Is the Addition of CO2 Laser to β3-Adrenoceptor Agonist Mirabegron Effective in the Management of Overactive Bladder? Results of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization
2.3. Intervention
2.4. Blinding
2.5. Outcomes
2.6. Sample Size Calculation
2.7. Statistical Analysis
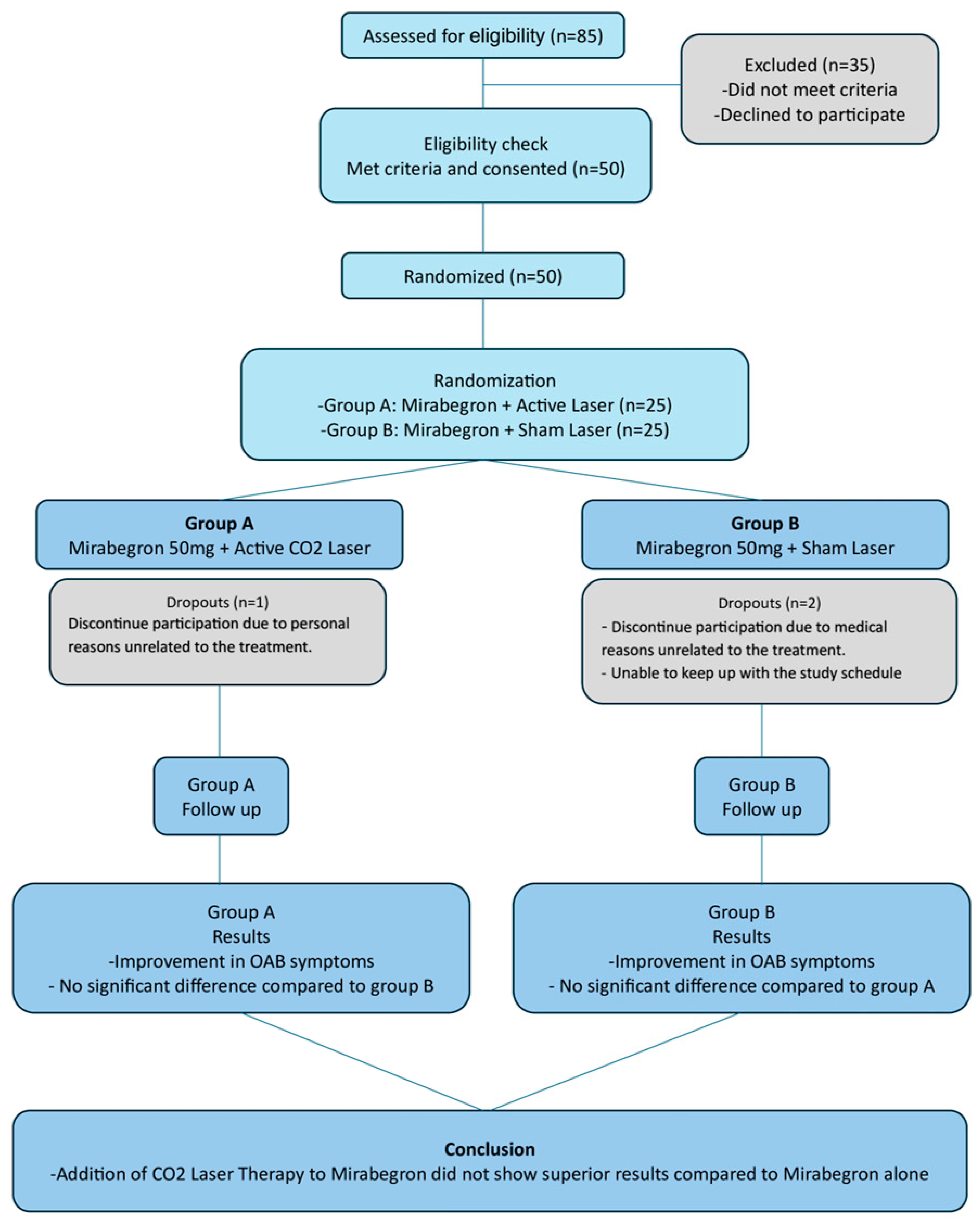
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OAB | Overactive Bladder |
| GSM | Genitourinary Syndrome of Menopause |
| ICIQ-FLUTS | International Consultation on Incontinence Questionnaire for the evaluation of the Female Lower Urinary Tract |
| PPiUS | Patient Perception intensity of Urgency Scale |
| OAB-q | Overactive Bladder Questionnaire |
| KHQ | King’s Health Questionnaire |
| UDI-6 | Urinary Distress Inventory-6 |
| PFIQ-7 | Pelvic Floor Impact Questionnaire-7 |
| PGI-I | Patient Global Impression of Improvement |
| ANOVA | Analysis of Variance |
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Qudah, S.; Abufaraj, M.; Farah, R.; Almazeedi, A.; Ababneh, A.; Alnabulsi, M.; Qatawneh, A.; Hyassat, D.; Ajlouni, K. The prevalence of overactive bladder and its impact on the quality of life: A cross-sectional study. Arab. J. Urol. 2024, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Milsom, I.; Kopp, Z.; Abrams, P.; Artibani, W.; Herschorn, S. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: Impact of overactive bladder. Eur. Urol. 2009, 56, 14–20. [Google Scholar] [CrossRef]
- Rubin, N.; Cerentini, T.M.; Schlöttgen, J.; do Nascimento Petter, G.; Bertotto, A.; La Verde, M.; Gullo, G.; Telles da Rosa, L.H.; Viana da Rosa, P.; Della Méa Plentz, R. Urinary incontinence and quality of life in high-performance swimmers: An observational study. Health Care Women Int. 2024, 45, 1446–1455. [Google Scholar] [CrossRef]
- Laganà, A.S.; La Rosa, V.L.; Palumbo, M.; Rapisarda, A.; Noventa, M.; Vitagliano, A.; Gullo, G.; Vitale, S.G. The impact of stress urinary incontinence on sexual function and quality of life. Gazz. Medica Ital. Arch. Sci. Mediche 2018, 177, 415–416. [Google Scholar] [CrossRef]
- Babin, C.P.; Catalano, N.T.; Yancey, D.M.; Pearl, N.Z.; Koonce, E.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Update on Overactive Bladder Therapeutic Options. Am. J. Ther. 2024, 31, e410–e419. [Google Scholar] [CrossRef]
- Sartori, L.G.F.; Nunes, B.M.; Farah, D.; Oliveira, L.M.; Novoa, C.C.T.; Sartori, M.G.F.; Fonseca, M.C.M. Mirabegron and Anticholinergics in the Treatment of Overactive Bladder Syndrome: A Meta-analysis. Rev. Bras. Ginecol. Obstet. 2023, 45, 337–346. [Google Scholar] [CrossRef]
