Association Between Human Embryo Culture Conditions, Cryopreservation, and the Potential Risk of Birth Defects in Children Conceived Through Assisted Reproduction Technology
Abstract
1. Introduction
2. Historical Landscape of Human Embryo Development
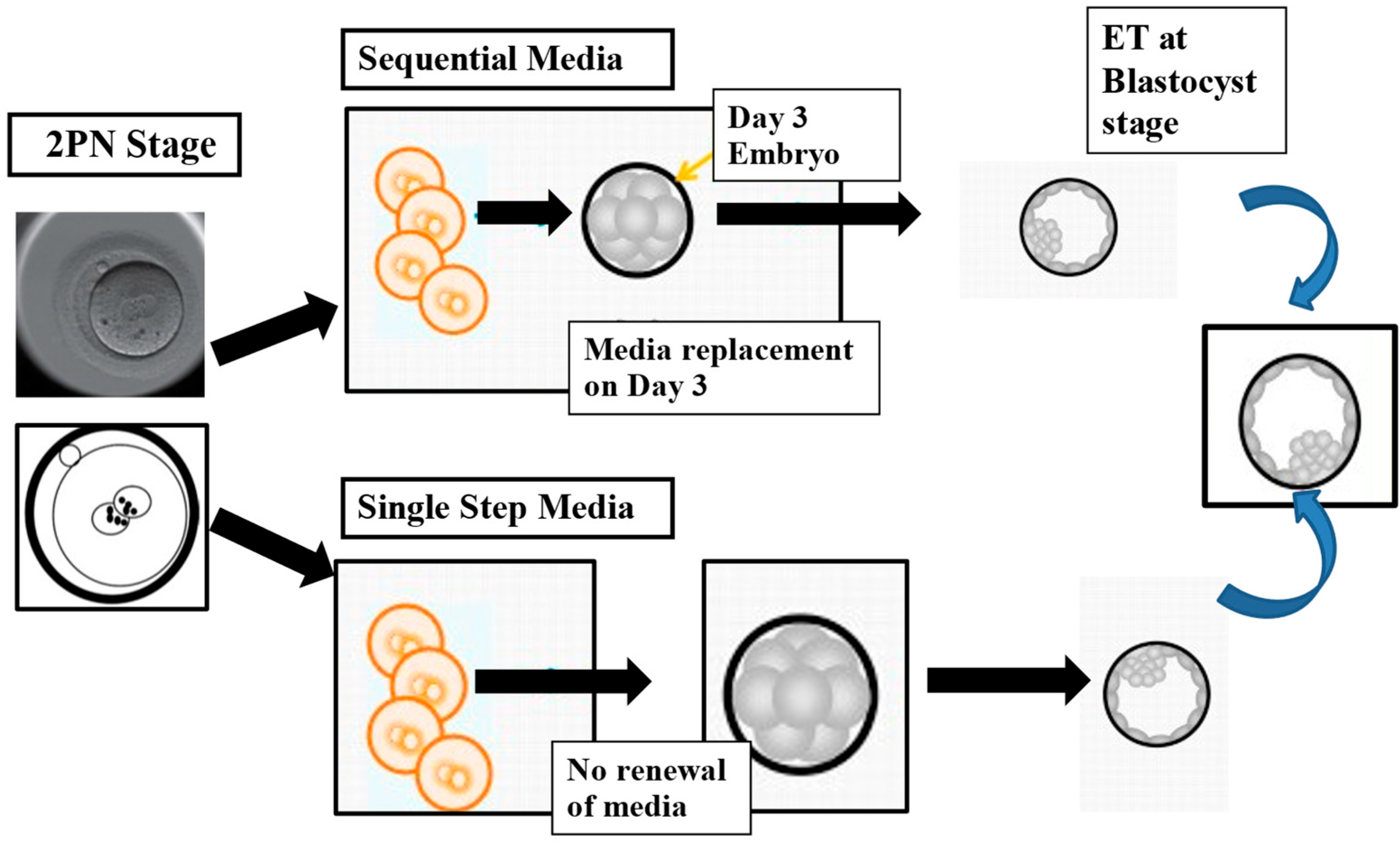
Evolution of Embryo Culture Media
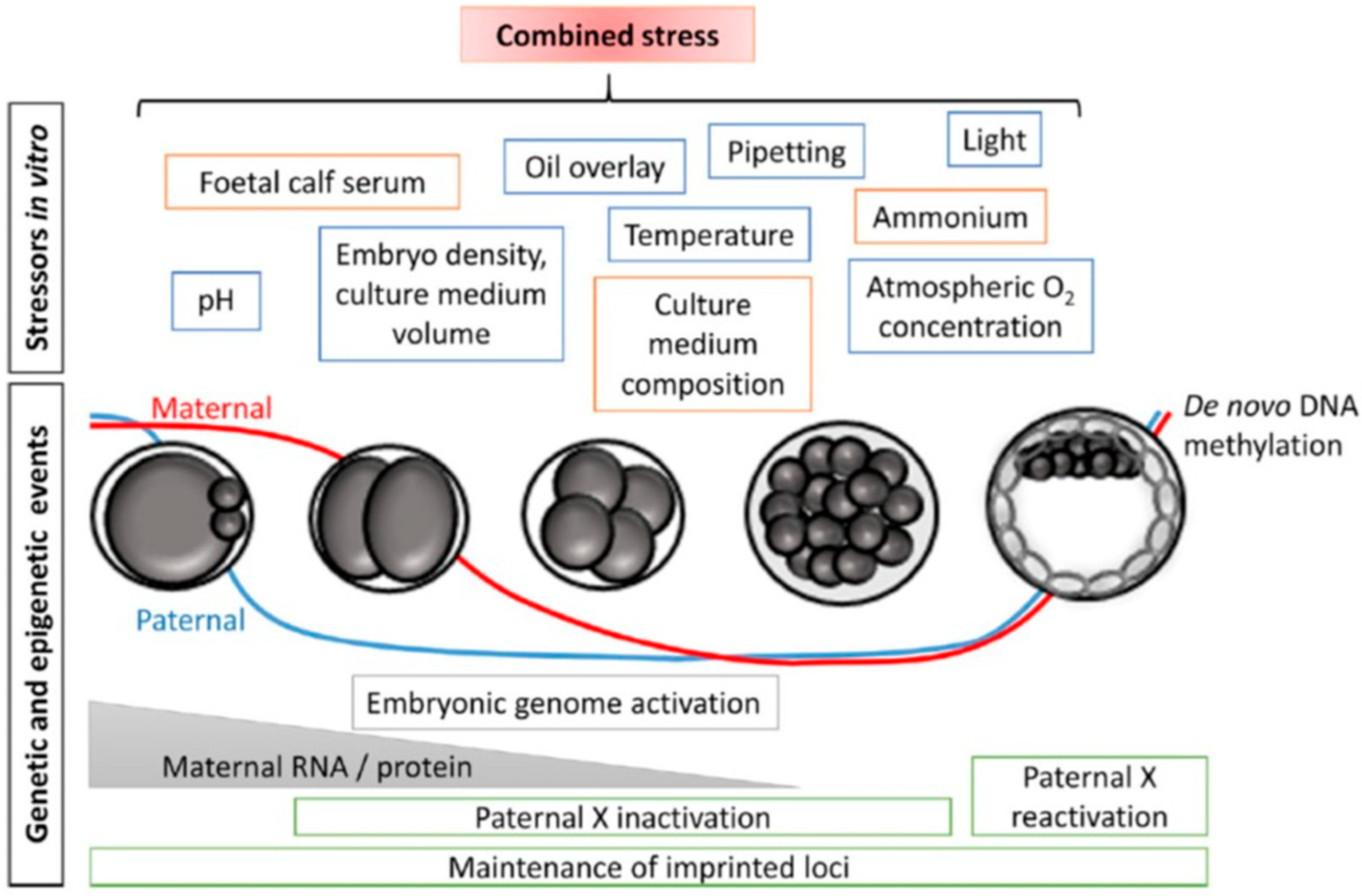
3. Media Composition and Embryo Development
3.1. Amino Acids and Protein Supplementation
3.2. pH and Osmolality
3.3. Temperature
4. Oxygen Tension and Oxidative Stress
5. Cryopreservation and Cryoprotectants
Application of Cryopreservation Procedures in ART
6. Epigenetics and the Embryonic Epigenome
6.1. Potential Impairment by Vitrification and Epigenetic Alterations
6.2. Human Studies
7. Potential Risk of ART Procedures and Epigenetic Dysfunction
ART Procedures, In Vitro Culture, and Birth Weight
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biggers, J.D. Thoughts on embryo culture conditions. Reprod. Biomed. Online 2002, 4, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Lane, M. Culture and selection of viable blastocysts: A feasible proposition for human IVF? Hum. Reprod. Update 1997, 3, 367–382. [Google Scholar] [CrossRef]
- Fouks, Y.; Yogev, Y. Twinning in ART: Single embryo transfer policy. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 84, 88–95. [Google Scholar] [CrossRef]
- De Neubourg, D.; Dancet, E.A.F.; Pinborg, A. Single-embryo transfer implies quality of care in reproductive medicine. Reprod. Biomed. Online 2022, 45, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Goossens, V.; et al. ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open. 2022, 2022, hoac022. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Cuevas Saiz, I.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; Rugescu, I.; et al. ART in Europe, 2019: Results generated from European registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar] [CrossRef] [PubMed]
- Toftager, M.; Bogstad, J.; Bryndorf, T.; Løssl, K.; Roskær, J.; Holland, T.; Prætorius, L.; Zedeler, A.; Nilas, L.; Pinborg, A. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum. Reprod. 2016, 31, 1253–1264. [Google Scholar] [CrossRef]
- Chen, C. Pregnancy after human oocyte cryopreservation. Lancet 1986, 327, 884–886. [Google Scholar] [CrossRef]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, A.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in art: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
- Potdar, N.; Gelbaya, T.A.; Nardo, L.G. Oocyte vitrification in the 21st century and post-warming fertility outcomes: A systematic review and meta-analysis. Reprod. Biomed. Online 2014, 29, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Norman, R.J. The longer-term health outcomes for children born as a result of Ivf treatment: Part I--general health outcomes. Hum. Reprod. Update 2013, 19, 232–243. [Google Scholar] [CrossRef]
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Chan, M.M.; Humm, K.C.; Karnik, R.; Mekhoubad, S.; Regev, A.; Eggan, K.; Meissner, A. DNA methylation dynamics of the human preimplantation embryo. Nature 2014, 511, 611–615. [Google Scholar] [CrossRef]
- Sciorio, R.; Rinaudo, P. Culture conditions in the IVF laboratory: State of the ART and possible new directions. J. Assist. Reprod. Genet. 2023, 40, 2591–2607. [Google Scholar] [CrossRef] [PubMed]
