring, and mackerel, as well as in fish oils [118].
Additionally, vitamin D3 is available through dietary supplements and is the form present in vitamin D-fortified foods such as milk, orange juice, and cereals [118]. Fortification with vitamin D is considered a promising and impactful strategy [120]. The recommended daily dose of vitamin D3 for adults between 19 and 50 is 600 UI/day, while for adults over 60, the International Osteoporosis Foundation recommends 800–1000 UI per day [41].
Vitamin D’s properties have been investigated for decades in the treatment of psoriasis [121], as it plays a crucial part in the maintenance of the homeostasis of the cutaneous barrier [118]. Various studies have explored the correlation between vitamin D-specific receptors and susceptibility to psoriasis [122,123]; it has been discovered that the A-1012G promoter polymorphism of the vitamin D receptor (VDR) gene is linked to an increased risk of psoriasis due to reduced expression of VDR mRNA, which may contribute to changes in the cutaneous barrier and the formation of psoriatic lesions [124]. Moreover, a reduced expression of VDR and decreased tight junction proteins are commonly observed on the skin of psoriatic patients [125,126]. Tight junctions are essential for regulating the adhesion and permeability of keratinocytes, polarizing cutaneous cell differentiation, and managing the extracellular calcium gradient. They interact with nuclear and cytoplasmic proteins and influence the regulation of specific genes involved in keratinocyte differentiation and proliferation [41]. Thus, maintaining the skin barrier homeostasis is essential for maintaining the tight junctions of epithelial and endothelial cells, which are under threat of being dysregulated due to psoriasis, and it reduces the pathological inflammatory response. As far as associated comorbidities are concerned, the study of Barrea et al. [41] suggests that vitamin D deficiency in psoriasis may be linked to both obesity and cardiovascular disease.
Topical vitamin D is commonly prescribed, either alone or with topical corticosteroids, for localized plaque psoriasis, showing good results [127]. Especially when combined with vitamin D analogues, corticosteroids are more effective and provide remission for longer periods of time when compared to monotherapy [94]. Those analogs are especially useful for sensitive areas like the face and do not lead to tolerance like corticosteroids do. They can be used indefinitely without serious side effects and are effective for both children and the elderly [41]. A recent analysis showed that topical vitamin D treatments have similar effectiveness to corticosteroids and even better results when combined with potent steroids, showing a “steroid-sparing” effect [118]. That being said, the findings of Ford et al. [81] suggest that oral vitamin D supplementation can help with psoriatic arthritis but not with the skin-related manifestations. In a similar vein, according to Formisano et al. [128], vitamin D supplementation does not affect PASI values in any notable way. Despite not affecting the PASI values, vitamin D deficiency is considered a risk factor for psoriasis [128], and, as mentioned earlier, while the cutaneous manifestations of psoriasis may be more concerning from the point of view of quality of life, other manifestations of the disease may benefit from such an intervention.
At any rate, in addition to its topical application, oral vitamin D supplementation serves as an important adjunctive treatment option for patients with psoriasis [118]. Secondly, vitamin D supplementation may play a crucial role in the prevention of psoriasis-related comorbidities, including hypertension [129] and metabolic syndrome [120], the latter being correlated with vitamin D deficiency [130]. Furthermore, its derivatives are capable of ameliorating the efficacy of phototherapy without causing any unwanted side effects [131].
3.4. The Role of Dietary Supplements in Psoriasis
Due to the potential side effects and benefits of simultaneous conventional and complementary and alternative medicine (CAM) practices, alternative or integrative therapies that may serve as substitutes or supplements to conventional treatment should be explored. The positive impact of macro- and micronutrient supplementation on improving psoriasis should be taken into account. The most frequently utilized oral dietary supplements among psoriasis patients are fish oil, selenium, and zinc [132]. Studies show that the evidence for zinc supplementation is controversial, yet supplements such as fish oil (omega-3 fatty acids) and selenium have been found beneficial for psoriasis patients. Results on the effectiveness of fish oil supplementation in psoriasis are ambivalent, with some reviews demonstrating a benefit [67] and some a lack of effectiveness [133]. The dosages varied, with the average being 4 g/day of eicosapentaenoic acid (EPA) and 2.6 g/day of docosahexaenoic acid (DHA). These supplements should be taken for an extended period (1–6 months) to achieve a noteworthy improvement in psoriasis. It was shown that consuming 6 ounces (170 g) of fatty fish daily improved psoriasis compared to the intake of white fish [67].
As for vitamin D, studies reveal that while topical vitamin D is effective [79,89] in counteracting inflammation at the skin level [79], oral supplementation is not recommended for psoriasis treatment in adults with normal vitamin D levels [73]. As mentioned before, vitamin D supplementation may be beneficial in selected cases, particularly in those with documented hypovitaminosis to prevent psoriasis-related comorbidities [76]. Topical application of phytoestrogens like genistein, present in soybeans, ameliorated the symptoms of psoriasiform dermatitis [99].
In addition, certain phytochemical compounds like Dunaliella bardawil (the richest known source of the antioxidant β-carotene), Tripterygium wilfordii (which contains bioactive triptolides and terpenoids), Azadirachta indica (the neem tree), and Curcuma longa (turmeric) are worth mentioning. Similarly, HESA-A is a compound produced in accordance with traditional Persian medicine, comprising mineral, herbal, and animal (marine shrimp) components, which has exhibited important benefits in clinical trials for improving psoriasis severity [73]. It is predominantly an herbal formulation, with all of its constituents being derived from the Apiaceae family; Kelussia odoratissima Mozaff. (wild celery) is paired with Cuminum cyminum L. (cumin), while Apium graveolens L. (cultivated celery) is paired with Carum carvi (C. carvi) L. (wild cumin) [73]. While more research is required in such traditional medical schemes, from the point of view of documenting their efficacy and their dosage and toxicity profiles, specifically in the case of HESA-A, there is a host of data documenting its use in certain pathological states [134,135].
Finally, prebiotics and probiotics may help by reducing lipopolysaccharide production and promoting a healthy gut microbiota, which can decrease inflammation [76,89,99,136,137,138].