- Culmone, S.; Speciale, P.; Guastella, E.; Puglisi, T.; Cucinella, G.; Piazza, F.; Morreale, C.; Buzzaccarini, G.; Gullo, G. Sacral neuromodulation for refractory lower urinary tract dysfunctions: A single-center retrospective cohort study. Ital. J. Gynaecol. Obstet. 2022, 34, 317–323. [Google Scholar] [CrossRef]
- Chughtai, B.; Forde, J.C.; Buck, J.; Asfaw, T.; Lee, R.; Te, A.E.; Kaplan, S.A. The concomitant use of fesoterodine and topical vaginal estrogen in the management of overactive bladder and sexual dysfunction in postmenopausal women. Post Reprod. Health 2016, 22, 34–40. [Google Scholar] [CrossRef]
- Araklitis, G.; Baines, G.; da Silva, A.S.; Robinson, D.; Cardozo, L. Recent advances in managing overactive bladder. F1000Research 2020, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.C.; Judice, L.; Riccetto, C.L.Z.; Toledo, L.G.M. Applicability of vaginal energy-based devices in urogynecology: Evidence and controversy. Rev. Assoc. Med. Bras. (1992) 2023, 69, e2023S2129. [Google Scholar] [CrossRef]
- Filippini, M.; Porcari, I.; Ruffolo, A.F.; Casiraghi, A.; Farinelli, M.; Uccella, S.; Franchi, M.; Candiani, M.; Salvatore, S. CO2-Laser therapy and Genitourinary Syndrome of Menopause: A Systematic Review and Meta-Analysis. J. Sex. Med. 2022, 19, 452–470. [Google Scholar] [CrossRef] [PubMed]
- Angelou, K.; Grigoriadis, T.; Diakosavvas, M.; Zacharakis, D.; Athanasiou, S. The Genitourinary Syndrome of Menopause: An Overview of the Recent Data. Cureus 2020, 12, e7586. [Google Scholar] [CrossRef]
- Pitsouni, E.; Grigoriadis, T.; Tsiveleka, A.; Zacharakis, D.; Salvatore, S.; Athanasiou, S. Microablative fractional CO2-laser therapy and the genitourinary syndrome of menopause: An observational study. Maturitas 2016, 94, 131–136. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Nitti, V.W.; Auerbach, S.; Martin, N.; Calhoun, A.; Lee, M.; Herschorn, S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J. Urol. 2013, 189, 1388–1395. [Google Scholar] [CrossRef]
- Khullar, V.; Cambronero, J.; Angulo, J.C.; Wooning, M.; Blauwet, M.B.; Dorrepaal, C.; Martin, N.E. Efficacy of mirabegron in patients with and without prior antimuscarinic therapy for overactive bladder: A post hoc analysis of a randomized European-Australian Phase 3 trial. BMC Urol. 2013, 13, 45. [Google Scholar] [CrossRef]
- Chapple, C.R.; Kaplan, S.A.; Mitcheson, D.; Klecka, J.; Cummings, J.; Drogendijk, T.; Dorrepaal, C.; Martin, N. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur. Urol. 2013, 63, 296–305. [Google Scholar] [CrossRef]