- Estudillo, E.; Jiménez, A.; Bustamante-Nieves, P.E.; Palacios-Reyes, C.; Velasco, I.; López-Ornelas, A. Cryopreservation of Gametes and Embryos and Their Molecular Changes. Int. J. Mol. Sci. 2021, 22, 10864. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Z.; Yu, P.; Wang, Y.; Pei, Y.; Dai, Y.; Liu, Y.; Yang, Y. The Association between Assisted Reproductive Technologies and Neurodevelopmental Disorders in Offspring: An Overview of Current Evidence. J. Integr. Neurosci. 2024, 23, 15. [Google Scholar] [CrossRef]
- Henningsen, A.K.; Pinborg, A. Birth perinatal outcomes complications for babies conceived following, A.R.T. Semin. Fetal Neonatal Med. 2014, 19, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, S.; Söderström-Anttila, V.; Wennerholm, U.B.; Laivuori, H.; Loft, A.; Oldereid, N.B.; Romundstad, L.B.; Bergh, C.; Pinborg, A.R. The health of children conceived by ART: ‘the chicken or the egg?’. Hum. Reprod. Update 2019, 25, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Schieve, L.A.; Meikle, S.F.; Ferre, C.; Peterson, H.B.; Jeng, G.; Wilcox, L.S. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N. Engl. J. Med. 2002, 346, 731–737. [Google Scholar] [CrossRef]
- Sunkara, S.K.; Antonisamy, B.; Redla, A.C.; Kamath, M.S. Female causes of infertility are associated with higher risk of preterm birth and low birth weight: Analysis of 117 401 singleton live births following IVF. Hum. Reprod. 2021, 36, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Terho, A.M.; Pelkonen, S.; Opdahl, S.; Romundstad, L.B.; Bergh, C.; Wennerholm, U.B.; Henningsen, A.A.; Pinborg, A.; Gissler, M.; Tiitinen, A. High birth weight and large-for-gestational-age in singletons born after frozen compared to fresh embryo transfer, by gestational week: A Nordic register study from the CoNARTaS group. Hum. Reprod. 2021, 36, 1083–1092. [Google Scholar] [CrossRef]
- Abe, K.; Schauer, T.; Torres-Padilla, M.E. Distinct patterns of RNA polymerase II and transcriptional elongation characterize mammalian genome activation. Cell Rep. 2022, 41, 111865. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Formation and function of oviduct fluid. J. Reprod. Fertil. 1988, 82, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef]
- Troncoso, C.; Bosch, E.; Rubio, C.; Remohí, J.; Simón, C.; Pellicer, A. The origin of biochemical pregnancies: Lessons learned from preimplantation genetic diagnosis. Fertil. Steril. 2003, 79, 449–450. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Calderon, I.; Leeton, J. Environment of the preimplantation human embryo in vivo: Metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil. Steril. 1996, 65, 349–353. [Google Scholar] [CrossRef]
- Barberet, J.; Bruno, C.; Valot, E.; Antunes-Nunes, C.; Jonval, L.; Chammas, J.; Choux, C.; Ginod, P.; Sagot, P.; Soudry-Faure, A.; et al. Can novel early non-invasive biomarkers of embryo quality be identified with time-lapse imaging to predict live birth? Hum. Reprod. 2019, 34, 1439–1449. [Google Scholar] [CrossRef]
- Sciorio, R.; Miranian, D.; Smith, G.D. Non-invasive oocyte quality assessment. Biol. Reprod. 2022, 106, 274–290. [Google Scholar] [CrossRef]
- Reignier, A.; Girard, J.M.; Lammers, J.; Chtourou, S.; Lefebvre, T.; Barriere, P.; Freour, T. Performance of Day 5 KIDScore™ morphokinetic prediction models of implantation and live birth after single blastocyst transfer. J. Assist. Reprod. Genet. 2019, 36, 2279–2285. [Google Scholar] [CrossRef]
- Rock, J.; Menkin, M.F. In vitro fertilization and cleavage of human ovarian eggs. Science 1944, 100, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Ruffenach, S.C. Landrum Brewer Shettles (1909–2003); Embryo Project Encyclopedia: Tempe, AZ, USA, 2009; ISSN 1940-5030. Available online: https://embryo.asu.edu/pages/landrum-brewer-shettles-1909-2003 (accessed on 26 June 2025).
