Clinical and Epidemiological Features and Antimicrobial Susceptibility Patterns of Chryseobacterium Species: A Scoping Review
Abstract
1. Introduction
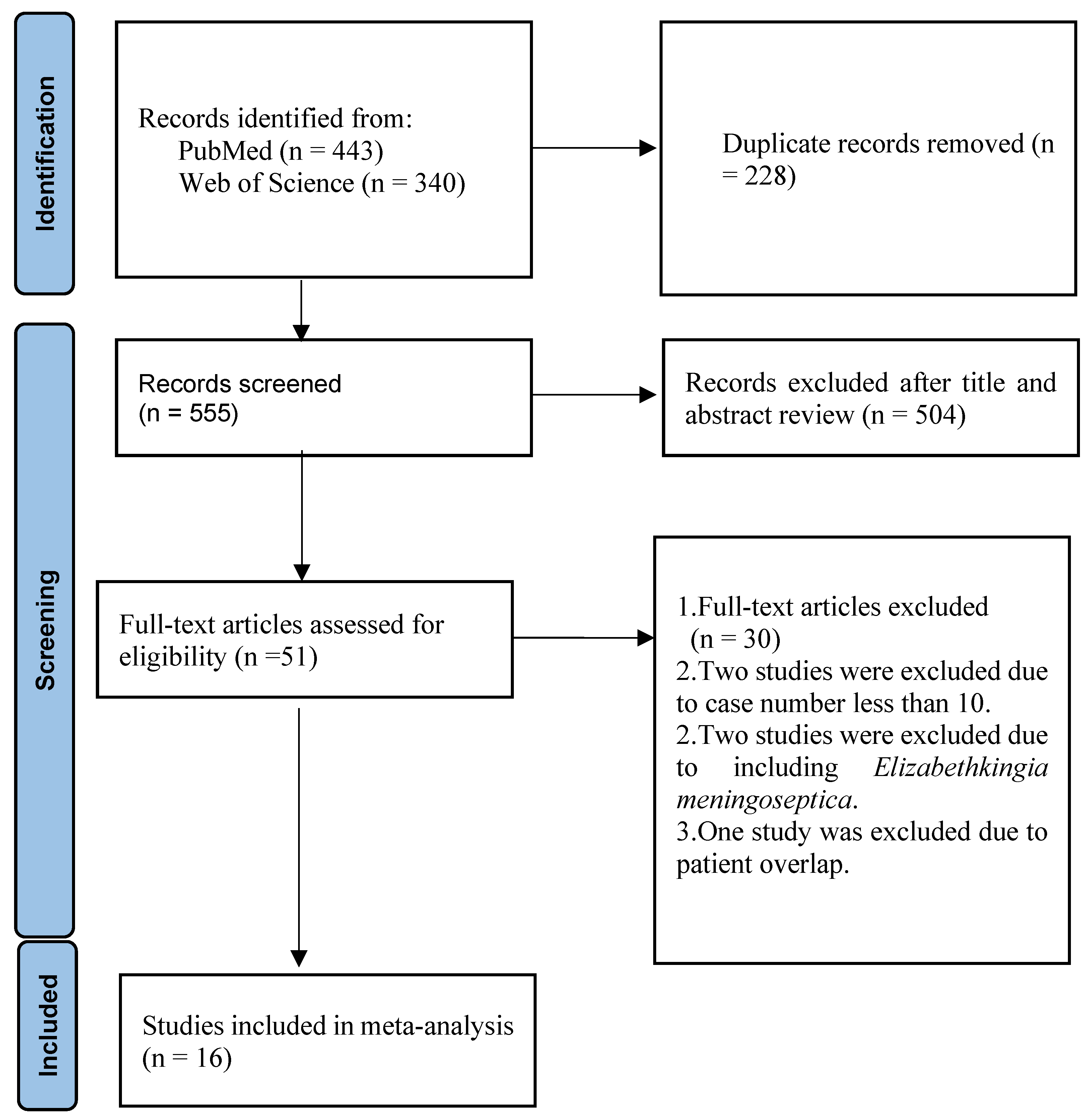
2. Materials and Methods
3. Results
4. Discussion
4.1. Clinical and Epidemiological Features of Patients with Chryseobacterium Species Infections
4.1.1. Incidence of Infection
4.1.2. Sites of Isolation
4.1.3. Clinical Characteristics of Patients with Chryseobacterium Species Infections
4.1.4. Mortality of Patients with Chryseobacterium Species Infections
4.2. Antimicrobial Susceptibility Patterns
4.2.1. Antimicrobial Susceptibility Patterns of Chryseobacterium Species
4.2.2. Antimicrobial Susceptibility Patterns of Chryseobacterium indologenes
4.2.3. Antimicrobial Susceptibility Patterns of Chryseobacterium gleum
4.2.4. Analysis of the Relationship Between Antimicrobial Susceptibility Rate and Geographical Location, Antimicrobial Susceptibility Test Method, and Study Period
5. Limitations
6. Conclusions
7. Future Directions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandamme, P.; Bernardet, J.F.; Segers, P.; Kersters, K.; Holmes, B. New perspectives in the classification of the Flavobacteria: De-scription of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int. J. Syst. Evol. Microbiol. 1994, 44, 827–831. [Google Scholar] [CrossRef]
- Euzéby, J.P. List of Prokaryotic Names with Standing in Nomenclature: Chryseobacterium. 2025. Available online: https://lpsn.dsmz.de/search?word=Chryseobacterium (accessed on 19 June 2025).
