Immunomodulatory Effects of Anesthetic Techniques in Lung Cancer Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Outcome Measurements
2.3. Data Collection and Extraction Process
2.4. Quality Evaluation
2.5. Statistical Analysis
3. Results
3.1. A Literature Search
3.2. Baseline Characteristics
3.3. Risk of Bias Assessment
3.4. Analysis of Outcomes
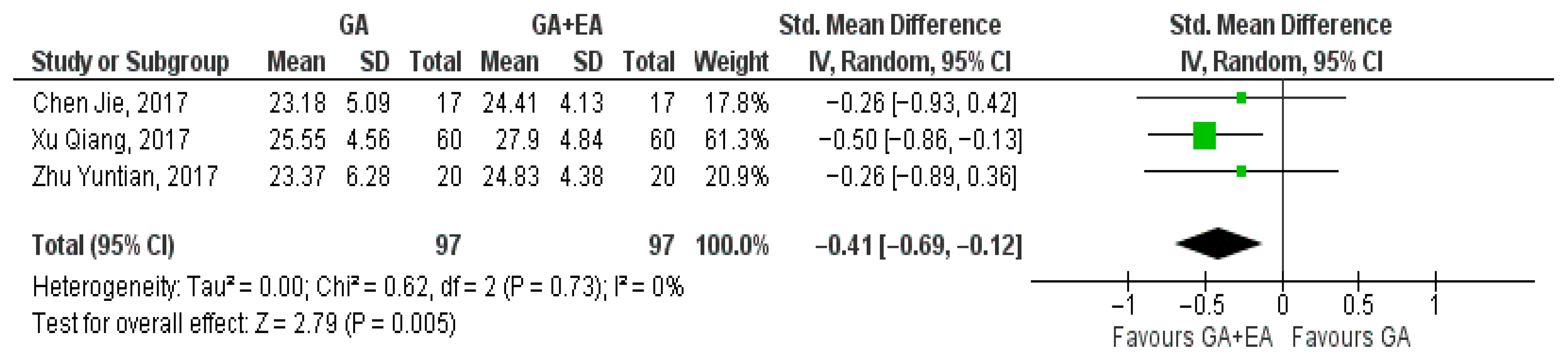
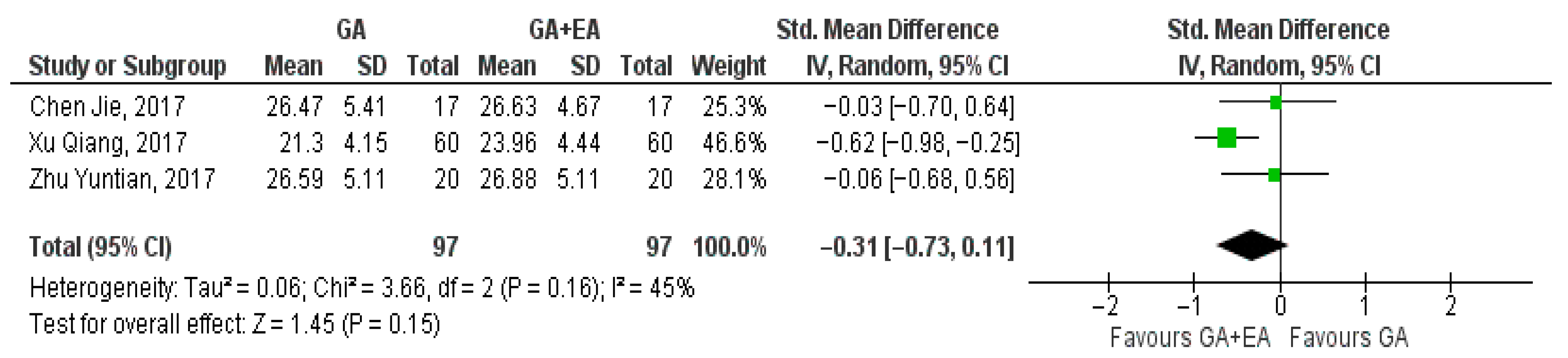
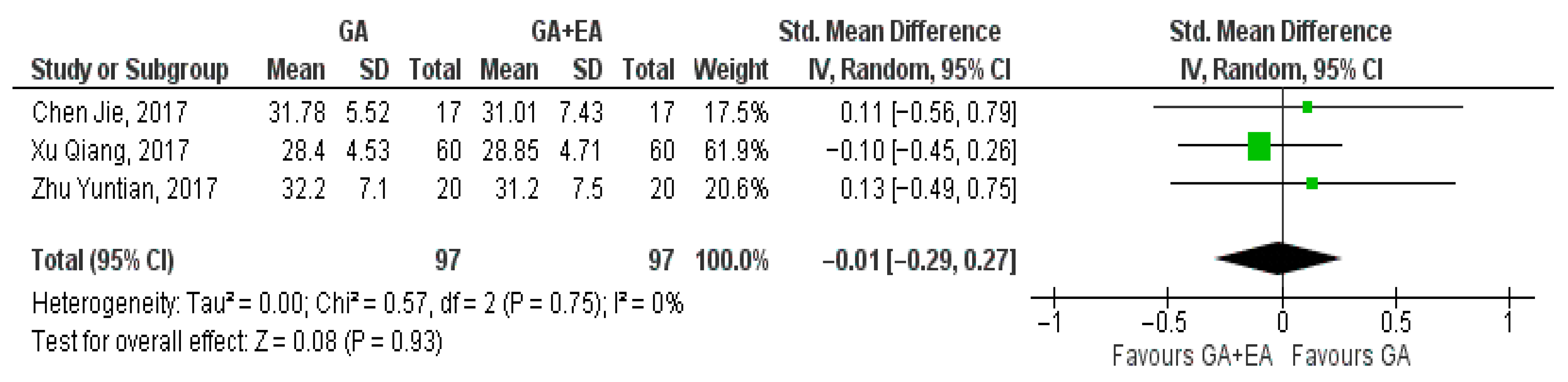
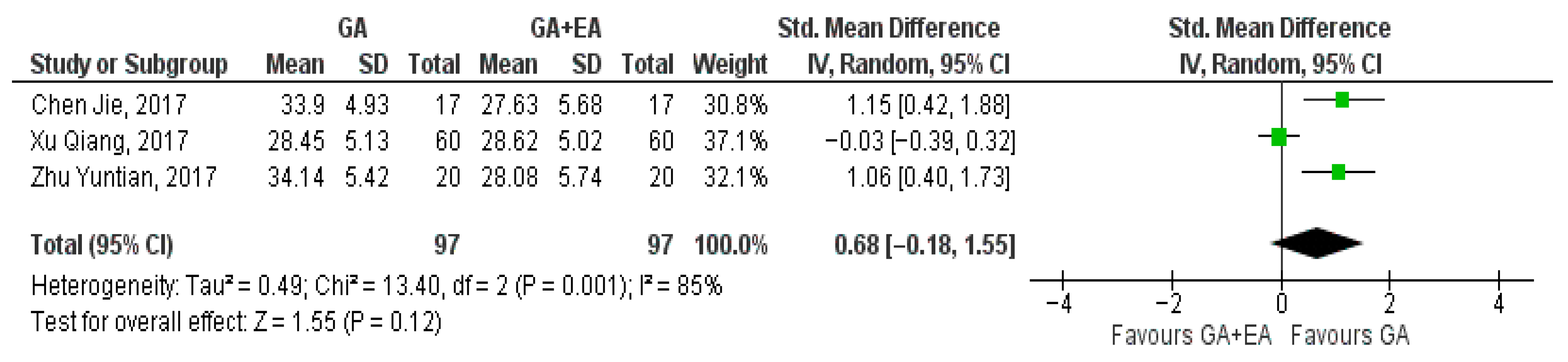
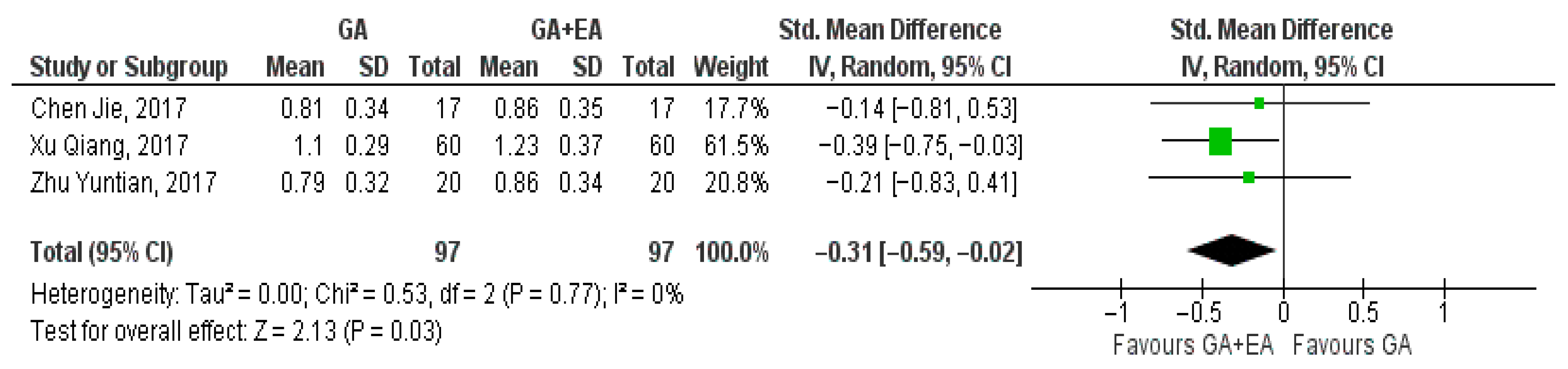