- González Isaza, P.; Jaguszewska, K.; Cardona, J.L.; Lukaszuk, M. Long-term effect of thermoablative fractional CO2 laser treatment as a novel approach to urinary incontinence management in women with genitourinary syndrome of menopause. Int. Urogynecol. J. 2018, 29, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Alsulihem, A.; Corcos, J. The use of vaginal lasers in the treatment of urinary incontinence and overactive bladder, systematic review. Int. Urogynecol. J. 2021, 32, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Cucinella, G.; Gugliotta, G.; Saitta, S.; Polito, S.; Adile, B.; Marci, R.; Calagna, G. Is vaginal fractional CO2 laser treatment effective in improving overactive bladder symptoms in post-menopausal patients? Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2491–2497. [Google Scholar]
- Aguiar, L.B.; Politano, C.A.; Costa-Paiva, L.; Juliato, C.R.T. Efficacy of Fractional CO2 Laser, Promestriene, and Vaginal Lubricant in the Treatment of Urinary Symptoms in Postmenopausal Women: A Randomized Clinical Trial. Lasers Surg. Med. 2020, 52, 713–720. [Google Scholar] [CrossRef]
- Okui, N. Efficacy and safety of non-ablative vaginal erbium:YAG laser treatment as a novel surgical treatment for overactive bladder syndrome: Comparison with anticholinergics and β3-adrenoceptor agonists. World J. Urol. 2019, 37, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Chiengthong, K.; Bunyavejchevin, S. Efficacy of Erbium YAG laser treatment in overactive bladder syndrome: A randomized controlled trial. Menopause 2023, 30, 414–420. [Google Scholar] [CrossRef]
- Athanasiou, S.; Pitsouni, E.; Grigoriadis, T.; Zacharakis, D.; Salvatore, S.; Serati, M. Mirabegron in female patients with overactive bladder syndrome: What’s new? A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 73–82. [Google Scholar] [CrossRef]
| Group | p | |||
|---|---|---|---|---|
| Active (Ν = 25; 50%) | Sham Ν = 25; 50%) | |||
| N (%) | N (%) | |||
| Age, mean (SD) | 61.5 (6.6) | 63.1 (6.3) | 0.372 + | |
| NVD, median (IQR) | 2 (1–2) | 2 (1–2) | 0.417 ++ | |
| BMI, mean (SD) | 28.6 (4.4) | 28.1 (4.8) | 0.711 + | |
| BMI | ||||
| Normal | 5 (20) | 5 (20) | 0.599 ‡ | |
| Overweight | 12 (48) | 15 (60) | ||
| Obese | 8 (32) | 5 (20) | ||
| Age at menopause, mean (SD) | 50.6 (4.2) | 49.8 (3.7) | 0.457 + | |
| Years of OAB symptoms, median (IQR) | 4 (3–7) | 4 (3–5) | 0.550 ++ | |
| History of abdominal surgery | 12 (48) | 12 (48) | 1.000 ‡ | |
| Hypertension | 6 (24) | 3 (12) | 0.463 ‡‡ | |
| Condition after CO2 laser treatment | Very much better | 4 (16) | 3 (12.5) | 0.796 ‡‡ |
| Much better | 12 (48) | 10 (41.7) | ||
| A little better | 9 (36) | 11 (45.8) | ||