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 312, 366. [Google Scholar] [CrossRef]
- Edwards, R.G. Test-tube babies. Nature 1981, 293, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.G.; Purdy, J.M.; Steptoe, P.C.; Walters, D.E. The growth of human preimplantation embryos in vitro. Am. J. Obstet. Gynecol. 1981, 141, 408–416. [Google Scholar] [CrossRef]
- Menezo, Y.; Testart, J.; Perone, D. Serum is not necessary in human in vitro fertilization and embryo development. Fertil. Steril. 1984, 42, 750–755. [Google Scholar] [CrossRef]
- Quinn, P.; Kerin, J.F.; Warnes, G.M. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil. Steril. 1985, 44, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Lawitts, J.A.; Biggers, J.D. Joint effects of sodium chloride, glutamine, and glucose in mouse preimplantation embryo culture media. Mol. Reprod. Dev. 1992, 31, 189–194. [Google Scholar] [CrossRef]
- Swain, J.E. Controversies in ART: Considerations and risks for uninterrupted embryo culture. Reprod. Biomed. Online 2019, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.C.; Biggers, J.D. Chemically defined media and the culture of mammalian preimplantation embryos: Historical perspective and current issues. Hum. Reprod. Update 2003, 9, 557–582. [Google Scholar] [CrossRef]
- Morbeck, D.E.; Krisher, R.L.; Herrick, J.R.; Baumann, N.A.; Matern, D.; Moyer, T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014, 102, 759–766. [Google Scholar] [CrossRef]
- Mantikou, E.; Youssef, M.A.; van Wely, M.; van der Veen, F.; Al-Inany, H.G.; Repping, S.; Mastenbroek, S. Embryo culture media and IVF/ICSI success rates: A systematic review. Hum. Reprod. Update 2013, 19, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.L.; Hamic, A.; Thompson, D.J.; Caperton, C.L. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: A sibling embryo study. Fertil. Steril. 2009, 92, 1783–1786. [Google Scholar] [CrossRef]
- Summers, M.C.; Bird, S.; Mirzai, F.M.; Thornhill, A.; Biggers, J.D. Human preimplantation embryo development in vitro: A morphological assessment of sibling zygotes cultured in a single medium or in sequential media. Hum. Fertil. 2013, 16, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Pool, T.B.; Schoolfield, J.; Han, D. Human embryo culture media comparisons. Methods Mol. Biol. 2012, 912, 367–386. [Google Scholar]
- Campo, R.; Binda, M.M.; Van Kerkhoven, G.; Frederickx, V.; Serneels, A.; Roziers, P.; Lopes, A.S.; Gordts, S.; Puttemans, P. Critical reappraisal of embryo quality as a predictive parameter for pregnancy outcome: A pilot study. Facts Views Vis. ObGyn 2010, 2, 289–295. [Google Scholar] [PubMed]
- Nelissen, E.C.; Van Montfoort, A.P.; Coonen, E.; Derhaag, J.G.; Geraedts, J.P.; Smits, L.J.; Land, J.A.; Evers, J.L.; Dumoulin, J.C. Further evidence that culture media affect perinatal outcome: Findings after transfer of fresh and cryopreserved embryos. Hum. Reprod. 2012, 27, 1966–1976. [Google Scholar] [CrossRef]
- Biggers, J.D.; McGinnis, L.K.; Raffin, M. Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol. Reprod. 2000, 63, 281–293. [Google Scholar] [CrossRef]
- Lane, M. Mechanisms for managing cellular and homeostatic stress in vitro. Theriogenology 2001, 55, 225–236. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Alleviation of the ‘‘2-cell block’’ and development to the blastocyst of CF1 mouse embryos: Role of amino acids, EDTA and physical parameters. Hum. Reprod. 1996, 11, 2703–2712. [Google Scholar] [CrossRef]
- Menezo, Y.; Lichtblau, I.; Elder, K. New insights into human pre-implantation metabolism in vivo and in vitro. J. Assist. Reprod. Genet. 2013, 30, 293–303. [Google Scholar] [CrossRef]
- Clare, C.E.; Pestinger, V.; Kwong, W.Y.; Tutt, D.A.R.; Xu, J.; Byrne, H.M.; Barrett, D.A.; Emes, R.A.; Sinclair, K.D. Interspecific Variation in One-Carbon Metabolism within the Ovarian Follicle, Oocyte, and Preimplantation Embryo: Consequences for Epigenetic Programming of DNA Methylation. Int. J. Mol. Sci. 2021, 22, 1838. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. Oxygen affects the ability of mouse blastocysts to regulate ammonium. Biol. Reprod. 2013, 89, 75. [Google Scholar] [CrossRef]
- Summers, M.C.; McGinnis, L.K.; Lawitts, J.A.; Biggers, J.D. Mouse embryo development following IVF in media containing either L-glutamine or glycyl-Lglutamine. Hum. Reprod. 2005, 20, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Gardner, D.K. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol. Reprod. 2003, 69, 1109–1117. [Google Scholar] [CrossRef]
- Pool, T.B.; Martin, J.E. High continuing pregnancy rates after in vitro fertilization-embryo transfer using medium supplemented with a plasma protein fraction containing alpha- and beta-globulins. Fertil. Steril. 1994, 61, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Weathersbee, P.S.; Pool, T.B.; Ord, T. Synthetic serum substitute (SSS): A globulin-enriched protein supplement for human embryo culture. J. Assist. Reprod. Genet. 1995, 12, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.I.; Rutherford, A.J.; Killick, S.R.; Maguiness, S.D.; Partridge, R.J.; Leese, H.J. Human tubal fluid: Production, nutrient composition and response to adrenergic agents. Hum. Reprod. 1997, 12, 2451–2456. [Google Scholar] [CrossRef]
- Meintjes, M.; Chantilis, S.J.; Ward, D.C.; Douglas, J.D.; Rodriguez, A.J.; Guerami, A.R. A randomized controlled study of human serum albumin and serum substitute supplement as protein supplements for IVF culture and the effect on live birth rates. Hum. Reprod. 2009, 24, 782–789. [Google Scholar] [CrossRef]
