Mapping the Maxillary Artery and Lateral Pterygoid Muscle Relationship: Insights from Radiological and Meta-Analytic Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Computed Tomography Angiography (CTA) Study
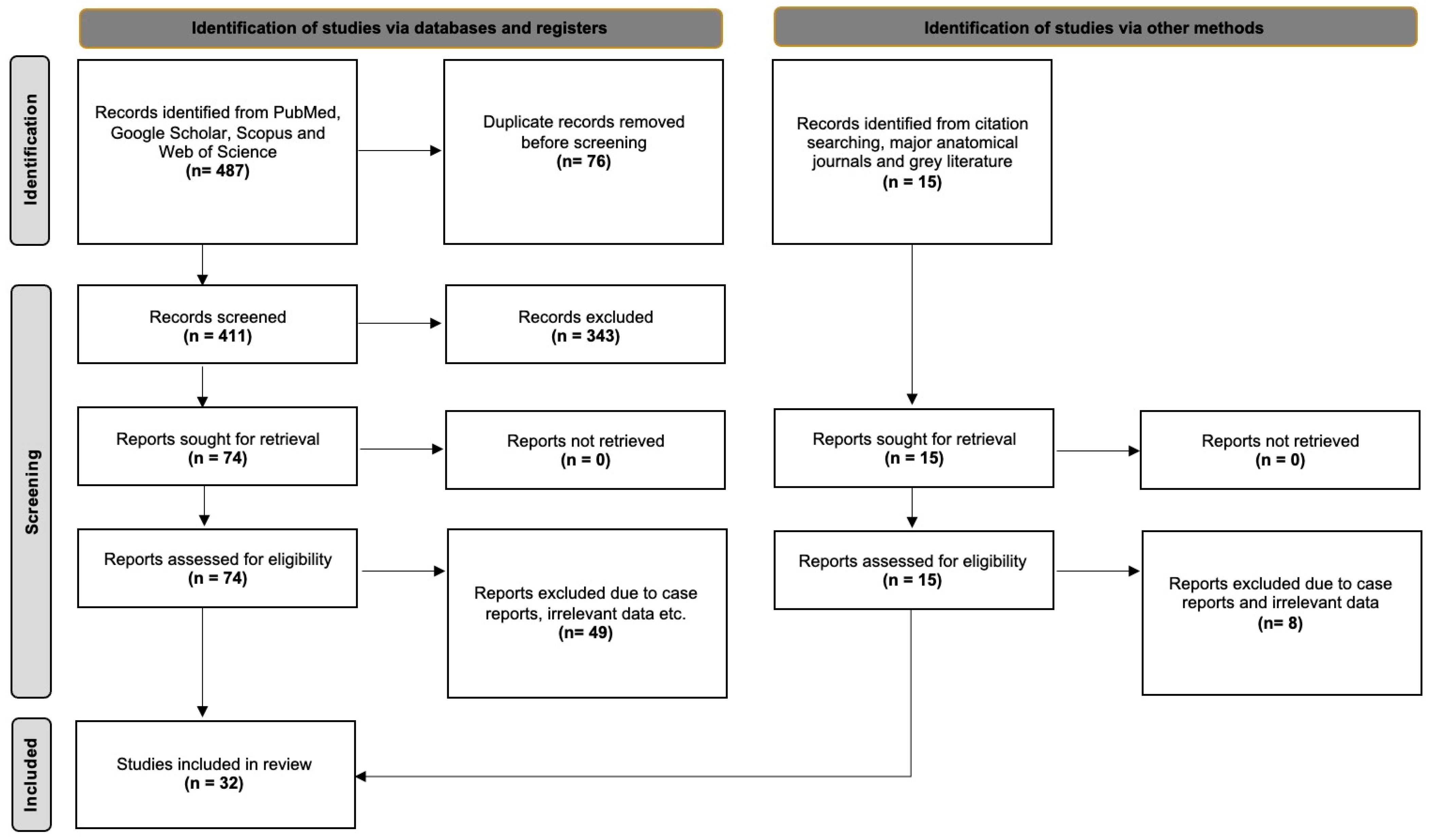
2.2. Systematic Review with Meta-Analysis
3. Results
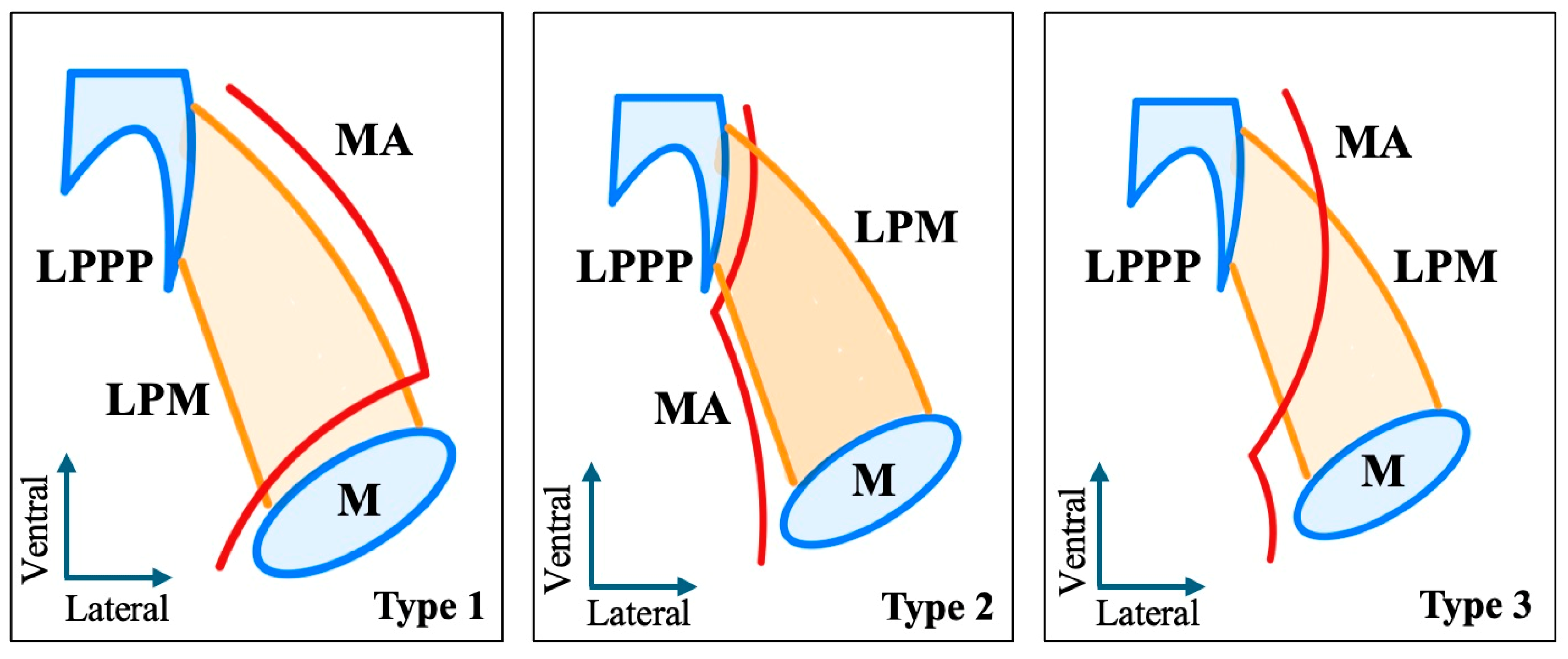
3.1. Original Radioanatomical Study
- -
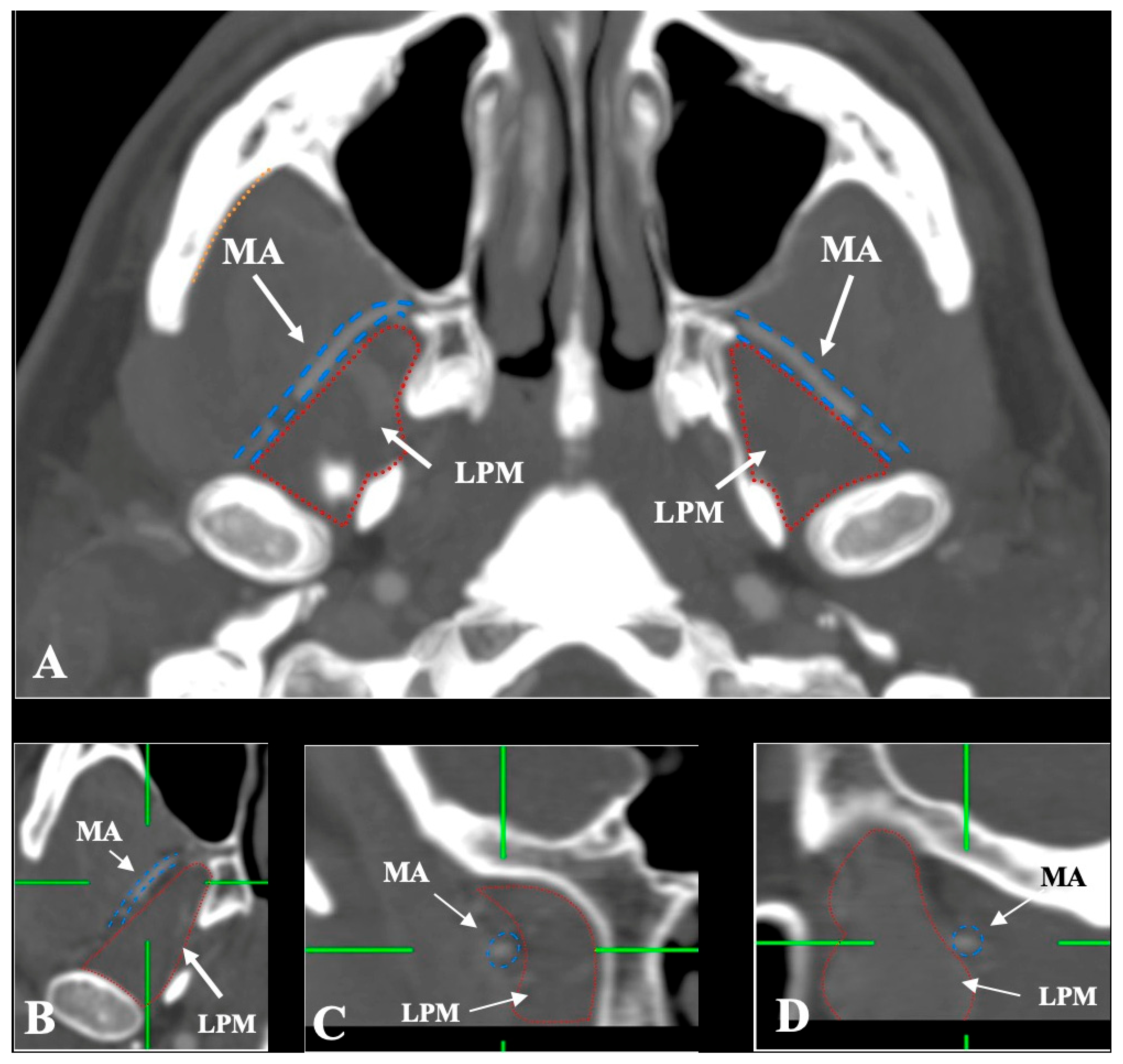