4. Discussion
From all the aforementioned, it is evident that there exist numerous extrinsic factors that can be modified in the context of psoriasis management. Perhaps the most important aspect, from the point of view of pathophysiology and inflammation, is oxidative stress. Oxidative stress, which can be caused by numerous factors [139,140,141,142], is under research for its importance in several different pathologies [143]. Based on current evidence, in psoriatic patients there is an impairment of most antioxidant mechanisms [18], and the adjustment of lipid peroxidation may lead to improved outcomes [71]. Regarding the role of diet, there are several interventions that can contribute to more favorable outcomes, such as phytocannabinoid oil intake [106], reduced calorie intake, or elimination diets; specialized diets may be of benefit for some patients (Table 1).

Table 1.
Dietary and nutritional interventions and supplements, and their effects on psoriatic patients, and some foods or dietary patterns that aggravating the disease are also presented.
Probably the most important role may be that of substances, phytochemicals in particular, which have concurrent antioxidant and anti-inflammatory properties; some such compounds have already been explored as a potential management solution [73]. Other such phytochemicals may be tried in the management of psoriasis, like kaempferol [144,145], pinosylvin [146], compounds derived from black pepper [147,148], capsaicin [149,150], and thymol [151]. Other recent concepts concern the role of probiotics and of gut microbiota in inflammation and disease [75,137,152,153]; the modification of gut microbiota may be useful in the management of psoriasis [136,154,155,156,157] and surely represents an interesting avenue for future research. Intestinal microbial dysbiosis has already been documented in psoriatic patients [158,159], while the dysregulation of the skin microbiome has also been proposed as a contributing factor [160].
In the introduction we have mentioned the influence of certain risk factors such as smoking and stress on mortality and morbidity. Stress and obesity are interconnected in many cases [161,162], and smoking often coexists with higher body mass index (BMI) [163,164], even though this relationship is not causal. Therefore, it can be seen that there is, most likely, an interplay between all these factors, directly or indirectly [165]; stress leads to psoriasis exacerbation [166], in turn leading to more stress [167], in a vicious cycle scheme. Therefore, the need for multidisciplinary management [168], including psychological management, in such patients becomes apparent. Similar multidisciplinary approaches have already been successfully tried in psoriatic arthritis [169,170]. Apart from such factors, the multidisciplinary aspect should be focused on other aspects such as joint involvement in psoriasis [171]; advanced modern concepts such as 3D printing applications [172,173,174,175] can be considered in such cases.
A proper diet and exercise regimen will lead, in the absence of other metabolic pathologies, to weight loss and the maintenance of a healthy physique. This is important when considering the interplay between obesity and inflammation [176,177]. Even in psoriatic patients, where there is persistent systemic inflammation, it follows that weight loss will be correlated with an improvement of disease status. Finally, the role of hormonal imbalances and treatment in psoriasis must be considered. In particular, hormonal imbalances may alter the presentation and clinical course of psoriasis [178,179], while hormonal treatment may be effective. For example, somatostatin treatment has been tried in the past with mixed results [180,181]; perhaps the use of more modern somatostatin analogs [182,183] should be more eagerly investigated in the future.
Even though the pathophysiology of psoriasis is extremely complex [184,185], there exists a host of modifiable factors that can influence its development, severity, and impact on quality of life. Altering dietary habits can greatly enhance the quality of life for patients, benefiting both psoriatic lesions and decreasing the likelihood of other associated diseases. Though there are no national or international guidelines recommending a specific nutritional approach to the management of psoriasis, several approaches may be tried based on the data reported in this review, either as a general rule or on an individualized patient-by-patient basis.
In considering the clinical significance of all the data herein presented, we must, at first, emphasize that while the data on the reduction of oxidative stress using dietary interventions are promising, in most cases there are additional steps which that be taken in order to obtain statistically significant correlations in wide and diverse patient samples. On the other hand, the wealth of encouraging data enables us to suggest that, pending further research, the implementation of dietary interventions in a patient-by-patient case may be of use at the moment. However, in certain cases, for example, when implementing ketogenic diet schemes or administering herbal or traditional supplements, the medical personnel should be mindful of potential adverse effects or toxicity.
The improvement in patient quality of life may be further increased by stress management and the elimination or reduction of other risk factors, which have already been presented. Modifying such factors, especially based on patient decision, may lead to self-empowerment, which can lead to better treatment outcomes [186,187].
5. Conclusions
It can be concluded that the management and clinical course of psoriasis, while in part depending on genetic factors, is mostly attributable to extrinsic factors. An important aspect of such factors is nutrition; in the context of nutrition, there are various approaches that have been tried, ranging from adopting different diet patterns to altering caloric intake or administering supplements. Perhaps the most crucial implicated factors, which can be modified by said interventions, are oxidative stress and obesity. Both are linked to and influence inflammation, which is arguably the most prominent component in the majority of psoriasis cases. Future studies should aim to expand on quantifying the influence of such factors in the management and quality of life of such patients and on examining the potential beneficial effects of other antioxidants, namely phytochemicals, in oral or topical administration.