- Yabuuchi, E.; Hashimoto, Y.; Ezaki, T.; Ido, Y.; Takeuchi, N. Genotypic and phenotypic differentiation of Flavobacterium indologenes Yabuuchi et al. 1983 from Flavobacterium gleum Holmes et al. 1984. Microbiol. Immunol. 1990, 34, 73–76. [Google Scholar] [CrossRef]
- Kirby, J.T.; Sader, H.S.; Walsh, T.R.; Jones, R.N. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp.: Report from the SENTRY Antimicrobial Surveillance Program (1997–2001). J. Clin. Microbiol. 2004, 42, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.; van Tiel, F.H.; van der Geest, S.; Smeets, H.G.; Stobberingh, E.E.; Gaillard, C.A. Topical antimicrobial prophylaxis of nosocomial pneumonia in mechanically ventilated patients. Microbiological observations. Infection 1993, 21, 137–139. [Google Scholar] [CrossRef]
- Lin, J.N.; Lai, C.H.; Yang, C.H.; Huang, Y.H. Differences in clinical manifestations, antimicrobial susceptibility patterns, and mutations of fluoroquinolone target genes between Chryseobacterium gleum and Chryseobacterium indologenes. Antimicrob. Agents Chemother. 2019, 63, e02256-18. [Google Scholar] [CrossRef]
- Won, D.; Byun, J.H.; Kim, M.; Yong, D. A case of Chryseobacterium hominis isolated from human blood drawn through peripherally inserted central catheter. Lab Med. 2019, 9, 246–248. [Google Scholar] [CrossRef]
- Green, B.T.; Nolan, P.E. Cellulitis and bacteraemia due to Chryseobacterium indologenes. J. Infect. 2001, 42, 219–220. [Google Scholar] [CrossRef]
- Nulens, E.; Bussels, B.; Bols, A.; Gordts, B.; Van Landuyt, H.W. Recurrent bacteremia by Chryseobacterium indologenes in an oncology patient with a totally implanted intravascular device. Clin. Microbiol. Infect. 2001, 7, 391–393. [Google Scholar] [CrossRef]
- Bomb, K.; Arora, A.; Trehan, N. Endocarditis due to Chryseobacterium meningosepticum. Indian J. Med. Microbiol. 2007, 25, 161–162. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Zahraldin, K. Chryseobacterium indologenes meningitis in a healthy newborn: A case report. Oman Med. J. 2013, 28, 133–134. [Google Scholar] [CrossRef]
- Atıcı, S.; Ünkar, Z.A.; Erdem, K.; Kadayifci, E.K.; Karaaslan, A.; Memişoğlu, A.Ç.; Soysal, A.; Toprak, N.Ü.; Söyletir, G.; Özek, E.; et al. Ventilator-associated pneumonia caused by Chryseobacterium indologenes: A rare infant case and review of the literature. Springerplus 2016, 5, 1741. [Google Scholar] [CrossRef] [PubMed]
- Bhagawati, G.; Bhardwaj, A.; Sajikumar, R.; Singh, S.P.; Prajapati, S. Bacteremia by Chryseobacterium indologenes in a patient with lung cancer: A clinical and microbiological investigation. Indian J. Crit. Care Med. 2019, 23, 157–159. [Google Scholar] [CrossRef]
- Bloom, A.H.; Perry, H.D.; Donnenfeld, E.D.; Davis, R.G. Chryseobacterium meningosepticum keratitis. Am. J. Ophthalmol. 2003, 136, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Garcia, G.; Sylvester, T.; Sunenshine, R. Chryseobacterium indologenes in a woman with metastatic breast cancer in the United States of America: A case report. J. Med. Case Rep. 2013, 7, 190. [Google Scholar] [CrossRef]
