Vitamin D Deficiency and Supplementation in Irritable Bowel Syndrome: Retrospective Evaluation of Subtype and Sex-Based Differences
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Baylink, D.J.; Cao, H.; Xiao, J.; Abdalla, M.; Wasnik, S.; Tang, X.; Tang, X. Inflammation- and Gut-Homing Macrophages, Engineered to De Novo Overexpress Active Vitamin D, Promoted the Regenerative Function of Intestinal Stem Cells. Int. J. Mol. Sci. 2021, 22, 9516. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Minervini, G.; Giordano, M. Vitamin D: Can Gender Medicine Have a Role? Biomedicines 2023, 11, 1762. [Google Scholar] [CrossRef] [PubMed]
- Bhoora, S.; Punchoo, R. Policing Cancer: Vitamin D Arrests the Cell Cycle. Int. J. Mol. Sci. 2020, 21, 9296. [Google Scholar] [CrossRef]
- Ali, S.; Kamal Mohamed, I.; Zaki, Z.E.; Elsayed, A.S. Clinical Correlation between Serum Vitamin D Level and Irritable Bowel Syndrome. El-Minia Med. Bull. 2022, 33, 228–232. [Google Scholar] [CrossRef]
- Al-Shemery, M.K.; Al Kafhage, F.A.; Al-Masaoodi, R.A. Vitamin D3 and Irritable Bowel Syndrome: Review. Int. Sci. Health J. 2024, 2, 117–127. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2020, 10, 3141. [Google Scholar] [CrossRef]
- Lacy, B.E.; Cangemi, D.J. Irritable Bowel Syndrome. In Handbook of Gastrointestinal Motility and Disorders of Gut-Brain Interactions, 2nd ed.; Rao, S.S.C., Parkman, H.P., McCallum, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 245–261. [Google Scholar] [CrossRef]
- Defrees, D.N.; Bailey, J. Irritable Bowel Syndrome: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Prim. Care 2017, 44, 655–671. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Global Burden of Irritable Bowel Syndrome: Trends, Predictions and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Shorey, S.; Demutska, A.; Chan, V.; Siah, K.T.H. Adults Living with Irritable Bowel Syndrome (IBS): A Qualitative Systematic Review. J. Psychosom. Res. 2021, 140, 110289. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef]
