Laparoscopic Radical Colectomy with Complete Mesocolic Excision Offers Similar Results Compared with Open Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Surgical Technique
2.2.1. Right Colectomy with CME Was Used for Tumors Situated in the Cecum and Ascending Colon up to Right Third of the Transverse Colon
2.2.2. Transverse Colectomy with CME Was Used for Tumors in the Vicinity of the Middle Third of the Transverse Colon
2.2.3. Left Colectomy with CME Was Used for Tumors Located at the Splenic Flexure, and Descending and Sigmoid Colon
2.3. Statistical Analysis
Calculation of Long-Term Survival
3. Results
3.1. Characteristics of the Study Cohort
3.2. Pathological Characteristics of the Surgical Specimen
3.3. Postoperative Outcome in the Study Cohort
3.4. Comparison of the Open vs. Laparoscopic Surgical Groups
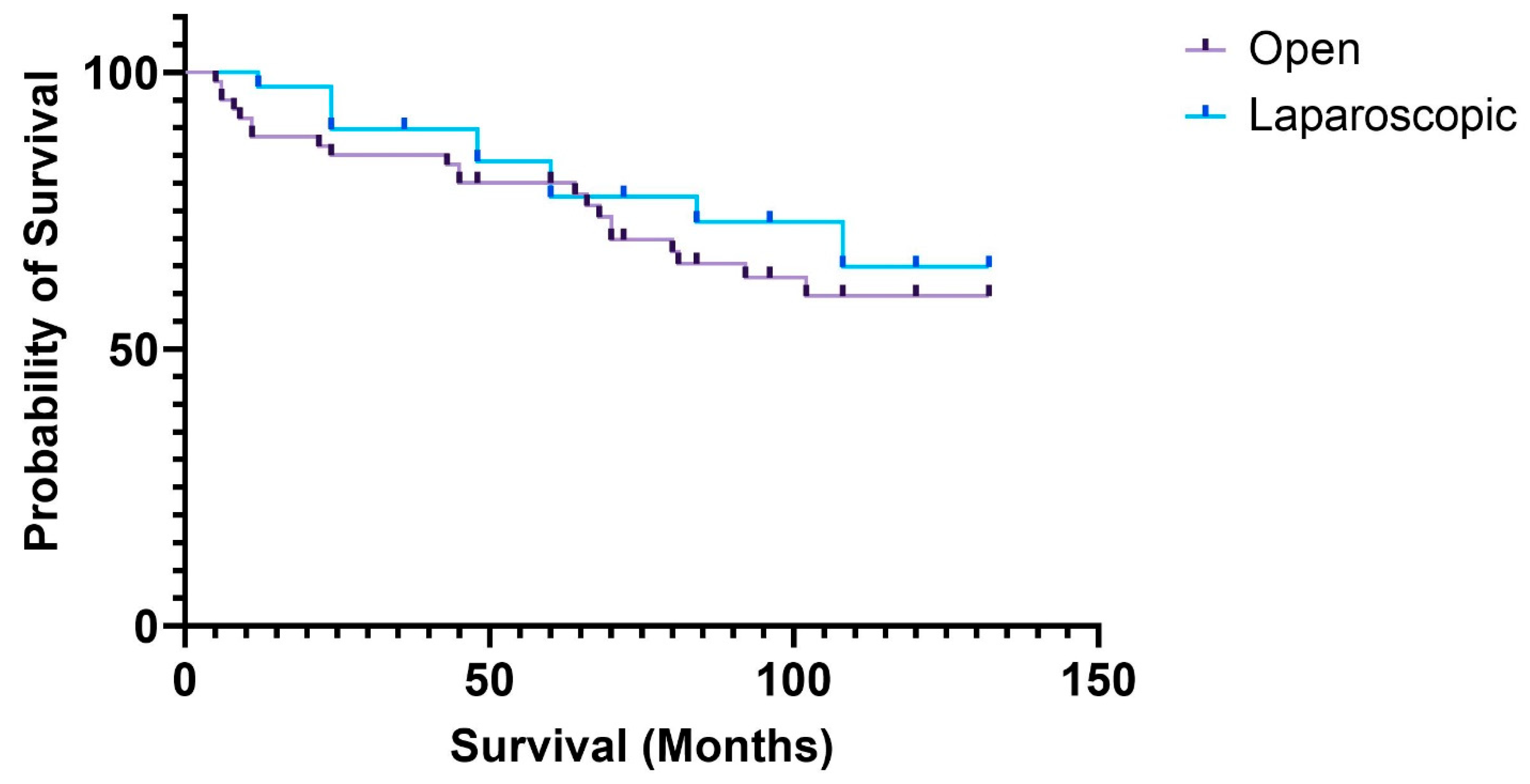
3.5. Long-Term Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Heald, R.J. The ‘Holy Plane’ of rectal surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—Technical notes and outcome. Color. Dis. 2009, 11, 354–364; discussion 364–365. [Google Scholar] [CrossRef] [PubMed]
- Le Voyer, T.E.; Sigurdson, E.R.; Hanlon, A.L.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J. Clin. Oncol. 2003, 21, 2912–2919. [Google Scholar] [CrossRef]
- Bertelsen, C.A.; Neuenschwander, A.U.; Jansen, J.E.; Wilhelmsen, M.; Kirkegaard-Klitbo, A.; Tenma, J.R.; Bols, B.; Ingeholm, P.; Rasmussen, L.A.; Jepsen, L.V.; et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: A retrospective, population-based study. Lancet Oncol. 2015, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Merkel, S.; Weber, K.; Matzel, K.E.; Agaimy, A.; Göhl, J.; Hohenberger, W. Prognosis of patients with colonic carcinoma before, during and after implementation of complete mesocolic excision. Br. J. Surg. 2016, 103, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.M.; Bos, A.C.R.K.; Lemmens, V.E.P.P.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.W.; Verhoeven, R.H.A. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Fleshman, J.; Sargent, D.J.; Green, E.; Anvari, M.; Stryker, S.J.; Beart, R.W., Jr.; Hellinger, M.; Flanagan, R., Jr.; Peters, W.; Nelson, H.; et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann. Surg. 2007, 246, 655–662; discussion 662–664. [Google Scholar] [CrossRef]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M.; MRC CLASICC Trial Group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef]
- Kitano, S.; Inomata, M.; Mizusawa, J.; Katayama, H.; Watanabe, M.; Yamamoto, S.; Ito, M.; Saito, S.; Fujii, S.; Konishi, F.; et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2017, 2, 261–268. [Google Scholar] [CrossRef]
- Fagarasan, V.; Cordos, A.; Petrisor, C.; Bintintan, A.; Chira, R.; Nickel, F.; Surlin, V.; Dindelegan, G.; Bintintan, V. Which is the Optimal Method of Reconstruction After Laparoscopic Right Hemicolectomy, the Intracorporeal or Extracorporeal Anastomosis Technique? Chirurgia 2020, 115, 493–504. [Google Scholar] [CrossRef]
- Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma, 2nd ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2010. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Torre, L.; Bray, F.; Siegel, R.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Søndenaa, K.; Quirke, P.; Hohenberger, W.; Sugihara, K.; Kobayashi, H.; Kessler, H.; Brown, G.; Tudyka, V.; D’Hoore, A.; Kennedy, R.H.; et al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery: Proceedings of a consensus conference. Int. J. Color. Dis. 2014, 29, 419–428. [Google Scholar] [CrossRef]
- Storli, K.E.; Søndenaa, K.; Furnes, B.; Nesvik, I.; Gudlaugsson, E.; Bukholm, I.; Eide, G.E. Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I–II. Tech. Coloproctol. 2014, 18, 557–564. [Google Scholar] [CrossRef]
- Galizia, G.; Lieto, E.; De Vita, F.; Ferraraccio, F.; Zamboli, A.; Mabilia, A.; Auricchio, A.; Castellano, P.; Napolitano, V.; Orditura, M. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int. J. Color. Dis. 2014, 29, 89–97. [Google Scholar] [CrossRef]
- Killeen, S.; Mannion, M.; Devaney, A.; Winter, D.C. Complete mesocolic resection and extended lymphadenectomy for colon cancer: A systematic review. Color. Dis. 2014, 16, 577–594. [Google Scholar] [CrossRef]
- Seow-En, I.; Chen, W.T. Complete mesocolic excision with central venous ligation/D3 lymphadenectomy for colon cancer—A comprehensive review of the evidence. Surg. Oncol. 2022, 42, 101755. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Bilchik, A.J. More extensive nodal dissection improves survival for stages I to III of colon cancer: A population-based study. Ann. Surg. 2006, 244, 602–610. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Lee, G.R.; Kim, J.H.; Lee, Y.S. Laparoscopic complete mesocolic excision with D3 lymph node dissection for right colon cancer in elderly patients. Sci. Rep. 2020, 10, 12633. [Google Scholar] [CrossRef]
- Ouyang, M.; Luo, Z.; Wu, J.; Zhang, W.; Tang, S.; Lu, Y.; Hu, W.; Yao, X. Comparison of outcomes of complete mesocolic excision with conventional radical resection performed by laparoscopic approach for right colon cancer. Cancer Manag. Res. 2019, 11, 8647–8656. [Google Scholar] [CrossRef]
- Reddavid, R.; Osella, G.; Evola, F.; Puca, L.; Spidalieri, L.; Rorato, L.; Sangiuolo, F.; Solej, M.; Degiuli, M. Complete mesocolic excision for right colon cancer—State of art: A systematic review of the literature. Ann. Laparosc. Endosc. Surg. 2020, 5, 42. [Google Scholar] [CrossRef]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar] [CrossRef]
- Colon Cancer Laparoscopic or Open Resection Study Group; Buunen, M.; Veldkamp, R.; Hop, W.C.; Kuhry, E.; Jeekel, J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef]