- Cone, L.A.; Morrow, A.A.; Benson, M.; Benson, M.; Younes, B.; Gade-Andavolu, R. Chryseobacterium indologenes sepsis due to an infected central catheter in a patient with metastatic breast cancer to the skin. Infect. Dis. Clin. Pract. 2007, 15, 403–405. [Google Scholar] [CrossRef]
- Al-Tatari, H.; Asmar, B.I.; Ang, J.Y. Lumboperitoneal shunt infection due to Chryseobacterium indologenes. Pediatr. Infect. Dis. J. 2007, 26, 657–659. [Google Scholar] [CrossRef]
- Monteen, M.R.; Ponnapula, S.; Wood, G.C.; Croce, M.A.; Swanson, J.M.; Boucher, B.A.; Fabian, T.C. Treatment of Chryseobacterium indologenes ventilator-associated pneumonia in a critically ill trauma patient. Ann. Pharmacother. 2013, 47, 1736–1739. [Google Scholar] [CrossRef]
- Afshar, M.; Nobakht, E.; Lew, S.Q. Chryseobacterium indologenes peritonitis in peritoneal dialysis. BMJ Case Rep. 2013, 2013, bcr2013009410. [Google Scholar] [CrossRef]
- Bhuyar, G.; Jain, S.; Shah, H.; Mehta, V.K. Urinary tract infection by Chryseobacterium indologenes. Indian J. Med. Microbiol. 2012, 30, 370–372. [Google Scholar] [CrossRef]
- Lin, J.N.; Lai, C.H.; Yang, C.H.; Huang, Y.H.; Lin, H.F.; Lin, H.H. Comparison of four automated microbiology systems with 16S rRNA gene sequencing for identification of Chryseobacterium and Elizabethkingia species. Sci. Rep. 2017, 7, 13824. [Google Scholar] [CrossRef]
- Lin, J.N.; Teng, S.H.; Lai, C.H.; Yang, C.H.; Huang, Y.H.; Lin, H.F.; Lin, H.H. Comparison of the VITEK MS and Bruker matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of Chryseobacterium isolated from clinical specimens and report of uncommon Chryseobacterium infections in humans. J. Clin. Microbiol. 2018, e00712-18. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Steigerwalt, A.G.; Nicholson, A.C. DNA-DNA hybridization study of strains of Chryseobacterium, Elizabethkingia and Empedobacter and of other usually indole-producing non-fermenters of CDC groups IIc, IIe, IIh and IIi, mostly from human clinical sources, and proposals of Chryseobacterium bernardetii sp. nov., Chryseobacterium carnis sp. nov., Chryseobacterium lactis sp. nov., Chryseobacterium nakagawai sp. nov. and Chryseobacterium taklimakanense comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 4639–4662. [Google Scholar]
- Chang, J.C.; Hsueh, P.R.; Wu, J.J.; Ho, S.W.; Hsieh, W.C.; Luh, K.T. Antimicrobial susceptibility of flavobacteria as determined by agar dilution and disk diffusion methods. Antimicrob. Agents Chemother. 1997, 41, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.L.; Jorgensen, J.H. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob. Agents Chemother. 1997, 41, 2738–2741. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Alon, D.; Karniel, E.; Zohar, I.; Gideon, Y.; Stein, G.Y. Chryseobacterium indologenes bacteremia: Clinical and microbiological characteristics of an emerging infection. Int. J. Clin. Med. 2018, 9, 520–527. [Google Scholar] [CrossRef]
- Aykac, K.; Ozsurekci, Y.; Tuncer, O.; Sancak, B.; Cengiz, A.B.; Kara, A.; Ceyhan, M. Six cases during 2012–2015 and literature review of Chryseobacterium indologenes infections in pediatric patients. Can. J. Microbiol. 2016, 62, 812–819. [Google Scholar] [CrossRef]
