Targeting the Roots of Kidney Disease: Systematic Review of the Therapies Targeting the Complement System
Abstract
1. Introduction
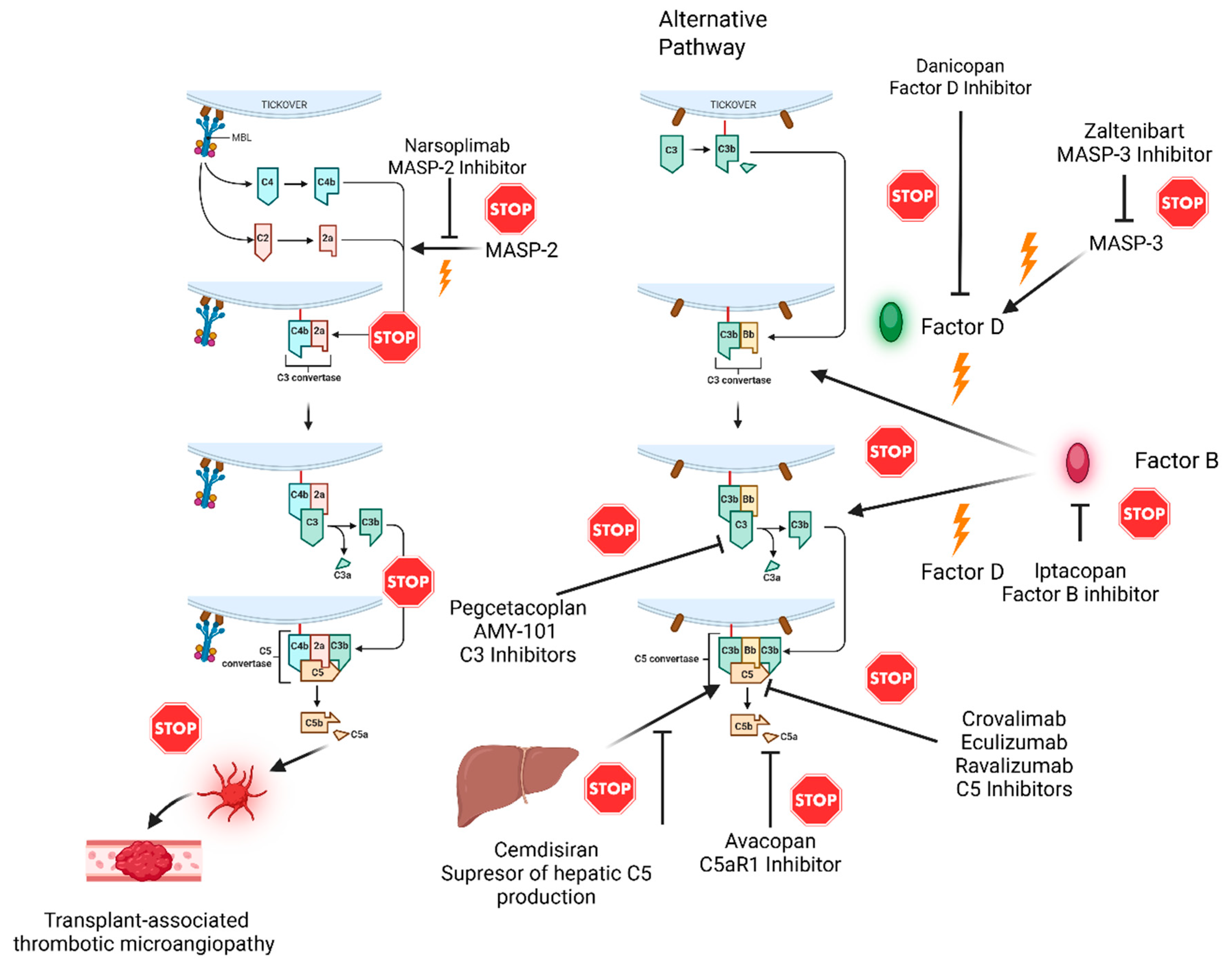
2. Sites of Action of Already Approved Drugs and Candidate Molecules in the Complement System
3. Materials and Methods
4. Complement-Related Kidney Diseases for Which Drugs Are Already Available or Are in Late-Phase Clinical Trials
5. History of Approvals and Clinical Development of Complement-Targeting Therapies for Kidney Diseases
6. Limitations
7. Future Research Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Pio, R.; Ajona, D.; Lambris, J.D. Complement inhibition: A promising concept for cancer treatment. Semin. Immunol. 2013, 25, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Biopharma PEG. Complement Inhibitors as Therapeutic Agents. Available online: https://www.biochempeg.com/article/281.html (accessed on 9 January 2025).
