High-Risk PNPLA3 rs738409 Genotype Is Associated with Higher Concentrations of CCL2 in Liver Transplant Candidates with Alcoholic End-Stage Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. DNA Isolation and Genotyping
2.3. Cytometric Bead Array
2.4. Statistical Analysis
3. Results
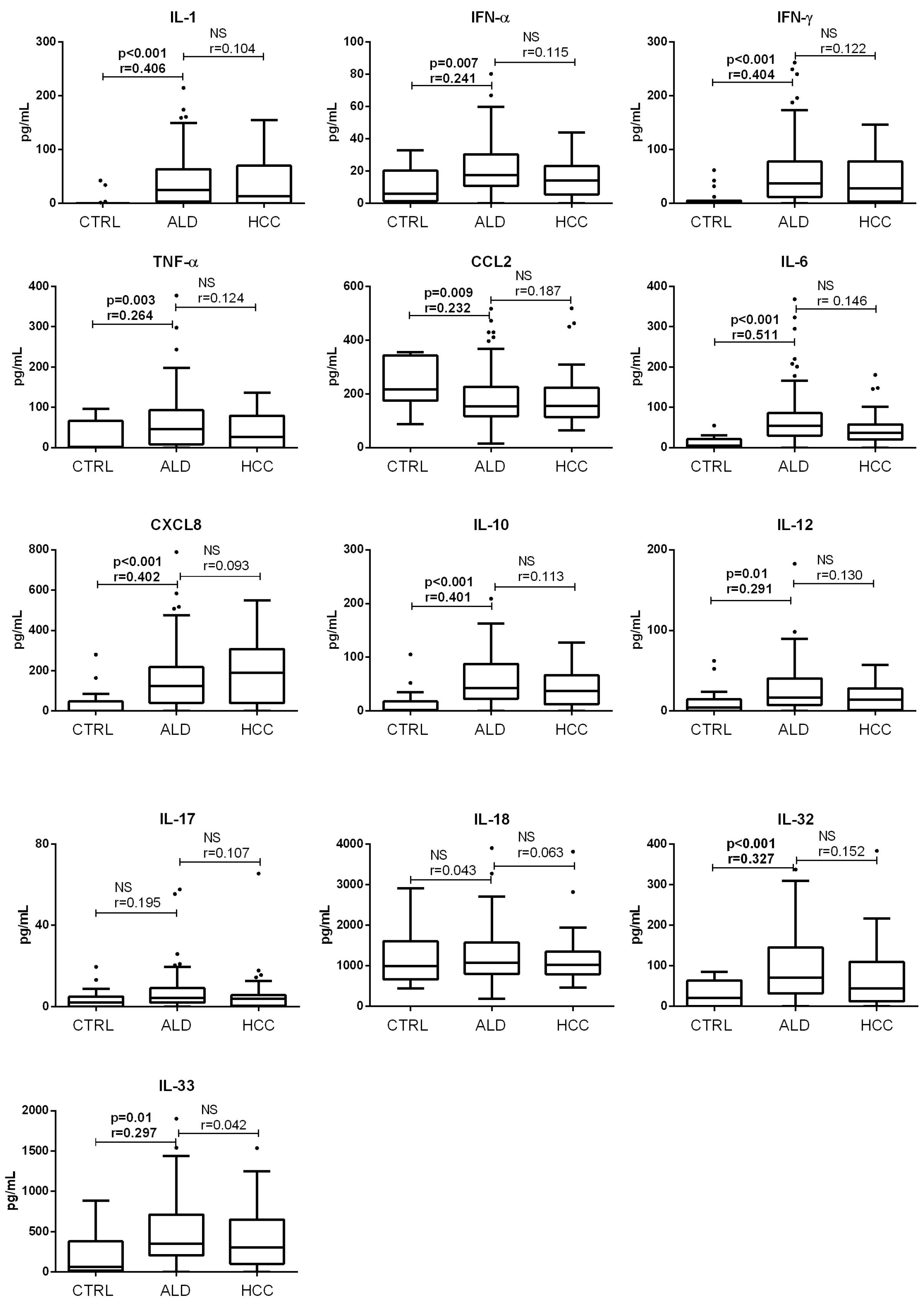
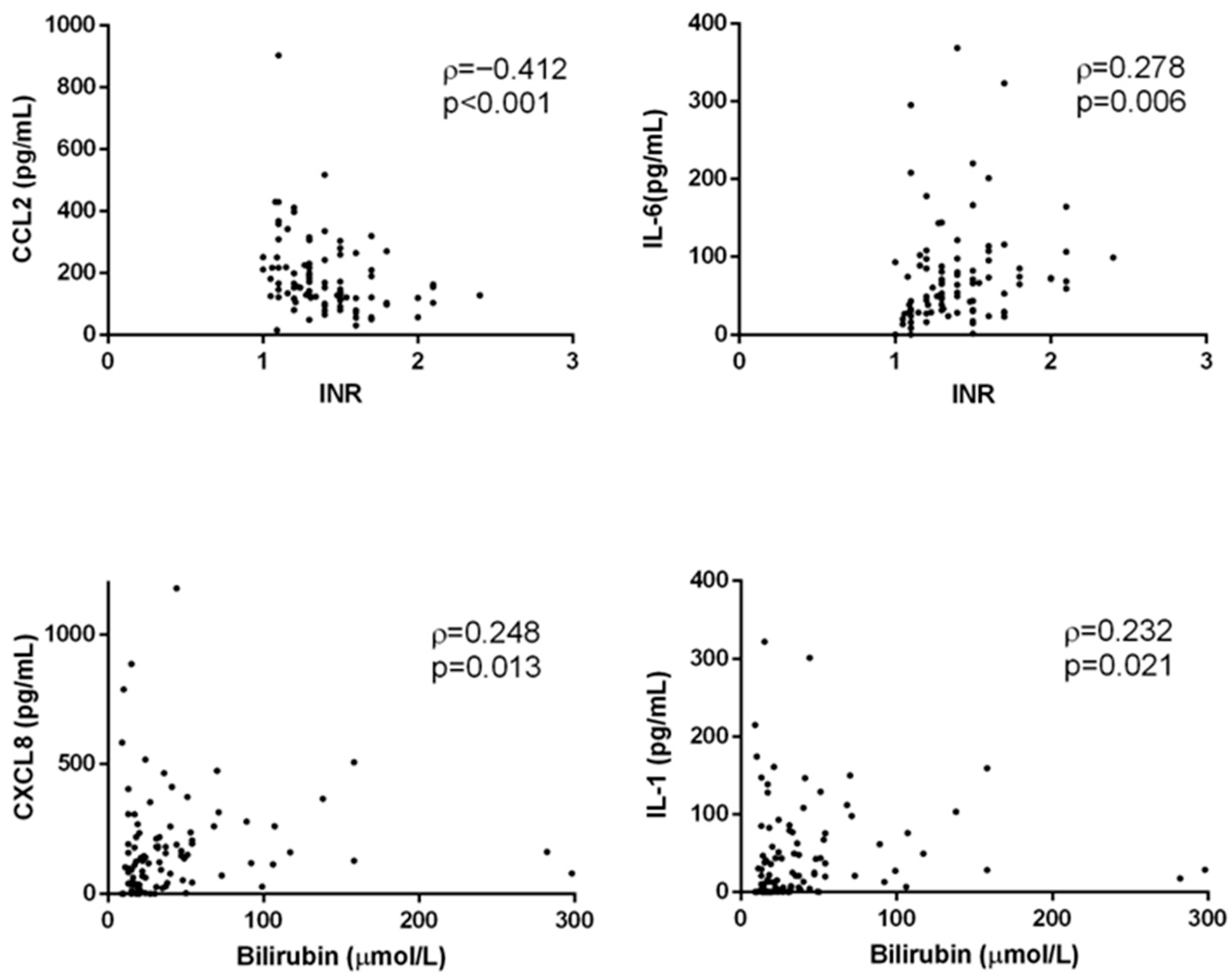
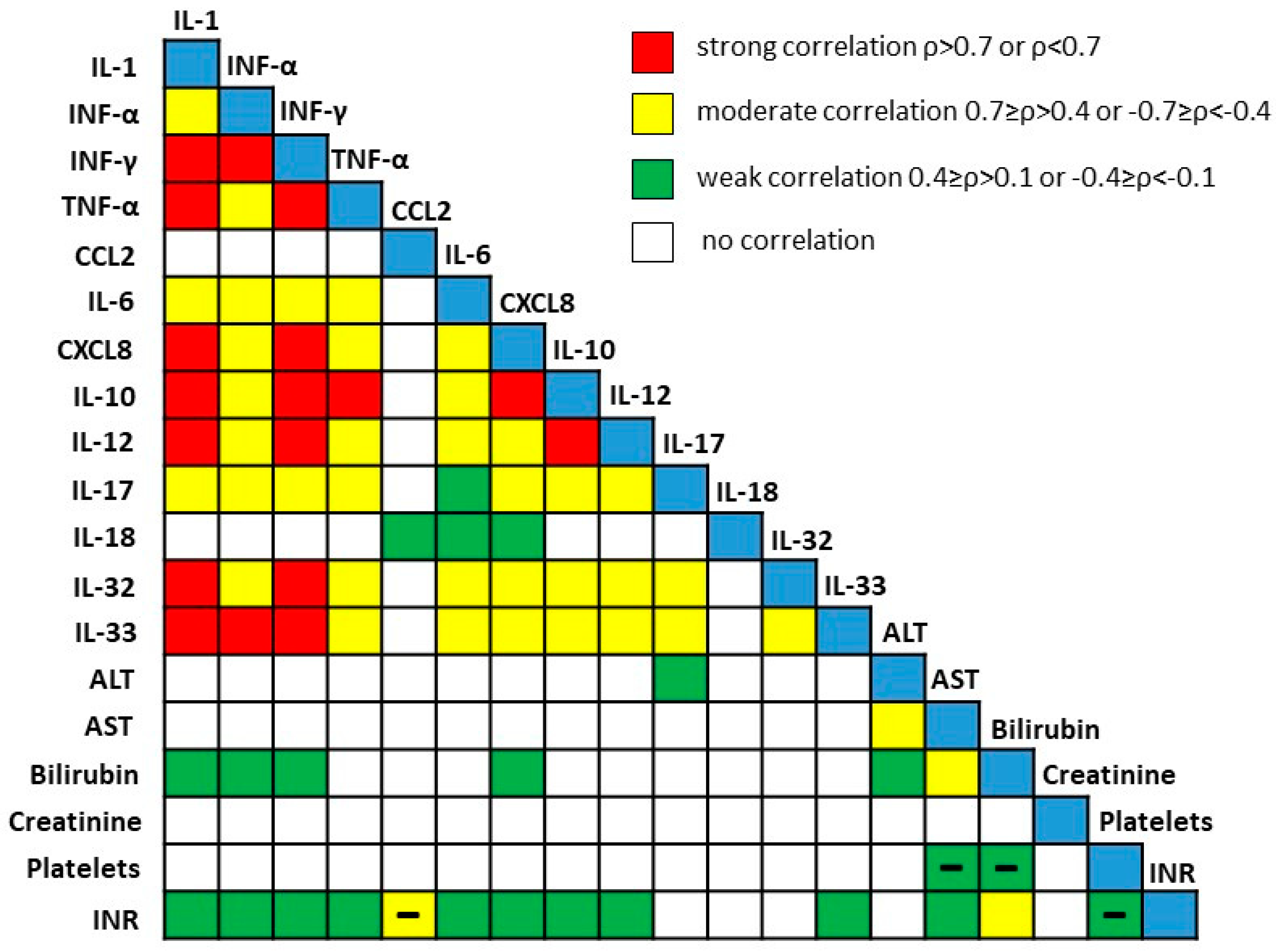
3.1. Concentration of Acute Proinflammatory Cytokines in End-Stage Alcoholic Liver Disease
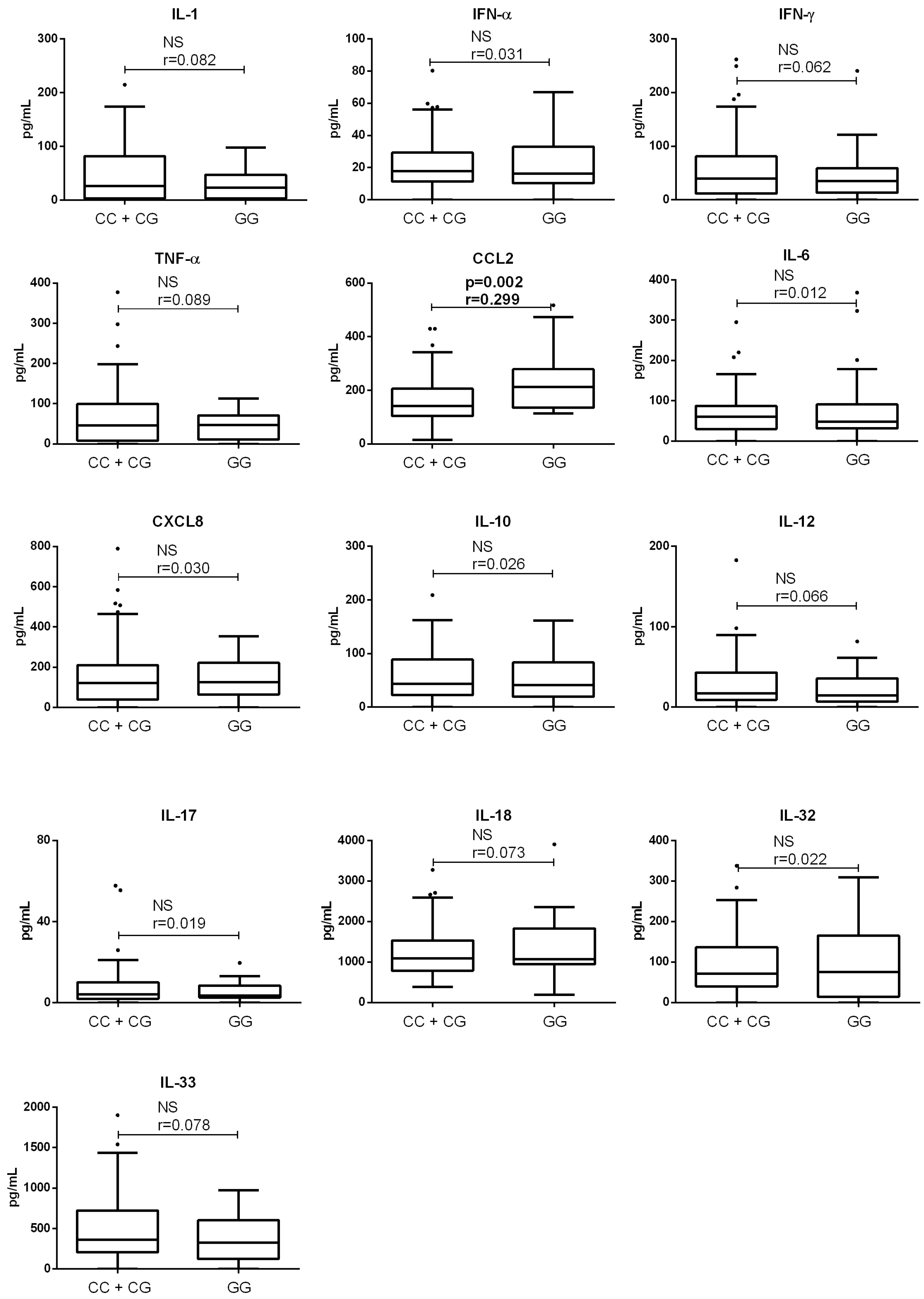
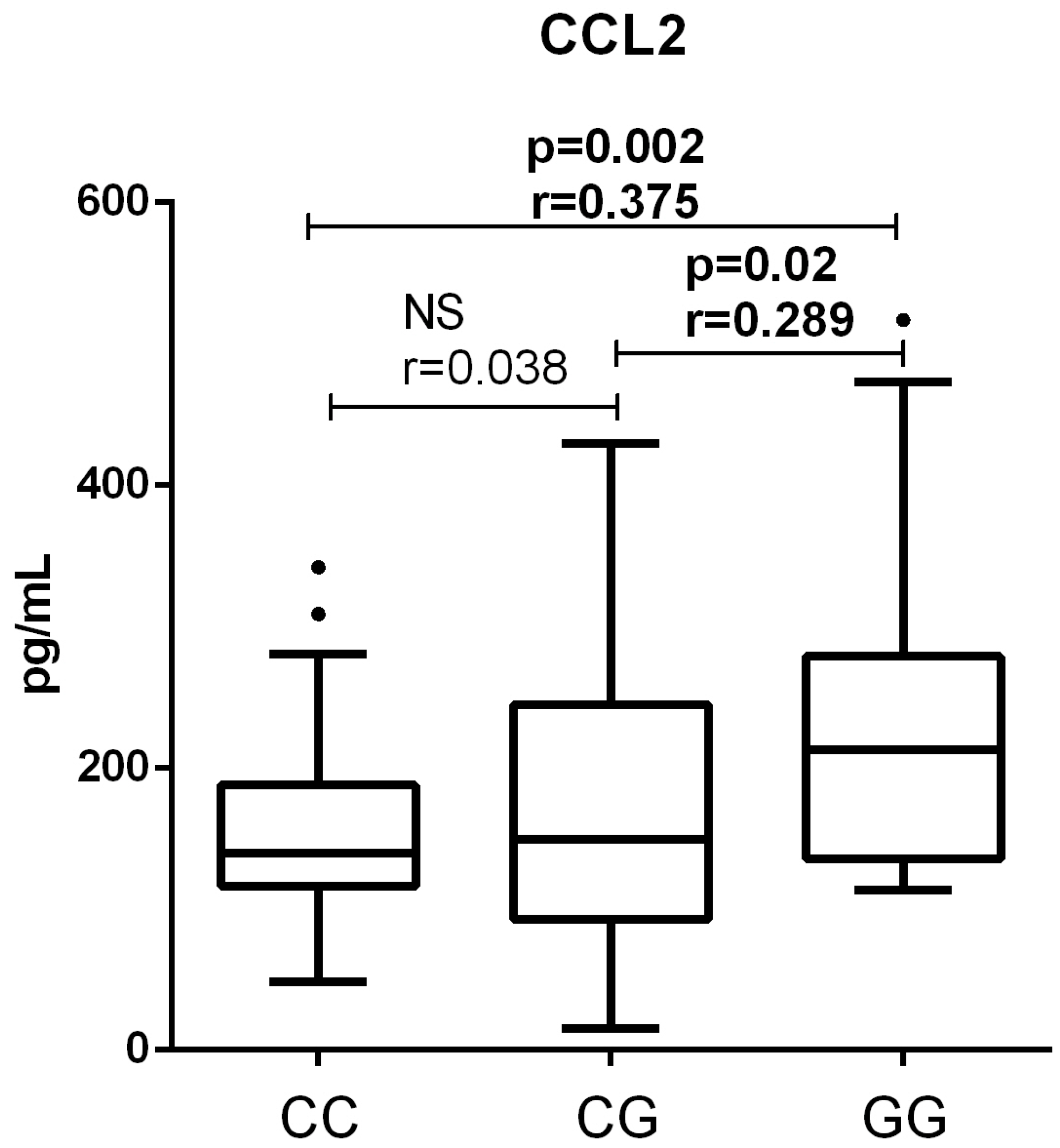
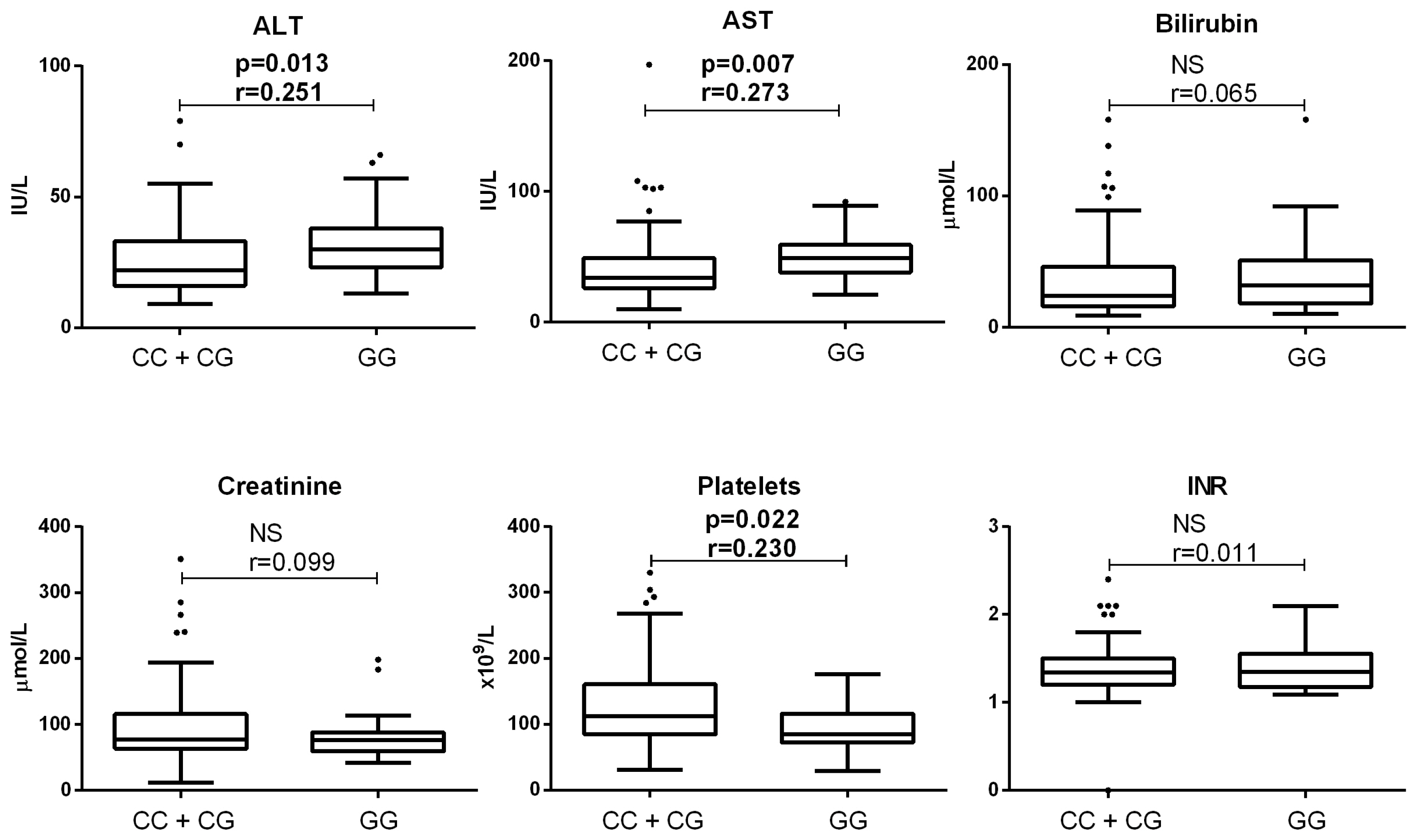
3.2. Association of PNPLA3 Genotype with Clinical Parameters of Patients with End-Stage ALD
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | alpha-fetoprotein |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ALD | alcoholic liver disease |
| CCL2 | chemokine (C-C motif) ligand 2 |
| CTRL | control group |
| CXCL8 | chemokine (C-X-C motif) ligand 8 |
| HCC | hepatocellular carcinoma |
| IFN | interferon |
| IL | interleukin |
| PNPLA3 | patatin-like phospholipase domain-containing protein 3 |
| TNF-α | tumor necrosis factor α |
References
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marroni, C.A.; Fleck, A.M., Jr.; Fernandes, S.A.; Galant, L.H.; Mucenic, M.; de Mattos Meine, M.H.; Mariante-Neto, G.; de Mello Brandão, A.B. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J. Gastroenterol. 2018, 24, 2785–2805. [Google Scholar] [CrossRef] [PubMed]