- Cooper, S.; Levy, I.; Ben-Zvi, H.; Ashkenazi-Hoffnung, L.; Ben-Shimol, S.; Shachor-Meyouhas, Y.; Grisaru-Soen, G.; Kriger, O.; Yahav, D.; Scheuerman, O. Flavobacteriaceae bacteremia in children: A multicenter study. Pediatr. Infect. Dis. J. 2019, 38, 1096–1099. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Hsiue, T.R.; Wu, J.J.; Teng, L.J.; Ho, S.W.; Hsieh, W.C.; Luh, K.T. Flavobacterium indologenes bacteremia: Clinical and microbiological characteristics. Clin. Infect. Dis. 1996, 23, 550–555. [Google Scholar] [CrossRef]
- Lambiase, A.; Del Pezzo, M.; Raia, V.; Sepe, A.; Ferri, P.; Rossano, F. Chryseobacterium respiratory tract infections in patients with cystic fibrosis. J. Infect. 2007, 55, 518–523. [Google Scholar] [CrossRef]
- Mirza, H.C.; Tuncer, Ö.; Ölmez, S.; Şener, B.; Tuğcu, G.D.; Özçelik, U.; Gürsoy, N.C.; Otlu, B.; Büyükçam, A.; Kara, A.; et al. Clinical strains of Chryseobacterium and Elizabethkingia spp. isolated from pediatric patients in a university hospital: Performance of MALDI-TOF MS-based identification, antimicrobial susceptibilities, and baseline patient characteristics. Microb. Drug Resist. 2018, 24, 816–821. [Google Scholar] [CrossRef]
- Koh, M.C.Y.; Ngiam, J.N.; Chew, K.L.; Smitasin, N.; Lum, L.H.; Allen, D.M. Clinical presentation and outcomes of bloodstream infection with intrinsically carbapenem-resistant non-fermenting gram-negative organisms: Stenotrophomonas maltophilia, Elizabethkingia spp. and Chryseobacterium spp. in Singapore, from 2012 to 2024. BMC Infect. Dis. 2025, 25, 273. [Google Scholar] [CrossRef]
- Kaur, H.; Mohan, B.; Hallur, V.; Raj, A.; Basude, M.; Mavuduru, R.S.; Taneja, N. Increased recognition of Chryseobacterium species as an emerging cause of nosocomial urinary tract infection following introduction of matrix-assisted laser desorption/ionisation-time of flight for bacterial identification. Indian J. Med. Microbiol. 2017, 35, 610–616. [Google Scholar] [CrossRef]
- Yadav, V.S.; Das, B.K.; Mohapatra, S.; Ahmed, M.N.; Gautam, H.; Kapil, A.; Sood, S.; Dhawan, B.; Chaudhry, R. Clinical correlation and antimicrobial susceptibility pattern of Chryseobacterium spp.: A three year prospective study. Intractable Rare Dis. Res. 2021, 10, 37–41. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Teng, L.J.; Yang, P.C.; Ho, S.W.; Hsieh, W.C.; Luh, K.T. Increasing incidence of nosocomial Chryseobacterium indologenes infections in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 568–574. [Google Scholar] [CrossRef]
- Lin, Y.T.; Jeng, Y.Y.; Lin, M.L.; Yu, K.W.; Wang, F.D.; Liu, C.Y. Clinical and microbiological characteristics of Chryseobacterium indologenes bacteremia. J. Microbiol. Immunol. Infect. 2010, 43, 498–505. [Google Scholar] [CrossRef]
- Chou, D.W.; Wu, S.L.; Lee, C.T.; Tai, F.T.; Yu, W.L. Clinical characteristics, antimicrobial susceptibilities, and outcomes of patients with Chryseobacterium indologenes bacteremia in an intensive care unit. Jpn. J. Infect. Dis. 2011, 64, 520–524. [Google Scholar] [CrossRef]