- Yu, X.L.; Wu, Q.Q.; He, L.P.; Zheng, Y.F. Role of in Vitamin D in Irritable Bowel Syndrome. World J. Clin. Cases 2023, 11, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.-Y.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.I.H.; Yaow, C.Y.L.; Loh, C.Y.L.; Teoh, S.E.; Masuda, Y.; Ng, W.K.; Ng, Q.X. Vitamin D Supplementation for Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2022, 37, 993–1003. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The Efficacy of Vitamin D Supplementation for Irritable Bowel Syndrome: A Systematic Review with Meta-Analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef]
- Williams, C.; Williams, E.; Corfe, B. Vitamin D Supplementation in People with IBS Has No Effect on Symptom Severity and Quality of Life: Results of a Randomised Controlled Trial. Eur. J. Nutr. 2022, 61, 299–308. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Husseiny, A.; Zamili, S.; Shala, H. Prevalence of IBS and Its Relationship to Vitamin D Deficiency in Al-Nasiriyah City, Iraq. Rawal Med. J. 2023, 48, 630–634. [Google Scholar] [CrossRef]
- Matthews, S.W.; Plantinga, A.; Burr, R.L.; Cain, K.C.; Savidge, T.C.; Kamp, K.; Heitkemper, M.M. Exploring the Role of Vitamin D and the Gut Microbiome: A Cross-Sectional Study of Individuals with Irritable Bowel Syndrome and Healthy Controls. Biol. Res. Nurs. 2023, 25, 436–443. [Google Scholar] [CrossRef]
- Abuelazm, M.; Muhammad, S.; Gamal, M.; Labieb, F.; Amin, M.A.; Abdelazeem, B.; Brašić, J.R. The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2618. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, A. Sex Differences in Vitamin D Metabolism, Serum Levels and Action. Br. J. Nutr. 2022, 128, 2115–2130. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, F.; Al-Mahroos, F.; Al-Sahlawi, H.S.; Al-Amer, E.A. The Impact of Dietary Intake and Sun Exposure on Vitamin D Deficiency Among Couples. Bahrain Med. Bull. 2014, 36, 33–37. [Google Scholar] [CrossRef][Green Version]
- Ning, Z.; Song, S.; Miao, L.; Zhang, P.; Wang, X.; Liu, J.; Hu, Y.; Xu, Y.; Zhao, T.; Liang, Y.; et al. High Prevalence of Vitamin D Deficiency in Urban Health Checkup Population. Clin. Nutr. 2016, 35, 859–863. [Google Scholar] [CrossRef]