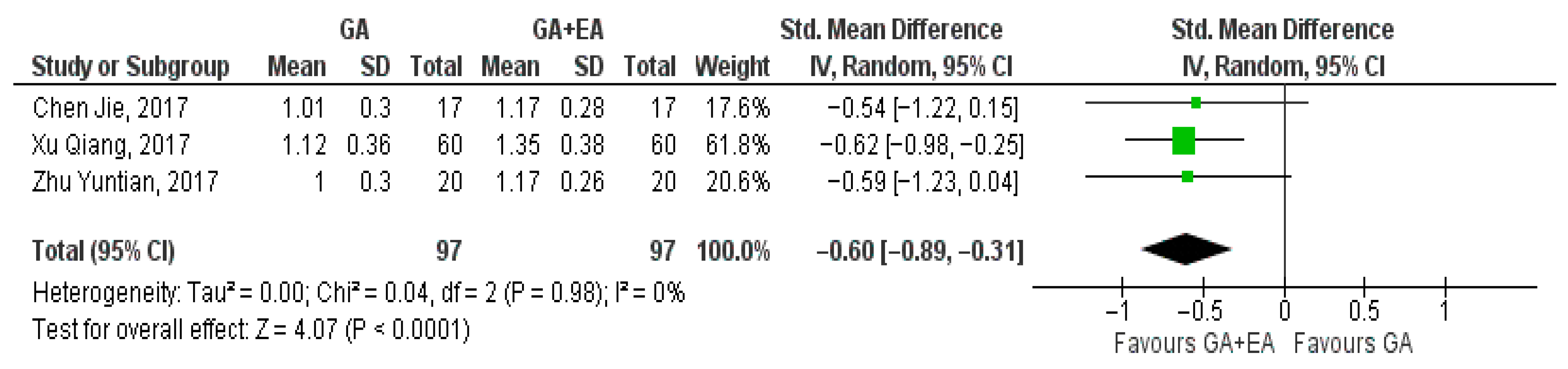
3.4.1. General Anesthesia Versus General Anesthesia Plus Epidural Anesthesia
- CD3+ cell counts
- b.
- CD4+ cell counts
- c.
- CD8+ cell counts
- d.
- CD4+/CD8+ ratio
- e.
- NK cell counts
3.4.2. Volatile-Based Anesthesia Versus Propofol
- CD4+ cell counts
- b.