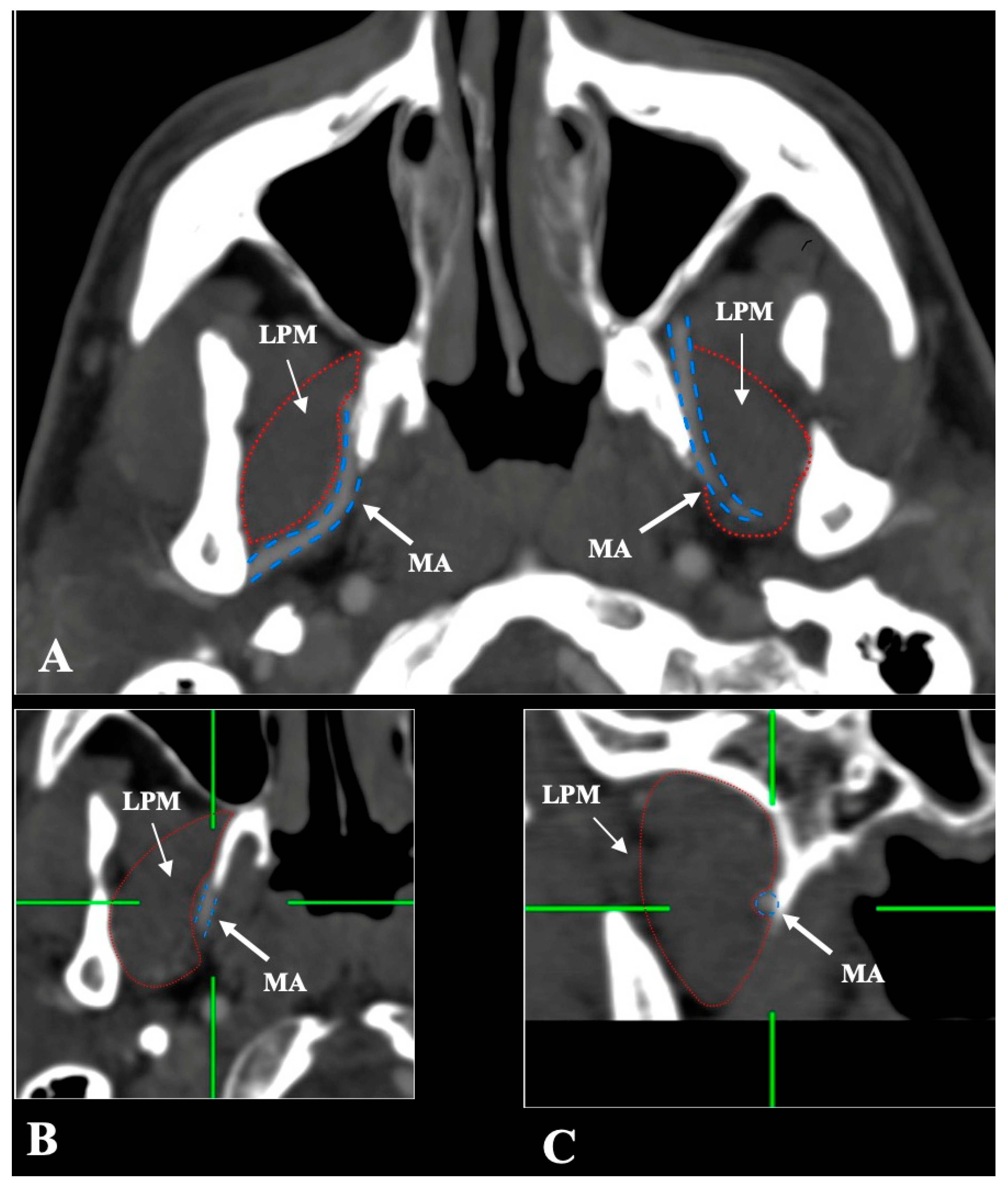
- Lateral (superficial) to the LPM: Detected in 321 sides (64.2%). This configuration was consistently visualized in axial, coronal, and sagittal planes (Figure 2).