- Harmon, Q.E.; Umbach, D.M.; Baird, D.D. Use of Estrogen-Containing Contraception Is Associated with Increased Concentrations of 25-Hydroxy Vitamin D. J. Clin. Endocrinol. Metab. 2016, 101, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yao, J.; Hu, L.; Liu, Y.; Hocher, J.; Zhang, X.; Hasan, A.; Lin, G.; Gong, F.; Hocher, B. Vitamin D Binding Protein Correlate with Estrogen Increase After Administration of Human Chorionic Gonadotropin but Do Not Affect Ovulation, Embryo, or Pregnancy Outcomes. Front. Endocrinol. 2024, 15, 1401975. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Potential Role of Vitamin D Supplementation as a Gut Microbiota Modifier in Healthy Individuals. Sci. Rep. 2020, 10, 21641. [Google Scholar] [CrossRef]
- O’Mahony, C.; Clooney, A.; Clarke, S.F.; Aguilera, M.; Gavin, A.; Simnica, D.; Ahern, M.; Fanning, A.; Stanley, M.; Rubio, R.C.; et al. Dietary-Induced Bacterial Metabolites Reduce Inflammation and Inflammation-Associated Cancer via Vitamin D Pathway. Int. J. Mol. Sci. 2023, 24, 1864. [Google Scholar] [CrossRef]
- Gryaznova, M.; Smirnova, Y.; Burakova, I.; Morozova, P.; Lagutina, S.; Chizhkov, P.; Korneeva, O.; Syromyatnikov, M. Fecal Microbiota Characteristics in Constipation-Predominant and Mixed-Type Irritable Bowel Syndrome. Microorganisms 2024, 12, 1414. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex Differences in the Phylum-Level Human Gut Microbiota Composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef]
- Almario, C.V.; Sharabi, E.; Chey, W.D.; Lauzon, M.; Higgins, C.S.; Spiegel, B. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results from a Nationwide Cross-Sectional Study. Gastroenterology 2023, 165, 1475–1487. [Google Scholar] [CrossRef]
- Aslam, A. Irritable Bowel Syndrome in Western and Non-Western Populations: Prevalence, Psychological Comorbidities, Quality of Life and Role of Nutrition. Glob. Immunol. Infect. Dis. Rev. 2024, IX, 64–76. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, N. Sex-Gender Differences in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Heitkemper, M.M. Gender Differences in Irritable Bowel Syndrome. Gastroenterology 2002, 123, 1686–1701. [Google Scholar] [CrossRef] [PubMed]