Author Contributions
Conceptualization, O.-G.V., V.M.V. and C.G.; methodology, O.-G.V., A.-T.P. and R.E.D.; validation, O.-G.V. and A.-T.P.; formal analysis, O.-G.V., A.-T.P. and R.E.D.; investigation, O.-G.V. and A.-T.P.; resources, R.E.D., V.M.V. and C.G.; writing—original draft preparation, O.-G.V., A.-T.P., R.E.D., V.M.V. and C.G.; writing—review and editing, O.-G.V., A.-T.P. and R.E.D.; visualization, R.E.D., V.M.V. and C.G.; supervision, V.M.V. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 25(OH)D3 | 25-hydroxyvitamin D |
| 8H2D | 8-hydroxy 2′-deoxyguanosine |
| CAM | Complementary and alternative medicine |
| CAT | Catalase |
| CBD | Cannabidiol |
| CRP | C-reactive protein |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FOX | Ferroxidase |
| HLA | Human leucocyte antigen |
| IMA | Ischemia-modified albumin |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| MUFA | Monounsaturated fatty acids |
| NAFLD | Nonalcoholic fatty liver disease |
| OSI | Oxidative stress index |
| PASI | Psoriasis area and severity index |
| PON-1 | Paraoxonase-1 |
| PUFA | Polyunsaturated fatty acids |
| SFAs | Saturated fatty acids |
| SOD | Superoxide dismutase |
| TAS | Total antioxidant status |
| TOS | Total oxidative stress |
| VDR | Vitamin D receptor |
| WHO | World Health Organization |
References
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Lories, R.J.; D’Agostino, M.A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Haneke, E. Nail psoriasis: Clinical features, pathogenesis, differential diagnoses, and management. Psoriasis 2017, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Tan, A.L.; Watad, A.; Helliwell, P. Pathophysiology, assessment and treatment of psoriatic dactylitis. Nat. Rev. Rheumatol. 2019, 15, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, A.C.; Bucur, Ș.; Giurcăneanu, C.; Gheucă-Solovăstru, L.; Constantin, T.; Furtunescu, F.; Ancuța, I.; Constantin, M.M. Prevalence and characteristics of psoriasis in Romania—First study in overall population. J. Pers. Med. 2021, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Kirby, B.; FitzGerald, O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann. Rheum. Dis. 2013, 72, 736–740. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef]
- Morariu, S.H.; Cotoi, O.S.; Tiucă, O.M.; Baican, A.; Gheucă-Solovăstru, L.; Decean, H.; Brihan, I.; Silaghi, K.; Biro, V.; Șerban-Pescar, D.; et al. Blood-Count-Derived Inflammatory Markers as Predictors of Response to Biologics and Small-Molecule Inhibitors in Psoriasis: A Multicenter Study. J. Clin. Med. 2024, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Cunningham, K.; Perlmutter, J.; Gottlieb, A. Systematic Review of Health-Related Quality of Life in Adolescents with Psoriasis. Dermatology 2016, 232, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Marangell, L.B.; Nakamura, M.; Armstrong, A.; Jeon, C.; Bhutani, T.; Wu, J.J. Depression and suicidality in psoriasis: Review of the literature including the cytokine theory of depression. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.; Karki, C.; Mason, M.; Guo, N.; Holmgren, S.H.; Greenberg, J.D.; Lebwohl, M. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: Results from the Corrona Psoriasis Registry. J. Am. Acad. Dermatol. 2018, 78, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, T.; Wang, Y.; Hu, Y.; Yin, S.; Qin, Z.; Yu, H. Pathophysiology and Treatment of Psoriasis: From Clinical Practice to Basic Research. Pharmaceutics 2025, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Nowowiejska, J.; Baran, A.; Grabowska, P.; Lewoc, M.; Kaminski, T.W.; Flisiak, I. Assessment of life quality, stress and physical activity among patients with psoriasis. Dermatol. Ther. 2022, 12, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Capon, F. The Genetic Basis of Psoriasis. Int. J. Mol. Sci. 2017, 18, 2526. [Google Scholar] [CrossRef] [PubMed]
- Balato, N.; Di Costanzo, L.; Patruno, C.; Patrì, A.; Ayala, F. Effect of weather and environmental factors on the clinical course of psoriasis. Occup. Environ. Med. 2013, 70, 600. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Luo, S.; Huang, Y.; Lu, Q. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017, 44, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef] [PubMed]
- Wroński, A.; Wójcik, P. Impact of ROS-Dependent Lipid Metabolism on Psoriasis Pathophysiology. Int. J. Mol. Sci. 2022, 23, 12137. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Ringen, J.; Fischer, B.; Bieler, T.; Perius, K.; Knopp, T.; Kommoss, K.S.; Korn, T.; Heikenwälder, M.; Oelze, M.; et al. Reactive oxygen species produced by myeloid cells in psoriasis as a potential biofactor contributing to the development of vascular inflammation. BioFactors 2023, 49, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.; Croxford, A.L.; Oelze, M.; Schüler, R.; Minwegen, D.; Wegner, J.; Koukes, L.; Yogev, N.; Nikolaev, A.; Reißig, S.; et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arter. Thromb. Vasc. Biol. 2014, 34, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Martens, W.J.M. Climate change, thermal stress and mortality changes. Soc. Sci. Med. 1998, 46, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Puttonen, S.; Härmä, M.; Hublin, C. Shift work and cardiovascular disease—Pathways from circadian stress to morbidity. Scand. J. Work Environ. Health 2010, 36, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Poulin, M.J.; Brown, S.L.; Dillard, A.J.; Smith, D.M. Giving to others and the association between stress and mortality. Am. J. Public Health 2013, 103, 1649–1655. [Google Scholar] [CrossRef]
- Hammond, E.C. Smoking in Relation to Mortality and Morbidity. Findings in First Thirty-Four Months of Follow-Up in a Prospective Study Started in 19592. JNCI J. Natl. Cancer Inst. 1964, 32, 1161–1188. [Google Scholar] [CrossRef] [PubMed]