- Kelava, T.; Turcic, P.; Markotic, A.; Ostojic, A.; Sisl, D.; Mrzljak, A. Importance of genetic polymorphisms in liver transplantation outcomes. World J. Gastroenterol. 2020, 26, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Bainrauch, A.; Šisl, D.; Markotić, A.; Ostojić, A.; Gašparov, S.; Lang, V.B.; Kovačić, N.; Grčević, D.; Mrzljak, A.; Kelava, T. NOTCH3 rs1043996 Polymorphism Is Associated with the Occurrence of Alcoholic Liver Cirrhosis Independently of PNPLA3 and TM6SF2 Polymorphisms. J. Clin. Med. 2021, 10, 4621. [Google Scholar] [CrossRef] [PubMed]
- Falleti, E.; Fabris, C.; Cmet, S.; Cussigh, A.; Bitetto, D.; Fontanini, E.; Fornasiere, E.; Bignulin, S.; Fumolo, E.; Bignulin, E.; et al. PNPLA3 rs738409C/G polymorphism in cirrhosis: Relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int. 2011, 31, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Kanwal, F.; Lim, H.; Duong, H.; Liu, Y.; Singal, A.G.; Khaderi, S.; Asrani, S.K.; Amos, C.I.; El-Serag, H.B. PNPLA3, obesity and heavy alcohol use in cirrhosis patients may exert a synergistic increase hepatocellular carcinoma risk. Clin. Gastroenterol. Hepatol. 2024, 22, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Xiao, X.; Byun, J.; Jun, G.; DeSantis, S.M.; Chen, H.; Thrift, A.P.; El-Serag, H.B.; Kanwal, F.; Amos, C.I. Synergistic Associations of PNPLA3 I148M Variant, Alcohol Intake, and Obesity With Risk of Cirrhosis, Hepatocellular Carcinoma, and Mortality. JAMA Netw. Open 2022, 5, e2234221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, L.; Chen, L.; Jiang, S.; Xin, Y.; Xuan, S. A Meta-Analysis of the Association Between the I148M Variant of Patatin-Like Phospholipase Domain Containing 3 Gene and the Presence of Chronic Hepatitis C. Hepat. Mon. 2015, 15, e31987. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Wannhoff, A.; Kattner, S.; Brune, M.; Hov, J.R.; Weiss, K.H.; Antoni, C.; Dollinger, A.; Neumann-Haefelin, C.; Seufferlein, T.; et al. PNPLA3 in end-stage liver disease: Alcohol consumption, hepatocellular carcinoma development, and transplantation-free survival. J. Gastroenterol. Hepatol. 