Unveiling the Causes of Acute and Non-Acute Myocardial Ischemic Syndromes: The Role of Optical Coherence Tomography
Abstract
1. Introduction
The one who leaves the cave and removes the blindfold from his eyes begins to see.
And in seeing, he comes to understand.
And in understanding, he may marvel at the light and beauty he no longer dared to believe in.
-Allegory of the Cave, Plato (Republic, Book VII)
2. Technical Principles and Clinical Role of OCT: Strengths and Limitations in Coronary Artery Disease Assessment
3. Myocardial Ischemic Syndromes: A New Nomenclature
4. The Role of OCT in Acute Myocardial Ischemic Syndromes
4.1. Exploring the Pathophysiology of Acute Coronary Syndromes: The Pivotal Role of OCT
4.1.1. Plaque Rupture and “Plaque Vulnerability”
4.1.2. Plaque Erosion
4.1.3. Eruptive Calcified Nodule
4.2. OCT for Qualitative and Quantitative Assessment of Intracoronary Thrombosis
4.3. Stent Thrombosis as a Cause of ACS: The Role of OCT in Detection and Understanding Underlying Mechanisms of Early and Late Stent Failure
4.4. The Role of OCT in Myocardial Infarction with Non-Obstructive Coronary Arteries
4.4.1. OCT Findings in Type 1 MINOCA
4.4.2. OCT Findings in Type 2 MINOCA
Spontaneous Coronary Artery Dissection
Epicardial Coronary Spasm
Coronary Embolism
5. The Role of OCT in Non-Acute Myocardial Ischemic Syndromes
5.1. OCT Assessment of Anatomic Severity of Epicardial Stenoses and Plaque Phenotype
5.2. OCT Findings in Ischemia with Non-Obstructive Coronary Arteries
Epicardial Coronary Spasm
6. Additional Information Provided by OCT Beyond AMIS and NAMIS
6.1. Plaque Vulnerability
6.2. Plaque Healing
6.3. Myocardial Bridge
OCT Ad Guidance for PCI in Myocardial Bridge
7. Beyond the Potential of OCT: The Role of Functional Coronary Assessment for a Definitive Diagnosis in AMIS and NAMIS with No-Obstructive Coronary Arteries
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMIS | Acute Myocardial Ischemic Syndromes |
| NAMIS | Non-Acute Myocardial Ischemic Syndromes |
| OCT | Optical Coherence Tomography |
| CAD | Coronary artery disease |
| ACS | acute coronary syndromes |
| PCI | Percutaneous Coronary Intervention |
| PR | Plaque rupture |
| PE | Plaque erosion |
| CN | Calcified nodule |
| MINOCA | Myocardial Infarction with Non-Obstructive Coronary Arteries |
| SCAD | Spontaneous coronary artery dissection |
| INOCA | Ischemia with Non-Obstructive Coronary Arteries |
| MB | Myocardial bridges |
| IVUS | Intravascular ultrasound |
| TD-OCT | Time-domain OCT |
| OFD | Optical frequency domain |
| IVI | Intravascular imaging |
| AI | Artificial intelligence |
| NIRS | Near-infrared spectroscopy |
| CCS | Chronic coronary syndromes |
| LM | Left main |
| CMVD | Coronary microvascular dysfunction |
| CFT | Coronary functional tests |
| TCFA | Thin-cap fibroatheroma |
| MACE | Major adverse cardiac event |
| MI | Myocardial infarction |
| BVS | Bioresorbable vascular scaffold |
| MLA | Minimal lumen area |
| QCA | Quantitative coronary angiography |
| DES | Drug-eluting stent |
| ST | Stent thrombosis |
| ARC | Academic Research Consortium |
| DAPT | Dual antiplatelet therapy |
| AMI | Acute myocardial infarction |
| LM | Left main |
| SED | Stent edge dissection |
| MSA | Minimum stent area |
| EAPCI | European Association of Percutaneous Cardiovascular Intervention |
| SM | Stent malapposition |
| ASM | Acute stent malapposition |
| LSM | Late stent malapposition |
| TMV | Total malapposition volume |
| CMR | Cardiac magnetic resonance |
| LCBI | Lipid core burden index |
| TL | True lumen |
| FL | False lumen |
| EEL | External elastic lamina |
| VV | Vasa vasorum |
| ECG | Electrocardiogram |
| Ach | Acetylcholine |
| VSA | Vasospastic angina |
| FFR | Fractional Flow Reserve |
| IFR | Instantaneous Wave-Free Ratio |
| MLD | Minimal lumen diameter |
| AICLs | Angiographically intermediate coronary lesions |
| AS | Area stenosis |
| OFR | Optical Flow Ratio |
| CCs | Cholesterol crystals |
| VSMC | Vascular smooth muscle cell |
| MFR | Myocardial flow reserve |
| MPR | Myocardial perfusion reserve |
| NHPRs | Non-hyperemic pressure ratios |
| RFR | Resting Full-cycle Ratio |
| ANOCA | Angina with No Obstructive Coronary Artery disease |
| IMR | Index of Microcirculatory Resistance |
| IVUS-VH | IVUS virtual histology |
| CCTA | Coronary computed tomography angiography |
| FAI | Fatty attenuation index |
| PVAT | Perivascular adipose tissue |
| PET | Positron emission tomography |
| LAD | Left anterior descending artery |
| dFFR | Diastolic FFR |
| WSS | Wall shear stress |
| ISR | In-stent restenosis |
| CABG | Coronary artery bypass grafting |
| CFR | Coronary flow reserve |
| IMR | Index of Microcirculatory Resistance |
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Boden, W.E.; De Caterina, R.; Kaski, J.C.; Merz, N.B.; Berry, C.; Marzilli, M.; Pepine, C.J.; Barbato, E.; Stefanini, G.; Prescott, E.; et al. Myocardial Ischemic Syndromes: A New Nomenclature to Harmonize Evolving International Clinical Practice Guidelines. Circulation 2024, 150, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K. Cardiovascular OCT Imaging; Springer: Cham, Switzerland, 2015; p. 216. ISBN 978-3-319-10801-8. [Google Scholar]
- Araki, M.; Park, S.J.; Dauerman, H.L.; Uemura, S.; Kim, J.S.; Di Mario, C.; Johnson, T.W.; Guagliumi, G.; Kastrati, A.; Joner, M.; et al. Optical Coherence Tomography in Coronary Atherosclerosis Assessment and Intervention. Nat. Rev. Cardiol. 2022, 19, 684–703. [Google Scholar] [CrossRef]