- -
- Medial (deep) to the LPM: Identified in 148 sides (29.6%), best appreciated on axial and coronal reconstructions (Figure 3).
- -
- Intramuscular (transversing the LPM fibers): Found in 31 sides (6.2%), clearly depicted in all planes (Figure 4).
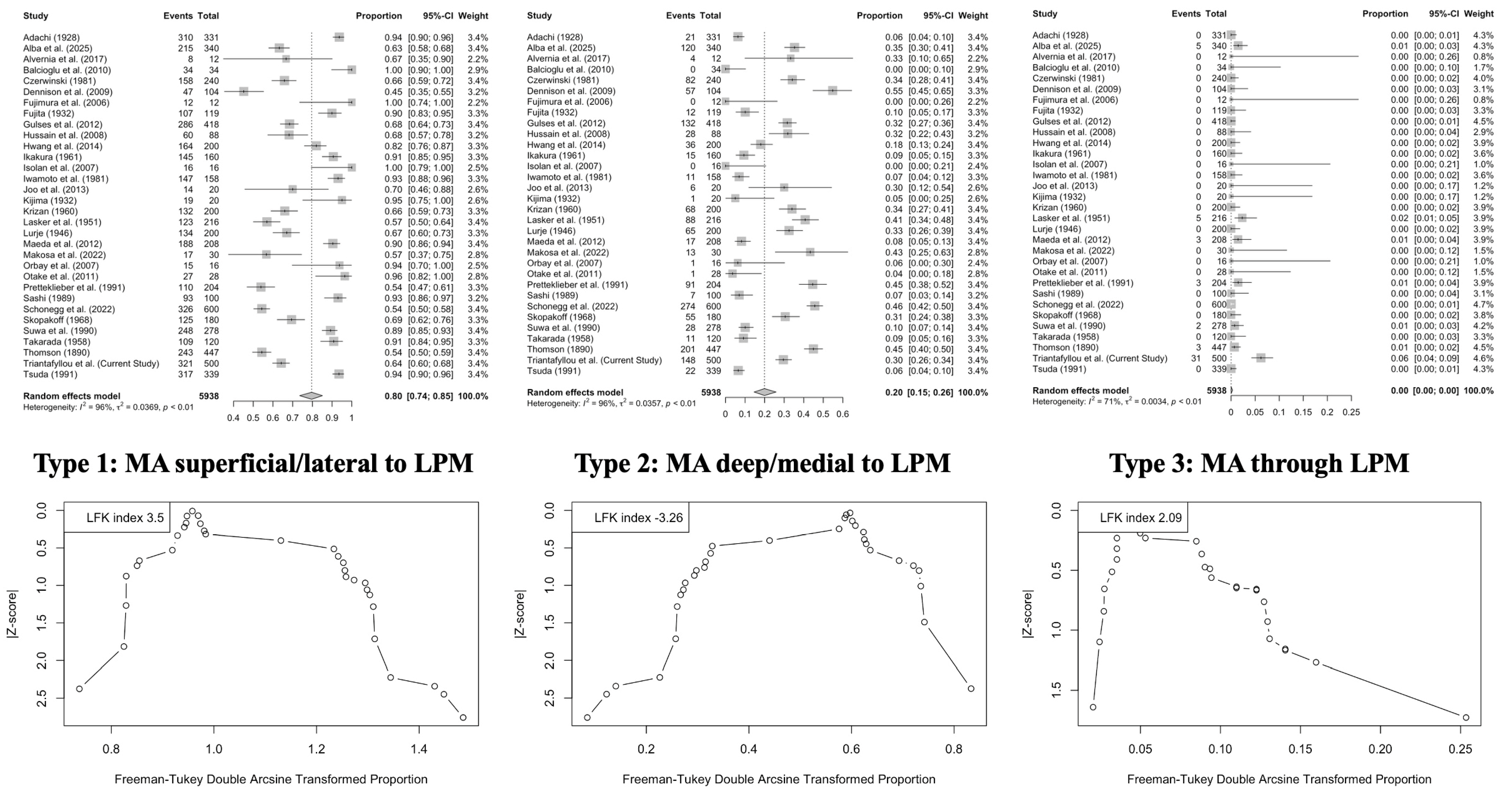
3.2. Systematic Review with Meta-Analysis
| Study | Year | Population | Type of Study | No. of Arteries | Risk of Bias |
|---|---|---|---|---|---|
| Thomson [9] | 1890 | Europe | Cadaveric | 447 | Low |
| Adachi [10] | 1928 | Asia | Cadaveric | 331 | High |
| Fujita [11] | 1932 | Asia | Cadaveric | 119 | High |
| Kijima [12] | 1932 | Asia | Cadaveric | 20 | High |
| Lurje [13] | 1946 | Europe | Cadaveric | 200 | High |
| Lasker et al. [14] | 1951 | America | Cadaveric | 216 | High |
| Takarada [15] | 1958 | Asia | Cadaveric | 120 | High |
| Krizan [16] | 1960 | Europe | Cadaveric | 200 | Low |
| Ikakura [17] | 1961 | Asia | Cadaveric | 160 | High |
| Skopakoff [18] | 1968 | Europe | Cadaveric | 180 | High |
| Czerwinski [19] | 1981 | Europe | Cadaveric | 240 | Low |
| Iwamoto et al. [20] | 1981 | Asia | Cadaveric | 158 | Low |
| Sashi [21] | 1989 | Asia | Cadaveric | 100 | Low |
| Suwa et al. [22] | 1990 | Asia | Cadaveric | 278 | Low |
| Pretteklieber et al. [23] | 1991 | Europe | Cadaveric | 204 | Low |
| Tsuda [24] | 1991 | Asia | Cadaveric | 339 | Low |
| Fujimura et al. [25] | 2006 | Asia | Cadaveric | 12 | High |
| Isolan et al. [26] | 2007 | America | Cadaveric | 16 | High |
| Orbay et al. [27] | 2007 | Europe | Cadaveric | 16 | High |
| Hussain et al. [28] | 2008 | America | Cadaveric | 88 | Low |
| Dennison et al. [29] | 2009 | America | Cadaveric | 104 | Low |
| Balcioglu et al. [30] | 2010 | Asia | Cadaveric | 34 | Low |
| Otake et al. [31] | 2011 | Asia | Cadaveric | 28 | Low |
| Gulses et al. [32] | 2012 | Asia | Imaging | 418 | Low |
| Maeda et al. [33] | 2012 | Asia | Cadaveric | 208 | Low |
| Joo et al. [34] | 2013 | Asia | Cadaveric | 20 | High |
| Hwang et al. [35] | 2014 | Asia | Imaging | 200 | Low |
| Alvernia et al. [36] | 2017 | America | Cadaveric | 12 | High |
| Makosa et al. [37] | 2022 | Africa | Cadaveric | 30 | High |
| Schonegg et al. [38] | 2022 | Europe | Imaging | 600 | Low |
| Albu et al. [4] | 2025 | Europe | Imaging | 340 | Low |
| Current Study | 2025 | Europe | Imaging | 500 | - |
- Lateral (superficial) course: 79.61% (95% CI: 73.53–85.11);
- Medial (deep) course: 19.94% (95% CI: 14.57–25.88);
- Intramuscular course: 0.01% (95% CI: 0.00–0.30).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Standring, S.; Anand, N.; Birch, R.; Collins, P.; Crossman, A.; Gleeson, M.; Jawaheer, G.; Smith, A.; Spratt, J.; Stringer, M.; et al. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier: London, UK, 2016. [Google Scholar]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ottone, N.E.; Sandoval, C.; Cid-Gutierrez, P.; Vásquez-Balboa, M.L.; Tubbs, R.S.; Fuentes, R. Systematic Review and Meta-Analysis of the Anatomy of the Maxillary Artery Using the Anatomical Quality Assurance (AQUA) Checklist. Surg. Radiol. Anat. 2021, 43, 1875–1886. [Google Scholar] [CrossRef]