- Fredrickson, J.; Krisher, R.; Morbeck, D.E. The impact of the protein stabilizer octanoic acid on embryonic development and fetal growth in a murine model. J. Assist. Reprod. Genet. 2015, 32, 1517–1524. [Google Scholar] [CrossRef]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Hentemann, M.; Mousavi, K.; Bertheussen, K. Differential pH in embryo culture. Fertil. Steril. 2011, 95, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Liu, X.; Jimenez-Morales, D.; Rinaudo, P.F. Murine blastocysts generated by in vitro fertilization show increased Warburg metabolism and altered lactate production. Elife 2022, 11, e79153. [Google Scholar] [CrossRef]
- Swain, J.E.; Carrell, D.; Cobo, A.; Meseguer, M.; Rubio, C.; Smith, G.D. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil. Steril. 2016, 105, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Pool, T.B. New pH-buffering system for media utilized during gamete and embryo manipulations for assisted reproduction. Reprod. Biomed. Online 2009, 18, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Noda, Y.; Goto, Y.; Mori, T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology 1994, 41, 499–510. [Google Scholar] [CrossRef]
- Baltz, J.; Shou, C. Cell volume regulation in mammalian oocytes and preimplantation embryos. Mol. Reprod. Dev. 2012, 79, 821–831. [Google Scholar] [CrossRef]
- Brinster, R.L. Studies on the development of mouse embryos in vitro. The effect of osmolarity and hydrogen ion concentration. J. Exp. Zool. 1965, 158, 49–57. [Google Scholar] [CrossRef]
- Swain, J.E.; Cabrera, L.; Xu, X.; Smith, G.D. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod. Biomed. Online 2012, 24, 142–147. [Google Scholar] [CrossRef]
- Setti, A.S.; de Almeida Ferreira Braga, D.A.; Vingris, L.; Iaconelli, A.; Borges, E.; Setti, A.S. Improved embryonic development and utilization rates with EmbryoScope: A within-subject comparison versus a benchtop incubator. Zygote 2022, 30, 633–637. [Google Scholar] [CrossRef]
- Minasi, M.G.; Colasante, A.; Riccio, T.; Ruberti, A.; Casciani, V.; Scarselli, F.; Spinella, F.; Fiorentino, F.; Varricchio, M.T.; Greco, E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: A consecutive case series study. Hum. Reprod. 2016, 31, 2245–2254. [Google Scholar] [CrossRef]
- Gorbsky, G.J. The spindle checkpoint and chromosome segregation in meiosis. FEBS J. 2015, 282, 2471–2487. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Meng, L.; Hackett, R.J.; Oldenbourg, R.; Keefe, D.L. Rigorous thermal control during intracytoplasmic sperm injection stabilizes the meiotic spindle and improves fertilization and pregnancy rates. Fertil. Steril. 2002, 77, 1274–1277. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, W.H.; Keefe, D.L. Overheating is detrimental to meiotic spindles within in vitro matured human oocytes. Zygote 2004, 12, 65–70. [Google Scholar] [CrossRef]
- Swearman, H.; Koustas, G.; Knight, E.; Liperis, G.; Grupen, C.; Sjoblom, C. pH: The silent variable significantly impacting meiotic spindle assembly in mouse oocytes. Reprod. Biomed. Online 2018, 37, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.J.; Braude, P.R.; Johnson, M.H.; Cant, A.; Currie, J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil. Steril. 1990, 54, 102–108. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- De Munck, N.; Janssens, R.; Santos-Ribeiro, S.; Tournaye, H.; Velde, H.; Verheyen, G. The effect of different temperature conditions on human embryos in vitro: Two sibling studies. Reprod. Biomed. Online 2019, 38, 508–515. [Google Scholar]
- Fawzy, M.; Emad, M.; Gad, M.A.; Sabry, M.; Kasem, H.; Mahmoud, M.; Bedaiwy, M.A. Comparing 36.5 °C with 37 °C for human embryo culture: A prospective randomized controlled trial. Reprod. Biomed. Online 2018, 36, 620–626. [Google Scholar] [CrossRef]
- Hong, K.; Forman, E.; Lee, H.; Ferry, K.M.; Treff, N.; Scott, R. Optimizing the temperature for embryo culture in IVF: A randomized controlled trial (RCT) comparing standard culture temperature of 37C to the reduced more physiologic temperature of 36C. Fertil. Steril. 2012, 98, s167. [Google Scholar] [CrossRef]
- Fischer, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. Nov. 1993, 99, 673–679. [Google Scholar] [CrossRef]
- Kovačič, B. Culture systems: Low-oxygen culture. Methods Mol. Biol. 2012, 912, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Catt, J.W.; Henman, M. Toxic effects of oxygen on human embryo development. Hum. Reprod. 2000, 15 (Suppl. 2), 199–206. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, U.; Engström, A.B.; Hellberg, D.; Nilsson, S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil. Steril. 2009, 91, 2461–2465. [Google Scholar] [CrossRef]
- Meintjes, M.; Chantilis, S.J.; Douglas, J.D.; Rodriguez, A.J.; Guerami, A.R.; Bookout, D.M.; Barnett, B.D.; Madden, J.D. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Hum. Reprod. 2009, 24, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.F.; Giritharan, G.; Talbi, S.; Dobson, A.T.; Schultz, R.M. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil. Steril. 2006, 86 (Suppl. 4), 1252–1265. [Google Scholar] [CrossRef]
- Kaltsa, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Zhang, L.; Liu, X.; Donjacour, A.; Ruggeri, E.; Palmerini, M.G.; Nottola, S.A.; Macchiarelli, G.; Rinaudo, P. Oxygen concentration alters mitochondrial structure and function in in vitro fertilized preimplantation mouse embryos. Hum. Reprod. 2019, 34, 601–611. [Google Scholar] [CrossRef]
- Li, W.; Goossens, K.; Van Poucke, M.; Foreir, K.; Braeckmans, K.; Van Soom, A.; Peelman, L.J. High oxygen tension increases global methylation in bovine 4-cell embryos and blastocysts but does not affect general retrotransposon expression. Reprod. Fertil. Dev. 2024, 28, 948–959. [Google Scholar] [CrossRef]