2014, 29, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Balcar, L.; Semmler, G.; Oberkofler, H.; Zandanell, S.; Strasser, M.; Datz, L.; Niederseer, D.; Feldman, A.; Stickel, F.; Datz, C.; et al. PNPLA3 is the dominant SNP linked to liver disease severity at time of first referral to a tertiary center. Dig. Liver Dis. 2022, 54, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cohen, J.C.; Hobbs, H.H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem. 2011, 286, 37085–37093. [Google Scholar] [CrossRef] [PubMed]
- Mitsche, M.A.; Hobbs, H.H.; Cohen, J.C. Patatin-like phospholipase domain-containing protein 3 promotes transfer of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. J. Biol. Chem. 2018, 293, 9232. [Google Scholar] [CrossRef] [PubMed]
- Ruhanen, H.; Perttilä, J.; Hölttä-Vuori, M.; Zhou, Y.; Yki-Järvinen, H.; Ikonen, E.; Käkelä, R.; Olkkonen, V.M. PNPLA3 mediates hepatocyte triacylglycerol remodeling. J. Lipid Res. 2014, 55, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.; Mancina, R.M.; Merlo, A.; Dongiovanni, P.; Rametta, R.; Montalcini, T.; Valenti, L.; Albanes, D.; Romeo, S. PNPLA3 I148M Variant Influences Circulating Retinol in Adults with Nonalcoholic Fatty Liver Disease or Obesity. J. Nutr. 2015, 145, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Darvishi, N.; Mansouri, K.; Ghasemi, H.; Hosseinian-Far, M.; Darvishi, F.; Mohammadi, M. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease: A systematic review and meta-analysis. BMC Endocr. Disord. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, H.; Yuan, D.; Xu, J.; Chen, Y.; Xu, X.; Xu, F.; Liang, H. PNPLA3 I148M mediates the regulatory effect of NF-kB on inflammation in PA-treated HepG2 cells. J. Cell Mol. Med. 2020, 24, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-K.; Kim, H.S.; Chung, Y.-H. Clinical aspects of tumor necrosis factor-α signaling in hepatocellular carcinoma. Curr. Pharm. Des. 2014, 20, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Nenu, I.; Toadere, T.M.; Topor, I.; Țichindeleanu, A.; Bondor, D.A.; Trella, Ș.E.; Sparchez, Z.; Filip, G.A. Interleukin-6 in Hepatocellular Carcinoma: A Dualistic Point of View. Biomedicines 2023, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Gao, B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J. Gastroenterol. Hepatol. 2012, 27, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Markotic, A.; Flegar, D.; Grcevic, D.; Sucur, A.; Lalic, H.; Turcic, P.; Kovacic, N.; Lukac, N.; Pravdic, D.; Vukojevic, K.; et al. LPS-induced inflammation desensitizes hepatocytes to Fas-induced apoptosis through Stat3 activation-The effect can be reversed by ruxolitinib. J. Cell Mol. Med. 2020, 24, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Degré, D.; Lemmers, A.; Gustot, T.; Ouziel, R.; Trépo, E.; Demetter, P.; Verset, L.; Quertinmont, E.; Vercruysse, V.; Le Moine, O.; et al. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin. Exp. Immunol. 2012, 169, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.-Y.; Han, J.; Zhang, X.; Hsu, S.-H.; He, S.; Wani, N.A.; Barajas, J.M.; Snyder, L.A.; Frankel, W.L.; Caligiuri, M.A.; et al. Blocking the CCL2-CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model. Mol. Cancer Ther. 2017, 16, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Cao, G.; Kou, C.; Liu, T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol. Rep. 