- Dudler, T.; Yaseen, S.; Cummings, W.J. Development and characterization of narsoplimab, a selective MASP-2 inhibitor, for the treatment of lectin-pathway–mediated disorders. Front. Immunol. 2023, 14, 1297352. [Google Scholar] [CrossRef]
- Li, Y.; Yabuki, M.; Cummings, W.J. Alternative pathway Masp-3 inhibitor OMS906 effectively and potently inhibits complement-mediated hemolysis in preclinical models mechanistically similar to paroxysmal nocturnal hemoglobinuria. Blood 2023, 142 (Suppl. S1), 4082. [Google Scholar] [CrossRef]
- Wong, R.S.M.; Pullon, H.W.H.; Amine, I.; Bogdanovic, A.; Deschatelets, P.; Francois, C.G.; Ignatova, K.; Issaragrisil, S.; Niparuck, P.; Numbenjapon, T.; et al. Inhibition of C3 with Pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria. Ann. Hematol. 2022, 101, 1971–1986. [Google Scholar] [CrossRef]
- Dixon, B.P.; Greenbaum, L.A.; Huang, L.; Rajan, S.; Ke, C.; Zhang, Y.; Li, L. Clinical safety and efficacy of Pegcetacoplan in a phase 2 study of patients with C3 glomerulopathy and other complement-mediated glomerular diseases. Kidney Int. Rep. 2023, 8, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 118126201, Amy-101. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amy-101 (accessed on 23 January 2025).
- Nester, C.; Appel, G.B.; Bomback, A.S.; Bouman, K.P.; Cook, H.T.; Daina, E.; Dixon, B.P.; Rice, K.; Najafian, N.; Hui, J.; et al. Clinical outcomes of patients with C3G or IC-MPGN treated with the factor D inhibitor Danicopan: Final results from two phase 2 studies. Am. J. Nephrol. 2023, 53, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves first complement factor D inhibitor. Nat. Rev. Drug Discov. 2024, 23, 329. [Google Scholar] [CrossRef]
- Nowicki, M.; Printza, N. Ravulizumab in adults and children with atypical hemolytic uremic syndrome: A plain language summary of three studies. J. Comp. Eff. Res. 2024, 13, e240103. [Google Scholar] [CrossRef]
- Scheinberg, P.; Clé, D.V.; Kim, J.S.; Nur, E.; Yenerel, M.N.; Barcellini, W.; Bonito, D.; Giai, V.; Hus, M.; Lee, Y.; et al. Phase 3 randomized COMMODORE 1 trial: Crovalimab versus Eculizumab in complement inhibitor-experienced patients with paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 2024, 99, 1757–1767. [Google Scholar] [CrossRef]
- Werion, A.; Rondeau, E. Application of C5 inhibitors in glomerular diseases in 2021. Kidney Res. Clin. Pract. 2022, 41, 412–421. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves First Interchangeable Biosimilar for Two Rare Diseases. 28 July 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-interchangeable-biosimilar-two-rare-diseases (accessed on 23 January 2025).