- Albu, A.C.; Tudose, R.C.; Vrapciu, A.D.; Rusu, M.C. Beyond Two Heads: An Imaging-Based Analysis of the Lateral Pterygoid Muscle’s Heads. Ann. Anat. Anat. Anz. 2025, 259, 152387. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Tomaszewski, K.A.; Walocha, J.A. Methods of Evidence-Based Anatomy: A Guide to Conducting Systematic Reviews and Meta-Analysis of Anatomical Studies. Ann. Anat. 2016, 205, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Henry, B.M.; Tomaszewski, K.A.; Ramakrishnan, P.K.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the Anatomical Quality Assessment (AQUA) Tool for the Quality Assessment of Anatomical Studies Included in Meta-Analyses and Systematic Reviews. Clin. Anat. 2017, 30, 6–13. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A New Improved Graphical and Quantitative Method for Detecting Bias in Meta-Analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A. Report of the Committee of Collective Investigation of the Anatomical Society of Great Britain and Ireland for the Year 1889–90. J. Anat. Physiol. 1891, 25, 89–101. [Google Scholar]
- Adachi, B. Adachi, B. A Maxillaris Interna. In Das Arterinsystem der Japaner; Maruzen: Kyoto, Japan, 1928; pp. 85–96. [Google Scholar]
- Fujita, T. Ueber Einen Fall von Beiderseitig Medial Vom N. Mandibularis Verlaufender A. Maxillaries Interna, Nebst Einer Statistikder Verlaufsvariatioon Der Arterie. J. Stomatol. Soc. Jpn. 1932, 6, 250–252. (In Japanese) [Google Scholar] [CrossRef]
- Kijima, N. On Distribution of the Artery over the Mandibular-Joint. Med. J. Kagoshima Univ. 1932, 10, 71–83. (In Japanese) [Google Scholar]
- Lurje, A. On the Topographical Anatomy of the Internal Maxillary Artery. Acta Anat. 1947, 2, 219–231. [Google Scholar] [CrossRef]
- Lasker, G.W.; Opdyke, D.L.; Miller, H. The Position of the Internal Maxillary Artery and Its Questionable Relation to the Cephalic Index. Anat. Rec. 1951, 109, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T. Anatomical Studies on the Maxillary Artery. J. Tokyo Dent. Coll. Soc. 1958, 53, 1–20. (In Japanese) [Google Scholar]
- Križan, Z. Beirtraege Zur Deskriptiven Und Topographischen Anatomie der A. Maxillaris. Acta Anat. 1960, 41, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ikakura, K. On the Origin Course and Distribution of the Maxillary Artery in Japanese. Kouku Kaibou Kenkyu 1961, 18, 91–122. (In Japanese) [Google Scholar]
- Skopakoff, C. Ueber Die Varialitaet Im Verlauf Der a.Maxillaris. Anat. Anz. 1968, 123, 534–546. [Google Scholar]
- Czerwinski, F. Variability of the Course of External Carotid Artery and Its Rami in Man in the Light of Anatomical and Radiological Studies. Folia Morphol. 1981, 40, 449–453. [Google Scholar]
- Iwamoto, S.; Konishi, M.; Takahashi, Y.; Kimura, K. Some Variations in the Course of the Maxillary Artery. J. Natl. Def. Med. Coll. 1981, 6, 75–78. (In Japanese) [Google Scholar]