2018, 39, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cvitkovic, K.; Sesar, A.; Sesar, I.; Pusic-Sesar, A.; Pejic, R.; Kelava, T.; Sucur, A.; Cavar, I. Concentrations of Selected Cytokines and Vascular Endothelial Growth Factor in Aqueous Humor and Serum of Diabetic Patients. Semin. Ophthalmol. 2020, 35, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, T.; Richelsen, B.; Bruun, J.M. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int. J. Obes. 2005, 29, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcu-taneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Miquel, R.; Colmenero, J.; Moreno, M.; García–Pagán, J.; Bosch, J.; Arroyo, V.; Ginès, P.; Caballería, J.; Bataller, R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology 2009, 136, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Rawa, T. Circulating Monocyte Chemoattractant Protein-1 (MCP-1) in Patients with Primary Biliary Cholangitis. Int. J. Mol. Sci. 2024, 25, 1333. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Cestari, M.; Okano, S.; Patel, P.J.; Horsfall, L.U.; Keshvari, S.; Hume, D.A.; Williams, S.; Russell, A.; Powell, E.E.; Irvine, K.M. Serum CC-Chemokine Ligand 2 Is Associated with Visceral Adiposity but Not Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Dig. Dis. 2023, 41, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.B.; Louvet, A.; Ntandja-Wandji, L.C.; Boleslawski, E.; Gnemmi, V.; Lassailly, G.; Truant, S.; Maggiotto, F.; Ningarhari, M.; Artru, F.; et al. Loss of hepatocyte identity following aberrant YAP activation: A key mechanism in alcoholic hepatitis. J. Hepatol. 