- CD8+ cell counts
- c.
- CD4+/CD8+ ratio
- d.
- T-reg cell counts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RCT | Randomized Controlled Trial |
| HPA axis | Hypothalamic–Pituitary–Adrenal axis |
| SNS | Sympathetic Nervous System |
| NK cells | Natural Killer cells |
| NKT cells | Natural Killer T cells |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| Th17 | T helper 17 |
| Treg cells | T regulatory cells |
| TIVA | Total Intravenous Anesthesia |
| ASA | American Association of Anesthesiologists |
| RoB | Risk of Bias |
| SMD | Standardized Mean Difference |
| Cl | Confidence Interval |
| RevMan | Review Manager |
| TNM | Tumor–Node–Metastasis |
| TNF-a | Tumor Necrosis Factor-a |
| IL | Interleukin |
| N/A | Not Available |
| TCR | T-cell receptor |
| MHC | Major Histocompatibility Complex |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, P.; Sun, Z.; Wang, Y.; Chen, J.; Miao, C. Surgical Trauma Induces Postoperative T-Cell Dysfunction in Lung Cancer Patients through the Programmed Death-1 Pathway. Cancer Immunol. Immunother. 2015, 64, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Calvano, S.E.; Lowry, S.F. Inflammatory Cytokines and Cell Response in Surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef]
- Kim, R. Effects of Surgery and Anesthetic Choice on Immunosuppression and Cancer Recurrence. J. Transl. Med. 2018, 16, 8. [Google Scholar] [CrossRef]
- Niwa, H.; Rowbotham, D.J.; Lambert, D.G.; Buggy, D.J. Can Anesthetic Techniques or Drugs Affect Cancer Recurrence in Patients Undergoing Cancer Surgery? J. Anesth. 2013, 27, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shao, X. Isoflurane Promotes Non-Small Cell Lung Cancer Malignancy by Activating the Akt-Mammalian Target of Rapamycin (Mtor) Signaling Pathway. Med. Sci. Monit. 2016, 22, 4644. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, X.; Wei, G.; Meng, Y. Exposure to Desflurane Anesthesia Confers Colorectal Cancer Cells Metastatic Capacity through Deregulation of Mir-34a/Loxl3. Eur. J. Cancer Prev. 2021, 30, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Loop, T.; Dovi-Akue, D.; Frick, M.; Roesslein, M.; Egger, L.; Humar, M.; Hoetzel, A.; Schmidt, R.; Borner, C.; Pahl, H.L.; et al. Volatile Anesthetics Induce Caspase-Dependent, Mitochondria-Mediated Apoptosis in Human T Lymphocytes In Vitro. Anesthesiology 2005, 102, 1147–1157. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Asada, A.; Arita, K.; Utsumi, T.; Yoshida, T.; Sato, E.; Utsumi, K.; Inoue, M. Induction and Mechanism of Apoptotic Cell Death by Propofol in Hl-60 Cells. Acta Anaesthesiol. Scand. 2002, 46, 1068–1074. [Google Scholar] [CrossRef]
- Wu, K.C.; Yang, S.T.; Hsia, T.C.; Yang, J.S.; Chiou, S.M.; Lu, C.C.; Wu, R.S.; Chung, J.G. Suppression of Cell Invasion and Migration by Propofol Are Involved in Down-Regulating Matrix Metalloproteinase-2 and P38 Mapk Signaling in A549 Human Lung Adenocarcinoma Epithelial Cells. Anticancer Res. 2012, 32, 4833–4842. [Google Scholar]
- Bar-Yosef, S.; Melamed, R.; Page, G.G.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Attenuation of the Tumor-Promoting Effect of Surgery by Spinal Blockade in Rats. Anesthesiology 2001, 94, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Seki, S.; Takahashi, T.; Kawarabayashi, N.; Higuchi, H.; Habu, Y.; Sugahara, S.; Kazama, T. Combined Spinal and General Anesthesia Attenuates Liver Metastasis by Preserving Th1/Th2 Cytokine Balance. Anesthesiology 2007, 106, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhang, D.; Sun, K.; Zhu, Y.; Xu, J.; Kang, Y.; Zhang, G.; Cai, Y.; Mao, S.; Long, R.; et al. Propofol Maintains Th17/Treg Cell Balance in Elderly Patients Undergoing Lung Cancer Surgery through Gabaa Receptor. BMC Immunol. 2022, 23, 58. [Google Scholar] [CrossRef]