- Brown, K.G.M.; Ng, K.S.; Solomon, M.J.; Chapuis, P.H.; Koh, C.E.; Ahmadi, N.; Austin, K.K.S. Complete mesocolic excision for colon cancer: Current status and controversies. ANZ J. Surg. 2024, 94, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Swanson, R.S.; Compton, C.C.; Stewart, A.K.; Bland, K.I. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann. Surg. Oncol. 2003, 10, 65–71. [Google Scholar] [CrossRef]
- Shin, J.K.; Kim, H.C.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A.; Chun, H.-K. Laparoscopic modified mesocolic excision with central vascular ligation in right-sided colon cancer shows better short- and long-term outcomes compared with the open approach in propensity score analysis. Surg. Endosc. 2018, 32, 2721–2731. [Google Scholar] [CrossRef]
- Bae, S.U.; Saklani, A.P.; Lim, D.R.; Kim, D.W.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann. Surg. Oncol. 2014, 21, 2288–2294. [Google Scholar] [CrossRef]
- Huang, J.L.; Wei, H.B.; Fang, J.F.; Zheng, Z.H.; Chen, T.F.; Wei, B.; Huang, Y.; Liu, J.P. Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int. J. Surg. 2015, 23, 12–17. [Google Scholar] [CrossRef]
- Zheng, Z.; Jemal, A.; Lin, C.C.; Hu, C.Y.; Chang, G.J. Comparative effectiveness of laparoscopy vs open colectomy among nonmetastatic colon cancer patients: An analysis using the National Cancer Data Base. J. Natl. Cancer Inst. 2015, 107, dju491. [Google Scholar] [CrossRef]
- West, N.P.; Kennedy, R.H.; Magro, T.; Luglio, G.; Sala, S.; Jenkins, J.T.; Quirke, P. Morphometric analysis and lymph node yield in laparoscopic complete mesocolic excision performed by supervised trainees. Br. J. Surg. 2014, 101, 1460–1467. [Google Scholar] [CrossRef]
- Gouvas, N.; Pechlivanides, G.; Zervakis, N.; Kafousi, M.; Xynos, E. Complete mesocolic excision in colon cancer surgery: A comparison between open and laparoscopic approach. Color. Dis. 2012, 14, 1357–1364. [Google Scholar] [CrossRef]
- Zmora, O.; Bar-Dayan, A.; Khaikin, M.; Lebeydev, A.; Shabtai, M.; Ayalon, A.; Rosin, D. Laparoscopic colectomy for transverse colon carcinoma. Tech. Coloproctol. 2010, 14, 25–30. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Liu, H.; Chen, H.; Deng, H.; Yu, J.; Xue, Q.; Li, G. Long-Term Outcomes of Laparoscopic Surgery for Advanced Transverse Colon Cancer. J. Gastrointest. Surg. 2014, 18, 1003–1009. [Google Scholar] [CrossRef]
| Open (Number of Cases) | Laparoscopic (Number of Cases) | ||
|---|---|---|---|
| Age (mean + 95%CI) | 66.2097 ± 2.415 | 63.0938 ± 3.518 | |
| Sex | Male | 30 | 19 |
| Female | 31 | 20 | |
| Charlson Comorbidity Index (mean + 95%CI) | 6.4561 ± 0.604 | 5.7391 ± 0.656 | |
| ASA score | 1 | 0 | 3 |
| 2 | 23 | 19 | |
| 3 | 36 | 17 | |
| 4 | 2 | 0 | |
| Tumor location | Cecum | 7 | 6 |
| Ascending colon | 15 | 8 | |
| Transverse colon | 19 | 4 | |
| Descending colon | 11 | 1 | |
| Sigmoid colon | 9 | 20 | |
| Average tumor size (cm) (25–75 percentiles) | 5 | 4 | |
| (4–6.5) | (3–4.8) | ||
| pT staging | Tis–3 | Tis–4 | |