- Buonpane, A.; Trimarchi, G.; Ciardetti, M.; Coceani, M.A.; Alagna, G.; Benedetti, G.; Berti, S.; Andò, G.; Burzotta, F.; De Caterina, A.R. Optical Coherence Tomography in Myocardial Infarction Management: Enhancing Precision in Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 5791. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical Use of Intracoronary Imaging. Part 1: Guidance and Optimization of Coronary Interventions. An Expert Consensus Document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef] [PubMed]
- Borzillo, I.; De Filippo, O.; Manai, R.; Bruno, F.; Ravetti, E.; Galanti, A.A.; Vergallo, R.; Porto, I.; De Ferrari, G.M.; D’Ascenzo, F. Role of Intracoronary Imaging in Myocardial Infarction with Non-Obstructive Coronary Disease (MINOCA): A Review. J. Clin. Med. 2023, 12, 2129. [Google Scholar] [CrossRef]
- Vergallo, R.; Park, S.-J.; Stone, G.W.; Erlinge, D.; Porto, I.; Waksman, R.; Mintz, G.S.; D’Ascenzo, F.; Seitun, S.; Saba, L.; et al. Vulnerable or High-Risk Plaque. JACC Cardiovasc. Imaging 2025, 18, 709–740. [Google Scholar] [CrossRef]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef]
- Fracassi, F.; Crea, F.; Sugiyama, T.; Yamamoto, E.; Uemura, S.; Vergallo, R.; Porto, I.; Lee, H.; Fujimoto, J.; Fuster, V.; et al. Healed Culprit Plaques in Patients With Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2019, 73, 2253–2263. [Google Scholar] [CrossRef]
- Ong, P.; Aziz, A.; Hansen, H.S.; Prescott, E.; Athanasiadis, A.; Sechtem, U. Structural and Functional Coronary Artery Abnormalities in Patients With Vasospastic Angina Pectoris. Circ. J. 2015, 79, 1431–1438. [Google Scholar] [CrossRef]
- Aoi, S.; Maehara, A.; Takahashi, T.; Latib, A.; Kobayashi, Y. OCT-Defined Myocardial Bridge as a Homogenous Band: Validation With a Hybrid IVUS-OCT Catheter. Cardiovasc. Revasc. Med. 2023, 46, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, M.E.; Tearney, G.J.; Weissman, N.J.; Boppart, S.A.; Bouma, B.E.; Hee, M.R.; Weyman, A.E.; Swanson, E.A.; Southern, J.F.; Fujimoto, J.G. Assessing Atherosclerotic Plaque Morphology: Comparison of Optical Coherence Tomography and High Frequency Intravascular Ultrasound. Heart 1997, 77, 397–403. [Google Scholar] [CrossRef]
- Vakhtin, A.B.; Kane, D.J.; Wood, W.R.; Peterson, K.A. Common-Path Interferometer for Frequency-Domain Optical Coherence Tomography. Appl. Opt. 2003, 42, 6953–6958. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Brezinski, M.E.; Tearney, G.J.; Bouma, B.E.; Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Southern, J.F.; Fujimoto, J.G. Optical Coherence Tomography for Optical Biopsy. Properties and Demonstration of Vascular Pathology. Circulation 1996, 93, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Brezinski, M.E.; Boppart, S.A.; Bouma, B.E.; Weissman, N.; Southern, J.F.; Swanson, E.A.; Fujimoto, J.G. Images in Cardiovascular Medicine. Catheter-Based Optical Imaging of a Human Coronary Artery. Circulation 1996, 94, 3013. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies: A Report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Ono, M.; Kawashima, H.; Hara, H.; Gao, C.; Wang, R.; Kogame, N.; Takahashi, K.; Chichareon, P.; Modolo, R.; Tomaniak, M.; et al. Advances in IVUS/OCT and Future Clinical Perspective of Novel Hybrid Catheter System in Coronary Imaging. Front. Cardiovasc. Med. 2020, 7, 119. [Google Scholar] [CrossRef]
- Waksman, R.; Di Mario, C.; Torguson, R.; Ali, Z.A.; Singh, V.; Skinner, W.H.; Artis, A.K.; Cate, T.T.; Powers, E.; Kim, C.; et al. Identification of Patients and Plaques Vulnerable to Future Coronary Events with Near-Infrared Spectroscopy Intravascular Ultrasound Imaging: A Prospective, Cohort Study. Lancet 2019, 394, 1629–1637. [Google Scholar] [CrossRef]
- Oemrawsingh, R.M.; Cheng, J.M.; García-García, H.M.; Van Geuns, R.-J.; De Boer, S.P.M.; Simsek, C.; Kardys, I.; Lenzen, M.J.; Van Domburg, R.T.; Regar, E.; et al. Near-Infrared Spectroscopy Predicts Cardiovascular Outcome in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 64, 2510–2518. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the Management of Chronic Coronary Syndromes: Developed by the Task Force for the Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Khattab, E.; Karelas, D.; Pallas, T.; Kostakis, P.; Papadopoulos, C.H.; Sideris, S.; Patsourakos, N.; Kadoglou, N.P.E. MINOCA: A Pathophysiological Approach of Diagnosis and Treatment—A Narrative Review. Biomedicines 2024, 12, 2457. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, M. On the Way to Language; Harper & Row: New York, NY, USA, 1971; Volume 81. [Google Scholar]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons From Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients with Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; Finn, A.V.; Virmani, R. The Thin-Cap Fibroatheroma: A Type of Vulnerable Plaque: The Major Precursor Lesion to Acute Coronary Syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Virmani, R.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Finn, A.V.; Gold, H.K. Pathologic Assessment of the Vulnerable Human Coronary Plaque. Heart 2004, 90, 1385–1391. [Google Scholar] [CrossRef]
- Cheng, J.M.; Garcia-Garcia, H.M.; De Boer, S.P.M.; Kardys, I.; Heo, J.H.; Akkerhuis, K.M.; Oemrawsingh, R.M.; Van Domburg, R.T.; Ligthart, J.; Witberg, K.T.; et al. In Vivo Detection of High-Risk Coronary Plaques by Radiofrequency Intravascular Ultrasound and Cardiovascular Outcome: Results of the ATHEROREMO-IVUS Study. Eur. Heart J. 2014, 35, 639–647. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between Coronary Plaque Morphology of the Left Anterior Descending Artery and 12 Months Clinical Outcome: The CLIMA Study. Eur. Heart J. 2020, 41, 383–391. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Calvert, P.A.; Obaid, D.R.; O’Sullivan, M.; Shapiro, L.M.; McNab, D.; Densem, C.G.; Schofield, P.M.; Braganza, D.; Clarke, S.C.; Ray, K.K.; et al. Association Between IVUS Findings and Adverse Outcomes in Patients With Coronary Artery Disease. JACC Cardiovasc. Imaging 2011, 4, 894–901. [Google Scholar] [CrossRef]