- Chen, F.L.; Wang, G.C.; Teng, S.O.; Ou, T.Y.; Yu, F.L.; Lee, W.S. Clinical and epidemiological features of Chryseobacterium indologenes infections: Analysis of 215 cases. J. Microbiol. Immunol. Infect. 2013, 46, 425–432. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lo, H.H.; Hsieh, H.Y.; Chang, S.M. Identification, epidemiological relatedness, and biofilm formation of clinical Chryseobacterium indologenes isolates from central Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 559–564. [Google Scholar] [CrossRef]
- Deng, L.; Li, M.F.; Li, Y.H.; Yang, J.L.; Zhou, X. Chryseobacterium indologenes in four patients with leukemia. Transpl. Infect. Dis. 2015, 17, 583–587. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Yang, Y.; Su, J.; Xu, X.; Wang, M.; Chen, Y.; Li, Y. Clinical and molecular characteristics of Chryseobacterium indologenes isolates at a teaching hospital in Shanghai, China. Ann. Transl. Med. 2021, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Sahu, C.; Afzal Hussain, N.A.F.; Ghar, M.; Prasad, K.N. The era of device colonizers: Chryseobacterium indologenes infections from a tertiary care center in North India. Indian J. Crit. Care Med. 2018, 22, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.H.; Chang, S.M. Identification, characterization, and biofilm formation of clinical Chryseobacterium gleum isolates. Diagn. Microbiol. Infect. Dis. 2014, 79, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Sarwar, U.; King, E.A.; Lat, A. Chryseobacterium indologenes subcutaneous port-related bacteremia in a liver transplant patient. Transpl. Infect. Dis. 2012, 14, 398–402. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yeh, K.M.; Chiu, S.K.; Shang, S.T.; Kan, L.P.; Yu, C.M.; Lin, J.C. Chryseobacterium indologenes peritonitis in a patient with malignant ascites. Int. Med. Case Rep. J. 2011, 4, 13–15. [Google Scholar] [CrossRef]
- Ozcan, N.; Dal, T.; Tekin, A.; Kelekci, S.; Can, S.; Ezin, O.; Kandemir, I.; Gul, K. Is Chryseobacterium indologenes a shunt-lover bacterium? A case report and review of the literature. Infez. Med. 2013, 21, 312–316. [Google Scholar]
- Lin, J.T.; Wang, W.S.; Yen, C.C.; Liu, J.H.; Chiou, T.J.; Yang, M.H.; Chao, T.C.; Chen, P.M. Chryseobacterium indologenes bacteremia in a bone marrow transplant recipient with chronic graft-versus-host disease. Scand. J. Infect. Dis. 2003, 35, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Mutcali, S.I.; Yemisen, M.; Soylu, H.; Balkan, I.I.; Mete, B.; Saltoglu, N. Recurrent port infection due to Chryseobacterium indologenes. Eurasian J. Med. 2013, 45, 60–61. [Google Scholar] [CrossRef]
| Author /Period | Confounding | Selection | Interventions Classification | Interventions Deviations | Missing Data | Measurement of Outcomes | Selective Results |
|---|---|---|---|---|---|---|---|
| Kirby JT/1997–2001 [4] | low risk | high risk | moderate risk | moderate risk | high risk | low risk | moderate risk |
| Lin LN/2005–2017 [6] | low risk | low risk | moderate risk | moderate risk | low risk | low risk | low risk |
| Lambiase A/2002–2006 [31] | moderate risk | moderate risk | high risk | high risk | high risk | serious risk | high risk |