- Gaspar, R.B.; Arnold, D.R.; Corrêa, C.A.P.; da Rocha, C.V., Jr.; Penteado, J.C.; Del Collado, M.; Vantini, R.; Garcia, J.M.; Lopes, F.L. Oxygen tension affects histone remodeling of in vitro-produced embryos in a bovine model. Theriogenology 2015, 83, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.; Schultz, R.M. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction 2004, 128, 301–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katz-Jaffe, M.G.; Linck, D.W.; Schoolcraft, W.B.; Gardner, D.K. A proteomic analysis of mammalian preimplantation embryonic development. Reproduction 2005, 130, 899–905. [Google Scholar] [CrossRef]
- Christianson, M.S.; Zhao, Y.; Shoham, G.; Granot, I.; Safran, A.; Khafagy, A.; Leong, M.; Shoham, Z. Embryo catheter loading and embryo culture techniques: Results of a worldwide Web-based survey. J. Assist. Reprod. Genet. 2014, 31, 1029–1036. [Google Scholar] [CrossRef]
- Trounson, A.; Mohr, L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983, 305, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, T.; Wada, S.; Takahashi, K.; Pedro, P.B.; An, T.Z.; Kasai, M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum. Reprod. 1998, 13, 2874–2879. [Google Scholar] [CrossRef]
- Ferreux, L.; Bourdon, M.; Sallem, A.; Santulli, P.; Barraud-Lange, V.; Le Foll, N.; Maignien, C.; Chapron, C.; de Ziegler, D.; Wolf, J.P.; et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on Day 5 than on Day 6. Hum. Reprod. 2018, 33, 390–398. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.A.; Ledger, W.; Edgar, D.H.; Sullivan, E.A. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: A population-based cohort study. Hum. Reprod. 2014, 29, 2794–2801. [Google Scholar] [CrossRef]
- Bourdon, M.; Pocate-Cheriet, K.; Finet de Bantel, A.; Grzegorczyk-Martin, V.; Amar Hoffet, A.; Arbo, E.; Poulain, M.; Santulli, P. Day 5 versus Day 6 blastocyst transfers: A systematic review and meta-analysis of clinical outcomes. Hum. Reprod. 2019, 34, 1948–1964. [Google Scholar] [CrossRef]
- Seki, S.; Mazur, P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology 2009, 59, 75–82. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo. Lett. 2004, 25, 375–388. [Google Scholar]
- Konc, J.; Kanyó, K.; Kriston, R.; Somoskői, B.; Cseh, S. Cryopreservation of embryos and oocytes in human assisted reproduction. Biomed. Res. Int. 2014, 2014, 307268. [Google Scholar] [CrossRef]
- Liebermann, J.; Hrvojevic, K.; Hirshfeld-Cytron, J.; Brohammer, R.; Wagner, Y.; Susralski, A.; Jasulaitis, S.; Chan, S.; Takhsh, E.; Uhler, M. Fast and furious: Pregnancy outcome with one-step rehydration in the warming protocol for human blastocysts. Reprod. Biomed. Online 2023, 48, 103731. [Google Scholar] [CrossRef] [PubMed]
- Vriens, I.J.H.; Ter Welle-Butalid, E.M.; de Boer, M.; de Die-Smulders, C.E.M.; Derhaag, J.G.; Geurts, S.M.E.; van Hellemond, I.E.G.; Luiten, E.J.T.; Dercksen, M.W.; Lemaire, B.M.D.; et al. Preserving fertility in young women undergoing chemotherapy for early breast cancer; the Maastricht experience. Breast Cancer Res. Treat. 2020, 181, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Vigano, P.; Filippi, F.; Papaleo, E.; Benaglia, L.; Candiani, M.; Vercellini, P. Fertility preservation in women with endometriosis: For all, for some, for none? Hum. Reprod. 2015, 30, 1280–1286. [Google Scholar] [CrossRef]
- Santos-Ribeiro, S.; Polyzos, N.P.; Haentjens, P.; Smitz, J.; Camus, M.; Tournaye, H.; Blockeel, C. Live birth rates after IVF are reduced by both low and high progesterone levels on the day of human chorionic gonadotrophin administration. Hum. Reprod. 2014, 29, 1698–1705. [Google Scholar] [CrossRef]
- Lawrenz, B.; Coughlan, C.; Melado, L.; Fatemi, H.M. The ART of frozen embryo transfer: Back to nature! Gynecol. Endocrinol. 2020, 36, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, M.; Li, Z.; Wang, L.; Hu, J.; Zhao, B.; Feng, Y.; Chen, X.; Sun, L. Fresh versus frozen embryo transfer for full-term singleton birth: A retrospective cohort study. J. Ovarian Res. 2018, 11, 59. [Google Scholar] [CrossRef]
- Ventura-Juncá, P.; Irarrázaval, I.; Rolle, A.J.; Gutiérrez, J.I.; Moreno, R.D.; Santos, M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015, 48, 68. [Google Scholar] [CrossRef]
- Hwang, S.S.; Dukhovny, D.; Gopal, D.; Cabral, H.; Diop, H.; Coddington, C.C.; Stern, J.E. Health outcomes for Massachusetts infants after fresh versus frozen embryo transferr. Fertil. Steril. 2019, 112, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Belva, F.; Bonduelle, M.; Roelants, M.; Verheyen, G.; Van Landuyt, L. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum. Reprod. 2016, 31, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Pandey, S.; Raja, E.A.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef] [PubMed]
- von Versen-Höynck, F.; Narasimhan, P.; Selamet Tierney, E.S.; Martinez, N.; Conrad, K.P.; Baker, V.L.; Winn, V.D. Absent or Excessive Corpus Luteum Number Is Associated With Altered Maternal Vascular Health in Early Pregnancy. Hypertension 2019, 73, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Henningsen, A.A.; Loft, A.; Malchau, S.S.; Forman, J.; Andersen, A.N. Large baby syndrome in singletons born after frozen embryo transfer (FET): Is it due to maternal factors or the cryotechnique? Hum. Reprod. 2014, 29, 618–627. [Google Scholar] [CrossRef]
- Chronopoulou, E.; Raperport, C.; Sfakianakis, A.; Srivastava, G.; Homburg, R. Elective oocyte cryopreservation for age-related fertility decline. J. Assist. Reprod. Genet. 2021, 38, 1177–1186. [Google Scholar] [CrossRef]
- Seyhan, A.; Akin, O.D.; Ertaş, S.; Ata, B.; Yakin, K.; Urman, B. A Survey of Women Who Cryopreserved Oocytes for Non-medical Indications (Social Fertility Preservation). Reprod. Sci. 2021, 28, 2216–2222. [Google Scholar] [CrossRef]