- Bellino, M.; Silverio, A.; Esposito, L.; Cancro, F.P.; Ferruzzi, G.J.; Di Maio, M.; Rispoli, A.; Vassallo, M.G.; Di Muro, F.M.; Galasso, G.; et al. Moving toward Precision Medicine in Acute Coronary Syndromes: A Multimodal Assessment of Non-Culprit Lesions. J. Clin. Med. 2023, 12, 4550. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Ali, Z.A.; Held, C.; Matsumura, M.; Kjøller-Hansen, L.; Bøtker, H.E.; Maeng, M.; Engstrøm, T.; Wiseth, R.; et al. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. J. Am. Coll. Cardiol. 2020, 76, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.-Q.; Volleberg, R.H.J.A.; Belkacemi, A.; Hermanides, R.S.; Meuwissen, M.; Protopopov, A.V.; Laanmets, P.; Krestyaninov, O.V.; Dennert, R.; Oemrawsingh, R.M.; et al. Fractional Flow Reserve–Negative High-Risk Plaques and Clinical Outcomes After Myocardial Infarction. JAMA Cardiol. 2023, 8, 1013–1021. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahn, J.-M.; Kang, D.-Y.; Yun, S.-C.; Ahn, Y.-K.; Kim, W.-J.; Nam, C.-W.; Jeong, J.-O.; Chae, I.-H.; Shiomi, H.; et al. Preventive Percutaneous Coronary Intervention versus Optimal Medical Therapy Alone for the Treatment of Vulnerable Atherosclerotic Coronary Plaques (PREVENT): A Multicentre, Open-Label, Randomised Controlled Trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.P.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. J. Am. Med. Assoc. 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- De Luca, L.; Halasz, G. The PACMAN-AMI Trial: A Revolution in the Treatment of Acute Coronary Syndromes. Eur. Heart J. Suppl. 2023, 25, C90–C95. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.P.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef]
- Farb, A.; Burke, A.P.; Tang, A.L.; Liang, T.Y.; Mannan, P.; Smialek, J.; Virmani, R. Coronary Plaque Erosion without Rupture into a Lipid Core. A Frequent Cause of Coronary Thrombosis in Sudden Coronary Death. Circulation 1996, 93, 1354–1363. [Google Scholar] [CrossRef]
- Burke, A.P.; Morbini, P.; Dal Bello, B.; Bocciarelli, M.; Virmani, R.; Arbustini, E.; Specchia, G. Plaque Erosion Is a Major Substrate for Coronary Thrombosis in Acute Myocardial Infarction. Heart 1999, 82, 269–272. [Google Scholar]
- Kramer, M.C.A.; Rittersma, S.Z.H.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.-H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of Thrombus Healing to Underlying Plaque Morphology in Sudden Coronary Death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Partida, R.A.; Libby, P.; Crea, F.; Jang, I.-K. Plaque Erosion: A New In Vivo Diagnosis and a Potential Major Shift in the Management of Patients with Acute Coronary Syndromes. Eur. Heart J. 2018, 39, 2070–2076. [Google Scholar] [CrossRef]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective Anti-Thrombotic Therapy without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion (the EROSION Study). Eur. Heart J. 2017, 38, 792–800. [Google Scholar] [CrossRef]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography–Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef]
- Shin, D.; Karimi Galougahi, K.; Spratt, J.C.; Maehara, A.; Collet, C.; Barbato, E.; Ribichini, F.L.; Gonzalo, N.; Sakai, K.; Mintz, G.S.; et al. Calcified Nodule in Percutaneous Coronary Intervention: Therapeutic Challenges. JACC Cardiovasc. Interv. 2024, 17, 1187–1199. [Google Scholar] [CrossRef]
- Vergallo, R.; Lombardi, M.; Besis, G.; Migliaro, S.; Ricchiuto, A.; Maino, A.; Buonpane, A.; Bianchini, E.; Annibali, G.; Galli, M.; et al. Pre-Stenting Residual Thrombotic Volume Assessed by Dual Quantitative Coronary Angiography Predicts Microvascular Obstruction in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Minerva Cardiol. Angiol. 2023, 71, 421–430. [Google Scholar] [CrossRef]
- Prati, F.; Capodanno, D.; Pawlowski, T.; Ramazzotti, V.; Albertucci, M.; La Manna, A.; Di Salvo, M.; Gil, R.J.; Tamburino, C. Local Delivery Versus Intracoronary Infusion of Abciximab in Patients with Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2010, 3, 928–934. [Google Scholar] [CrossRef]
- Kajander, O.A.; Koistinen, L.S.; Eskola, M.; Huhtala, H.; Bhindi, R.; Niemelä, K.; Jolly, S.S.; Sheth, T.; Kassam, S.; Vijayraghavan, R.; et al. Feasibility and Repeatability of Optical Coherence Tomography Measurements of Pre-Stent Thrombus Burden in Patients with STEMI Treated with Primary PCI. Eur. Heart J. 2015, 16, 96–107. [Google Scholar] [CrossRef]
- Amabile, N.; Hammas, S.; Fradi, S.; Souteyrand, G.; Veugeois, A.; Belle, L.; Motreff, P.; Caussin, C. Intra-Coronary Thrombus Evolution during Acute Coronary Syndrome: Regression Assessment by Serial Optical Coherence Tomography Analyses. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Condello, F.; Spaccarotella, C.; Sorrentino, S.; Indolfi, C.; Stefanini, G.G.; Polimeni, A. Stent Thrombosis and Restenosis with Contemporary Drug-Eluting Stents: Predictors and Current Evidence. J. Clin. Med. 2023, 12, 1238. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; Van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Sun, W.; Tian, T.; Zhou, S.; Zhang, Z.; Gao, M.; Qiao, B.; Zheng, Y. Very Late Stent Thrombosis in Drug-Eluting Stents New Observations and Clinical Implications. Cardiol. Rev. 2019, 27, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Windecker, S. Early Stent Thrombosis: Past, Present, and Future. Circulation 2009, 119, 657–659. [Google Scholar] [CrossRef]