- Barratt, J.; Liew, A.; Yeo, S.C.; Fernström, A.; Barbour, S.J.; Sperati, C.J.; Villanueva, R.; Wu, M.-J.; Wang, D.; Borodovsky, A.; et al. Phase 2 trial of Cemdisiran in adult patients with IgA nephropathy: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kang, B.; Chen, J.; Li, S.; Zhou, J. Factor B inhibitor iptacopan for the treatment of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2024, 66, 101210. [Google Scholar] [CrossRef]
- Bomback, A.S.; Herlitz, L.C.; Kedia, P.P.; Petersen, J.; Yue, H.; Lafayette, R.A.; ACCOLADE Study Group. Safety and efficacy of Avacopan in patients with C3 glomerulopathy. J. Am. Soc. Nephrol. 2025, 36, 487–499. [Google Scholar] [CrossRef]
- Schmidt, C.Q.; Lambris, J.D.; Ricklin, D. Protection of host cells by complement regulators. Nat. Rev. Immunol. 2016, 16, 132–147. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7956994/ (accessed on 23 January 2025). [CrossRef]
- Apellis Pharmaceuticals. Apellis Dosed First Patient in Phase 3 Study of APL-2 for Treatment-Naive Patients with Geographic Atrophy. 14 November 2018. Available online: https://investors.apellis.com/news-releases/news-release-details/apellis-dosed-first-patient-phase-3-study-apl-2-treatment-naive (accessed on 23 January 2025).
- Wijnsma, K.L.; Duineveld, C.; Wetzels, J.F.M.; van de Kar, N.C.A.J. Eculizumab in atypical hemolytic uremic syndrome: Strategies toward restrictive use. Pediatr. Nephrol. 2018, 34, 2261–2277. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Eculizumab Therapy for Dense Deposit Disease and C3 Nephropathy. 2024. Available online: https://clinicaltrials.gov/study/NCT01221181 (accessed on 9 January 2025).
- Ariceta, G.; Dixon, B.P.; Kim, S.H.; Kapur, G.; Mauch, T.; Ortiz, S.; Vallee, M.; Denker, A.E.; Kang, H.G.; Greenbaum, L.A.; et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2021, 100, 225–237. [Google Scholar] [CrossRef]
- Mastellos, D.C.; Reis, E.S.; Ricklin, D.; Smith, R.J.; Lambris, J.D. Complement C3-targeted therapy: Replacing long-held assertions with evidence-based discovery. Trends Immunol. 2017, 38, 383–394. [Google Scholar] [CrossRef]
- Perkovic, V.; Barratt, J.; Rovin, B.; Kashihara, N.; Maes, B.; Zhang, H.; Trimarchi, H.; Kollins, D.; Papachristofi, O.; Jacinto-Sanders, S.; et al. Alternative complement pathway inhibition with iptacopan in IgA nephropathy. N. Engl. J. Med. 2025, 392, 531–543. [Google Scholar] [CrossRef]
- PRISMA Group. Transparent Reporting of Systematic Reviews and Meta-Analyses. PRISMA Statement; PRISMA Group: Raleigh, CA, USA, 2024. [Google Scholar]
- Schaefer, F.; Al-Dakkak, I.; Anokhina, K.; Cohen, D.; Greenbaum, L.A.; Ariceta, G. Global aHUS Registry Analysis of Patients Switching to Ravulizumab from Eculizumab. Kidney Int. Rep. 2024, 9, 2648–2656. [Google Scholar] [CrossRef]
- National Library of Medicine. A Study Evaluating the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of Crovalimab in Pediatric Participants with Atypical Hemolytic Uremic Syndrome (aHUS) (COMMUTE-p). 2024. Available online: https://clinicaltrials.gov/study/NCT04958265 (accessed on 9 January 2025).