- Sashi, R. X-Ray Anatomy of the Maxillary Artery. Akita J. Med. 1989, 16, 817–831. (In Japanese) [Google Scholar]
- Suwa, F.; Takemura, A.; Ehara, Y.; Takeda, N.; Masu, M. On the Arteria Maxillaris Which Passes Medial to the Pterygoideus Lateralis Muscle of the Japanese Patterns of Origin of the Inferior Alveolar, the Masseteric and the Posterior Temporal Arteries. Okajimas Folia Anat. Jpn. 1990, 67, 303–308. [Google Scholar] [CrossRef]
- Pretterklieber, M.L.; Skopakoff, C.; Mayr, R. The Human Maxillary Artery Reinvestigated: I. Topographical Relations in the Infratemporal Fossa. Cells Tissues Organs 1991, 142, 281–287. [Google Scholar] [CrossRef]
- Tsuda, K. Three-Dimensional Analysis of Arteriographs of the Maxillary Artery in Man—Part 1: The Maxillary Artery and Its Branches. J. Jpn. PRS 1991, 11, 188–198. (In Japanese) [Google Scholar]
- Fujimura, K.; Segami, N.; Kobayashi, S. Anatomical Study of the Complications of Intraoral Vertico-Sagittal Ramus Osteotomy. J. Oral Maxillofac. Surg. 2006, 64, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Isolan, G.; Rowe, R.; Al-Mefty, O. Microanatomy and Surgical Approaches to the Infratemporal Fossa: An Anaglyphic Three-Dimensional Stereoscopic Printing Study. Skull Base 2007, 17, 285–302. [Google Scholar] [CrossRef]
- Orbay, H.; Kerem, M.; Ünlü, R.E.; Cömert, A.; Tüccar, E.; Şensöz, Ö. Maxillary Artery: Anatomical Landmarks and Relationship with the Mandibular Subcondyle. Plast. Reconstr. Surg. 2007, 120, 1865–1870. [Google Scholar] [CrossRef]
- Hussain, A.; Binahmed, A.; Karim, A.; Sándor, G.K.B. Relationship of the Maxillary Artery and Lateral Pterygoid Muscle in a Caucasian Sample. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 32–36. [Google Scholar] [CrossRef]
- Dennison, J.; Batra, A.; Herbison, P. The Maxillary Artery and the Lateral Pterygoid Muscle: The New Zealand Story. Oral Surg. Oral Med. Oral Pathol Oral Radiol. Endodontol. 2009, 108, e26–e29. [Google Scholar] [CrossRef] [PubMed]
- Balcioglu, H.A.; Kilic, C.; Varol, A.; Ozan, H.; Kocabiyik, N.; Yildirim, M. A Morphometric Study of the Maxillary Artery and Lingula in Relation to Mandibular Ramus Osteotomies and TMJ Surgery. Eur. J. Dent. 2010, 4, 166–170. [Google Scholar] [CrossRef]
- Otake, I.; Kageyama, I.; Mataga, I. Clinical Anatomy of the Maxillary Artery. Okajimas Folia Anat. Jpn. 2011, 87, 155–164. [Google Scholar] [CrossRef]
- Gulses, A.; Oren, C.; Altug, H.A.; Ilica, T.; Sencimen, M. Radiologic Assessment of the Relationship Between the Maxillary Artery and the Lateral Pterygoid Muscle. J. Craniofac. Surg. 2012, 23, 1465–1467. [Google Scholar] [CrossRef]
- Maeda, S.; Aizawa, Y.; Kumaki, K.; Kageyama, I. Variations in the Course of the Maxillary Artery in Japanese Adults. Anat. Sci. Int. 2012, 87, 187–194. [Google Scholar] [CrossRef]
- Joo, W.; Funaki, T.; Yoshioka, F.; Rhoton, A.L. Microsurgical Anatomy of the Infratemporal Fossa. Clin. Anat. 2013, 26, 455–469. [Google Scholar] [CrossRef]