- Li, M.H.; Xu, Z.Z.; Huang, S.M.; Li, T.; Li, X.Y.; Wang, D.X. Effect of Combined Epidural Anaesthesia on Tumor-Infiltrating Lymphocytes in Lung Adenocarcinoma: A Prospective Exploratory Sub-Analysis. Acta Anaesthesiol. Scand. 2018, 62, 687–700. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, N.-J.; Zhang, H.; Zhu, Y.-M. Effects of Combined General-Epidural Anesthesia and Total Intravenous Anesthesia on Cellular Immunity and Prognosis in Patients with Non-Small Cell Lung Cancer: A Comparative Study. Mol. Med. Rep. 2017, 16, 4445–4454. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kawagoe, I.; Inoue, S.; Kochiyama, T.; Fukuda, M.; Saito, M.; Hayashida, M. Propofol Decreases Cd8+ T Cells and Sevoflurane Increases Regulatory T Cells after Lung Cancer Resection: A Randomized Controlled Trial. J. Thorac. Dis. 2021, 13, 5430–5438. [Google Scholar] [CrossRef]
- Yuan, X.; Qu, P.; Fang, J.; Cai, S.; Fu, S. Effect of Epidural Anesthesia on the Immune Function of Patients with Non-Small Cell Lung Cancer. Int. J. Pharmacol. 2018, 14, 826–834. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, R.; Fang, J. Effects of Different Types of Anesthesia and Analgesia on the Cellular Immunity of Patients after Radical Resection for Lung Cancer. Biomed. Res. 2017, 28, 5981–5986. [Google Scholar]
- Chen, J.; Luo, F.; Lei, M.; Chen, Z. A Study on Cellular Immune Function of Patients Treated with Radical Resection of Pulmonary Carcinoma with Two Different Methods of Anesthesia and Analgesia. J. BUON Off. J. Balk. Union Oncol. 2017, 22, 1416–1421. [Google Scholar]
- Philip, M.; Schietinger, A. Cd8(+) T Cell Differentiation and Dysfunction in Cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef]
- Margolick, J.B.; Donnenberg, A.D.; Chu, C.; O’Gorman, M.R.; Giorgi, J.V.; Muñoz, A. Decline in Total T Cell Count Is Associated with Onset of Aids, Independent of Cd4(+) Lymphocyte Count: Implications for Aids Pathogenesis. Clin. Immunol. Immunopathol. 1998, 88, 256–263. [Google Scholar] [CrossRef]
- Richardson, J.R.; Schöllhorn, A.; Gouttefangeas, C.; Schuhmacher, J. Cd4+ T Cells: Multitasking Cells in the Duty of Cancer Immunotherapy. Cancers 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Sigel, K.; Wisnivesky, J.; Crothers, K.; Gordon, K.; Brown, S.T.; Rimland, D.; Rodriguez-Barradas, M.C.; Gibert, C.; Goetz, M.B.; Bedimo, R.; et al. Immunological and Infectious Risk Factors for Lung Cancer in Us Veterans with Hiv: A Longitudinal Cohort Study. Lancet HIV 2017, 4, e67–e73. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, J.D.; Gray, S.; Richard, D.; Fennell, D.; O’Leary, J.J.; Blackhall, F.H.; O’Byrne, K.J. Circulating Tumour Cells, Their Role in Metastasis and Their Clinical Utility in Lung Cancer. Lung Cancer 2012, 76, 19–25. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, F.; Li, H.; Yang, L.; Lv, T.; Gu, W.; Song, Y. Circulating Tumor Cells Were Associated with the Number of T Lymphocyte Subsets and Nk Cells in Peripheral Blood in Advanced Non-Small-Cell Lung Cancer. Dis. Markers 2017, 2017, 5727815. [Google Scholar] [CrossRef] [PubMed]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour Antigens Recognized by T Lymphocytes: At the Core of Cancer Immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Gao, Z.; Han, Y. Prognostic Value of Peripheral Naive Cd8(+) T Cells in Oligometastatic Non-Small-Cell Lung Cancer. Future Oncol. 2022, 18, 55–65. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Principe, D.R.; Chiec, L.; Mohindra, N.A.; Munshi, H.G. Regulatory T-Cells as an Emerging Barrier to Immune Checkpoint Inhibition in Lung Cancer. Front. Oncol. 2021, 11, 684098. [Google Scholar] [CrossRef] [PubMed]
- Glasner, A.; Rose, S.A.; Sharma, R.; Gudjonson, H.; Chu, T.; Green, J.A.; Rampersaud, S.; Valdez, I.K.; Andretta, E.S.; Dhillon, B.S.; et al. Conserved Transcriptional Connectivity of Regulatory T Cells in the Tumor Microenvironment Informs New Combination Cancer Therapy Strategies. Nat. Immunol. 2023, 24, 1020–1035. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, G.C.; Liu, Z.P.; Chen, X.; Zhao, Y.X.; Ding, Q.; Huang, S. The Prevalence of Foxp3+ Regulatory T-Cells in Peripheral Blood of Patients with Nsclc. Cancer Biother. Radiopharm. 2009, 24, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| First Author, Year, Country | Number of Participants (Male/Total) | Lung Cancer Subtype | TNM Classification Group 1 | TNM Classification Group 2 | TNM Classification Group 3 | Type of Surgery | ASA | Age of Participants (Years) |
|---|---|---|---|---|---|---|---|---|
| Cui, C. [13] 2022, China | 36 Propofol group: 13/18 Sevoflurane group: 12/18 | N/A | N/A | N/A | N/A | Lobectomy and lymph node dissection, excluding unilateral pneumonectomy | I–II | N/A |
| Li, M.-H. [14] 2018, China | 64 General anesthesia group: 10/27 General plus EA group: 20/37 | Adenocarcinoma | General anesthesia group T1: 16, T2: 9, T3: 1, T4: 1, N0: 24, N1: 0, N2: 3, M0: 27 | General anesthesia plus EA group T1: 23, T2: 10, T3: 3, T4: 1, N0: 27, N1: 2, N2: 8, M0: 37 | N/A | General anesthesia group Thoracoscopic surgery: pneumonectomy, 0; lobectomy, 26; less resection, 1; General anesthesia plus EA group Thoracoscopic surgery: pneumonectomy, 1; lobectomy, 35; less resection, 1; | General anesthesia group: ASA I: 2, ASA II: 26, ASA III: - General anesthesia plus EA group: ASA I: 0, ASA II: 37, ASA III: - | General anesthesia group: 62.0 ± 10.9 General anesthesia plus EA group: 61.1 ± 8.5 |
| Xu Q. [15] 2017, China | 120 General anesthesia group: 43/60 General plus EA group: 44/60 | General anesthesia group; squamous cell carcinoma: 18, adenocarcinoma: 31, large cell carcinoma: 5, adenosquamous carcinoma: 6 General plus EA group; squamous cell carcinoma: 20, adenocarcinoma: 30, large cell carcinoma: 5, adenosquamous carcinoma: 5 | General anesthesia group Stage II: 27, Stage IIA: 33 | General anesthesia plus EA group Stage II: 26 Stage IIA: 34 | N/A | Video-assisted thoracoscopic surgery for radical resection: right upper lobectomy, 34; right middle lobectomy, 16; right lower lobectomy, 15; left upper lobectomy, 30; left lower lobectomy, 22; total left lung resection, 3; | N/A | General anesthesia group: 56.2 ± 6.4 General anesthesia plus EA group: 56.1 ± 5.8 |