| T1–1 | T1–2 | ||
| T2–7 | T2–6 | ||
| T3–39 | T3–23 | ||
| T4a–7 | T4a–4 | ||
| T4b–4 | T4b–0 | ||
| Complete R0 resection | 100% | 96.5% | |
| Resection margin (cm) (25–75 percentiles) | Distal | 11 | 9 |
| (5.27–14.75) | (5.12–13.37) | ||
| Proximal | 11.5 | 10 | |
| (7.5–17) | (5.12–12.37) | ||
| Radial | 6 | 5 | |
| (3–9.75) | (2.25–7.75) | ||
| Average lymph node count Median (min–max) | 23 | 20 | |
| (8–48) | (7–49) | ||
| Length of hospital stay (days) (25–75 percentiles) | 10 | 8 | |
| (7–13.25) | (7–11.5) | ||
| Open | Laparoscopic | p-Value | |
|---|---|---|---|
| Right colon (40 cases) Lymph node count (min–max) Radial margin (25–75 percentile) | 24 (8–30) 5.5 (2–10) | 24 (13–49) 5.5 (3–7) | p = 0.631 p = 0.702 |
| Transverse colon (11 cases) Lymph node count (min–max) Radial margin (25–75 percentile) | 24 (11–33) 6 (2.5–7) | 17 (16–18) 5 (5–5) | p = 0.405 p = 0.613 |
| Left colon (49 cases) Lymph node count (min–max) Radial margin (25–75 percentile) | 21 (8–40) 6 (3–9.62) | 18 (7–47) 4.5 (1–9) | p = 0.225 p = 0.52 |
| Open | Laparoscopic | p-Value | |
|---|---|---|---|
| Radial resection margin (cm) (25–75 percentiles) | 8 (5–11) | 4 (1–5.5) | 0.01 |
| Distal resection margin (cm) (25–75 percentiles) | 13 (7.12–15.75) | 10 (7–15.25) | 0.5 |
| Proximal resection margin (cm) (25–75 percentiles) | 10.5 (7–19.5) | 10.5 (9.35–12) | 0.77 |
| Complete resection (R0) | 95% | 100% | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bintintan, V.V.; Fagarasan, V.; Seicean, R.I.; Andras, D.; Ene, A.I.; Chira, R.; Bintintan, A.; Nagy, G.; Petrisor, C.; Cocu, S.; et al. Laparoscopic Radical Colectomy with Complete Mesocolic Excision Offers Similar Results Compared with Open Surgery. Medicina 2025, 61, 1231. https://doi.org/10.3390/medicina61071231
Bintintan VV, Fagarasan V, Seicean RI, Andras D, Ene AI, Chira R, Bintintan A, Nagy G, Petrisor C, Cocu S, et al. Laparoscopic Radical Colectomy with Complete Mesocolic Excision Offers Similar Results Compared with Open Surgery. Medicina. 2025; 61(7):1231. https://doi.org/10.3390/medicina61071231
Chicago/Turabian StyleBintintan, Vasile V., Vlad Fagarasan, Radu I. Seicean, David Andras, Alexandru I. Ene, Romeo Chira, Adriana Bintintan, Georgiana Nagy, Cristina Petrisor, Simona Cocu, and et al. 2025. "Laparoscopic Radical Colectomy with Complete Mesocolic Excision Offers Similar Results Compared with Open Surgery" Medicina 61, no. 7: 1231. https://doi.org/10.3390/medicina61071231
APA StyleBintintan, V. V., Fagarasan, V., Seicean, R. I., Andras, D., Ene, A. I., Chira, R., Bintintan, A., Nagy, G., Petrisor, C., Cocu, S., Stefanescu, E., Negoi, I., Calborean, A., Dindelegan, G. C., Silaghi, C., Lupan, I., & Samasca, G. (2025). Laparoscopic Radical Colectomy with Complete Mesocolic Excision Offers Similar Results Compared with Open Surgery. Medicina, 61(7), 1231. https://doi.org/10.3390/medicina61071231