- Ergenç, Z.; Ergenç, H.; Yıldırım, İ.; Öztürk, A.; Usanmaz, M.; Karacaer, C.; Günay, S.; Kaya, G. Relationship Between Vitamin D Levels and Hematological Parameters. Med. Sci. Discov. 2023, 10, 1–7. [Google Scholar] [CrossRef]
- Mobarki, A.A.; Dobie, G.; Saboor, M.; Madkhali, A.M.; Habibullah, M.; Nahari, M.H.; Kamli, H.; Hakamy, A.; Hamali, H.A. Prevalence and Correlation of Vitamin D Levels with Hematological and Biochemical Parameters in Young Adults. Ann. Clin. Lab. Sci. 2022, 52, 815–824. [Google Scholar] [PubMed]
- Aggeletopoulou, I.; Geramoutsos, G.; Pastras, P.; Triantos, C. Vitamin D in Irritable Bowel Syndrome: Exploring Its Role in Symptom Relief and Pathophysiology. Nutrients 2025, 17, 1028. [Google Scholar] [CrossRef]
- Young, M.F.; Ou, J.; Duong, C.; Luo, H.; Beyh, Y.; Meng, J.; Gernand, A.D.; Roth, D.E.; Suchdev, P.S. Assessment of Vitamin D Status and Association with Inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Am. J. Clin. Nutr. 2022, 117, 175–181. [Google Scholar] [CrossRef]
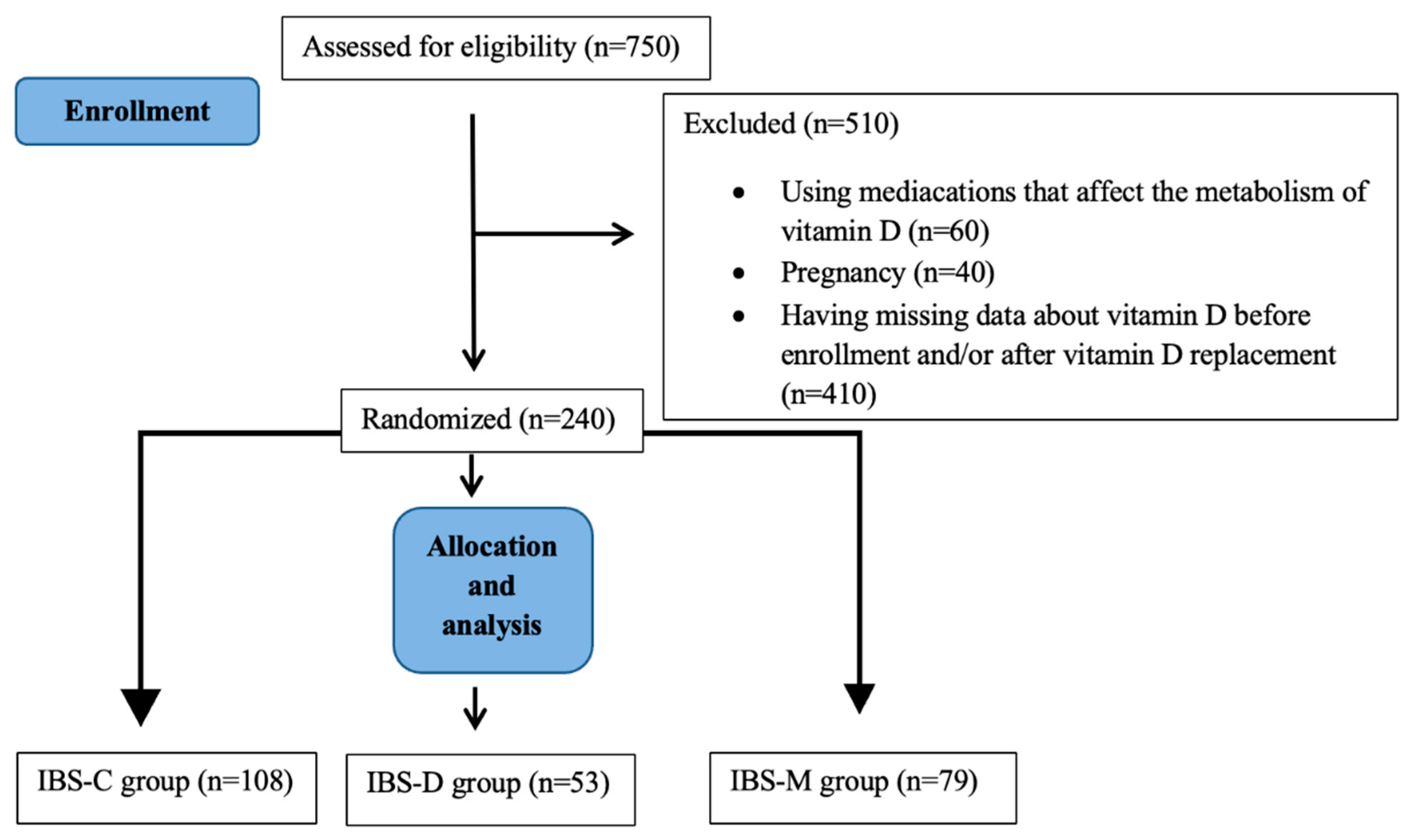

| IBS-D * Group (N = 53) | IBS-C * Group (N = 108) | IBS-M * Group (N = 79) | p Value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Sex | ||||
| Female | 40 | 74 | 45 | 0.488 |
| Male | 13 | 34 | 34 | |
| Age (years) | 44 (35–57) | 46 (37–60) | 44 (30–61) | 0.598 |
| Hematological Parameters | ||||
| Hemoglobin (g/dL) | 14.2 (13.1–15.9) | 14.4 (13.4–15.8) | 14.4 (13.2–15.7) | 0.728 |
| White blood cell (×103) | 8.0 (6.59–8.89) | 7.3 (6.2–8.7) | 7.3 (6.0–9.3) | 0.114 |
| Mean corpuscular volume (fL) | 84.8 (81.6–88.0) | 84.9 (83.1–89.8) | 85.9 (83.1–88.0) | 0.357 |
| Biochemical Parameters | ||||
| Platelet (×103) | 294 (235–336) | 274 (235–316) | 280 (237–327) | 0.579 |
| Calcium (mg/dL) | 9.53 (8.91–9.81) | 9.56 (9.21–9.80) | 9.60 (9.20–9.90) | 0.498 |
| Magnesium (mg/dL) | 1.96 (1.84–2.11) | 1.95 (1.85–2.11) | 1.93 (1.80–2.08) | 0.609 |
| Iron (μg/dL) | 74.1 (51.6–94.1) | 78.3 (58.3–103) | 76.0 (52.0–96.6) | 0.659 |
| Ferritin (ng/mL) | 48.5 (18.8–85.3) | 50.6 (18.8–98.2) | 55.3 (23.0–121.8) | 0.082 |
| Vitamin Levels | ||||
| Vitamin B12 (pg/mL) | 277 (234–378) | 315 (241–389) | 280 (237–327) | 0.163 |
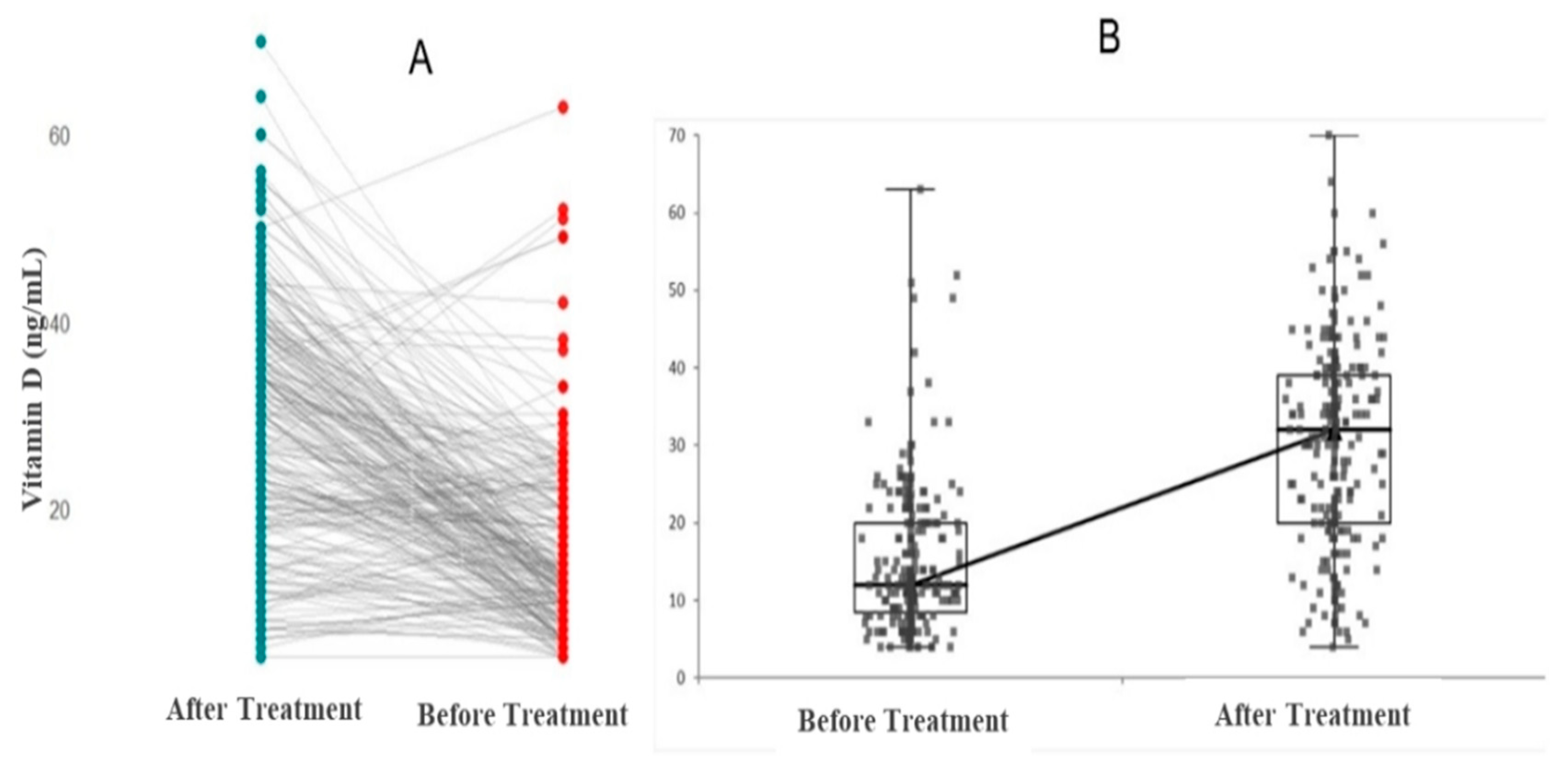