- Aoki, J.; Lansky, A.J.; Mehran, R.; Moses, J.; Bertrand, M.E.; McLaurin, B.T.; Cox, D.A.; Lincoff, A.M.; Ohman, E.M.; White, H.D.; et al. Early Stent Thrombosis in Patients With Acute Coronary Syndromes Treated With Drug-Eluting and Bare Metal Stents: The Acute Catheterization and Urgent Intervention Triage Strategy Trial. Circulation 2009, 119, 687–698. [Google Scholar] [CrossRef]
- Belguidoum, S.; Meneveau, N.; Motreff, P.; Ohlman, P.; Boussaada, M.; Silvain, J.; Guillon, B.; Descotes-Genon, V.; Lefrançois, Y.; Morel, O.; et al. Relationship between Stent Expansion and Fractional Flow Reserve after Percutaneous Coronary Intervention: A Post Hoc Analysis of the DOCTORS Trial. EuroIntervention 2021, 17, E132–E139. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; Burzotta, F.; Limbruno, U.; Gatto, L.; Manna, A.L.; Versaci, F.; Marco, V.; Vito, L.D.; Imola, F.; et al. Clinical Impact of OCT Findings During PCI The CLI-OPCI II Study. JACC Cardiovasc. Imaging 2015, 8, 1297–1305. [Google Scholar] [CrossRef]
- Im, E.; Kim, B.K.; Ko, Y.G.; Shin, D.H.; Kim, J.S.; Choi, D.; Jang, Y.; Hong, M.K. Incidences, Predictors, and Clinical Outcomes of Acute and Late Stent Malapposition Detected by Optical Coherence Tomography after Drug-Eluting Stent Implantation. Circ. Cardiovasc. Interv. 2014, 7, 88–96. [Google Scholar] [CrossRef]
- Guo, N.; Maehara, A.; Mintz, G.S.; He, Y.; Xu, K.; Wu, X.; Lansky, A.J.; Witzenbichler, B.; Guagliumi, G.; Brodie, B.; et al. Incidence, Mechanisms, Predictors, and Clinical Impact of Acute and Late Stent Malapposition After Primary Intervention in Patients With Acute Myocardial Infarction: An Intravascular Ultrasound Substudy of the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Circulation 2010, 122, 11. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mintz, G.S.; Kim, J.S.; Kim, B.K.; Jang, Y.; Hong, M.K. Long-Term Clinical Outcomes of Drug-Eluting Stent Malapposition. Korean Circ. J. 2020, 50, 880–889. [Google Scholar] [CrossRef]
- Kim, B.G.; Kachel, M.; Kim, J.S.; Guagliumi, G.; Kim, C.; Kim, I.S.; Lee, Y.J.; Lee, O.H.; Byun, Y.S.; Kim, B.O.; et al. Clinical Implications of Poststent Optical Coherence Tomographic Findings: Severe Malapposition and Cardiac Events. JACC Cardiovasc. Imaging 2022, 15, 126–137. [Google Scholar] [CrossRef]
- Ali, Z.A.; Maehara, A.; Généreux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical Coherence Tomography Compared with Intravascular Ultrasound and with Angiography to Guide Coronary Stent Implantation (ILUMIEN III: OPTIMIZE PCI): A Randomised Controlled Trial. Lancet 2016, 388, 2618–2628. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Joner, M.; Godschalk, T.C.; Malik, N.; Alfonso, F.; Xhepa, E.; De Cock, D.; Komukai, K.; Tada, T.; Cuesta, J.; et al. Optical Coherence Tomography Findings in Patients with Coronary Stent Thrombosis: A Report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation 2017, 136, 1007–1021. [Google Scholar] [CrossRef]
- Souteyrand, G.; Amabile, N.; Mangin, L.; Chabin, X.; Meneveau, N.; Cayla, G.; Vanzetto, G.; Barnay, P.; Trouillet, C.; Rioufol, G.; et al. Mechanisms of Stent Thrombosis Analysed by Optical Coherence Tomography: Insights from the National PESTO French Registry. Eur. Heart J. 2016, 37, 1208–1216. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Otsuka, F.; Sato, Y.; Bhoite, R.R.; Sakamoto, A.; Torii, S.; Yahagi, K.; Cornelissen, A.; Mori, M.; Kawakami, R.; et al. Healthy Strut Coverage after Coronary Stent Implantation: An Ex Vivo Human Autopsy Study. Circ. Cardiovasc. Interv. 2020, 13, 5. [Google Scholar] [CrossRef]
- Won, H.; Shin, D.H.; Kim, B.K.; Mintz, G.S.; Kim, J.S.; Ko, Y.G.; Choi, D.; Jang, Y.; Hong, M.K. Optical Coherence Tomography Derived Cut-off Value of Uncovered Stent Struts to Predict Adverse Clinical Outcomes after Drug-Eluting Stent Implantation. Int. J. Cardiovasc. Imaging 2013, 29, 1255–1263. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging–Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography–Guided versus Angiography-Guided PCI. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef]
- Holm, N.R.; Andreasen, L.N.; Neghabat, O.; Laanmets, P.; Kumsars, I.; Bennett, J.; Olsen, N.T.; Odenstedt, J.; Hoffmann, P.; Dens, J.; et al. OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions. N. Engl. J. Med. 2023, 389, 1477–1487. [Google Scholar] [CrossRef]
- Stone, G.W.; Christiansen, E.H.; Ali, Z.A.; Andreasen, L.N.; Maehara, A.; Ahmad, Y.; Landmesser, U.; Holm, N.R. Holm. Intravascular Imaging-Guided Coronary Drug-Eluting Stent Implantation: An Updated Network Meta-Analysis. Lancet 2024, 403, 824–837. [Google Scholar] [CrossRef]
- Kang, D.Y.; Ahn, J.M.; Yun, S.C.; Hur, S.H.; Cho, Y.K.; Lee, C.H.; Hong, S.J.; Lim, S.; Kim, S.W.; Won, H.; et al. Park Optical Coherence Tomography-Guided or Intravascular Ultrasound-Guided Percutaneous Coronary Intervention: The OCTIVUS Randomized Clinical Trial. Circulation 2023, 148, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Truesdell, A.G.; Alasnag, M.A.; Kaul, P.; Rab, S.T.; Riley, R.F.; Young, M.N.; Batchelor, W.B.; Maehara, A.; Welt, F.G.; Kirtane, A.J. Intravascular Imaging During Percutaneous Coronary Intervention: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 590–605. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC Working Group Position Paper on Myocardial Infarction with Non-Obstructive Coronary Arteries. Eur. Heart J. 2016, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; Spertus, J.A.; et al. Myocardial Infarction with Non-Obstructive Coronary Arteries as Compared with Myocardial Infarction and Obstructive Coronary Disease: Outcomes in a Medicare Population. Eur. Heart J. 2020, 41, 870–878. [Google Scholar] [CrossRef]