- Greenbaum, L.A.; Al-Dakkak, I.; Anokhina, E.; Ardissino, G.; Ariceta, G.; Kumar, G.; Licht, C.; Siedlecki, A.M.; Walle, J.V. Long-term outcomes in eculizumab-treated patients enrolled in the global AHUS registry. J. Am. Soc. Nephrol. 2022, 33, 189. [Google Scholar] [CrossRef]
- Sheerin, N.; Greenbaum, L.; Shuichi, I.; Loirat, C.; Maruyama, S.; Zhao, M.; Benkali, K.; Pieterse, C.; Shah; Sostelly, A.; et al. Two phase III trials evaluating crovalimab in patients with atypical haemolytic uraemic syndrome (aHUS): COMMUTE-a and COMMUTE-p. Kidney Int. Rep. 2022, 7 (Suppl. S6), S460–S461. [Google Scholar] [CrossRef]
- Gentile, B.; Stefani, J.; Buri, M.; Leon, P. Crovalimab: Patient preferences and ongoing phase III COMMUTE trials in acute haemolytic uraemic syndrome (aHUS). Nephrol. Dial. Transplant. 2024, 39 (Suppl. S1), gfae069-1195-1222. [Google Scholar] [CrossRef]
- Kaplan, B.S.; Ruebner, R.L.; Spinale, J.M.; Copelovitch, L. Current treatment of atypical hemolytic uremic syndrome. Intractable Rare Dis. Res. 2014, 3, 34–45. [Google Scholar] [CrossRef]
- Wong, E.; Nester, C.; Cavero, T.; Karras, A.; Le Quintrec, M.; Lightstone, L.; Eisenberger, U.; Soler, M.J.; Kavanagh, D.; Daina, E.; et al. Efficacy and safety of Iptacopan in patients with C3 glomerulopathy. Kidney Int. Rep. 2023, 8, 2754–2764. [Google Scholar] [CrossRef]
- Bomback, A.S.; Kavanagh, D.; Vivarelli, M.; Meier, M.; Wang, Y.; Webb, N.J.; Trapani, A.J.; Smith, R.J. Alternative complement pathway inhibition with Iptacopan for the treatment of C3 glomerulopathy: Study design of the APPEAR-C3G trial. Kidney Int. Rep. 2022, 7, 2150–2159. [Google Scholar] [CrossRef]
- Fierce Pharma. Amyndas’ Lead Candidate AMY-101 Receives Orphan Drug Status from the FDA and the EMA for the Treatment of C3 Glomerulopathy. 2016. Available online: https://www.fiercepharma.com/pharma/amyndas-lead-candidate-amy-101-receives-orphan-drug-status-from-fda-and-ema-for-treatment-c3 (accessed on 9 January 2025).
- Novartis. Novartis Investigational Iptacopan Phase III Study Demonstrates Clinically Meaningful and Highly Statistically Significant Proteinuria Reduction in Patients with IgA Nephropathy (IgAN). 2024. Available online: https://www.novartis.com/news/media-releases/novartis-investigational-iptacopan-phase-iii-study-demonstrates-clinically-meaningful-and-highly-statistically-significant-proteinuria-reduction-patients-iga-nephropathy-igan (accessed on 9 January 2025).
- Bruchfeld, A.; Magin, H.; Nachman, P.; Parikh, S.; Lafayette, R.; Potarca, A.; Miao, S.; Bekker, P. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy—An open-label pilot study. Clin. Kidney J. 2022, 15, 922–928. [Google Scholar] [CrossRef]
- Omeros Corporation. Omeros Corporation Provides Update on Interim Analysis of ARTEMIS-IGAN Phase 3 Trial of Narsoplimab in IgA Nephropathy. 2023. Available online: https://investor.omeros.com/news-releases/news-release-details/omeros-corporation-provides-update-interim-analysis-artemis-igan (accessed on 9 January 2025).
- Castelli, M.; Micò, M.C.; Grassi, A.; Algarotti, A.; Lussana, F.; Finazzi, M.C.; Rambaldi, B.; Pavoni, C.; Rizzuto, G.; Tebaldi, P.; et al. Safety and efficacy of Narsoplimab in pediatric and adult patients with transplant-associated thrombotic microangiopathy: A real-world experience. Bone Marrow Transplant. 2024, 59, 1161–1168. [Google Scholar] [CrossRef]
- Rondeau, E.; Scully, M.; Ariceta, G.; Barbour, T.; Cataland, S.; Heyne, N.; Miyakawa, Y.; Ortiz, S.; Swenson, E.; Vallee, M.; et al. Ravulizumab for the treatment of adult patients with atypical hemolytic uremic syndrome: A 52-week interim analysis of a phase 3 study. Kidney Int. Rep. 2020, 5, 939–949. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujita, N.; Hibino, S. Prophylactic amoxicillin for the prevention of meningococcal infection in infants with atypical hemolytic uremic syndrome under treatment with eculizumab: A report of two cases. CEN Case Rep. 2020, 9, 247–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Library of Medicine. Single Patient Expanded Access Treatment Plan for the Investigational Product Narsoplimab. 2024. Available online: https://clinicaltrials.gov/study/NCT04247906 (accessed on 9 January 2025).