| Mirza HC/2012–2016 [32] | high risk | serious risk | high risk | high risk | serious risk | serious risk | high risk |
| Koh MCY/2012–2024 [33] | high risk | serious risk | high risk | high risk | serious risk | serious risk | high risk |
| Kaur H/2013–2013 [34] | serious risk | serious risk | serious risk | high risk | serious risk | serious risk | serious risk |
| Yadav VS/2017–2019 [35] | serious risk | serious risk | serious risk | serious risk | serious risk | serious risk | serious risk |
| Hsueh PR/1993–1995 [36] | moderate risk | moderate risk | high risk | high risk | high risk | serious risk | high risk |
| Lin YT/2002–2008 [37] | serious risk | high risk | serious risk | serious risk | serious risk | serious risk | serious risk |
| Chou DW/2004–2008 [38] | serious risk | Serious risk | high risk | serious | serious risk | serious risk | serious risk |
| Chen FL/2004–2011 [39] | low risk | low risk | high risk | moderate risk | low risk | moderate risk | moderate risk |
| Chang YC/2007–2011 [40] | high risk | moderate risk | moderate risk | moderate risk | moderate risk | moderate risk | moderate risk |
| Deng L/2010–2013 [41] | high risk | high risk | high risk | high risk | high risk | high risk | high risk |
| Zhang Y/2010–2018 [42] | low risk | low risk | low risk | moderate risk | low risk | low risk | low risk |
| Jain V/2016–2016 [43] | high risk | high risk | high risk | moderate risk | high risk | high risk | high risk |
| Lo HH/2007–2011 [44] | high risk | moderate risk | high risk | high risk | high risk | high risk | high risk |
| (A) | ||||||||||
| Author/Period | Country/No. | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Lambiase A/2002–2006 [31] %* | Italy/30 | 45.5% | 45.5% | 45.5% | 100% | 0% | 4.6% | NA | 0% | 0% |
| Lin LN/2005–2017 [6] * | Taiwan/126 | 19.8% | 18.3% | 32.5% | 47.6% | 0% | 0% | 73.0% | 13.5% | 17.5% |
| Mirza HC/2012–2016 [32] & | Turkey/18 | 100% | 94.4% | 94.4% | 100% | 16.6% | 16.6% | NA | 100% | 100% |
| Koh MCY/2012–2024 [33] & | Singapore/25 | 80% | NA | 77.3% | 95.7% | NA | NA | 100% | 25% | NA |
| Kaur H/2013–2013 [34] % | India/19 | 5.27% | 0% | 0% | 73.6% $ | 0% | 5.27% | 100% | 0% | 0% |
| Yadav VS/ 2017–2019 [35] % | India/20 | 45% | NA | NA | 33% $ | 33% | 33% | NA | NA | NA |
| (B) | ||||||||||
| Author/Period | Country | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Lambiase A/2002–2006 [31] %* | Italy | 10/22 | 10/22 | 10/22 | 22/22 | 0/22 | 1/22 | NA | 0/22 | 0/22 |
| Lin LN/2005–2017 [5] * | Taiwan | 25/126 | 23/126 | 41/126 | 60/126 | 0/126 | 0/126 | 92/126 | 17/126 | 22/126 |
| Koh MCY/2012–2024 [33] & | Singapore | 12/15 | NA | 17/22 | 22/23 | NA | NA | 17/17 | 1/4 | NA |
| Kaur H/2013–2013 [34] % | India | 1/19 | 0/19 | 0/19 | 14 $/19 | 0/19 | 1/19 | 19/19 | 0/19 | 0/19 |
| Yadav VS /2017–2019 [35] % | India | 9/20 | NA | NA | 7 $/20 | 7/20 | 7/20 | NA | NA | NA |
| pooled rate | 57/202 | 33/167 | 68/189 | 125/210 | 7/187 | 9/187 | 128/162 | 18/171 | 22/167 | |
| percentage | 28.2% | 19.8% | 36.0% | 59.5% | 3.7% | 4.8% | 79.0% | 10.5% | 13.2% | |
| (A) | ||||||||||