- Cobo, A.; Garcia-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Stearns, V.; Schneider, B.; Henry, N.L.; Hayes, D.F.; Flockhart, D.A. Breast cancer treatment and ovarian failure: Risk factors and emerging genetic determinants. Nat. Rev. Cancer 2006, 6, 886–893. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
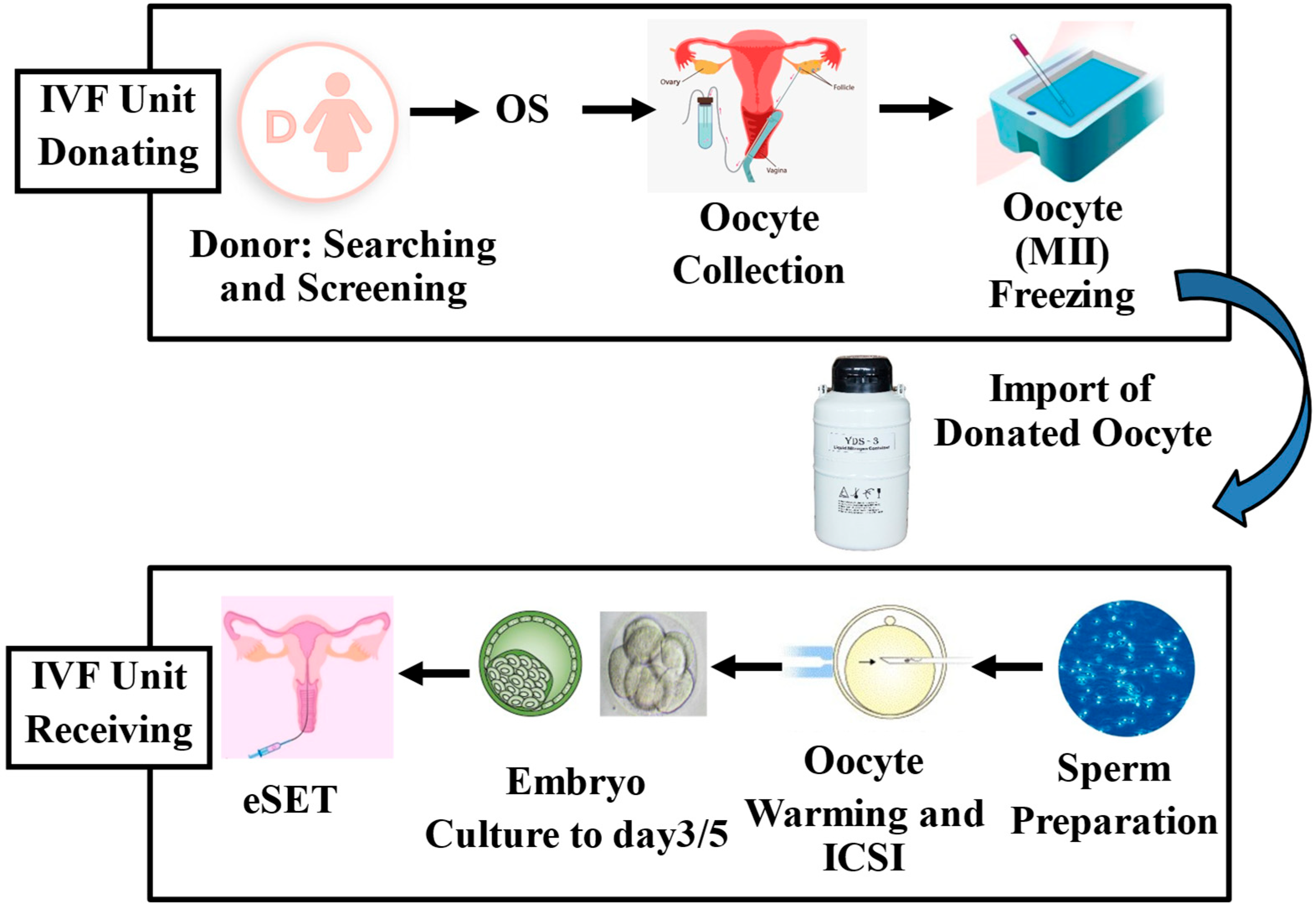
- Sauer, M.V.; Kavic, S.M. Oocyte and embryo donation 2006: Reviewing two decades of innovation and controversy. Reprod. Biomed. Online 2006, 12, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; Garrido, N.; Pellicer, A.; Remohí, J. Six years’ experience in ovum donation using vitrified oocytes: Report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil. Steril. 2015, 104, 1426–1434. [Google Scholar] [CrossRef]
- Seshadri, S.; Saab, W.; Exeter, H.; Drew, E.; Petrie, A.; Davies, M.; Serhal, P. Clinical outcomes of a vitrified donor oocyte programme: A single UK centre experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Cimadomo, D.; Maggiulli, R.; Vaiarelli, A.; Dusi, L.; Buffo, L.; Amendola, M.G.; Colamaria, S.; Giuliani, M.; Bruno, G.; et al. Definition of a clinical strategy to enhance the efficacy, efficiency and safety of egg donation cycles with imported vitrified oocytes. Hum. Reprod. 2020, 35, 785–795. [Google Scholar] [CrossRef]
- Waddington, C.H. The epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.E.A.; Martienssen, R.A.; Riggs, A.D. Epigenetic Mechanisms of Gene Regulation; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1996; Monograph 32. [Google Scholar]
- Skinner, M.K. Environmental epigenomics and disease susceptibility. EMBO Rep. 2011, 12, 620–622. [Google Scholar] [CrossRef]
- Mak, W.; Weaver, J.R.; Bartolomei, M.S. Is ART changing the epigenetic landscape of imprinting? Anim. Reprod. 2010, 7, 168–176. [Google Scholar]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Rivera, C.M.; Ren, B. Mapping human epigenomes. Cell 2013, 155, 39–55. [Google Scholar] [CrossRef]
- Hirst, M.; Marra, M.A. Epigenetics and human disease. Int. J. Biochem. Cell Biol. 2009, 41, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Weber, W. Cancer epigenetics. Prog. Mol. Biol. Transl. Sci. 2010, 95, 299–349. [Google Scholar]
- Iwatani, M.; Ikegami, K.; Kremenska, Y.; Hattori, N.; Tanaka, S.; Yagi, S.; Shiota, K. Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body. Stem Cells 2006, 24, 2549–2556. [Google Scholar] [CrossRef]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Quinn, P.J. Dimethyl sulphoxide: A review of its applications in cell biology. Biosci. Rep. 1994, 14, 259–281. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Long, C.; Liu, G.; Bai, H.; Ma, L.; Bai, T.; Zuo, Y.; Li, S. WGBS combined with RNA-seq analysis revealed that Dnmt1 affects the methylation modification and gene expression changes during mouse oocyte vitrification. Theriogenology 2022, 177, 11–21. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Deng, T.; Zou, P.; Wang, Y.; Quan, F.; Zhang, Y. Effects of oocyte vitrification on epigenetic status in early bovine embryos. Theriogenology 2016, 86, 868–878. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Wang, Z.; Chang, H.; Xie, X.; Fu, L.; Zhang, Y.; Quan, F. Resveratrol improved the developmental potential of oocytes after vitrification by modifying the epigenetics. Mol. Reprod. Dev. 2019, 86, 862–870. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; He, F. Embryo vitrification affects the methylation of the H19/Igf2 differentially methylated domain and the expression of H19 and Igf2. Fertil. Steril. 2010, 93, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Wang, J.J.; Zhang, P.P.; Hao, H.S.; Pang, Y.W.; Wang, H.Y.; Du, W.H.; Zhao, S.J.; Ruan, W.M.; Zou, H.Y.; et al. Oocyte IVM or vitrification significantly impairs DNA methylation patterns in blastocysts as analysed by single-cell whole-genome methylation sequencing. Reprod. Fertil. Dev. 2020, 32, 676–689. [Google Scholar] [CrossRef]
- Ying, L.; Xiang-Wei, F.; Jun-Jie, L.; Dian-Shuai, Y.; Shi-En, Z. DNA methylation pattern in mouse oocytes and their in vitro fertilized early embryos: Effect of oocyte vitrification. Zygote 2014, 22, 138–145. [Google Scholar]
- Cheng, K.R.; Fu, X.W.; Zhang, R.N.; Jia, G.X.; Hou, Y.P.; Zhu, S.E. Effect of oocyte vitrification on deoxyribonucleic acid methylation of H19, Peg3, and Snrpn differentially methylated regions in mouse blastocysts. Fertil. Steril. 2014, 102, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Aksu, D.A.; Agca, C.; Aksu, S.; Bagis, H.; Akkoc, T.; Caputcu, A.T.; Arat, S.; Taskin, A.C.; Kizil, S.H.; Karasahin, T.; et al. Gene expression profiles of vitrified in vitro- and in vivo-derived bovine blastocysts. Mol. Reprod. Dev. 2012, 79, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Al-Khtib, M.; Perret, A.; Khoueiry, R.; Ibala-Romdhane, S.; Blachère, T.; Greze, C.; Lornage, J.; Lefèvre, A. Vitrification at the germinal vesicle stage does not affect the methylation profile of H19 and KCNQ1OT1 imprinting centers in human oocytes subsequently matured in vitro. Fertil. Steril. 2011, 95, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhou, W.H.; Chu, D.P.; Fu, L.; Sha, W.; Li, Y. Ultrastructural changes and methylation of human oocytes vitrified at the germinal vesicle stage and matured in vitro after thawing. Gynecol. Obstet. Investig. 2017, 82, 252–261. [Google Scholar] [CrossRef]
- De Munck, N.; Petrussa, L.; Verheyen, G.; Staessen, C.; Vandeskelde, Y.; Sterckx, J.; Bocken, G.; Jacobs, K.; Stoop, D.; De Rycke, M. Chromosomal meiotic segregation, embryonic developmental kinetics and DNA (hydroxy) methylation analysis consolidate the safety of human oocyte vitrification. Basic. Sci. Reprod. Med. 2015, 21, 535–544. [Google Scholar] [CrossRef]