- Poikolainen, K. Alcohol and mortality: A review. J. Clin. Epidemiol. 1995, 48, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Gmel, G.; Sempos, C.T.; Trevisan, M. Alcohol-related morbidity and mortality. Alcohol. Res. Health 2003, 27, 39–51. [Google Scholar] [PubMed]
- White, W.B. Smoking-Related Morbidity and Mortality in the Cardiovascular Setting. Prev. Cardiol. 2007, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hingson, R.W.; Zha, W.; Weitzman, E.R. Magnitude of and trends in alcohol-related mortality and morbidity among US college students ages 18–24, 1998–2005. J. Stud. Alcohol Drugs Suppl. 2009, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.D.; Abnet, C.C.; Feskanich, D.; Freedman, N.D.; Hartge, P.; Lewis, C.E.; Ockene, J.K.; Prentice, R.L.; Speizer, F.E.; Thun, M.J. Smoking and mortality—Beyond established causes. N. Engl. J. Med. 2015, 372, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Importance of diet on disease prevention. Int. J. Med. Med. Sci. 2013, 5, 55–59. [Google Scholar]
- Sakurai, M.; Nakamura, K.; Miura, K.; Takamura, T.; Yoshita, K.; Nagasawa, S.Y.; Morikawa, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Dietary carbohydrate intake, presence of obesity and the incident risk of type 2 diabetes in Japanese men. J. Diabetes Investig. 2016, 7, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Ornella, R.; Maria, G.C.; Elisa, S.; Maria, N.; Chiara, M.; Antonio, N.; Tiziana, M.; Gian, L.d.A.; Francesco, D.M. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. Atenei Parm. 2018, 89, 60. [Google Scholar]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The association between psoriasis and obesity: A systematic review and meta-analysis of observational studies. Nutr. Diabetes 2012, 2, e54. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Nappi, F.; Di Somma, C.; Savanelli, M.C.; Falco, A.; Balato, A.; Balato, N.; Savastano, S. Environmental Risk Factors in Psoriasis: The Point of View of the Nutritionist. Int. J. Environ. Res. Public Health 2016, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Debbaneh, M.; Millsop, J.W.; Bhatia, B.K.; Koo, J.; Liao, W. Diet and psoriasis, part I: Impact of weight loss interventions. J. Am. Acad. Dermatol. 2014, 71, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Garbicz, J.; Całyniuk, B.; Górski, M.; Buczkowska, M.; Piecuch, M.; Kulik, A.; Rozentryt, P. Nutritional Therapy in Persons Suffering from Psoriasis. Nutrients 2021, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Katta, R.; Desai, S.P. Diet and dermatology: The role of dietary intervention in skin disease. J. Clin. Aesthet. Dermatol. 2014, 7, 46–51. [Google Scholar] [PubMed]
- Katta, R.; Kramer, M.J. Skin and Diet: An Update on the Role of Dietary Change as a Treatment Strategy for Skin Disease. Ski. Ther. Lett. 2018, 23, 1–5. [Google Scholar]
- Martínez-González, M.A.; Martínez, J.A.; Hu, F.B.; Gibney, M.J.; Kearney, J. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Mendonça, C.R.; Delpino, F.M.; Elias Souza, G.V.; Pereira de Souza Rosa, L.; de Oliveira, C.; Noll, M. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 50, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Black, M.H.; Watanabe, R.M.; Trigo, E.; Takayanagi, M.; Lawrence, J.M.; Buchanan, T.A.; Xiang, A.H. High-fat diet is associated with obesity-mediated insulin resistance and β-cell dysfunction in Mexican Americans. J. Nutr. 2013, 143, 479–485. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Bolocan, A.; Ion, D. A review of innovation in medicine. Technol. Innov. Life Sci. 2022, 1, 42–48. [Google Scholar] [CrossRef]
- Meenan, F.O. A note on the history of psoriasis. Ir. J. Med. Sci. 1955, 30, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Mrowietz, U.; Radtke, M.A.; Schäfer, I.; von Kiedrowski, R.; Strömer, K.; Enk, A.; Maul, J.-T.; Reich, K.; Zander, N.; et al. What is psoriasis?—Perception and assessment of psoriasis among the German population. JDDG J. Der Dtsch. Dermatol. Ges. 2018, 16, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-L.; Gong, Y.; Qi, Y.-J.; Shao, Z.-M.; Jiang, Y.-Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S. An Evidence-based Look at the Effects of Diet on Health. Cureus 2019, 11, e4715. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Giovannucci, E. Healthful Plant-Based Diet and Incidence of Type 2 Diabetes in Asian Population. Nutrients 2022, 14, 3078. [Google Scholar] [CrossRef] [PubMed]
- Giosuè, A.; Calabrese, I.; Vitale, M.; Riccardi, G.; Vaccaro, O. Consumption of Dairy Foods and Cardiovascular Disease: A Systematic Review. Nutrients 2022, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Basiri, R.; Spicer, M.; Levenson, C.; Ledermann, T.; Akhavan, N.; Arjmandi, B. Improving Dietary Intake of Essential Nutrients Can Ameliorate Inflammation in Patients with Diabetic Foot Ulcers. Nutrients 2022, 14, 2393. [Google Scholar] [CrossRef] [PubMed]
- Lewis Luján, L.M.; McCarty, M.F.; Di Nicolantonio, J.J.; Gálvez Ruiz, J.C.; Rosas-Burgos, E.C.; Plascencia-Jatomea, M.; Iloki Assanga, S.B. Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration. Nutrients 2022, 14, 1985. [Google Scholar] [CrossRef]
- Bartimoccia, S.; Cammisotto, V.; Nocella, C.; Del Ben, M.; D’Amico, A.; Castellani, V.; Baratta, F.; Pignatelli, P.; Loffredo, L.; Violi, F.; et al. Extra Virgin Olive Oil Reduces Gut Permeability and Metabolic Endotoxemia in Diabetic Patients. Nutrients 2022, 14, 2153. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.; Borges-Canha, M.; Pimentel-Nunes, P. Microbiota Modulation in Patients with Metabolic Syndrome. Nutrients 2022, 14, 4490. [Google Scholar] [CrossRef] [PubMed]
- Cozma, E.C.; Avram, I.; Voiculescu, V.M.; Mihai, M.M.; Găman, A.M. Correlations between Gut Microbiota and Hematological, Inflammatory, Biochemical and Oxidative Stress Parameters in Treatment-Naïve Psoriasis Patients. Int. J. Mol. Sci. 2024, 25, 6649. [Google Scholar] [CrossRef] [PubMed]