| Vitamin D level at admission (ng/mL) | 12.0 (10.0–20.0) | 13.0 (9.01–22.0) | 11.0 (8.00–40) | 0.193 |
| Vitamin D level after 2 months (ng/mL) | 33.0 (26.0–36.0) | 32.0 (20.0–40.0) | 28.0 (18.0–40.0) | 0.652 |
| IBS-D * Group (N = 53) | |||
| Before Treatment | After Treatment | p Value | |
| Vitamin D (ng/mL) | 12 (10–20) | 33 (25–36) | 0.001 |
| IBS-C * Group (N = 108) | |||
| Vitamin D (ng/mL) | 13 (9–22) | 32 (20–40) | 0.001 |
| IBS-M * Group (N = 79) | |||
| Vitamin D (ng/mL) | 11 (8–18) | 28 (18–40) | 0.001 |
| Female | Male | p Value | |
|---|---|---|---|
| IBS-D * Group (N = 53) | |||
| Before Vitamin D treatment (ng/mL) | 15 (10.2–20) | 11 (7.51–16.5) | 0.291 |
| After Vitamin D treatment (ng/mL) | 33 (21.2–33.7) | 34 (26.5–39.5) | 0.223 |
| IBS-C * Group (N = 108) | |||
| Before Vitamin D treatment (ng/mL) | 12 (8.75–20) | 16 (11–23.5) | 0.150 |
| After Vitamin D treatment (ng/mL) | 32 (19–40) | 32.5 (20–40.5) | 0.372 |
| IBS-M * Group (N = 79) | |||
| Before Vitamin D treatment (ng/mL) | 10 (7–13.5) | 13.5 (8–22.5) | 0.025 |
| After Vitamin D treatment (ng/mL) | 26 (16–39) | 34 (18.7–40.5) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Düzen Oflas, N.; Yılmaz Ürün, Y. Vitamin D Deficiency and Supplementation in Irritable Bowel Syndrome: Retrospective Evaluation of Subtype and Sex-Based Differences. Medicina 2025, 61, 1229. https://doi.org/10.3390/medicina61071229
Düzen Oflas N, Yılmaz Ürün Y. Vitamin D Deficiency and Supplementation in Irritable Bowel Syndrome: Retrospective Evaluation of Subtype and Sex-Based Differences. Medicina. 2025; 61(7):1229. https://doi.org/10.3390/medicina61071229
Chicago/Turabian StyleDüzen Oflas, Nur, and Yonca Yılmaz Ürün. 2025. "Vitamin D Deficiency and Supplementation in Irritable Bowel Syndrome: Retrospective Evaluation of Subtype and Sex-Based Differences" Medicina 61, no. 7: 1229. https://doi.org/10.3390/medicina61071229
APA StyleDüzen Oflas, N., & Yılmaz Ürün, Y. (2025). Vitamin D Deficiency and Supplementation in Irritable Bowel Syndrome: Retrospective Evaluation of Subtype and Sex-Based Differences. Medicina, 61(7), 1229. https://doi.org/10.3390/medicina61071229