| Session 0 | Session 1 | Session 2 | Session 3 | Change | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p2 | p3 | |
| Frequency/Day | A | 12.2 (3) | 10.4 (2.3) | 9.1 (1.4) | 8.5 (1.6) | −3.7 (2) | <0.001 | 0.639 |
| Β | 11.6 (3.2) | 10.3 (2.9) | 8.7 (2.4) | 8.4 (2.1) | −3.2 (2.3) | <0.001 | ||
| p1 | 0.359 | 0.771 | 0.292 | 0.705 | ||||
| Urgency/Day | A | 5.31 (3.62) | 2.23 (1.75) | 1.58 (2.02) | 1.11 (1.74) | −4.2 (3.49) | <0.001 | 0.091 |
| Β | 4.05 (3.24) | 2.35 (1.44) | 1.61 (1.37) | 0.93 (1.23) | −3.12 (2.68) | <0.001 | ||
| p1 | 0.900 | 0.840 | 0.573 | 0.869 | ||||
| Nocturia/day | A | 1.91 (1.03) | 1.54 (0.78) | 1.25 (0.62) | 1.11 (0.77) | −0.8 (0.71) | <0.001 | 0.316 |
| Β | 1.87 (1.02) | 1.35 (0.72) | 1.04 (0.66) | 1.17 (0.67) | −0.7 (0.85) | <0.001 | ||
| p1 | 0.961 | 0.489 | 0.276 | 0.595 | ||||
| Mean Total Volume | A | 2027.6 (681.1) | 1953.4 (607.3) | 1770.5 (631.4) | 1723.1 (687.6) | −304.4 (577.1) | 0.005 | 0.374 |
| Β | 2085.7 (597.4) | 1927.8 (476.5) | 1772.8 (549.8) | 1843.3 (558.6) | −242.5 (503.1) | 0.005 | ||
| p1 | 0.643 | 0.911 | 0.794 | 0.304 | ||||
| Mean Urinary Volume | A | 177.4 (69.2) | 195 (71.8) | 197.2 (74.1) | 205.2 (78.8) | 27.8 (57.3) | 0.100 | 0.455 |
| Β | 191.7 (61.8) | 194.6 (59.3) | 212.2 (64.3) | 230.9 (90.9) | 39.2 (84.3) | 0.022 | ||
| p1 | 0.389 | 0.873 | 0.365 | 0.255 | ||||
| Incontinence Episodes/Day | A | 2.19 (4.12) | 0.68 (1.54) | 0.43 (1.41) | 0.4 (1.43) | −1.79 (3.78) | 0.002 | 0.431 |
| Β | 1.44 (2.33) | 0.76 (1.56) | 0.66 (1.69) | 0.62 (1.69) | −0.82 (1.34) | 0.019 | ||
| p1 | 0.737 | 0.770 | 0.460 | 0.588 |
| Session 0 | Session 1 | Session 2 | Session 3 | Change | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p2 | p3 | |
| UDI6 score | A | 49.2 (18.1) | 36 (19.2) | 29.5 (18.9) | 23.7 (15.3) | −25.5 (21.1) | <0.001 | 0.896 |
| Β | 52.2 (23.1) | 39.2 (17.1) | 28.5 (14.9) | 25.7 (20.6) | −26.5 (27.6) | 0.001 | ||
| p1 | 0.839 | 0.384 | 0.520 | 0.900 | ||||
| UIQ-7 score | A | 53.1 (21.9) | 38.7 (24.2) | 28.8 (22.1) | 21.5 (19.3) | −31.6 (24) | <0.001 | 0.611 |
| Β | 56.6 (24) | 32.6 (22.6) | 22.3 (20.6) | 20 (20.3) | −36.6 (25) | <0.001 | ||
| p1 | 0.760 | 0.464 | 0.371 | 0.543 |
| Session 0 | Session 1 | Session 2 | Session 3 | Change | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p2 | p3 | |
| General health perception | A | 31 (24.2) | 27 (19) | 25 (16.1) | 25 (17.7) | −6 (23.1) | 0.969 | 0.103 |
| Β | 27 (20.3) | 23 (22.7) | 20 (19.1) | 14 (17.8) | −13 (21.8) | 0.001 | ||
| p1 | 0.674 | 0.275 | 0.170 | 0.010 | ||||
| Incontinence impact | A | 77.3 (20.9) | 56 (23) | 46.7 (31.9) | 37.3 (24.2) | −40 (30.4) | 0.001 | 0.674 |