| Author/Period | Country/No. | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Kirby JT/1997–2001 [4] * | World/20 | 90% | 85% | 100% | 95% | 15% | 10% | NA | 85% | 85% |
| Hsueh PR/1993–1995 [36] # | Taiwan/36 | NA | 18% | NA | NA | 0% | NA | 74% | 42% | NA |
| Lin YT/2002–2008 [37] % | Taiwan/16 | 50% | 43.7% | 62.5% | 75% | 0% | 0% | NA | 0% | 12.5% |
| Chou DW/2004–2008 [38] * | Taiwan/10 | 20% | 30% | 30% | 100% | 10% | 0% | 100% | 0% | 0% |
| Chen FL/2004–2011 [39] *% | Taiwan/177 | 29.3% | 31.6% | 34.4% | 87.5% | 3.9% | 8.4% | NA | 3.3% | 3.3% |
| Lin JN/2005–2017 [6] * | Taiwan/84 | 13.1% | 16.7% | 19.0% | 42.9% | 0% | 0% | 67.9% | 8.3% | 16.7% |
| Chang YC/2007–2011 [40] * | China/34 | 2.9% | 14.7% | NA | 52.9% | 0% | NA | 100% | 2.9% | 2.9% |
| Deng L/2010–2013 [41] *% | China/23 | 26.0% | 4.34% | 8.69% | 73.9% | 8.69% | NA | 30.4% | NA | 13.0% |
| Zhang Y/2010–2018 [42] * | China/135 | 37.0% | 12.6% | 14.8% | 97.8% | 0.7% | 0% | 98.5% | 6.7% | NA |
| Mirza HC/2012–2016 [32] & | Turkey/16 | 100% | 100% | 100% | 100% | 18.8% | 18.8% | NA | 100% | 100% |
| Jain V/2016–2016 [43] * | India/12 | 16.7% | 41.6% | 75.0% | 91.6% | NA% | 0% | NA | NA | 0% |
| Yadav VS /2017–2019 [35] % | India/18 | 44.4% | 16.6% | NA | 33.3% $ | 33.3% | 38.8% | NA | 16.6% | NA |
| (B) | ||||||||||
| Author/ Period | Country | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Hsueh PR/1993–1995 [36] # | Taiwan | NA | 6/36 | NA | NA | 0/36 | NA | 27/36 | 15/36 | NA |
| Lin YT/2002–2008 [37] % | Taiwan | 8/16 | 7/16 | 10/16 | 12/16 | 0/16 | 0/16 | NA | 0/16 | 2/16 |
| Chou DW/2004–2008 [38] * | Taiwan | 2/10 | 3/10 | 3/10 | 10/10 | 1/10 | 0/10 | 10/10 | 0/10 | 0/10 |
| Chen FL/2004–2011 [39] *% | Taiwan | 52/177 | 56/177 | 61/177 | 155/177 | 7/177 | 15/177 | NA | 6/177 | 6/177 |
| Lin JN/2005–2017 [5] * | Taiwan | 11/84 | 14/84 | 16/84 | 36/84 | 0/84 | 0/84 | 57/84 | 7/84 | 14/84 |
| Chang YC/2007–2011 [40] * | China | 10/34 | 5/34 | NA | 18/34 | 0/34 | NA | 34/34 | 1/34 | 1/34 |
| Deng L/2010–2013 [41] *% | China | 6/23 | 1/23 | 2/23 | 17/23 | 2/23 | NA | 7/23 | NA | 3/23 |
| Zhang Y/2010–2018 [42] * | China | 50/135 | 17/135 | 20/135 | 132/135 | 1/135 | 0/135 | 133/135 | 9/135 | NA |
| Jain V/2016–2016 [43] * | India | 2/12 | 5/12 | 9/12 | 11/12 | NA | 0/12 | NA | NA | 0/12 |
| Yadav VS /2017–2019 [35] % | India | 8/18 | 3/18 | NA | 6 $/18 | 6/18 | 7/18 | NA | 3/18 | NA |
| pooled rate | 149/509 | 117/545 | 121/457 | 397/509 | 17/533 | 22/452 | 268/322 | 41/510 | 26/356 | |
| percentage | 29.3% | 21.5% | 26.5% | 78.0% | 3.2% | 4.9% | 83.2% | 8.09% | 7.3% | |
| (A) | ||||||||||
| Author/Period | Country/No. | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Lin JN/2005–2017 [6] * | Taiwan/42 | 33.3% | 21.4% | 59.5% | 57.1% | 2.4% | 0% | 83.3% | 23.8% | 19.0% |
| Lo HH/2007–2011 [44] * | Taiwan/15 | 0% | 40.0% | NA | 93.3% | 0% | NA | 100% | 0% | 0% |
| (B) | ||||||||||
| Author/Period | Country | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Lin JN/2005–2017 [5] * | Taiwan | 14/42 | 9/42 | 25/42 | 24/42 | 1/42 | 0/42 | 35/42 | 10/42 | 8/42 |
| Lo HH/2007–2011 [44] * | Taiwan | 0/15 | 6/15 | NA | 14/15 | 0/15 | NA | 15/15 | 0/15 | 0/15 |