2021, 75, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rosenthal, S.B.; Meshgin, N.; Baglieri, J.; Musallam, S.G.; Diggle, K.; Lam, K.; Wu, R.; Pan, S.Q.; Chen, Y.; et al. Primary Alcohol-Activated Human and Mouse Hepatic Stellate Cells Share Similarities in Gene-Expression Profiles. Hepatol. Commun. 2020, 4, 606–626. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Schrammen, P.L.; Möckel, D.; Govaere, O.; Liepelt, A.; Krenkel, O.; Ergen, C.; McCain, M.V.; Eulberg, D.; Luedde, T.; et al. The CCR2+ Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signaling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Živković, L.; Asare, Y.; Bernhagen, J.; Dichgans, M.; Georgakis, M.K. Pharmacological targeting of the CCL2/CCR2 axis for atheroprotection: A meta-analysis of preclinical studies. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e131–e144. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 axis in cancer immunotherapy: One stone, three birds? Front. Immunol. 2021, 12, 771210. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, E.; Ramezani, M.; Sadeghi, M. Evaluation of serum interleukin-6 levels in hepatocellular carcinoma patients: A systematic review and meta-analysis. Clin. Exp. Hepatol. 2018, 4, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, E.; Sadeghi, M.; Shakiba, M. A systematic review and meta-analysis of evaluation of serum interleukin 8 levels in hepatocellular carcinoma. Clin. Exp. Hepatol. 2019, 5, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Eder, C. Mechanisms of interleukin-1beta release. Immunobiology 2009, 214, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Plesch, E.; Chen, C.-C.; Butz, E.; Rosato, A.S.; Krogsaeter, E.K.; Yinan, H.; Bartel, K.; Keller, M.; Robaa, D.; Teupser, D.; et al. Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells. Elife 2018, 7, e39720. [Google Scholar] [CrossRef] [PubMed]