- Bakhshi, H.; Gibson, C.M. MINOCA: Myocardial Infarction No Obstructive Coronary Artery Disease. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 33, 100312. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Srichai, M.B.; Iqbal, S.N.; Slater, J.N.; Mancini, G.B.J.; Feit, F.; Pena-Sing, I.; Axel, L.; Attubato, M.J.; Yatskar, L.; et al. Mechanisms of Myocardial Infarction in Women Without Angiographically Obstructive Coronary Artery Disease. Circulation 2011, 124, 1414–1425. [Google Scholar] [CrossRef]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1440–1450. [Google Scholar] [CrossRef]
- Saw, J. Coronary Angiogram Classification of Spontaneous Coronary Artery Dissection. Catheter. Cardiovasc. Interv. 2014, 84, 1115–1122. [Google Scholar] [CrossRef]
- Agwuegbo, C.C.; Ahmed, E.N.; Olumuyide, E.; Moideen Sheriff, S.; Waduge, S.A. Spontaneous Coronary Artery Dissection: An Updated Comprehensive Review. Cureus 2024, 16, e55106. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.M.; Rihal, C.S.; et al. Clinical Features, Management, and Prognosis of Spontaneous Coronary Artery Dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Al-Hussaini, A.; Joseph, S.; Van Soest, G.; Wood, A.; Macaya, F.; Gonzalo, N.; Cade, J.; Caixeta, A.; Hlinomaz, O.; et al. Spontaneous Coronary Artery Dissection. JACC Cardiovasc. Imaging 2019, 12, 2475–2488. [Google Scholar] [CrossRef]
- Mehmedbegović, Z.; Ivanov, I.; Čanković, M.; Perišić, Z.; Kostić, T.; Maričić, B.; Krljanac, G.; Beleslin, B.; Apostolović, S. Invasive Imaging Modalities in a Spontaneous Coronary Artery Dissection: When “Believing Is Seeing”. Front. Cardiovasc. Med. 2023, 10, 1270259. [Google Scholar] [CrossRef]
- Offen, S.; Yang, C.; Saw, J. Spontaneous Coronary Artery Dissection (SCAD): A Contemporary Review. Clin. Cardiol. 2024, 47, e24236. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Campo, G.; Serafino, L.; ZANON, S.; Rubino, F.; MONIZZI, G.; Biscaglia, S.; ANCONA, M.; Polimeni, A.; NICCOLI, G.; et al. #FullPhysiology: A Systematic Step-by-Step Guide to Implement Intracoronary Physiology in Daily Practice. Minerva Cardiol. Angiol. 2023, 71, 504–514. [Google Scholar] [CrossRef]
- Tanaka, A.; Shimada, K.; Tearney, G.J.; Kitabata, H.; Taguchi, H.; Fukuda, S.; Kashiwagi, M.; Kubo, T.; Takarada, S.; Hirata, K.; et al. Conformational Change in Coronary Artery Structure Assessed by Optical Coherence Tomography in Patients with Vasospastic Angina. J. Am. Coll. Cardiol. 2011, 58, 1608–1613. [Google Scholar] [CrossRef]
- Nishimiya, K.; Matsumoto, Y.; Takahashi, J.; Uzuka, H.; Wang, H.; Tsuburaya, R.; Hao, K.; Ohyama, K.; Odaka, Y.; Miyata, S.; et al. Enhanced Adventitial Vasa Vasorum Formation in Patients With Vasospastic Angina. J. Am. Coll. Cardiol. 2016, 67, 598–600. [Google Scholar] [CrossRef]
- Pijls, N.H.J.; van Schaardenburgh, P.; Manoharan, G.; Boersma, E.; Bech, J.-W.; van’t Veer, M.; Bär, F.; Hoorntje, J.; Koolen, J.; Wijns, W.; et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis: 5-Year Follow-Up of the DEFER Study. J. Am. Coll. Cardiol. 2007, 49, 2105–2111. [Google Scholar] [CrossRef]
- Volleberg, R.; Mol, J.Q.; van der Heijden, D.; Meuwissen, M.; van Leeuwen, M.; Escaned, J.; Holm, N.; Adriaenssens, T.; van Geuns, R.J.; Tu, S.; et al. Optical Coherence Tomography and Coronary Revascularization: From Indication to Procedural Optimization. Trends Cardiovasc. Med. 2023, 33, 92–106. [Google Scholar] [CrossRef]
- Burzotta, F.; Leone, A.M.; Aurigemma, C.; Zambrano, A.; Zimbardo, G.; Arioti, M.; Vergallo, R.; De Maria, G.L.; Cerracchio, E.; Romagnoli, E.; et al. Fractional Flow Reserve or Optical Coherence Tomography to Guide Management of Angiographically Intermediate Coronary Stenosis. JACC Cardiovasc. Interv. 2020, 13, 49–58. [Google Scholar] [CrossRef]
- Burzotta, F.; Zito, A.; Aurigemma, C.; Romagnoli, E.; Bianchini, F.; Bianchini, E.; Paraggio, L.; Ierardi, C.; Crea, F.; Leone, A.M.; et al. Fractional Flow Reserve or Optical Coherence Tomography for Angiographically Intermediate Coronary Stenoses: 5-Year Outcomes in the FORZA Trial. Eur. Heart J. 2024, 45, 2785–2788. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Crociani, M.F.; Nardi, G.; Ciardetti, N.; Biagiotti, L.; Bigi, E.; Meucci, F.; Stolcova, M.; Ristalli, F.; Cecchi, E.; et al. Coronary Plaque Characteristics Assessed by Optical Coherence Tomography and Plasma Lipoprotein(a) Levels in Patients With Acute Coronary Syndrome. Cathet Cardio Interv. 2024, 103, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Uemura, S.; Soeda, T.; Minami, Y.; Cho, J.M.; Ong, D.S.; Aguirre, A.D.; Gao, L.; Biasucci, L.M.; Crea, F.; et al. Prevalence and Predictors of Multiple Coronary Plaque Ruptures: In Vivo 3-Vessel Optical Coherence Tomography Imaging Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2229–2238. [Google Scholar] [CrossRef]
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.-K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of Macrophage Content in Atherosclerotic Plaques by Optical Coherence Tomography. Circulation 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Uemura, S.; Ishigami, K.I.; Soeda, T.; Okayama, S.; Sung, J.H.; Nakagawa, H.; Somekawa, S.; Takeda, Y.; Kawata, H.; Horii, M.; et al. Thin-Cap Fibroatheroma and Microchannel Findings in Optical Coherence Tomography Correlate with Subsequent Progression of Coronary Atheromatous Plaques. Eur. Heart J. 2012, 33, 78–85. [Google Scholar] [CrossRef]