| pooled rate | 14/57 | 15/57 | 25/42 | 38/57 | 1/57 | 0/42 | 50/57 | 10/57 | 8/57 | |
| percentage | 24.6% | 26.3% | 59.5% | 66.7% | 1.8% | 0% | 87.7% | 17.5% | 14.0% | |
| (A) | |||||||||
| Country | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Italy [31] | 45.5% | 45.5% | 45.5% | 100% | 0% | 4.6% | NA | 0% | 0% |
| Taiwan [5] | 19.8% | 18.3% | 32.5% | 47.6% | 0% | 0% | 73.0% | 13.5% | 17.5% |
| Singapore [33] | 80% | NA | 77.3% | 95.7% | NA | NA | 100% | 25% | NA |
| India [34,35] | 25.6% | 0% | 0% | 53.8% | 17.9% | 20.5% | 100% | 0% | 0% |
| (B) | |||||||||
| Country/No | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Taiwan [6,36,37,38,39] | 25.4% | 26.6% | 31.4% | 74.2% | 2.5% | 5.2% | 72.3% | 8.7% | 7.7% |
| China [40,41,42] | 34.4% | 12.0% | 13.9% | 87.0% | 1.6% | 0% | 90.6% | 5.9% | 7.0% |
| India [35,43] | 33.3% | 26.7% | 75.0% | 56.7% | 33.3% | 23.3% | NA | 16.7% | 0% |
| (A) | |||||||||
| Method | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| E-test [33] | 80.0% | NA | 77.3% | 95.7% | NA | NA | 100% | 25.0% | NA |
| Disc diffusion [34,35] | 25.6% | 0% | 0% | 53.8% | 17.9% | 20.5% | 100% | 0% | 0% |
| Broth microdilution [5] | 19.8% | 18.3% | 32.5% | 47.6% | 0% | 0% | 73.0% | 13.5% | 17.5% |
| (B) | |||||||||
| Country | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Agar dilution [36] | NA | 16.7% | NA | NA | 0% | NA | 75.0% | 41.7% | NA |
| Disc diffusion [35,37] | 47.1% | 29.4% | 62.5% | 52.9% | 17.6% | 20.6% | NA | 8.8% | 12.5% |
| Broth microdilution [5,38,40,42,43] | 27.3% | 16.0% | 19.9% | 75.3% | 0.8% | 0% | 89.0% | 6.5% | 10.7% |
| (A) | |||||||||
| Period | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| before 2010 [5,31] | 24.0% | 22.6% | 34.9% | 56.2% | 0% | 0.7% | 73.0% | 11.6% | 15.1% |
| After 2010 [33,34,35] | 40.7% | 0% | 41.5% | 69.4% | 17.9% | 20.5% | 100% | 4.3% | 0% |
| (B) | |||||||||
| Period | TZP | CIP | LVX | SXT | IMI | MPM | MIN | CAZ | FEM |
| Before 2000 [36] | NA | 16.7% | NA | NA | 0% | NA | 75.0% | 41.7% | NA |
| 2000-2010 [5,37,38,39,40] | 25.9% | 26.5% | 31.4% | 72.0% | 2.5% | 5.2% | 78.9% | 4.4% | 7.2% |
| After 2010 [35,41,42,43] | 35.1% | 13.8% | 18.2% | 88.3% | 5.1% | 4.2% | 88.6% | 7.8% | 8.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C. Clinical and Epidemiological Features and Antimicrobial Susceptibility Patterns of Chryseobacterium Species: A Scoping Review. Medicina 2025, 61, 1197. https://doi.org/10.3390/medicina61071197
Huang C. Clinical and Epidemiological Features and Antimicrobial Susceptibility Patterns of Chryseobacterium Species: A Scoping Review. Medicina. 2025; 61(7):1197. https://doi.org/10.3390/medicina61071197
Chicago/Turabian StyleHuang, Chienhsiu. 2025. "Clinical and Epidemiological Features and Antimicrobial Susceptibility Patterns of Chryseobacterium Species: A Scoping Review" Medicina 61, no. 7: 1197. https://doi.org/10.3390/medicina61071197
APA StyleHuang, C. (2025). Clinical and Epidemiological Features and Antimicrobial Susceptibility Patterns of Chryseobacterium Species: A Scoping Review. Medicina, 61(7), 1197. https://doi.org/10.3390/medicina61071197