- Peregud, D.I.; Baronets, V.Y.; Lobacheva, A.S.; Ivanov, A.S.; Arisheva, O.S.; Garmash, I.V.; Kobalava, Z.D.; Pirozhkov, S.V.; Terebilina, N.N. PNPLA3 rs738409 associates with alcoholic liver cirrhosis but not with serum levels of IL6, IL10, IL8 or CCL2 in the Russian population. Ann. Hepatol. 2021, 20, 100247. [Google Scholar] [CrossRef] [PubMed]
- E Rinella, M.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Kirchmeyer, M.; Gaigneaux, A.; Servais, F.A.; Arslanow, A.; Casper, M.; Krawczyk, M.; Lammert, F.; Behrmann, I. Altered profiles of circulating cytokines in chronic liver diseases (NAFLD/HCC): Impact of the PNPLA3I148M risk allele. Hepatol. Commun. 2023, 7, e0306. [Google Scholar] [CrossRef] [PubMed]
- Kelava, T.; Bekan, I.B.; Šisl, D.; Šucur, A.; Bainrauch, A.; Bralic-Lang, V.; Planinić, P.; Kovacèic, N.; Grcèevic, D.; Sesa, V.; et al. SAT-514 High-risk PNPLA3 rs738409 genotype is associated with higher CCL2 concentrations in liver transplant candidates with alcohol-related liver disease. In Proceedings of the EASL Congress 2025, Amsterdam, The Netherlands, 7–10 May 2025. [Google Scholar]
| Control | ALD | HCC | p Value | |
|---|---|---|---|---|
| n | 19 | 106 | 35 | - |
| Age 1 | 58 (51–71) | 60 (55–65) | 60.5 (55–65) | 0.57 |
| Sex 2 | 0.04 | |||
| female | 5 (26.3%) | 18 (17%) | 1 (2.9%) | |
| male | 14 (73.7%) | 88 (83%) | 34 (97.1%) | |
| rs738409 PNPLA3 genotype 2 | ||||
| CC | 7 (36.8%) | 40 (37.7%) | 10 (28.6%) | 0.82 |
| CG | 7 (36.8%) | 40 (37.7%) | 17 (48.6%) | |
| GG | 5 (26.3%) | 26 (24.5%) | 8 (22.9%) |
| CC/CG | GG | p | CC | CG | GG | p | |
|---|---|---|---|---|---|---|---|
| n | 80 | 26 | 40 | 40 | 26 | - | |
| Age 1, years | 60.5 (55–65) | 58 (55–63) | 0.45 | 61 (55–65) | 59.5 (56–64.5) | 58 (55–63) | 0.75 |