| Yamaguchi, A. [16] 2021, Japan | 64 General anesthesia Desflurane group: 15/20 Sevoflurane group: 15/22 Propofol group: 15/22 | Desflurane group; adenocarcinoma: 16, squamous cell carcinoma: 3, metastatic tumor: 1 Sevoflurane group; adenocarcinoma: 15, squamous cell carcinoma: 3, small cell carcinoma: 1, sarcoma: 1, metastatic tumor: 2 Propofol group; adenocarcinoma: 14, squamous cell carcinoma: 7, metastatic tumor: 1 | Desflurane group Stage 0: 3, Stage I: 9, Stage II: 4, Stage III: 3, metastatic tumor: 1 | Sevoflurane group Stage 0: 2, Stage I: 12, Stage II: 5, Stage III: 1, metastatic tumor: 2 | Propofol group: Stage 0: 1, Stage I: 17, Stage II: 1, Stage III: 2. metastatic tumor: 1 | Desflurane group: lobectomy, 14; segment resection, 2; partial resection, 4; Sevoflurane group: lobectomy, 16; segment resection, 4; partial resection, 2; Propofol group: lobectomy, 14; segment resection, 7; partial resection, 1; | Desflurane group: ASA I: 0, ASA II: 14, ASA III: 6 Sevoflurane group: ASA I: 2, ASA II: 16, ASA III: 4 Propofol group: ASA I: 2, ASA II: 14, ASA III: | Desflurane group: 69.2 ± 8.9 Sevoflurane group: 69.0 ± 9.0 Propofol group: 68.8 ± 8.9 |
| Yuan, X. [17] 2018, China | 246 General anesthesia Sevoflurane group: 85/123 Propofol group: 78/123 | N/A | Sevoflurane group Stage I: 12, Stage II: 25, Stage III: 86 | Propofol group Stage I: 15, Stage II: 29, Stage III: 79 | N/A | Thoracotomy | Sevoflurane group: ASA I: 73, ASA II: 12, ASA III: 8 ASA IV: 30 Propofol group: ASA I: 69 ASA II: 13, ASA III: 9, ASA IV: 32 | Sevoflurane group: 43.95 ± 2.01 Propofol group: 46.39 ± 1.85 |
| Zhu, Y. [18] 2017, China | 40 General anesthesia group: 11/20 General anesthesia plus EA group: 13/20 | N/A | N/A | N/A | N/A | Radical resection of lung cancer, thoracotomy | I–II | General anesthesia group: 59 ± 7 General anesthesia plus EA: 58 ± 6 |
| Chen, J. [19] 2017, China | 34 General anesthesia group:13/17 General anesthesia plus EA group:12/17 | N/A | N/A | N/A | N/A | Radical resection | I–II | General anesthesia group: 56 ± 6 General anesthesia plus EA: 59 ± 7 |
| First Author, Year, Country | GA Maintenance | Epidural Catheter Characteristics | Duration of the Epidural Catheter | Epidural Medications Intraoperatively | Intraoperative Opioids | Postoperative Analgesia |
|---|---|---|---|---|---|---|
| Cui C. [13] 2022, China | General anesthesia Propofol group Sevoflurane group | N/A | N/A | N/A | Fentanyl 1 μg/kg (when heart rate or blood pressure was higher than 20% of the basic values) | N/A |
| Li, M.-H. [14] 2018, China | General anesthesia group: propofol General anesthesia plus EA group: epidural ropivacaine, IV propofol | The epidural catheter was placed at the T6–T8 level preoperatively | N/A | 0.5% ropivacaine (by continuous infusion at a rate of 4–6 mL/h), | iv sulfentanil/remifentanyl | General anesthesia group: PCIA with 0.5 mg/mL morphine General anesthesia with EA group: PCEA with 0.12% ropivacaine and 0.5 lg/mL sufentanil |
| Xu Q. [15] 2017, China | General anesthesia group: IV vecuronium General anesthesia plus EA group: epidural ropivacaine, IV vecuronium | The epidural catheter was placed at the T7–T8 level | N/A | 0.375% ropivacaine | 5 µg/kg fentanyl | N/A |
| Yamaguchi, A. [16] 2021, Japan | General anesthesia desflurane group sevoflurane group propofol group | The epidural catheter was placed at the T5–T7 level | N/A | 4 mL 0.25% levobupivacaine, 50 µg fentanyl, and 1–2 mg morphine, followed by continuous epidural infusion of levobupivacaine (3 mL/h) and morphine (2–3 mg/day) | 0.1–0.25 µg/kg/min ramifentanil according to blood pressure and heart rate | Levobupivacaine (3 mL/h) and morphine (2–3 mg/day) via the epidural catheter |