- Huo, Y.; Yuan, P.; Qin, Q.; Yan, Z.; Yan, L.; Liu, P.; Li, R.; Yan, J.; Qiao, J. Effects of vitrification and cryostorage duration on single-cell RNA-Seq profiling of vitrified-thawed human metaphase II oocytes. Front. Med. 2021, 15, 144–154. [Google Scholar] [CrossRef]
- Chatterjee, A.; Saha, D.; Niemann, H.; Gryshkov, O.; Glasmacher, B.; Hofmann, N. Effects of cryopreservation on the epigenetic profile of cells. Cryobiology 2017, 74, 1–7. [Google Scholar] [CrossRef]
- Stigliani, S.; Moretti, S.; Anserini, P.; Casciano, I.; Venturini, P.L.; Scaruffi, P. Storage time does not modify the gene expression profile of cryopreserved human metaphase II oocytes. Hum. Reprod. 2015, 30, 2519–2526. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Q.; Yang, L.; Zhou, W.; Ni, T.; Yan, J. Pregnancy and neonatal outcomes after long-term vitrification of blastocysts among 6,900 patients after their last live birth. Fertil. Steril. 2023, 119, 36–44. [Google Scholar] [CrossRef]
- Bouillon, C.; Léandri, R.; Desch, L.; Ernst, A.; Bruno, C.; Cerf, C.; Chiron, A.; Souchay, C.; Burguet, A.; Jimenez, C.; et al. Does Embryo Culture Medium Influence the Health and Development of Children Born after In Vitro Fertilization? PLoS ONE 2016, 11, e0150857. [Google Scholar] [CrossRef] [PubMed]
- Choufani, S.; Turinsky, A.L.; Melamed, N.; Greenblatt, E.; Brudno, M.; Bérard, A.; Fraser, W.D.; Weksberg, R.; Trasler, J.; Monnier, P. 3D cohort study group. Impact of assisted reproduction, infertility, sex and paternal factors on the placental DNA methylome. Hum. Mol. Genet. 2019, 28, 372–385. [Google Scholar] [CrossRef]
- Barberet, J.; Barry, F.; Choux, C.; Guilleman, M.; Karoui, S.; Simonot, R.; Bruno, C.; Fauque, P. What impact does oocyte vitrification have on epigenetics and gene expression? Clin. Epigenetics 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Marcho, C.; Cui, W.; Mager, J. Epigenetic dynamics during preimplantation development. Reproduction 2015, 150, R109–R120. [Google Scholar] [CrossRef]
- Tunster, S.J.; Jensen, A.B.; John, R.M. Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction 2013, 145, R117–R137. [Google Scholar] [CrossRef]
- Eggermann, T.; Perez de Nanclares, G.; Maher, E.R.; Temple, I.K.; Tumer, Z.; Monk, D.; Mackay, D.J.G.; Grønskov, K.; Riccio, A.; Linglart, A.; et al. Imprinting disorders: A group of congenital disorders with overlapping patterns of molecular changes affecting imprinted loci. Clin. Epigenetics 2015, 7, 123. [Google Scholar] [CrossRef]
- White, C.R.; Denomme, M.M.; Tekpetey, F.R.; Feyles, V.; Power, S.G.; Mann, M.R. High frequency of imprinted methylation errors in human preimplantation embryos. Sci. Rep. 2015, 5, 17311. [Google Scholar] [CrossRef]
- Huntriss, J.D.; Hemmings, K.E.; Hinkins, M.; Rutherford, A.J.; Sturmey, R.G.; Elder, K.; Picton, H.M. Variable imprinting of the MEST gene in human preimplantation embryos. Eur. J. Hum. Genet. 2013, 21, 40–47. [Google Scholar] [CrossRef]
- Chen, Z.; Robbins, K.M.; Wells, K.D.; Rivera, R.M. Large offspring syndrome: A bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics 2013, 8, 591–601. [Google Scholar] [CrossRef]
- Hiura, H.; Okae, H.; Chiba, H.; Miyauchi, N.; Sato, F.; Sato, A.; Arima, T. Imprinting methylation errors in ART. Reprod. Med. Biol. 2014, 13, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Lazaraviciute, G.; Kauser, M.; Bhattacharya, S.; Haggarty, P.; Bhattacharya, S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum. Reprod. Update 2014, 20, 840–852. [Google Scholar] [CrossRef]
- Vermeiden, J.P.; Bernardus, R.E. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil. Steril. 2013, 99, 642–651. [Google Scholar] [CrossRef]
- Henningsen, A.A.; Gissler, M.; Rasmussen, S.; Opdahl, S.; Wennerholm, U.B.; Spangsmose, A.L.; Tiitinen, A.; Bergh, C.; Romundstad, L.B.; Laivuori, H.; et al. Imprinting Disorders in Children Born After ART: A Nordic Study From the CoNARTaS Group. Hum. Reprod. 2020, 35, 1178–1184. [Google Scholar] [CrossRef]
- Hattori, H.; Hiura, H.; Kitamura, A.; Miyauchi, N.; Kobayashi, N.; Takahashi, S.; Okae, H.; Kyono, K.; Kagami, M.; Ogata, T.; et al. Association of four imprinting disorders and ART. Clin. Epigenetics 2019, 11, 21. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23 (Suppl. 6), 588S–595S. [Google Scholar] [CrossRef]
- Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; Simmers, T.A.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 2006, 84, 322–327. [Google Scholar] [CrossRef]
- Dumoulin, J.C.; Land, J.A.; Van Montfoort, A.P.; Nelissen, E.C.; Coonen, E.; Derhaag, J.G.; Schreurs, I.L.; Dunselman, G.A.; Kester, A.D.; Geraedts, J.P.; et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum. Reprod. 2010, 25, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Vergouw, C.G.; Kostelijk, E.H.; Doejaaren, E.; Hompes, P.G.; Lambalk, C.B.; Schats, R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum. Reprod. 2012, 27, 2619–2626. [Google Scholar] [CrossRef]
- Roberts, S.A.; Vail, A. On the appropriate interpretation of evidence: The example of culture media and birth weight. Hum. Reprod. 2017, 32, 1151–1154. [Google Scholar] [CrossRef]