- Wexler, M.S. Avacopan May Be Effective Option to Steroids for AAV, Phase 3 Trial Finds. 2021. Available online: https://ancavasculitisnews.com/news/avacopan-possible-effective-option-steroids-aav-phase-3-advocate-trial/ (accessed on 29 June 2025).
- Dixon, B.P.; Kavanagh, D.; Aris, A.D.M.; Adams, B.; Kang, H.G.; Wang, E.; Garlo, K.; Ogawa, M.; Amancha, P.; Chakravarty, S.; et al. Ravulizumab in Atypical Hemolytic Uremic Syndrome: An Analysis of 2-Year Efficacy and Safety Outcomes in 2 Phase 3 Trials. Kidney Med. 2024, 6, 100855. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Evaluate Efficacy and Safety of Iptacopan in Patients with C3 Glomerulopathy. 2024. Available online: https://clinicaltrials.gov/study/NCT04817618 (accessed on 9 January 2025).
- Mayo Clinic. A Study of Cemdisiran in Adults with Immunoglobulin A Nephropathy (IgAN). 2024. Available online: https://www.mayo.edu/research/clinical-trials/cls-20467506 (accessed on 9 January 2025).
- Drugs.com. Soliris (Eculizumab) FDA Approval History. 2024. Available online: https://www.drugs.com/history/soliris.html (accessed on 9 January 2025).
- Tarragon Estebanez, B.; Bomback, A.S. C3 glomerulopathy: Novel treatment paradigms. Kidney Int. Rep. 2023, 9, 569–579. [Google Scholar] [CrossRef]
- European Medicines Agency. Soliris: EPAR—Product Information; EMA: Amsterdam, The Netherlands, 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/soliris (accessed on 12 January 2025).
- Drugs.com. Ultomiris (Ravulizumab-cwvz) FDA Approval History. 2024. Available online: https://www.drugs.com/history/ultomiris.html (accessed on 9 January 2025).
- European Medicines Agency. Ultomiris: EPAR—Product Information; EMA: Amsterdam, The Netherlands, 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ultomiris (accessed on 12 January 2025).
- Alexion Pharmaceuticals. ULTOMIRIS (Ravulizumab) Receives Marketing Authorization from the European Commission for the Treatment of Adult and Pediatric Patients with aHUS; Alexion Pharmaceuticals: Boston, MA, USA, 2019; Available online: https://media.alexion.com/news-releases/news-release-details/ultomirisr-ravulizumab-receives-marketing-authorization-european (accessed on 12 January 2025).
- Ryan, C.; FDA Approves Crovalimab for Paroxysmal Nocturnal Hemoglobinuria. OncLive. 2024. Available online: https://www.onclive.com/view/fda-approves-crovalimab-for-paroxysmal-nocturnal-hemoglobinuria (accessed on 9 January 2025).
- National Library of Medicine. Evaluate Long-Term Efficacy and Safety of Investigational Treatment NCT04861259. 2024. Available online: https://clinicaltrials.gov/study/NCT04861259 (accessed on 9 January 2025).
- Roche. Positive Phase III Results for Roche’s Gazyva/Gazyvaro Show Superiority to Standard Therapy Alone in People with Lupus Nephritis. 2024. Available online: https://www.roche.com/media/releases/med-cor-2024-09-26 (accessed on 9 January 2025).
- GlobeNewswire. ChemoCentryx Announces EU Approval of Tavneos® (Avacopan) for the Treatment of ANCA-Associated Vasculitis. 2022. Available online: https://www.globenewswire.com/news-release/2022/01/19/2369344/19219/en/ChemoCentryx-Announces-EU-Approval-of-TAVNEOS-avacopan-for-the-Treatment-of-ANCA-Associated-Vasculitis.html (accessed on 9 January 2025).
- European Medicines Agency. Tavneos: EPAR—Product Information; EMA: Amsterdam, The Netherlands, 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tavneos (accessed on 12 January 2025).