- Hwan Hwang, S.; Hoon Joo, Y.; Hyun Seo, J.; Myung Kang, J. Proximity of the Maxillary Artery to the Mandibular Ramus: An Anatomic Study Using Three-dimensional Reconstruction of Computer Tomography. Clin. Anat. 2014, 27, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Alvernia, J.E.; Hidalgo, J.; Sindou, M.P.; Washington, C.; Luzardo, G.; Perkins, E.; Nader, R.; Mertens, P. The Maxillary Artery and Its Variants: An Anatomical Study with Neurosurgical Applications. Acta Neurochir. 2017, 159, 655–664. [Google Scholar] [CrossRef]
- Makosa, T.; Bere, S.; Chidavaenzi, R.; Mawera, G. Relationship of the Neurovascular Structures of the Infratemporal Fossae in Black Zimbabweans: A Cadaveric Study. Anat. J. Afr. 2022, 11, 2075–2085. [Google Scholar] [CrossRef]
- Schönegg, D.; Ferrari, R.; Ebner, J.; Blumer, M.; Lanzer, M.; Gander, T. Proximity of the Middle Meningeal Artery and Maxillary Artery to the Mandibular Head and Mandibular Neck as Revealed by Three-Dimensional Time-of-Flight Magnetic Resonance Angiography. Oral Maxillofac. Surg. 2022, 26, 139–146. [Google Scholar] [CrossRef]
- Mandal, G.; Montalbano, M.; Natsis, K.; Piagkou, M.; Tubbs, R.S.; Loukas, M. Musculus Pterygoideus Proprius: A Meta-analysis. Clin. Anat. 2023, 37, 859–868. [Google Scholar] [CrossRef]
- Rusu, M.C.; Toader, C.; Tudose, R.C.; Grigoriţă, L.O. Debate on the Morphological Variability of the Lateral Pterygoid Muscle—Discrepancies, Speculations and New Original Anatomical Samples. Medicina 2024, 60, 1913. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Pękala, P.A.; Frączek, P.A.; Pękala, J.R.; Natsis, K.; Piagkou, M.; Tomaszewski, K.A.; Tomaszewska, I.M. Prevalence, Morphology, and Morphometry of the Pterygospinous Bar: A Meta-Analysis. Surg. Radiol. Anat. 2020, 42, 497–507. [Google Scholar] [CrossRef]
- Pękala, P.A.; Henry, B.M.; Pękala, J.R.; Frączek, P.A.; Taterra, D.; Natsis, K.; Piagkou, M.; Skrzat, J.; Tomaszewska, I.M. The Pterygoalar Bar: A Meta-Analysis of Its Prevalence, Morphology and Morphometry. J. Cranio-Maxillofac. Surg. 2017, 45, 1535–1541. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Luzzi, S.; Olewnik, Ł.; Tsakotos, G.; Zielinska, N.; Galzio, R.; Tudose, R.C.; Rusu, M.C.; Piagkou, M. Foramen Ovale Morphology and Relationship with the Lateral Pterygoid Process Plate: Proposal for a New Classification System. Anat. Sci. Int. 2025, 100, 354–365. [Google Scholar] [CrossRef]
- Piagkou, M.; Demesticha, T.; Skandalakis, P.; Johnson, E.O. Functional Anatomy of the Mandibular Nerve: Consequences of Nerve Injury and Entrapment. Clin. Anat. 2011, 24, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Sakaguchi-Kuma, T.; Fukino, K.; Ono, T. Masticatory Muscles and Branches of Mandibular Nerve: Positional Relationships Between Various Muscle Bundles and Their Innervating Branches. Anat. Rec. 2019, 302, 609–619. [Google Scholar] [CrossRef]