- Dobrică, E.C.; Cozma, M.A.; Găman, M.A.; Voiculescu, V.M.; Găman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019, 53, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Kiełbowski, K.; Bakinowska, E.; Ostrowski, P.; Pala, B.; Gromowska, E.; Gurazda, K.; Dec, P.; Modrzejewski, A.; Pawlik, A. The Role of Adipokines in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 6390. [Google Scholar] [CrossRef] [PubMed]
- Bakry, O.A.; El Hefnawy, S.; Mariee, A.H.; El Gendy, Y. Urinary Biopyrrins: A New Marker of Oxidative Stress in Psoriasis. Indian J. Dermatol. 2016, 61, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Bhatia, B.K.; Debbaneh, M.; Koo, J.; Liao, W. Diet and psoriasis, part III: Role of nutritional supplements. J. Am. Acad. Dermatol. 2014, 71, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Torsekar, R.; Gautam, M.M. Topical Therapies in Psoriasis. Indian Dermatol. Online J. 2017, 8, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.; Lopes, D.; Łuczaj, W.; Neves, B.; Pinto, B.; Maurício, T.; Domingues, P.; Skrzydlewska, E.; Domingues, M.R. Algal Lipids as Modulators of Skin Disease: A Critical Review. Metabolites 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Bellinato, F.; Maurelli, M.; Geat, D.; Girolomoni, G.; Gisondi, P. Managing the Patient with Psoriasis and Metabolic Comorbidities. Am. J. Clin. Dermatol. 2024, 25, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Protein adducts with lipid peroxidation products in patients with psoriasis. Redox Biol. 2023, 63, 102729. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, E.; Oliveri, M.; Girometta, C.; Ratto, D.; Di Iorio, C.; Occhinegro, A.; Rossi, P. Nutritional strategies for psoriasis: Current scientific evidence in clinical trials. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8537–8551. [Google Scholar] [CrossRef] [PubMed]
- Adawi, M.; Damiani, G.; Bragazzi, N.L.; Bridgewood, C.; Pacifico, A.; Conic, R.R.Z.; Morrone, A.; Malagoli, P.; Pigatto, P.D.M.; Amital, H.; et al. The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study. Nutrients 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R. Western Diet and Psoriatic-Like Skin and Joint Diseases: A Potential Role for the Gut Microbiota. J. Investig. Dermatol. 2021, 141, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.L.; Nasca, M.R.; Boscaglia, S.; Micali, G. The role of lifestyle and nutrition in psoriasis: Current status of knowledge and interventions. Dermatol. Ther. 2022, 35, e15685. [Google Scholar] [CrossRef] [PubMed]
- Ingkapairoj, K.; Chularojanamontri, L.; Chaiyabutr, C.; Silpa-Archa, N.; Wongpraparut, C.; Bunyaratavej, S. Dietary habits and perceptions of psoriatic patients: Mediterranean versus Asian diets. J. Dermatol. Treat. 2022, 33, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Zanesco, S.; Hall, W.; Gibson, R.; Griffiths, C.; Maruthappu, T. Approaches to nutrition intervention in plaque psoriasis, a multi-system inflammatory disease-The Diet and Psoriasis Project (DIEPP). Nutr. Bull. 2022, 47, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Cintoni, M.; Palombaro, M.; Maramao, F.S.; Raoul, P.; Egidi, G.; Leonardi, E.; Bianchi, L.; Campione, E.; Rinninella, E.; Gasbarrini, A.; et al. Metabolic Disorders and Psoriasis: Exploring the Role of Nutritional Interventions. Nutrients 2023, 15, 3876. [Google Scholar] [CrossRef] [PubMed]
- Michalski, P.; Palazzo-Michalska, V.; Buda, P.; Michalska-Bańkowska, A.; Bańkowski, M.; Strojny, D.; Grabarek, B.O. A crossroads between dietary habits, alcohol consumption, and smoking in the clinical course of psoriasis: A narrative review. Postep. Dermatol. Alergol. 2023, 40, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.R.; Siegel, M.; Bagel, J.; Cordoro, K.M.; Garg, A.; Gottlieb, A.; Green, L.J.; Gudjonsson, J.E.; Koo, J.; Lebwohl, M.; et al. Dietary Recommendations for Adults With Psoriasis or Psoriatic Arthritis From the Medical Board of the National Psoriasis Foundation: A Systematic Review. JAMA Dermatol. 2018, 154, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Berna-Rico, E.; Fernandez-Nieto, D.; Gonzalez-Cantero, A. RF—Role of the Mediterranean Diet in the Treatment of Psoriasis. Actas Dermosifiliogr. 2023, 114, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Budu-Aggrey, A.; Brumpton, B.; Tyrrell, J.; Watkins, S.; Modalsli, E.H.; Celis-Morales, C.; Ferguson, L.D.; Vie, G.; Palmer, T.; Fritsche, L.G.; et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. PLoS Med. 2019, 16, e1002739. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Ghahremani, A.; Namdar Ahmadabad, H. Intermittent fasting: A promising dietary intervention for autoimmune diseases. Autoimmun. Rev. 2023, 22, 103408. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sim, N.; Fotouhi, A.; Daveluy, S. Vegan Diet in Dermatology: A Review. J. Clin. Med. 2023, 12, 5800. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.K.; Millsop, J.W.; Debbaneh, M.; Koo, J.; Linos, E.; Liao, W. Diet and psoriasis, part II: Celiac disease and role of a gluten-free diet. J. Am. Acad. Dermatol. 2014, 71, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current Evidence on the Efficacy of Gluten-Free Diets in Multiple Sclerosis, Psoriasis, Type 1 Diabetes and Autoimmune Thyroid Diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Duchnik, E.; Kruk, J.; Tuchowska, A.; Marchlewicz, M. The Impact of Diet and Physical Activity on Psoriasis: A Narrative Review of the Current Evidence. Nutrients 2023, 15, 840. [Google Scholar] [CrossRef] [PubMed]