- Syed, Y.Y. Correction: Iptacopan: First approval. Drugs. 2024, 84, 875. [Google Scholar] [CrossRef] [PubMed]
- Novartis. Novartis oral Fabhalta® (Iptacopan) Sustained Clinically Meaningful Results at One Year in Phase III C3 Glomerulopathy (C3G) Trial. 2024. Available online: https://www.novartis.com/news/media-releases/novartis-oral-fabhalta-iptacopan-sustained-clinically-meaningful-results-one-year-phase-iii-c3-glomerulopathy-c3g-trial (accessed on 9 January 2025).
- BioSpace. Omeros Shares Plummet on Phase III Kidney Disease Trial Failure. Available online: https://www.biospace.com/omeros-shares-plummet-on-phase-iii-kidney-disease-trial-failure (accessed on 25 January 2025).
- Drugs.com. Narsoplimab: What Is It and Is It FDA Approved? 2024. Available online: https://www.drugs.com/history/narsoplimab.html (accessed on 9 January 2025).
- Novartis. Evaluate Long-Term Safety, Tolerability, and Efficacy of Iptacopan in Study Participants with aHUS. 2024. Available online: https://www.novartis.com/clinicaltrials/study/nct05795140 (accessed on 9 January 2025).
- Ichgcp.net. Eculizumab to Cemdisiran Switch in aHUS (DANCE). 2019. Available online: https://ichgcp.net/clinical-trials-registry/NCT03999840 (accessed on 9 January 2025).
- GlobeNewswire. Regeneron Pharmaceuticals Announces Novel Combination of Pozelimab and Cemdisiran (Poze-Cemdi) Achieved Greater Control of Intravascular Hemolysis in Patients with Paroxysmal Nocturnal Hemoglobinuria Compared to Ravulizumab. 2024. Available online: https://www.globenewswire.com/news-release/2024/12/08/2993358/0/en/Novel-Combination-of-Pozelimab-and-Cemdisiran-Poze-Cemdi-Achieved-Greater-Control-of-Intravascular-Hemolysis-in-Patients-with-Paroxysmal-Nocturnal-Hemoglobinuria-Compared-to-Ravuli.html (accessed on 9 January 2025).
- Risitano, A.M.; Frieri, C.; Urciuoli, E.; Marano, L. The complement alternative pathway in paroxysmal nocturnal hemoglobinuria: From a pathogenic mechanism to a therapeutic target. Immunol. Rev. 2022, 313, 95–111. [Google Scholar] [CrossRef]
- European Union Clinical Trials Register. Clinical Trials Register. 2014. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-004220-11/PL (accessed on 9 January 2025).
- Fakhouri, F.; Bomback, A.; Kavanagh, D.; Remuzzi, G.; Sunder-Plassmann, G.; Kanellis, J.; Daina, E.; Walker, P.; Wang, Z.; Ahmad, Z. Pegcetacoplan for post-transplant recurrent C3 glomerulopathy or immune complex membranoproliferative glomerulonephritis in NOBLE: 12-week evolution. Nephrol. Dial. Transplant. 2024, 39 (Suppl. S1), gfae069-0442-1467. [Google Scholar] [CrossRef]
- European Medicines Agency. Aspaveli: EPAR—Product Information; EMA: Amsterdam, The Netherlands, 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/aspaveli (accessed on 12 January 2025).
- FDA Grants Rare Pediatric Disease Designation to Omeros’ MASP-3 Inhibitor Zaltenibart for Treatment of C3 Glomerulopathy. BioSpace. 2024. Available online: https://www.biospace.com/press-releases/fda-grants-rare-pediatric-disease-designation-to-omeros-masp-3-inhibitor-zaltenibart-for-treatment-of-c3-glomerulopathy/ (accessed on 9 January 2025).