| Β | 73.3 (23.6) | 50.7 (17) | 38.7 (26.7) | 32 (24.5) | −41.4 (35.1) | <0.001 | ||
| p1 | 0.466 | 0.486 | 0.740 | 0.336 | ||||
| Role limitations score | A | 58 (25.5) | 37.3 (29.4) | 30 (24.1) | 28 (27.1) | −30 (36.3) | 0.001 | 0.091 |
| Β | 55.3 (21.9) | 31.3 (18.2) | 22.7 (19.8) | 18.1 (26.0) | −37.2 (30.5) | <0.001 | ||
| p1 | 0.624 | 0.470 | 0.564 | 0.166 | ||||
| Physical limitations score | A | 56.7 (26.8) | 46.7 (34) | 37.3 (30.5) | 32 (29.2) | −24.7 (33.7) | 0.002 | 0.919 |
| Β | 56 (34) | 34.7 (22) | 25.3 (19.3) | 23.3 (26.4) | −32.7 (36.2) | 0.001 | ||
| p1 | 0.433 | 0.682 | 0.645 | 0.382 | ||||
| Social limitations score | A | 51.8 (27.6) | 36.2 (24.8) | 27.6 (23.5) | 20.4 (14.1) | −31.3 (27.1) | <0.001 | 0.951 |
| Β | 40.2 (26) | 27.3 (20.7) | 19.1 (15.1) | 16.9 (15) | −23.3 (26.7) | <0.001 | ||
| p1 | 0.158 | 0.190 | 0.410 | 0.259 | ||||
| Personal Relationships score | A | 39.3 (41.7) | 22.6 (27.4) | 8.8 (14.6) | 8.9 (13.9) | −30.4 (35.2) | 0.366 | 0.506 |
| Β | 23.5 (33.9) | 17.9 (31) | 3.3 (9.3) | 6 (18) | −17.6 (27.3) | 0.028 | ||
| p1 | 0.760 | 0.349 | 0.150 | 0.185 | ||||
| Emotions score | A | 48 (31.9) | 32.4 (25) | 25.8 (21.2) | 21.3 (21) | −26.7 (32.7) | 0.002 | 0.589 |
| Β | 46.2 (34.6) | 32.9 (27.7) | 16.4 (17.9) | 16.9 (20.8) | −29.3 (32.2) | <0.001 | ||
| p1 | 0.537 | 0.947 | 0.230 | 0.384 | ||||
| Sleep/Energy score | A | 43.3 (19.2) | 32 (18.6) | 28.7 (23.8) | 20.7 (21.7) | −22.7 (21.5) | <0.001 | 0.547 |
| Β | 40.7 (28.5) | 34.7 (28.8) | 22.7 (26.7) | 20.7 (25.1) | −20 (22.6) | 0.003 | ||
| p1 | 0.108 | 0.748 | 0.306 | 0.816 | ||||
| Severity measures score | A | 44 (26.2) | 34.7 (21.9) | 30.7 (22.1) | 27 (25.7) | −17 (19.5) | 0.001 | 0.954 |
| Β | 42 (22.6) | 31.7 (20.1) | 26 (17.6) | 21 (19.3) | −21 (18.5) | <0.001 | ||
| p1 | 1.000 | 0.979 | 0.904 | 0.812 | ||||
| Symptom severity scale (0-10) | A | 7.4 (1.89) | 5.88 (2.4) | 5.08 (2.33) | 3.84 (2.85) | −3.56 (2.38) | <0.001 | 0.789 |
| Β | 6.76 (2.05) | 5.2 (2.14) | 4.6 (2.02) | 3.56 (2.31) | −3.2 (2.4) | <0.001 | ||
| p1 | 0.235 | 0.450 | 0.493 | 0.997 |
| Session 0 | Session 1 | Session 2 | Session 3 | Change | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p2 | p3 | |
| Symptom severity | A | 43 (18.9) | 27.9 (12.9) | 19.8 (12) | 17.1 (12.2) | −25.9 (19.6) | <0.001 | 0.552 |
| Β | 43.3 (18.3) | 23.3 (14.1) | 18.5 (10.1) | 16.3 (11.3) | −27 (20.5) | <0.001 | ||
| p1 | 0.925 | 0.285 | 0.828 | 0.718 | ||||
| Coping | A | 57.5 (19) | 73.5 (21.1) | 79.8 (20.3) | 82.8 (18.6) | 25.3 (21.5) | 0.001 | 0.928 |
| Β | 61.5 (22.6) | 76.7 (16.3) | 83 (15.7) | 86 (16.5) | 24.5 (22.5) | 0.001 | ||