- Kranyak, A.; Haran, K.; Smith, P.; Johnson, C.; Liao, W.; Bhutani, T. The Mediterranean Diet as a Potential Solution to the Gut Microbiome Dysbiosis in Psoriasis Patients. J. Psoriasis Psoriatic Arthritis 2024, 9, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Molina-Leyva, A.; Cuenca-Barrales, C.; Vega-Castillo, J.J.; Ruiz-Carrascosa, J.C.; Ruiz-Villaverde, R. Adherence to Mediterranean diet in Spanish patients with psoriasis: Cardiovascular benefits? Dermatol. Ther. 2019, 32, e12810. [Google Scholar] [CrossRef] [PubMed]
- Aryanian, Z.; Asghari, M.; Zanousi, P.P.; Ghadimi, R.; Kebria, A.S.; Hatami, P. Adherence to the Mediterranean diet in patients with psoriasis and its relationship with the severity of the disease: A case-control study. Health Sci. Rep. 2024, 7, e70049. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, G.; Rastrelli, L.; Galdo, G.; Molettieri, P.; Rotondi Aufiero, F.; Cereda, E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: A proof-of-concept, single-arm, open-label clinical trial. Nutrition 2020, 74, 110757. [Google Scholar] [CrossRef]
- Lambadiari, V.; Katsimbri, P.; Kountouri, A.; Korakas, E.; Papathanasi, A.; Maratou, E.; Pavlidis, G.; Pliouta, L.; Ikonomidis, I.; Malisova, S.; et al. The Effect of a Ketogenic Diet versus Mediterranean Diet on Clinical and Biochemical Markers of Inflammation in Patients with Obesity and Psoriatic Arthritis: A Randomized Crossover Trial. Int. J. Mol. Sci. 2024, 25, 2475. [Google Scholar] [CrossRef] [PubMed]
- Katsimbri, P.; Korakas, E.; Kountouri, A.; Ikonomidis, I.; Tsougos, E.; Vlachos, D.; Papadavid, E.; Raptis, A.; Lambadiari, V. The Effect of Antioxidant and Anti-Inflammatory Capacity of Diet on Psoriasis and Psoriatic Arthritis Phenotype: Nutrition as Therapeutic Tool? Antioxidants 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, F.; Leitner, J.; Aminzadeh-Gohari, S.; Weber, D.D.; Sanio, P.; Koller, A.; Feichtinger, R.G.; Weiss, R.; Kofler, B.; Lang, R. The Influence of Ketogenic Diets on Psoriasiform-Like Skin Inflammation. J. Investig. Dermatol. 2020, 140, 707–710.e7. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Vining, E.P.G. Clinical Aspects of the Ketogenic Diet. Epilepsia 2007, 48, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef] [PubMed]
- Polo, T.C.F.; Corrente, J.E.; Miot, L.D.B.; Papini, S.J.; Miot, H.A. Dietary patterns of patients with psoriasis at a public healthcare institution in Brazil. Bras. Dermatol. 2020, 95, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.C.T.; Taghizadeh, M.; Nahavandi, M.; Jafarnejad, S. Efficacy of ω-3 supplementation in patients with psoriasis: A me-ta-analysis of randomized controlled trials. Clin. Rheumatol. 2019, 38, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Purzycka-Bohdan, D.; Nedoszytko, B.; Reich, A.; Szczerkowska-Dobosz, A.; Bartosiñska, J.; Batycka-Baran, A.; Czajkowski, R.; Dobrucki, I.T.; Dobrucki, L.W.; et al. Pathogenesis of psoriasis in the “omic” era. Part III. Metabolic disorders, metabolomics, nutrigenomics in psoriasis. Postep. Dermatol. Alergol. 2020, 37, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Sicińska, P.; Pytel, E.; Kurowska, J.; Koter-Michalak, M. Supplementation with omega fatty acids in various diseases. Postep. Hig. Med. Dosw. 2015, 69, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kiluk, P.; Myśliwiec, H.; Flisiak, I. The role of lipids in psoriasis. Dermatol. Rev./Przegląd Dermatol. 2017, 104, 619–635. [Google Scholar] [CrossRef]
- Antosik, K.; Krzęcio-Nieczyporuk, E.; Kurowska-Socha, B. Diet and nutrition in psoriasis treatment. Hygeia Public Health 2017, 52, 131–137. [Google Scholar]
- Puaratanaarunkon, T.; Sittisaksomjai, S.; Sivapornpan, N.; Pongcharoen, P.; Chakkavittumrong, P.; Ingkaninan, K.; Temkitthawon, P.; Promgool, T.; Waranuch, N.; Asawanonda, P. Topical cannabidiol-based treatment for psoriasis: A dual-centre randomized placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e718–e720. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Atalay, S.; Rogowska-Wrzesińska, A.; Skrzydlewska, E. The Effect of Cannabidiol on UV-Induced Changes in Intracellular Signaling of 3D-Cultured Skin Keratinocytes. Int. J. Mol. Sci. 2021, 22, 1501. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Gęgotek, A.; Skrzydlewska, E. Protective Effects of Cannabidiol on the Membrane Proteome of UVB-Irradiated Keratinocytes. Antioxidants 2021, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metab-olism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Engeli, B.E.; Lachenmeier, D.W.; Diel, P.; Guth, S.; Villar Fernandez, M.A.; Roth, A.; Lampen, A.; Cartus, A.T.; Wätjen, W.; Hengstler, J.G.; et al. Cannabidiol in Foods and Food Supplements: Evaluation of Health Risks and Health Claims. Nutrients 2025, 17, 489. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2016, 232, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Trojacka, E.; Zaleska, M.; Galus, R. Influence of exogenous and endogenous factors on the course of psoriasis. Pol. Merkur. Lek. 2015, 38, 169–173. [Google Scholar]
- Voiculescu, V.M.; Nelson Twakor, A.; Jerpelea, N.; Pantea Stoian, A. Vitamin D: Beyond Traditional Roles—Insights into Its Biochemical Pathways and Physiological Impacts. Nutrients 2025, 17, 803. [Google Scholar] [CrossRef] [PubMed]
- Bârsan, M.; Chelaru, V.F.; Râjnoveanu, A.G.; Popa, Ș.L.; Socaciu, A.I.; Bădulescu, A.V. Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 7584. [Google Scholar] [CrossRef] [PubMed]
- Santana, K.V.d.S.d.; Oliver, S.L.; Mendes, M.M.; Lanham-New, S.; Charlton, K.E.; Ribeiro, H. Association between vitamin D status and lifestyle factors in Brazilian women: Implications of Sun Exposure Levels, Diet, and Health. Eclinicalmedicine 2022, 47, 101400. [Google Scholar] [CrossRef] [PubMed]