- Baumer, Y.; Mehta, N.N.; Dey, A.K.; Powell-Wiley, T.M.; Boisvert, W.A. Cholesterol Crystals and Atherosclerosis. Eur. Heart J. 2020, 41, 2236–2239. [Google Scholar] [CrossRef]
- Fujiyoshi, K.; Minami, Y.; Ishida, K.; Kato, A.; Katsura, A.; Muramatsu, Y.; Sato, T.; Kakizaki, R.; Nemoto, T.; Hashimoto, T.; et al. Incidence, Factors, and Clinical Significance of Cholesterol Crystals in Coronary Plaque: An Optical Coherence Tomography Study. Atherosclerosis 2019, 283, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Liuzzo, G. Pathogenesis of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2013, 61, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.; Holtzman, J.N.; Juszczynski, C.; Khan, N.; Kaur, G.; Varma, B.; Gulati, M. Ischemia with No Obstructive Arteries (INOCA): A Review of the Prevalence, Diagnosis and Management. Curr. Probl. Cardiol. 2023, 48, 101420. [Google Scholar] [CrossRef]
- Mehta, P.K.; Huang, J.; Levit, R.D.; Malas, W.; Waheed, N.; Bairey Merz, C.N. Ischemia and No Obstructive Coronary Arteries (INOCA): A Narrative Review. Atherosclerosis 2022, 363, 8–21. [Google Scholar] [CrossRef]
- Shimokawa, H.; Suda, A.; Takahashi, J.; Berry, C.; Camici, P.G.; Crea, F.; Escaned, J.; Ford, T.; Yii, E.; Kaski, J.C.; et al. Clinical Characteristics and Prognosis of Patients with Microvascular Angina: An International and Prospective Cohort Study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur. Heart J. 2021, 42, 4592–4600. [Google Scholar] [CrossRef]
- Abramik, J.; Mariathas, M.; Felekos, I. Coronary Microvascular Dysfunction and Vasospastic Angina—Pathophysiology, Diagnosis and Management Strategies. J. Clin. Med. 2025, 14, 1128. [Google Scholar] [CrossRef] [PubMed]
- Yaker, Z.S.; Lincoff, A.M.; Cho, L.; Ellis, S.G.; Ziada, K.M.; Zieminski, J.J.; Gulati, R.; Gersh, B.J.; Holmes, D.; Raphael, C.E. Coronary Spasm and Vasomotor Dysfunction as a Cause of MINOCA. EuroIntervention 2024, 20, e123–e134. [Google Scholar] [CrossRef]
- Scalone, G.; Niccoli, G.; Crea, F. Editor’s Choice- Pathophysiology, Diagnosis and Management of MINOCA: An Update. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-K.; Lim, H.-S.; Fearon, W.F.; Yong, A.S.; Yamada, R.; Tanaka, S.; Lee, D.P.; Yeung, A.C.; Tremmel, J.A. Invasive Evaluation of Patients With Angina in the Absence of Obstructive Coronary Artery Disease. Circulation 2015, 131, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Ciliberti, G.; Restivo, A.; Laborante, R.; Migliaro, S.; Canonico, F.; Sangiorgi, G.M.; Tebaldi, M.; Porto, I.; Andreini, D.; et al. Myocardial Bridge Evaluation towards Personalized Medicine: Study Design and Preliminary Results of the RIALTO Registry. Eur. Heart J. Suppl. 2022, 24, H48–H56. [Google Scholar] [CrossRef]
- De Bruyne, B.; Hersbach, F.; Pijls, N.H.J.; Bartunek, J.; Bech, J.-W.; Heyndrickx, G.R.; Gould, K.L.; Wijns, W. Abnormal Epicardial Coronary Resistance in Patients With Diffuse Atherosclerosis but “Normal” Coronary Angiography. Circulation 2001, 104, 2401–2406. [Google Scholar] [CrossRef]
- Vergallo, R.; Ren, X.; Yonetsu, T.; Kato, K.; Uemura, S.; Yu, B.; Jia, H.; Abtahian, F.; Aguirre, A.D.; Tian, J.; et al. Pancoronary Plaque Vulnerability in Patients with Acute Coronary Syndrome and Ruptured Culprit Plaque: A 3-Vessel Optical Coherence Tomography Study. Am. Heart J. 2014, 167, 59–67. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Arbab-Zadeh, A.; Soeda, T.; Fuster, V.; Jang, I.-K. Optical Coherence Tomography of Plaque Vulnerability and Rupture. J. Am. Coll. Cardiol. 2021, 78, 1257–1265. [Google Scholar] [CrossRef]
- Vergallo, R.; Porto, I.; D’Amario, D.; Annibali, G.; Galli, M.; Benenati, S.; Bendandi, F.; Migliaro, S.; Fracassi, F.; Aurigemma, C.; et al. Coronary Atherosclerotic Phenotype and Plaque Healing in Patients with Recurrent Acute Coronary Syndromes Compared with Patients with Long-Term Clinical Stability: An In Vivo Optical Coherence Tomography Study. JAMA Cardiol. 2019, 4, 321–329. [Google Scholar] [CrossRef]
- Shimokado, A.; Matsuo, Y.; Kubo, T.; Nishiguchi, T.; Taruya, A.; Teraguchi, I.; Shiono, Y.; Orii, M.; Tanimoto, T.; Yamano, T.; et al. In Vivo Optical Coherence Tomography Imaging and Histopathology of Healed Coronary Plaques. Atherosclerosis 2018, 275, 35–42. [Google Scholar] [CrossRef]
- Ziada, K.M.; Misumida, N. In Vivo Identification of Healed Plaques in Culprit Lesions: Is What We’re Seeing Really There? J. Am. Coll. Cardiol. 2019, 73, 2264–2266. [Google Scholar] [CrossRef]
- Dai, J.; Fang, C.; Zhang, S.; Li, L.; Wang, Y.; Xing, L.; Yu, H.; Jiang, S.; Yin, Y.; Wang, J.; et al. Frequency, Predictors, Distribution, and Morphological Characteristics of Layered Culprit and Nonculprit Plaques of Patients With Acute Myocardial Infarction: In Vivo 3-Vessel Optical Coherence Tomography Study. Circ. Cardiovasc. Interv. 2020, 13, e009125. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Kim, H.O.; Kurihara, O.; Araki, M.; Shinohara, H.; Thondapu, V.; Yonetsu, T.; Soeda, T.; Minami, Y.; Higuma, T.; et al. Characteristics of Non-Culprit Plaques in Acute Coronary Syndrome Patients with Layered Culprit Plaque. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 1421–1430. [Google Scholar] [CrossRef]