- Dumitru, C.C.; Rusu, M.C.; Vrapciu, A.D. Maxillary Artery Traversing Through the Temporal Muscle. J. Craniofac. Surg. 2024, 35, e273–e274. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Fasil, M.; Murugan, M.; Sakkarai, J. Unique Variation in the Course of Maxillary Artery in Infratemporal Fossa: A Case Report. Surg. Radiol. Anat. 2014, 36, 507–509. [Google Scholar] [CrossRef]
- Aland, R.C.; Shaw, V. Divided Maxillary Artery in Relation to the Lateral Pterygoid Muscle. Anat. Sci. Int. 2016, 91, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Vrapciu, A.D.; Popescu, Ş.A. Fenestrated Maxillary Artery. J. Craniofac. Surg. 2022, 33, e861–e863. [Google Scholar] [CrossRef] [PubMed]


| MA-LPM Relationship | Total N = 500 (%) | Left n = 250 (%) | Right n = 250 (%) | p-Value | Males n = 276 (%) | Females n = 224 (%) | p-Value |
|---|---|---|---|---|---|---|---|
| Lateral/Superficial | 321 (64.2) | 164 (65.6) | 157 (62.8) | 0.320 | 176 (63.8) | 145 (64.7) | 0.117 |
| Medial/Deep | 148 (29.6) | 74 (29.6) | 74 (29.6) | 79 (28.6) | 69 (30.8) | ||
| Through | 31 (6.2) | 12 (4.8) | 19 (7.6) | 21 (7.6) | 10 (4.5) |
| MA-LPM Relationship | Lateral/Superficial n (%) | Medial/Deep n (%) | Through n (%) |
|---|---|---|---|
| Lateral/Superficial | 136 (54.4) | 19 (7.6) | 2 (0.8) |
| Medial/Deep | 22 (8.8) | 50 (20) | 2 (0.8) |
| Through | 6 (2.4) | 5 (2) | 8 (3.2) |
| Parameters | Superficial/Lateral | Deep/Medial | Through |
|---|---|---|---|
| Asia (n = 16) | 90.79% | 9.04% | 0.00% |
| Europe (n = 10) | 62.93% | 35.66% | 0.36% |
| Africa (n = 1) | 56.67% | 43.33% | 0.06% |
| America (n = 5) | 67.70% | 31.80% | 0.00% |
| p-value | <0.0001 * | <0.0001 * | 0.2335 |
| Cadaveric (n = 27) | 82.11% | 17.63% | 0.00% |
| Imaging (n = 5) | 66.54% | 31.89% | 0.73% |
| p-value | 0.0046 * | 0.0107 * | 0.6002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piagkou, M.; Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Demetriou, F.; Tsakotos, G.; Olewnik, Ł.; Duparc, F. Mapping the Maxillary Artery and Lateral Pterygoid Muscle Relationship: Insights from Radiological and Meta-Analytic Evidence. Medicina 2025, 61, 1201. https://doi.org/10.3390/medicina61071201
Piagkou M, Triantafyllou G, Papadopoulos-Manolarakis P, Demetriou F, Tsakotos G, Olewnik Ł, Duparc F. Mapping the Maxillary Artery and Lateral Pterygoid Muscle Relationship: Insights from Radiological and Meta-Analytic Evidence. Medicina. 2025; 61(7):1201. https://doi.org/10.3390/medicina61071201
Chicago/Turabian StylePiagkou, Maria, George Triantafyllou, Panagiotis Papadopoulos-Manolarakis, Fotis Demetriou, George Tsakotos, Łukasz Olewnik, and Fabrice Duparc. 2025. "Mapping the Maxillary Artery and Lateral Pterygoid Muscle Relationship: Insights from Radiological and Meta-Analytic Evidence" Medicina 61, no. 7: 1201. https://doi.org/10.3390/medicina61071201
APA StylePiagkou, M., Triantafyllou, G., Papadopoulos-Manolarakis, P., Demetriou, F., Tsakotos, G., Olewnik, Ł., & Duparc, F. (2025). Mapping the Maxillary Artery and Lateral Pterygoid Muscle Relationship: Insights from Radiological and Meta-Analytic Evidence. Medicina, 61(7), 1201. https://doi.org/10.3390/medicina61071201