- Karnabeda, O.; Moskalenko, V.; Lysak, Z.; Nangia, N.; Osborne, C.A.; Whitaker, J.S.; Gavriilaki, E.; Panse, J. OMS906, a novel alternative pathway MASP-3 inhibitor, normalizes hemoglobin levels and increases clone size in treatment-naïve PNH patients. Blood 2023, 142 (Suppl. S1), 573. [Google Scholar] [CrossRef]
- Griffin, M.; Kelly, R.J.; Gavillet, M.; Muus, P.; Hoechsmann, B.; Cummings, W.J.; Efthimiou, J.; Pullman, W.; Schrezenmeier, H.; Panse, J. Monotherapy treatment with Zaltenibart (OMS906), an alternative pathway Masp-3 inhibitor, improved key hematologic parameters in patients with PNH with a suboptimal response to Ravulizumab: Interim results from a phase 2 proof-of-concept study. Blood 2024, 144 (Suppl. S1), 4072. [Google Scholar] [CrossRef]
- Terriou, L.; Lee, J.W.; Forsyth, C.; Griffin, M.; Szer, J.; Röth, A.; Gustovic, P.; Metzger, J.; Patel, A.S.; Patriquin, C.J. Long-term effectiveness of eculizumab: Data from the International PNH Registry. Eur. J. Haematol. 2023, 111, 796–804. [Google Scholar] [CrossRef]
- Kulasekararaj, A.G.; Griffin, M.; Langemeijer, S.; Usuki, K.; Kulagin, A.; Ogawa, M.; Yu, J.; Mujeebuddin, A.; Nishimura, J.I.; Lee, J.W.; et al. Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies. Eur. J. Haematol. 2022, 109, 205–214. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9546219/ (accessed on 22 January 2025). [CrossRef]
- Peipert, J.D.; Kulasekararaj, A.G.; Gaya, A.; Langemeijer, S.M.C.; Yount, S.; Gonzalez-Fernandez, F.A.; Gutierrez, E.O.; Martens, C.; Sparling, A.; Webster, K.A.; et al. Patient preferences and quality of life implications of ravulizumab (every 8 weeks) and eculizumab (every 2 weeks) for the treatment of paroxysmal nocturnal hemoglobinuria. PLoS ONE 2020, 15, e0237497. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7473546/ (accessed on 22 January 2025). [CrossRef]
- European Medicines Agency. Piasky: EPAR—Risk Management Plan. Available online: https://www.ema.europa.eu/en/documents/rmp/piasky-epar-risk-management-plan_en.pdf (accessed on 22 January 2025).
- FabHALTA. Available online: https://www.fabhalta-hcp.com/ (accessed on 22 January 2025).
- Pegcetacoplan: Uses, Side Effects, and Warnings. Drugs.com. Available online: https://www.drugs.com/pegcetacoplan.html#side-effects (accessed on 22 January 2025).
- Mayo Clinic. Avacopan: Description, Uses, and Side Effects. Available online: https://www.mayoclinic.org/drugs-supplements/avacopan-oral-route/description/drg-20524765#drug-side-effects (accessed on 22 January 2025).
- Gaya, A.; Munir, T.; Urbano-Ispizua, A.; Griffin, M.; Taubel, J.; Bush, J.; Bhan, I.; Borodovsky, A.; Wang, Y.; Badri, P.; et al. Results of a phase 1/2 study of cemdisiran in healthy subjects and patients with paroxysmal nocturnal hemoglobinuria. EJHaem 2023, 4, 612–624. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10435727/ (accessed on 22 January 2025). [CrossRef] [PubMed]
- Khaled, S.K.; Claes, K.; Goh, Y.T.; Kwong, Y.L.; Leung, N.; Mendrek, W.; Nakamura, R.; Sathar, J.; Ng, E.; Nangia, N.; et al. Narsoplimab, a Mannan-Binding Lectin-Associated Serine Protease-2 Inhibitor, for the Treatment of Adult Hematopoietic Stem-Cell Transplantation–Associated Thrombotic Microangiopathy. J. Clin. Oncol. 2022, 40, 2447–2457. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9467678/ (accessed on 22 January 2025). [CrossRef] [PubMed]

| Drug | Target | Pathway | Mechanism |
|---|---|---|---|
| Pegcetacoplan [6,7] | C3 | Terminal | Inhibits C3 cleavage, blocking complement activation and MAC formation [6,7]. |