| p1 | 0.747 | 0.434 | 0.447 | 0.527 | ||||
| Concern | A | 68.5 (17.7) | 80.8 (17.6) | 84.6 (14.4) | 89.6 (12.6) | 21.1 (18.6) | <0.001 | 0.776 |
| Β | 71.4 (19.6) | 83.1 (19) | 88.7 (11.2) | 90.9 (12.5) | 19.4 (18.4) | 0.001 | ||
| p1 | 0.633 | 0.421 | 0.307 | 0.884 | ||||
| Sleep | A | 67.5 (16.7) | 79.5 (16.3) | 84.5 (17) | 87.4 (15) | 19.8 (13.6) | 0.003 | 0.654 |
| Β | 69 (24.2) | 84 (17.6) | 87.5 (15.2) | 87.7 (15) | 18.7 (22.6) | 0.001 | ||
| p1 | 0.802 | 0.511 | 0.531 | 0.972 | ||||
| Social interaction | A | 76.8 (19.6) | 92 (14.6) | 95.2 (8.6) | 94.1 (11.4) | 17.3 (22.4) | 0.004 | 0.917 |
| Β | 81.6 (23.5) | 93.4 (14.6) | 97.6 (4.8) | 97.4 (5.8) | 15.8 (22) | 0.010 | ||
| p1 | 0.652 | 0.788 | 0.235 | 0.186 | ||||
| Total OAB-q score | A | 66.4 (15) | 80.6 (16) | 85.2 (14.4) | 87.9 (13.8) | 21.4 (16) | <0.001 | 0.864 |
| Β | 71.1 (19.3) | 83.3 (15.1) | 88.4 (11.5) | 90 (11.6) | 18.9 (18) | <0.001 | ||
| p1 | 0.507 | 0.546 | 0.450 | 0.692 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kypriotis, K.; Prodromidou, A.; Athanasiou, S.; Zacharakis, D.; Kathopoulis, N.; Douligeris, A.; Athanasiou, V.; Michala, L.; Grigoriadis, T. Is the Addition of CO2 Laser to β3-Adrenoceptor Agonist Mirabegron Effective in the Management of Overactive Bladder? Results of a Randomized Controlled Trial. Medicina 2025, 61, 1198. https://doi.org/10.3390/medicina61071198
Kypriotis K, Prodromidou A, Athanasiou S, Zacharakis D, Kathopoulis N, Douligeris A, Athanasiou V, Michala L, Grigoriadis T. Is the Addition of CO2 Laser to β3-Adrenoceptor Agonist Mirabegron Effective in the Management of Overactive Bladder? Results of a Randomized Controlled Trial. Medicina. 2025; 61(7):1198. https://doi.org/10.3390/medicina61071198
Chicago/Turabian StyleKypriotis, Konstantinos, Anastasia Prodromidou, Stavros Athanasiou, Dimitrios Zacharakis, Nikolaos Kathopoulis, Athanasios Douligeris, Veatriki Athanasiou, Lina Michala, and Themos Grigoriadis. 2025. "Is the Addition of CO2 Laser to β3-Adrenoceptor Agonist Mirabegron Effective in the Management of Overactive Bladder? Results of a Randomized Controlled Trial" Medicina 61, no. 7: 1198. https://doi.org/10.3390/medicina61071198
APA StyleKypriotis, K., Prodromidou, A., Athanasiou, S., Zacharakis, D., Kathopoulis, N., Douligeris, A., Athanasiou, V., Michala, L., & Grigoriadis, T. (2025). Is the Addition of CO2 Laser to β3-Adrenoceptor Agonist Mirabegron Effective in the Management of Overactive Bladder? Results of a Randomized Controlled Trial. Medicina, 61(7), 1198. https://doi.org/10.3390/medicina61071198