| Sex 2 | 0.40 | 0.67 | |||||
| female | 15 (18.8%) | 3 (11.5%) | 8 (20%) | 7 (17.5%) | 3 (11.5%) | ||
| male | 65 (81.3%) | 23 (88.5%) | 32 (80%) | 33 (82.5%) | 23 (88.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budimir Bekan, I.; Šisl, D.; Šućur, A.; Bainrauch, A.; Bralić Lang, V.; Planinić, P.; Kovačić, N.; Grčević, D.; Mrzljak, A.; Kelava, T. High-Risk PNPLA3 rs738409 Genotype Is Associated with Higher Concentrations of CCL2 in Liver Transplant Candidates with Alcoholic End-Stage Liver Disease. Medicina 2025, 61, 1293. https://doi.org/10.3390/medicina61071293
Budimir Bekan I, Šisl D, Šućur A, Bainrauch A, Bralić Lang V, Planinić P, Kovačić N, Grčević D, Mrzljak A, Kelava T. High-Risk PNPLA3 rs738409 Genotype Is Associated with Higher Concentrations of CCL2 in Liver Transplant Candidates with Alcoholic End-Stage Liver Disease. Medicina. 2025; 61(7):1293. https://doi.org/10.3390/medicina61071293
Chicago/Turabian StyleBudimir Bekan, Ivan, Dino Šisl, Alan Šućur, Ana Bainrauch, Valerija Bralić Lang, Pavao Planinić, Nataša Kovačić, Danka Grčević, Anna Mrzljak, and Tomislav Kelava. 2025. "High-Risk PNPLA3 rs738409 Genotype Is Associated with Higher Concentrations of CCL2 in Liver Transplant Candidates with Alcoholic End-Stage Liver Disease" Medicina 61, no. 7: 1293. https://doi.org/10.3390/medicina61071293
APA StyleBudimir Bekan, I., Šisl, D., Šućur, A., Bainrauch, A., Bralić Lang, V., Planinić, P., Kovačić, N., Grčević, D., Mrzljak, A., & Kelava, T. (2025). High-Risk PNPLA3 rs738409 Genotype Is Associated with Higher Concentrations of CCL2 in Liver Transplant Candidates with Alcoholic End-Stage Liver Disease. Medicina, 61(7), 1293. https://doi.org/10.3390/medicina61071293