| AMY-101 [8] | C3 | Terminal | Inhibits C3, reducing inflammation and preventing MAC formation [8]. |
| Eculizumab [20,21] | C5 | Terminal | Blocks cleavage into C5a and C5b, preventing MAC formation [20,21]. |
| Ravulizumab [22] | C5 | Terminal | Long-acting C5 inhibitor offering extended dosing intervals [22]. |
| Crovalimab [12] | C5 | Terminal | Subcutaneous formulation; prevents MAC formation [12]. |
| Avacopan [13,23] | C5a receptor (C5aR1) | Alternative | Blocks C5a receptor on neutrophils, inhibiting inflammation [13,23]. |
| Iptacopan [24] | Factor B | Alternative | Inhibits alternative pathway amplification by targeting the C3 convertase [24]. |
| Narsoplimab [13,23] | MASP-2 | Lectin | Prevents activation of the lectin pathway by inhibiting MASP-2 [13,23]. |
| Cemdisiran [15] | C5 (gene level) | Terminal | RNA interference reduces C5 production in hepatocytes [13]. |
| Biosimilars [14] | C5 (e.g., eculizumab-aeeb) | Terminal | Cost-effective alternatives with identical mechanisms [12]. |
| Drug | Disease | Trial Phase | Outcome | NCT Number | Significance |
|---|---|---|---|---|---|
| Ravulizumab [43] | aHUS | Phase 3 | 61–90% TA-TMA response, improved renal function | NCT02949128 | Extended dosing intervals improve adherence. |
| Avacopan [42] | AAV | Phase 3 | 72.3% remission at 26 weeks | NCT02994927 | Steroid-sparing option reduces adverse effects. |
| Iptacopan [44] | C3G | Phase 3 | Reduced proteinuria, delayed disease progression | NCT04817618 | Oral therapy offers convenience. |
| Cemdisiran [45] | IgA Nephropathy (early stage) | Phase 1 | Safe, effective C5 suppression | NCT03841448 | First RNAi-based complement therapy. |
| Drugs | Diseases | Side Effects | Long-Term Data |
|---|---|---|---|
| Eculizumab | aHUS, PNH [6,11,21] | Risk of meningococcal infections [13,26] | Limited to 1 year efficacy [70] |
| Ravulizumab | aHUS, PNH [46,47] | Like eculizumab [13,23] | Well tolerated [71]; 93% of patients preferred ravulizumab to eculizumab [72] |
| Crovalimab | aHUS, PNH [12,27] | Like eculizumab [72,73] | Awaiting long-term outcomes |
| Iptacopan | PNH, C3G, IgAN [16,24,32,57] | Risk of severe infections [74] | Awaiting long-term outcomes |
| Pegcetacoplan | PNH, C3G [6,29] | Risk of severe infections [75] | Awaiting long-term outcomes |
| AMY-101 | C3G [34] | Not available yet | Awaiting long-term outcomes |
| Avacopan | IgAN, C3G [36] | Allergy symptoms, digestive problems [76,77] | Awaiting long-term outcomes |
| Cemdisiran | aHUS, IgAN, PNH [15,45,66] | Sleep disorders, nausea, headache [78] | Awaiting long-term outcomes |
| Narsoplimab | TA-TMA [4] | Digestive problems, neutropenia, nausea [79] | Awaiting long-term outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, M.; Nowicki, M. Targeting the Roots of Kidney Disease: Systematic Review of the Therapies Targeting the Complement System. Medicina 2025, 61, 1205. https://doi.org/10.3390/medicina61071205
Roman M, Nowicki M. Targeting the Roots of Kidney Disease: Systematic Review of the Therapies Targeting the Complement System. Medicina. 2025; 61(7):1205. https://doi.org/10.3390/medicina61071205
Chicago/Turabian StyleRoman, Maja, and Michał Nowicki. 2025. "Targeting the Roots of Kidney Disease: Systematic Review of the Therapies Targeting the Complement System" Medicina 61, no. 7: 1205. https://doi.org/10.3390/medicina61071205
APA StyleRoman, M., & Nowicki, M. (2025). Targeting the Roots of Kidney Disease: Systematic Review of the Therapies Targeting the Complement System. Medicina, 61(7), 1205. https://doi.org/10.3390/medicina61071205