- Mattozzi, C.; Paolino, G.; Richetta, A.G.; Calvieri, S. Psoriasis, vitamin D and the importance of the cutaneous barrier’s integrity: An update. J. Dermatol. 2016, 43, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Orgaz-Molina, J.; Magro-Checa, C.; Arrabal-Polo, M.A.; Raya-Álvarez, E.; Naranjo, R.; Buendía-Eisman, A.; Arias-Santiago, S. Association of 25-hydroxyvitamin D with metabolic syndrome in patients with psoriasis: A case-control study. Acta Derm. Venereol. 2014, 94, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Ajazuddin. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, B.; Relhan, V.; Goel, K.; Kochhar, A.M.; Garg, V.K. Vitamin D and skin diseases: A review. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Richetta, A.G.; Silvestri, V.; Giancristoforo, S.; Rizzolo, P.; D’Epiro, S.; Graziano, V.; Mattozzi, C.; Navazio, A.S.; Campoli, M.; D’Amico, C.; et al. A-1012G promoter polymorphism of vitamin D receptor gene is associated with psoriasis risk and lower allele-specific expression. DNA Cell Biol. 2014, 33, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Visconti, B.; Paolino, G.; Carotti, S.; Pendolino, A.L.; Morini, S.; Richetta, A.G.; Calvieri, S. Immunohistochemical expression of VDR is associated with reduced integrity of tight junction complex in psoriatic skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the skin: Focus on a complex relationship: A review. J. Adv. Res. 2015, 6, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Formisano, E.; Proietti, E.; Borgarelli, C.; Pisciotta, L. Psoriasis and Vitamin D: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3387. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Nie, X.; Cai, J. The effect of vitamin D supplementation on hypertension in non-CKD populations: A systemic review and meta-analysis. Int. J. Cardiol. 2017, 227, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef] [PubMed]
- Talbott, W.; Duffy, N. Complementary and alternative medicine for psoriasis: What the dermatologist needs to know. Am. J. Clin. Dermatol. 2015, 16, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Chi, C.C. Effects of fish oil supplement on psoriasis: A meta-analysis of randomized controlled trials. BMC Complement. Altern. Med. 2019, 19, 354. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Barikbin, B.; Naseri, M.; Mohagheghi, M. The effect of HESA-A on psoriasis vulgaris. J. Drugs Dermatol. 2008, 7, 559–561. [Google Scholar] [PubMed]
- Mousavi, S.R.; Moshiri, M.; Darchini-Maragheh, E.; Ghasempouri, S.K.; Dadpour, B.; Sardar Antighechi, F.; Balali-Mood, M. Therapeutic effects of HESA-A (a herbal-marine compound) in acute organophosphorus pesticide poisoning. Avicenna J. Phytomed 2020, 10, 235–242. [Google Scholar] [PubMed]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Sánchez-Pellicer, P.; Agüera-Santos, J.; Navarro-Moratalla, L. Probiotics in the Therapeutic Arsenal of Dermatologists. Microorganisms 2021, 9, 1513. [Google Scholar] [CrossRef] [PubMed]
- Buhaș, M.C.; Candrea, R.; Gavrilaș, L.I.; Miere, D.; Tătaru, A.; Boca, A.; Cătinean, A. Transforming Psoriasis Care: Probiotics and Prebiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 11225. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, A.M.; and Abomughaid, M.M. Oxidative stress: An overview of past research and future insights. All Life 2024, 17, 2316092. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Scheau, A.-E.; Savulescu-Fiedler, I.; Caruntu, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. Int. J. Mol. Sci. 2023, 24, 16299. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Georgatos-Garcia, S.; Touriki, G.; Dragosloveanu, C.D.M.; Caruntu, A.; Savulescu-Fiedler, I.; Dragosloveanu, S.; et al. Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential. Curr. Issues Mol. Biol. 2025, 47, 204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malhotra, S.; Prasad, A.K.; Van der Eycken, E.V.; Bracke, M.E.; Stetler-Stevenson, W.G.; Parmar, V.S.; Ghosh, B. Anti-inflammatory and antioxidant properties of Piper species: A perspective from screening to molecular mechanisms. Curr. Top. Med. Chem. 2015, 15, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Troumpata, L.; Periferakis, K.; Adalis, G.; Periferakis, A.; Georgatos-Garcia, S.; Maier, C.; Costache, A.; Garofil, D.; Costache, D. Traditional Ethnomedical and Ethnobotanical Applications and Uses of Piper Nigrum. Rom. J. Mil. Med. 2025, 128, 286–303. [Google Scholar] [CrossRef]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef] [PubMed]
- Petran, E.M.; Periferakis, A.; Troumpata, L.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Periferakis, K.; Caruntu, A.; Savulescu-Fiedler, I.; Sima, R.-M.; et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Curr. Issues Mol. Biol. 2024, 46, 7895–7943. [Google Scholar] [CrossRef] [PubMed]
- Gago, C.; Serralheiro, A.; Miguel, M.d.G. Anti-Inflammatory Activity of Thymol and Thymol-Rich Essential Oils: Mechanisms, Applications, and Recent Findings. Molecules 2025, 30, 2450. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Moldoveanu, E.-T.; Niculescu, A.-G.; Predescu, E.; Roza, E.; Tincu, I.F.; Grumezescu, A.M.; Teleanu, D.M. Liposomal and Lipid-Based Drug Delivery Systems: Bridging Gut Microbiota and Pediatric Disorder Treatments. Pharmaceutics 2025, 17, 707. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lin, Q.; Hou, S.; Cui, X.; Shou, N.; Yuan, X.; Xu, W.; Fu, K.; Wang, Q.; Shi, Z. Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation. Int. J. Mol. Sci. 2025, 26, 3332. [Google Scholar] [CrossRef] [PubMed]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome-Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wu, K.; Yang, Q.; Meng, B.; Niu, Y.; Zhao, F. Advances in psoriasis and gut microorganisms with co-metabolites. Front. Microbiol. 2023, 14, 1192543. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zou, X.; Gao, L.; Zhao, H. Gut microbiota and psoriasis: Pathogenesis, targeted therapy, and future directions. Front. Cell Infect. Microbiol. 2024, 14, 1430586. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, L.; Sun, T.; Guo, K.; Geng, S. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.J.E.; Kell, D.B.; Pretorius, E. Bacterial Dysbiosis and Translocation in Psoriasis Vulgaris. Front. Cell Infect. Microbiol. 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Rizvi, M.R.; Saraswat, S. Obesity and Stress: A Contingent Paralysis. Int. J. Prev. Med. 2022, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Goens, D.; Virzi, N.E.; Jung, S.E.; Rutledge, T.R.; Zarrinpar, A. Obesity, Chronic Stress, and Stress Reduction. Gastroenterol. Clin. N. Am. 2023, 52, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.; Augustson, E.M.; Patrick, H. Unraveling the Relationship between Smoking and Weight: The Role of Sedentary Behavior. J. Obes. 2012, 2012, 735465. [Google Scholar] [CrossRef] [PubMed]