- Mintz, G.S.; Nissen, S.E.; Anderson, W.D.; Bailey, S.R.; Erbel, R.; Fitzgerald, P.J.; Pinto, F.J.; Rosenfield, K.; Siegel, R.J.; Tuzcu, E.M.; et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus)33: A report of the American college of cardiology task force on clinical expert consensus documents developed in collaboration with the European society of cardiology endorsed by the society of cardiac angiography and interventions. J. Am. Coll. Cardiol. 2001, 37, 1478–1492. [Google Scholar] [CrossRef]
- Nurmohamed, N.S.; Van Rosendael, A.R.; Danad, I.; Ngo-Metzger, Q.; Taub, P.R.; Ray, K.K.; Figtree, G.; Bonaca, M.P.; Hsia, J.; Rodriguez, F.; et al. Atherosclerosis Evaluation and Cardiovascular Risk Estimation Using Coronary Computed Tomography Angiography. Eur. Heart J. 2024, 45, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Tzolos, E.; Williams, M.C.; Dey, D.; Berman, D.; Slomka, P.; Newby, D.E.; Dweck, M.R. Noninvasive Coronary Atherosclerotic Plaque Imaging. JACC Cardiovasc. Imaging 2023, 16, 1608–1622. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Angelopoulos, A.; Tsioufis, K.; Antoniades, C.; Tousoulis, D. Cardiovascular Risk Stratification by Coronary Computed Tomography Angiography Imaging: Current State-of-the-Art. Eur. J. Prev. Cardiol. 2022, 29, 608–624. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- The SCOT-HEART Investigators. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular Adipose Tissue as a Source of Therapeutic Targets and Clinical Biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-Invasive Detection of Coronary Inflammation Using Computed Tomography and Prediction of Residual Cardiovascular Risk (the CRISP CT Study): A Post-Hoc Analysis of Prospective Outcome Data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Duchnowski, P.; Śmigielski, W. Usefulness of Myocardial Damage Biomarkers in Predicting Cardiogenic Shock in Patients Undergoing Heart Valve Surgery. Pol. Heart J. 2024, 82, 423–426. [Google Scholar] [CrossRef]
- Yi, B.; He, L.; Zhang, D.; Zeng, M.; Zhao, C.; Meng, W.; Qin, Y.; Weng, Z.; Xu, Y.; Liu, M.; et al. Non-Culprit Plaque Healing on Serial OCT Imaging and Future Outcome in Patients with Acute Coronary Syndromes. Atherosclerosis 2025, 401, 119092. [Google Scholar] [CrossRef]
- Cao, H.-M.; Jiang, J.-F.; Deng, B.; Xu, J.-H.; Xu, W.-J. Evaluation of Myocardial Bridges with Optical Coherence Tomography. J. Int. Med. Res. 2010, 38, 681–685. [Google Scholar] [CrossRef]
- Sternheim, D.; Power, D.A.; Samtani, R.; Kini, A.; Fuster, V.; Sharma, S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management. J. Am. Coll. Cardiol. 2021, 78, 2196–2212. [Google Scholar] [CrossRef]
- Otsuka, T.; Ueki, Y.; Kawai, K.; Sato, Y.; Losdat, S.; Windecker, S.; Virmani, R.; Räber, L. Definition of Myocardial Bridge by Optical Coherence Tomography. JACC Cardiovasc. Imaging 2023, 16, 716–718. [Google Scholar] [CrossRef]
- Pargaonkar, V.S.; Kimura, T.; Kameda, R.; Tanaka, S.; Yamada, R.; Schwartz, J.G.; Perl, L.; Rogers, I.S.; Honda, Y.; Fitzgerald, P.; et al. Invasive Assessment of Myocardial Bridging in Patients with Angina and No Obstructive Coronary Artery Disease. EuroIntervention 2021, 16, 1070–1078. [Google Scholar] [CrossRef]
- Gould, K.L.; Kirkeeide, R.; Johnson, N.P. Coronary Branch Steal: Experimental Validation and Clinical Implications of Interacting Stenosis in Branching Coronary Arteries. Circ. Cardiovasc. Imaging 2010, 3, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Choi, G.; Chun, E.J.; Kim, H.J.; Park, J.; Jung, J.-H.; Lee, M.-H.; Otake, H.; Doh, J.-H.; Nam, C.-W.; et al. Computational Fluid Dynamic Measures of Wall Shear Stress Are Related to Coronary Lesion Characteristics. Heart 2016, 102, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.S.; Avati Nanjundappa, R.P.; Branch, J.R.; Taylor, W.R.; Quyyumi, A.A.; Jo, H.; McDaniel, M.C.; Suo, J.; Giddens, D.; Samady, H. Shear Stress and Plaque Development. Expert Rev. Cardiovasc. Ther. 2010, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Duerinckx, A.J. Wall Shear Stress and Early Atherosclerosis. Am. J. Roentgenol. 2000, 174, 1657–1665. [Google Scholar] [CrossRef]
- Nishimiya, K.; Matsumoto, Y.; Wang, H.; Piao, Z.; Ohyama, K.; Uzuka, H.; Hao, K.; Tsuburaya, R.; Takahashi, J.; Ito, K.; et al. Absence of Adventitial Vasa Vasorum Formation at the Coronary Segment with Myocardial Bridge—An Optical Coherence Tomography Study. Int. J. Cardiol. 2018, 250, 275–277. [Google Scholar] [CrossRef]
- Ciliberti, G.; Laborante, R.; Di Francesco, M.; Restivo, A.; Rizzo, G.; Galli, M.; Canonico, F.; Zito, A.; Princi, G.; Vergallo, R.; et al. Comprehensive Functional and Anatomic Assessment of Myocardial Bridging: Unlocking the Gordian Knot. Front. Cardiovasc. Med. 2022, 9, 970422. [Google Scholar] [CrossRef]
- Xu, T.; You, W.; Wu, Z.; Meng, P.; Ye, F.; Wu, X.; Chen, S. Retrospective Analysis of OCT on MB Characteristics and 1-Year Follow-up of the ISR Incidence after the DES Implantation in Patients with MB. Sci. Rep. 2022, 12, 534. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Ekenbäck, C.; Nickander, J.; Jokhaji, F.; Tornvall, P.; Engblom, H.; Spaak, J.; Persson, J. Coronary Microvascular Dysfunction in Takotsubo Syndrome and Associations with Left Ventricular Function. ESC Heart Fail. 2023, 10, 2395–2405. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Myftari, V.; Colombo, L.; Tomarelli, E.; Piccialuti, A.; Di Pietro, G.; Birtolo, L.I.; Maestrini, V.; et al. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility. J. Clin. Med. 2023, 12, 3586. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef] [PubMed]