- Kleijkers, S.H.; Mantikou, E.; Slappendel, E.; Consten, D.; van Echten-Arends, J.; Wetzels, A.M.; van Wely, M.; Smits, L.J.M.; van Montfoort, A.P.A.; Repping, S.; et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: A multicenter RCT. Hum. Reprod. 2016, 31, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Siargkas, A.; Tsakiridis, I.; Giouleka, S.; Chaveeva, P.; Mar Gil, M.; Plasencia, W.; De Paco Matallana, C.; Kolibianakis, E.M.; Dagklis, T. The Association of Assisted Reproductive Technology with Placental and Umbilical Abnormalities. J. Pers. Med. 2025, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Kleijkers, S.H.; Van Montfoort, A.P.; Smits, L.J.; Viechtbauer, W.; Roseboom, T.J.; Nelissen, E.C.; Coonen, E.; Derhaag, J.G.; Bastings, L.; Schreurs, I.E.L.; et al. IVF culture medium affects post-natal weight in humans during the first 2 years of life. Hum. Reprod. 2014, 29, 661–669. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wang, N.; Jin, F. Long-term follow-up of children conceived through assisted reproductive technology. J. Zhejiang Univ. Sci. B 2013, 14, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, M.; Lian, Y.; Chen, L.; Liu, P. No effect of embryo culture media on birthweight and length of newborns. Hum. Reprod. 2013, 28, 1762–1767. [Google Scholar] [CrossRef]
- De Vos, A.; Janssens, R.; Van de Velde, H.; Haentjens, P.; Bonduelle, M.; Tournaye, H.; Verheyen, G. The type of culture medium and the duration of in vitro culture do not influence birthweight of ART singletons. Hum. Reprod. 2015, 30, 20–27. [Google Scholar] [CrossRef]
- Eskild, A.; Monkerud, L.; Tanbo, T. Birthweight and placental weight; do changes in culture media used for IVF matter? Comparisons with spontaneous pregnancies in the corresponding time periods. Hum. Reprod. 2013, 28, 3207–3214. [Google Scholar] [CrossRef]
- Kleijkers, S.H.; Van Montfoort, A.P.; Smits, L.J.; Coonen, E.; Derhaag, J.G.; Evers, J.L.; Dumoulin, J.C. Age of G-1 PLUS v5 embryo culture medium is inversely associated with birthweight of the newborn. Hum. Reprod. 2015, 30, 1352–1357. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Chen, L.; Liu, P.; Qiao, J. The protein source in embryo culture media influences birthweight: A comparative study between G1 v5 and G1-PLUS v5. Hum. Reprod. 2014, 29, 1387–1392. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, S.; Li, M.; Chen, L.; Lian, Y.; Liu, P.; Qiao, J. Effect of in vitro culture period on birthweight of singleton newborns. Hum. Reprod. 2014, 29, 448–454. [Google Scholar] [CrossRef]
- Zandstra, H.; Van Montfoort, A.P.; Dumoulin, J.C. Does the type of culture medium used influence birthweight of children born after IVF? Hum. Reprod. 2015, 30, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Litzky, J.F.; Boulet, S.L.; Esfandiari, N.; Zhang, Y.; Kissin, D.M.; Theiler, R.N.; Marsit, C.J. Effect of frozen/thawed embryo transfer on birthweight, macrosomia, and low birthweight rates in US singleton infants. Am. J. Obstet. Gynecol. 2018, 218, 433.e1–433.e10. [Google Scholar] [CrossRef] [PubMed]

| Study [Ref] | Materials: Human or Animal | Oocytes or Embryo Analyzed (n) | Technology of Assessment | Studied Sequences or Genes | Main Findings |
|---|---|---|---|---|---|
| Al-Khtib et al., 2011 [147] | (Human) GV oocytes donated for research and IVM to MII | 77 MII after IVM from 184 vitrified GV stage, and 85 MII from 120 fresh GV | Pyrosequencing | Methylation profile of H19 and KCNQ1OT1, H19-DMR, and KvDMR1 | Oocyte vitrification at the GV stage does not affect the methylation profiles of H19-DMR and KvDMR1 |
| Zhao et al., 2020 [143] | (Bovine) Oocytes | Vitrified MII oocytes from matured in vitro | Single-cell whole-genome methylation sequencing | Global analysis | Peg3 methylation level was significantly decreased in derived blastocysts |
| Cheng et al., 2014 [145] | (Mouse) Blastocysts | Blastocysts from Vitrified MII oocytes | Bisulfite sequencing | H19, Peg3, Snrpn | No significant differences in oocytes. Decrease in blastocysts after oocyte vitrification. |
| De Munck et al., 2015 [149] | (Human) Mature (MII) donated oocytes | 31 embryos (Day-3) from 17 fresh oocytes and 14 after vitrification | Immunofluorescence (5mC, 5hmC) | Global Analysis | No differences in fluorescence intensities between embryos from fresh and vitrified oocytes |
| Liu et al., 2017 [148] | (Human) Vitrified mature oocytes (MII), and MII from GV matured in vitro | 56 in vivo MII, 106 MII from GV matured in vitro, 122 MII from vitrified GV | Immunofluorescence (5mC) | Global analysis | No significant differences in fluorescence intensities between the groups |
| Barberet et al., 2020 [156] | (Human) Placenta | Review manuscript | Pyrosequencing and q-PCR | H19, IGF2, KCNQ1OT1 SNURF | The placental DNA methylation levels of H19/IGF2 were lower in the fresh embryo transfer group than in the control (H19/IGF2-seq1) and frozen embryo transfer (H19/IGF2-seq2) groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciorio, R.; Tramontano, L.; Gullo, G.; Fleming, S. Association Between Human Embryo Culture Conditions, Cryopreservation, and the Potential Risk of Birth Defects in Children Conceived Through Assisted Reproduction Technology. Medicina 2025, 61, 1194. https://doi.org/10.3390/medicina61071194
Sciorio R, Tramontano L, Gullo G, Fleming S. Association Between Human Embryo Culture Conditions, Cryopreservation, and the Potential Risk of Birth Defects in Children Conceived Through Assisted Reproduction Technology. Medicina. 2025; 61(7):1194. https://doi.org/10.3390/medicina61071194
Chicago/Turabian StyleSciorio, Romualdo, Luca Tramontano, Giuseppe Gullo, and Steven Fleming. 2025. "Association Between Human Embryo Culture Conditions, Cryopreservation, and the Potential Risk of Birth Defects in Children Conceived Through Assisted Reproduction Technology" Medicina 61, no. 7: 1194. https://doi.org/10.3390/medicina61071194
APA StyleSciorio, R., Tramontano, L., Gullo, G., & Fleming, S. (2025). Association Between Human Embryo Culture Conditions, Cryopreservation, and the Potential Risk of Birth Defects in Children Conceived Through Assisted Reproduction Technology. Medicina, 61(7), 1194. https://doi.org/10.3390/medicina61071194