- Dare, S.; Mackay, D.F.; Pell, J.P. Relationship between smoking and obesity: A cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS ONE 2015, 10, e0123579. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, V.M.; Popa, L.G.; Bumbacea, R.S.; Nitipir, C.; Giurcaneanu, C. Genetics of psoriasis susceptibility and treatment response. Farmacia 2016, 64, 313–322. [Google Scholar]
- Snast, I.; Reiter, O.; Atzmony, L.; Leshem, Y.A.; Hodak, E.; Mimouni, D.; Pavlovsky, L. Psychological stress and psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 178, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Rigas, H.M.; Bucur, S.; Ciurduc, D.M.; Nita, I.E.; Constantin, M.M. Psychological Stress and Depression in Psoriasis Patients—A Dermatologist’s Perspective. Maedica 2019, 14, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea-Arana, A.; Serra Torres, M.; Hergueta Diaz, M.; González Guerrero, M.E.; Fariñas Padron, L.; Navarro Martín, S.; Vargas Osorio, K.; Palacios Abufón, A.; García de Yébenes, M.J.; Loza, E. Experience and satisfaction with a multidisciplinary care unit for patients with psoriasis and psoriatic arthritis. Reumatol. Clínica 2019, 15, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.X.; Zheng, M. A multidisciplinary team for the diagnosis and management of psoriatic arthritis. Chin. Med. J. 2021, 134, 1387–1389. [Google Scholar] [CrossRef]
- Jadon, D.R.; Helliwell, P.S. The role of the multidisciplinary team in the management of psoriatic arthritis. Musculoskelet. Care 2022, 20 (Suppl. S1), S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, H.; Hoede, N.; Eißner, D.; Hahn, K.; Hülse, R. Joint involvement in psoriasis. Arch. Für Dermatol. Forsch. 1974, 250, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.-A.; Georgatos-Garcia, S.; Scheau, A.-E.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; et al. Use of Biomaterials in 3D Printing as a Solution to Microbial Infections in Arthroplasty and Osseous Reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2024, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Timofticiuc, I.-A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Garofil, N.D.; Didilescu, A.C.; Caruntu, C.; Scheau, C. 3D Bioprinting in Limb Salvage Surgery. J. Funct. Biomater. 2024, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Timofticiuc, I.-A.; Caruntu, A.; Dragosloveanu, C.D.M.; Scheau, A.-E.; Badarau, I.A.; Periferakis, A.; Dragosloveanu, S.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Head and Neck 3D Bioprinting—A Review on Recent Advancements in Soft Tissue 3D Bioprinting and Medical Applications. J. Funct. Biomater. 2025, 16, 240. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Savulescu-Fiedler, I.; Dorobantu-Lungu, L.-R.; Dragosloveanu, S.; Benea, S.N.; Dragosloveanu, C.D.M.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Scheau, C. The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms. Curr. Issues Mol. Biol. 2025, 47, 115. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.I.; Constantin, A.M.; Marina, M.E.; Orasan, R.I. The role of hormones in the pathogenesis of psoriasis vulgaris. Clujul Med. 2016, 89, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cassalia, F.; Lunardon, A.; Frattin, G.; Danese, A.; Caroppo, F.; Fortina, A.B. How Hormonal Balance Changes Lives in Women with Psoriasis. J. Clin. Med. 2025, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Klughardt, G.; Neidhardt, M.; Galle, K.; Frey, H.; Geiger, A. Treatment of psoriasis with Somatostatin. Arch. Dermatol. Res. 1981, 272, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Guilhou, J.J.; Boulanger, A.; Barneon, G.; Vic, P.; Meynadier, J.; Tardieu, J.C.; Clot, J. Somatostatin treatment of psoriasis. Arch. Dermatol. Res. 1982, 274, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Tsigas, G.; Periferakis, A.-T.; Badarau, I.A.; Scheau, A.-E.; Tampa, M.; Georgescu, S.R.; Didilescu, A.C.; Scheau, C.; Caruntu, C. Antitumoral and Anti-inflammatory Roles of Somatostatin and Its Analogs in Hepatocellular Carcinoma. Anal. Cell. Pathol. 2021, 2021, 1840069. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Tsigas, G.; Periferakis, A.-T.; Tone, C.M.; Hemes, D.A.; Periferakis, K.; Troumpata, L.; Badarau, I.A.; Scheau, C.; Caruntu, A.; et al. Agonists, Antagonists and Receptors of Somatostatin: Pathophysiological and Therapeutical Implications in Neoplasias. Curr. Issues Mol. Biol. 2024, 46, 9721–9759. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Orzan, O.A.; Tutunaru, C.V.; Ianoși, S.L. Understanding the Intricate Pathophysiology of Psoriasis and Related Skin Disorders. Int. J. Mol. Sci. 2025, 26, 749. [Google Scholar] [CrossRef] [PubMed]
- Hickmann, E.; Richter, P.; Schlieter, H. All together now—Patient engagement, patient empowerment, and associated terms in personal healthcare. BMC Health Serv. Res. 2022, 22, 1116. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.J.; Gallamore, M.J.; Hansen, N.R.; Martin, D.C. Patient empowerment: A critical evaluation and prescription for a foundational definition. Front. Psychol. 2025, 15, 1473345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).