- Galante, D.; Viceré, A.; Marrone, A.; Verardi, F.M.; Viccaro, V.; Giuliana, C.; Pollio Benvenuto, C.; Todisco, S.; Biscaglia, S.; Aurigemma, C.; et al. Fractional Flow Reserve (FFR) and Index of Microcirculatory Resistance (IMR) Relationship in Patients with Chronic or Stabilized Acute Coronary Syndromes. Int. J. Cardiol. 2025, 422, 132978. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International Standardization of Diagnostic Criteria for Vasospastic Angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- El Farissi, M.; Zimmermann, F.M.; De Maria, G.L.; van Royen, N.; van Leeuwen, M.A.H.; Carrick, D.; Carberry, J.; Wijnbergen, I.F.; Konijnenberg, L.S.F.; Hoole, S.P.; et al. The Index of Microcirculatory Resistance After Primary PCI: A Pooled Analysis of Individual Patient Data. JACC Cardiovasc. Interv. 2023, 16, 2383–2392. [Google Scholar] [CrossRef]
- Rahman, H.; Demir, O.M.; Khan, F.; Ryan, M.; Ellis, H.; Mills, M.T.; Chiribiri, A.; Webb, A.; Perera, D. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 2538–2549. [Google Scholar] [CrossRef]
- Boerhout, C.B.; De Waard, G.D.W.; Lee, J.M.; Mejia-Renteria, H.; Lee, S.H.; Jung, J.-H.; Hoshino, M.; Echavarria-Pinto, M.; Meuwissen, M.; Matsuo, H.; et al. Prognostic Value of Structural and Functional Coronary Microvascular Dysfunction in Patients with Non-Obstructive Coronary Artery Disease; from the Multicentre International ILIAS Registry. EuroIntervention 2022, 18, 719–728. [Google Scholar] [CrossRef]
- Kelshiker, M.A.; Seligman, H.; Howard, J.P.; Rahman, H.; Foley, M.; Nowbar, A.N.; Rajkumar, C.A.; Shun-Shin, M.J.; Ahmad, Y.; Sen, S.; et al. Coronary Flow Reserve and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Eur. Heart J. 2022, 43, 1582–1593. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc. Interv. 2020, 13, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Rahman, H.; Douiri, A.; Demir, O.M.; De Silva, K.; Clapp, B.; Webb, I.; Gulati, A.; Pinho, P.; Dutta, U.; et al. ChaMP-CMD: A Phenotype-Blinded, Randomized Controlled, Cross-Over Trial. Circulation 2024, 149, 36–47. [Google Scholar] [CrossRef]
- Leone, A.M.; Galante, D.; Viceré, A.; Marrone, A.; Verardi, F.M.; Giuliana, C.; Pollio Benvenuto, C.; Viccaro, V.; Todisco, S.; Erriquez, A.; et al. Functional Coronary Assessment in Angina with Intermediate Coronary Stenosis: The #FullPhysiology Approach. Eur. Heart J. 2025, 46, 978–980. [Google Scholar] [CrossRef]

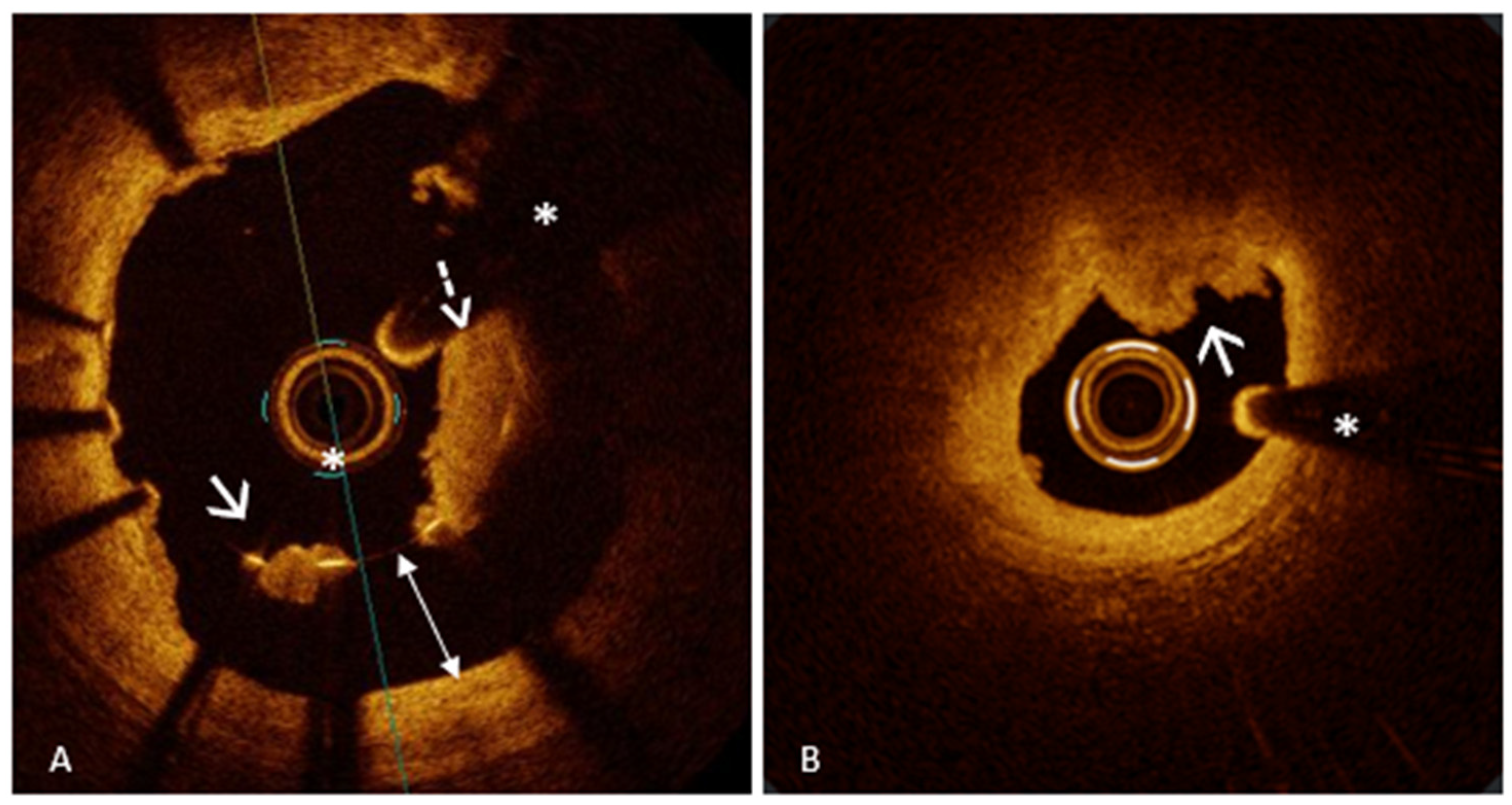

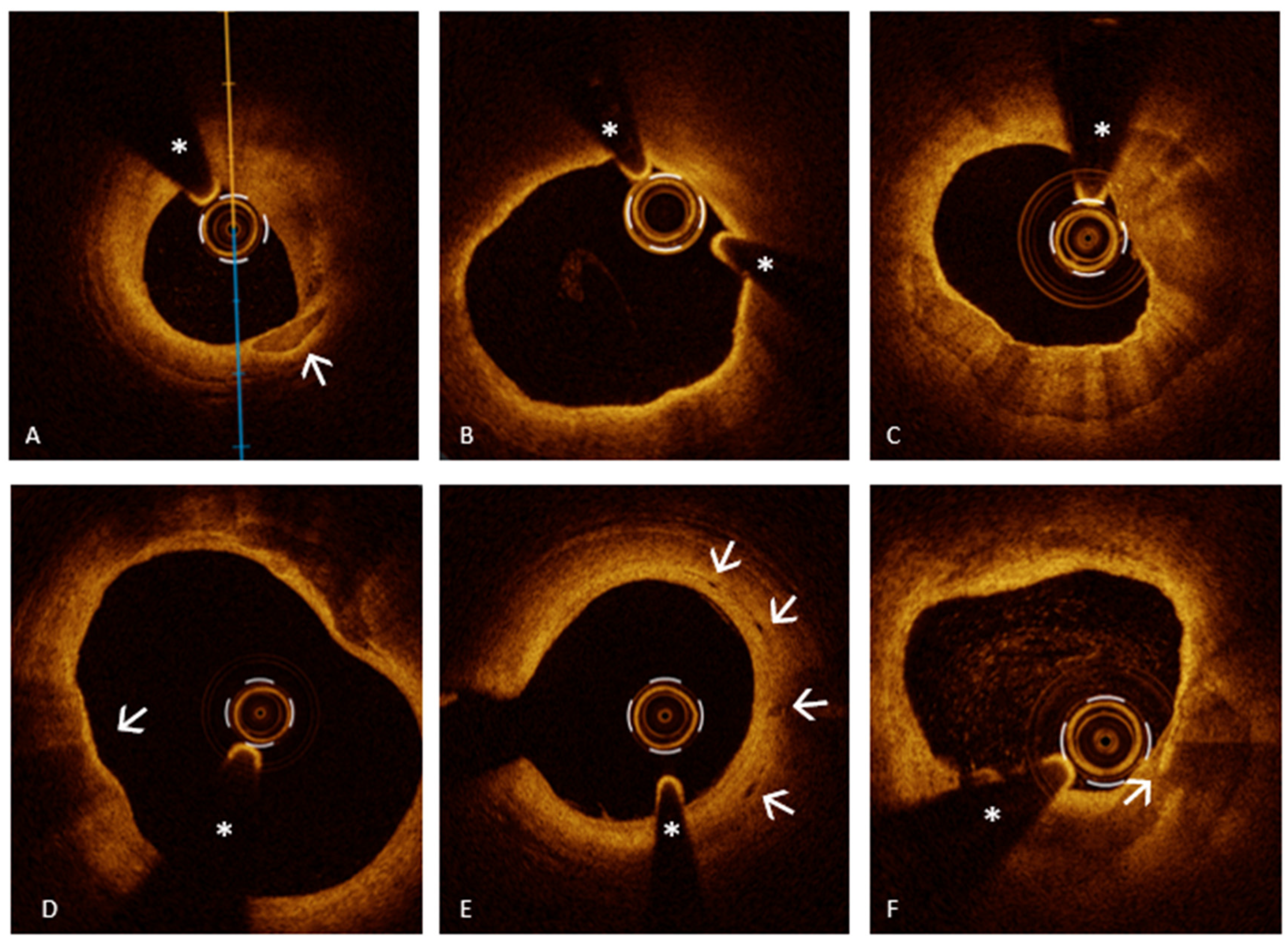
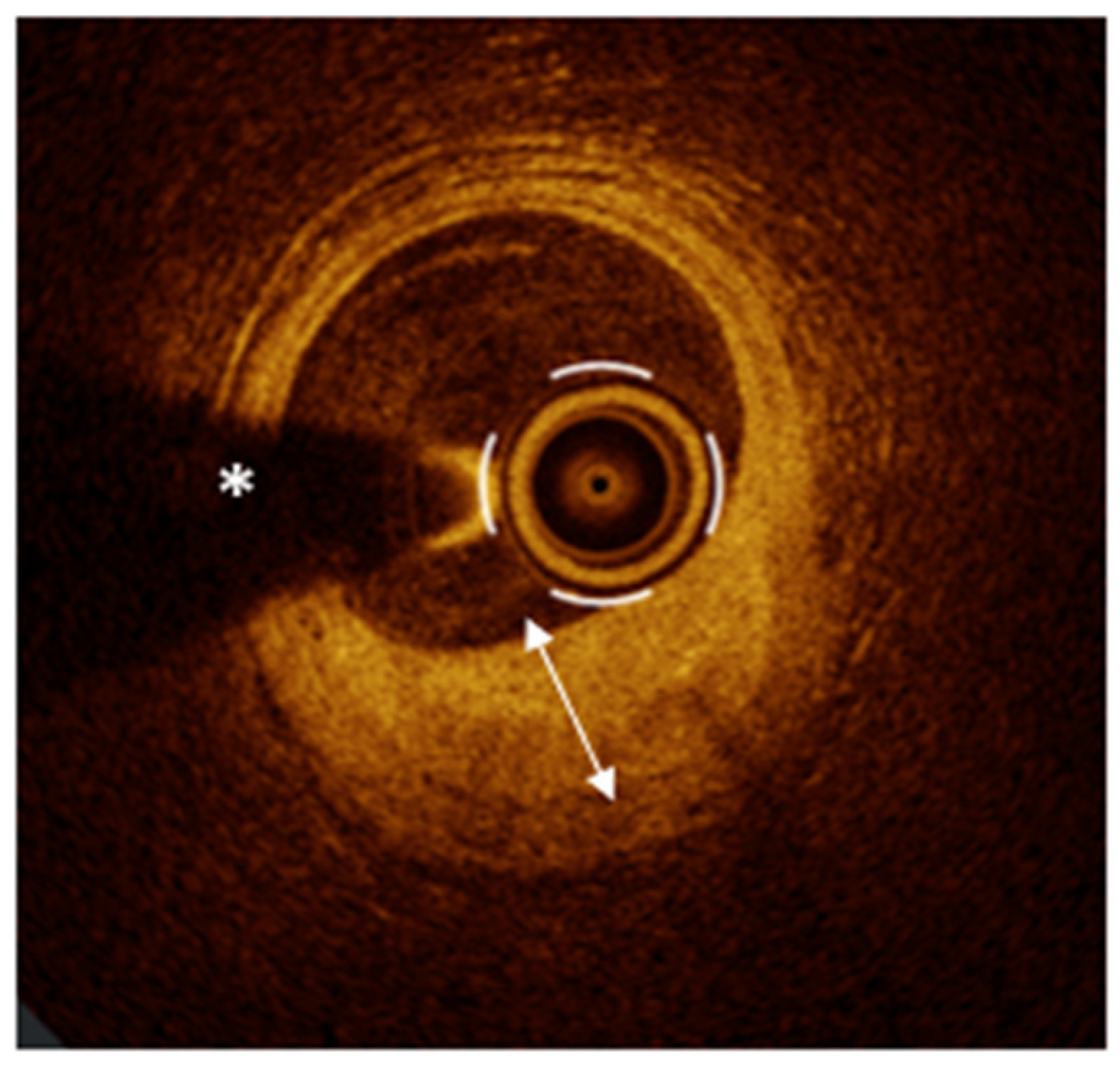
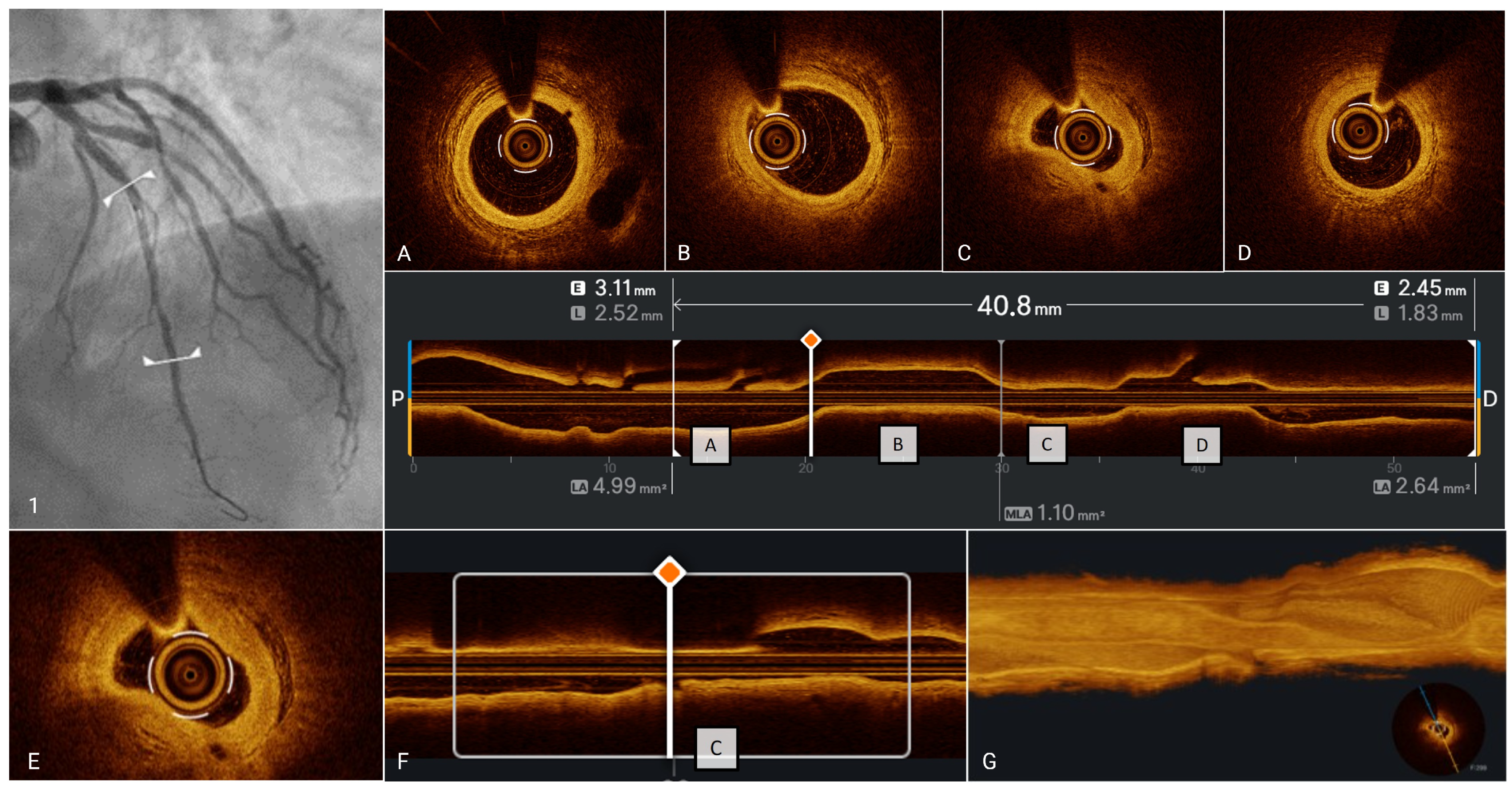
| Acute Myocardial Ischemic Syndromes | ||
|---|---|---|
| Syndrome | Mechanism | OCT Diagnostic Role |
| Acute Coronary Syndrome | Flow-limiting plaque rupture, plaque erosion, eruptive calcified nodule. | Characterizes culprit lesions; identifies mechanism of acute coronary syndromes; characterizes thrombus burden and type; assess underlying plaque phenotype and vulnerability. |
| Myocardial Infarction with Non-Obstructive Coronary Artery Disease type 1 | Non-flow limiting plaque rupture, plaque erosion, eruptive calcified nodule. | Characterizes culprit lesions; identifies mechanism of plaque destabilization; characterizes thrombus burden and type; assess underlying plaque phenotype and vulnerability. |
| Myocardial Infarction with Non-Obstructive Coronary Artery Disease type 2 | Spontaneous coronary artery dissection, coronary embolism, epicardial spasm | Identifies intimal flap and/or intramural hematoma; visualizes thrombus without underlying plaque; confirms vasospasm-related changes in vessel architecture. |
| Non-Acute Myocardial Ischemic Syndromes | ||
| Epicardial stenoses | Atherosclerotic plaque with different plaque phenotype | Assesses plaque phenotype and severity; identifies vulnerable plaque features such as thin fibrous cap, large lipid pool, macrophages, microchannels, cholesterol crystal and “plaque healing”. |
| Ischemia with Non-Obstructive Coronary Arteries | Epicardial spasm, myocardial bridge | Confirms vasospasm-related changes in vessel architecture; visualizes ‘half-moon’ appearance caused by muscle overlying the artery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonpane, A.; De Caterina, A.R.; Trimarchi, G.; Di Muro, F.M.; Galante, D.; Zella, S.; Pizzino, F.; Ciardetti, M.; Paradossi, U.; Concistrè, G.; et al. Unveiling the Causes of Acute and Non-Acute Myocardial Ischemic Syndromes: The Role of Optical Coherence Tomography. Medicina 2025, 61, 1218. https://doi.org/10.3390/medicina61071218
Buonpane A, De Caterina AR, Trimarchi G, Di Muro FM, Galante D, Zella S, Pizzino F, Ciardetti M, Paradossi U, Concistrè G, et al. Unveiling the Causes of Acute and Non-Acute Myocardial Ischemic Syndromes: The Role of Optical Coherence Tomography. Medicina. 2025; 61(7):1218. https://doi.org/10.3390/medicina61071218
Chicago/Turabian StyleBuonpane, Angela, Alberto Ranieri De Caterina, Giancarlo Trimarchi, Francesca Maria Di Muro, Domenico Galante, Samuela Zella, Fausto Pizzino, Marco Ciardetti, Umberto Paradossi, Giovanni Concistrè, and et al. 2025. "Unveiling the Causes of Acute and Non-Acute Myocardial Ischemic Syndromes: The Role of Optical Coherence Tomography" Medicina 61, no. 7: 1218. https://doi.org/10.3390/medicina61071218
APA StyleBuonpane, A., De Caterina, A. R., Trimarchi, G., Di Muro, F. M., Galante, D., Zella, S., Pizzino, F., Ciardetti, M., Paradossi, U., Concistrè, G., Berti, S., Leone, A. M., Crea, F., Trani, C., & Burzotta, F. (2025). Unveiling the Causes of Acute and Non-Acute Myocardial Ischemic Syndromes: The Role of Optical Coherence Tomography. Medicina, 61(7), 1218. https://doi.org/10.3390/medicina61071218