| Yuan, X. [17] 2018, China | General anesthesia sevoflurane group propofol group | N/A | N/A | N/A | 0.1–0.2 µg/kg/min remifentanil | N/A |
| Zhu, Y. [18] 2017, China | General anesthesia group: propofol General anesthesia plus EA group: rovipacaine epidural, propofol IV | The epidural catheter was placed at the T7–T8 level | N/A | A total of 0.375% ropivacaine (3–5 mL) was administered epidurally every 30 min during surgery, 30 min before the end of surgery, additional ropivacaine was administered | General anesthesia group: 4–6 ng/mL remifentanil (to control blood pressure and heart rate below 1.2 times the baseline), sulfentanil loading dose (5 mL; 0.1 μg/kg) followed by infusion 2 mL/h, sulfentanil through PCA (containing 3 μg/kg sufentanil/150 mL, 2 mL bolus at 15 min) General anesthesia plus EA group: ramifentanil | Epidural rovipacaine and sulfentanil through PCA pump [3 μg/kg sufentanil and 0.2% ropivacaine (300 mL)]- bolus 4 mL at 15 min |
| Chen J. [19] 2017, China | General anesthesia group: propofol General anesthesia plus EA group: rovipacaine epidural, propofol IV | The epidural catheter was placed at the T7–T8 level | N/A | Ropivacaine 0.375% and 3–5 mL before surgery, repeated every 30 min for 1–2 times during the operation, around 30 min before finishing surgery, ropivacaine was added in the epidural line for the last time as load capacity, analgesia pump was connected to epidural line | Remifentanyl through controlled infusion (target concentration at 3–6 μg/mL) | Epidural sufentanyl 3 μg/kg, ropivacaine 0.2%, flow rate 4 mL/h (dose 300 mL) |
| First Author, Year, Country | Randomisation Process | Deviation from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|
| Cui C. [13] 2022, China | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Li, M.-H. [14] 2018, China | Low | Some concerns | Low | Low | Low | Some concerns |
| Χu, Q. [15] 2017, China | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Yamaguchi, A. [16] 2021, Japan | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Yuan, X. [17] 2018, China | Some concerns | Low | Low | Low | Low | Some concerns |
| Zhu, Y. [18] 2017, China | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Chen, J. [19] 2017, China | Some concerns | Some concerns | Low | Low | Low | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantis, G.; Katsadouros, I.; Tsaousi, G.; Grosomanidis, V.; Pourzitaki, C. Immunomodulatory Effects of Anesthetic Techniques in Lung Cancer Surgery: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1263. https://doi.org/10.3390/medicina61071263
Konstantis G, Katsadouros I, Tsaousi G, Grosomanidis V, Pourzitaki C. Immunomodulatory Effects of Anesthetic Techniques in Lung Cancer Surgery: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(7):1263. https://doi.org/10.3390/medicina61071263
Chicago/Turabian StyleKonstantis, Georgios, Ilias Katsadouros, Georgia Tsaousi, Vasileios Grosomanidis, and Chryssa Pourzitaki. 2025. "Immunomodulatory Effects of Anesthetic Techniques in Lung Cancer Surgery: A Systematic Review and Meta-Analysis" Medicina 61, no. 7: 1263. https://doi.org/10.3390/medicina61071263
APA StyleKonstantis, G., Katsadouros, I., Tsaousi, G., Grosomanidis, V., & Pourzitaki, C. (2025). Immunomodulatory Effects of Anesthetic Techniques in Lung Cancer Surgery: A Systematic Review and Meta-Analysis. Medicina, 61(7), 1263. https://doi.org/10.3390/medicina61071263