Causes of Death in Childhood Acute Lymphoblastic Leukemia: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Relapsed Patient Characteristics
3.3. Treatment Outcomes and Survival Rates
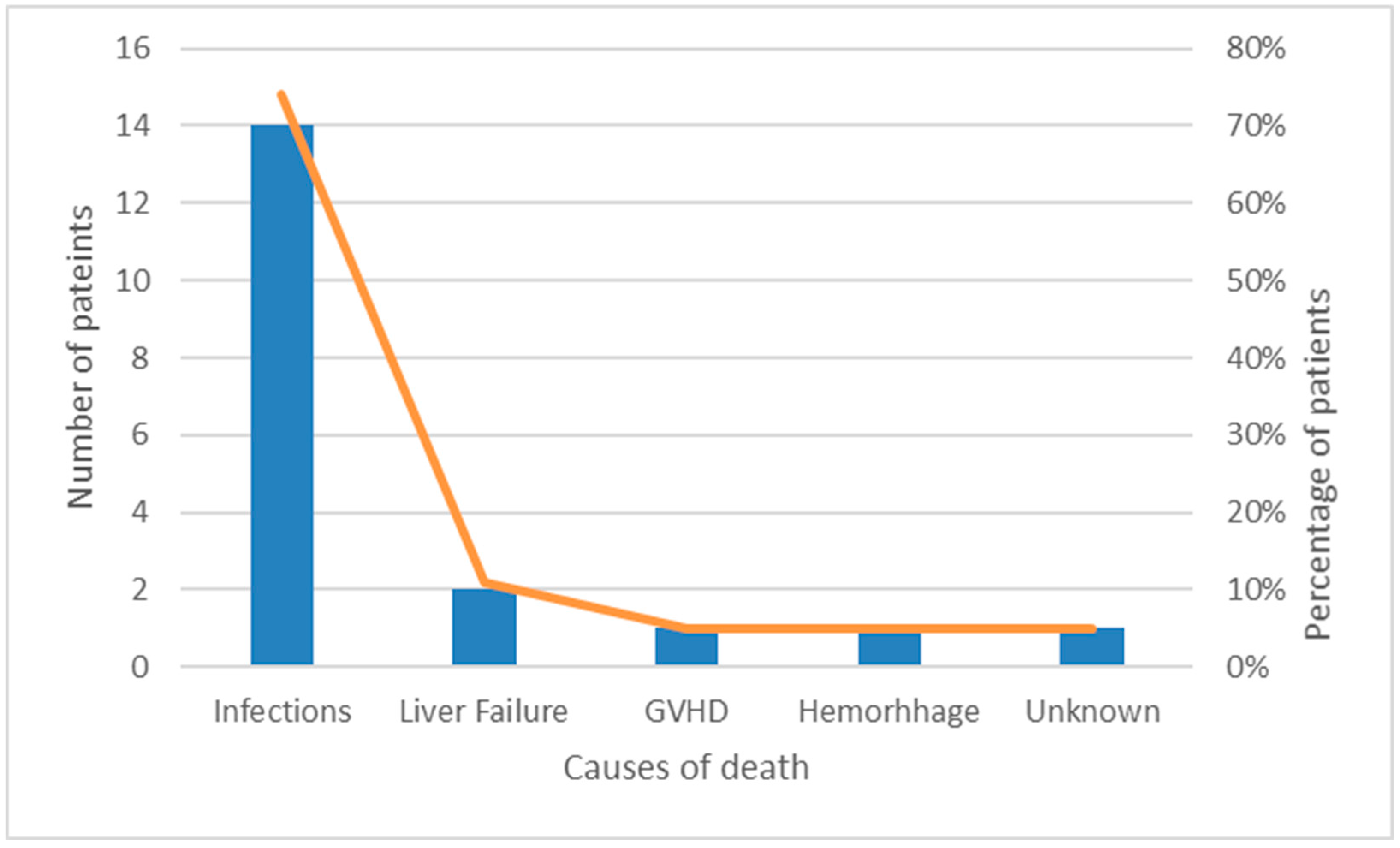
3.4. Mortality and Causes of Death
3.5. Treatment Protocols
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| OS | Overall survival |
| HSCT | Hematopoietic stem cell transplantation |
| CI | Confidence interval |
| EFS | Event-free survival |
| IFI | Invasive fungal infection |
References
- Seth, R.; Singh, A. Leukemias in Children. Indian J. Pediatr. 2015, 82, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukemia. Lancet 2014, 381, 1943–1955. [Google Scholar] [CrossRef]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.W.; Ward, K.C.; Bonaventure, A.; Siegel, D.A.; Coleman, M.P. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017, 123 (Suppl. S24), 5178–5189. [Google Scholar] [CrossRef]
- Campbell, M.; Kiss, C.; Zimmermann, M.; Riccheri, C.; Kowalczyk, J.; Felice, M.S.; Kuzmanovic, M.; Kovacs, G.; Kosmidis, H.; Gonzalez, A.; et al. Childhood Acute Lymphoblastic Leukemia: Results of the Randomized Acute Lymphoblastic Leukemia Intercontinental-Berlin-Frankfurt-Münster 2009 Trial. J. Clin. Oncol. 2023, 41, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Fu, J.; Liu, Y.; Wang, H.; Song, Y. Global, regional, and national burden of acute lymphoblastic leukemia in children: Epidemiological trends analysis from 1990 to 2021. IScience 2024, 27, 111356. [Google Scholar] [CrossRef]
- Hough, R.; Rowntree, C.; Goulden, N.; Mitchell, C.; Moorman, A.; Wade, R.; Vora, A. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: Results from UKALL 2003. Br. J. Haematol. 2016, 172, 439–451. [Google Scholar] [CrossRef]
- Stary, J.; Zimmermann, M.; Campbell, M.; Castillo, L.; Dibar, E.; Donska, S.; Gonzalez, A.; Izraeli, S.; Janic, D.; Jazbec, J.; et al. Intensive Chemotherapy for Childhood Acute Lymphoblastic Leukemia: Results of the Randomized Intercontinental Trial ALL IC-BFM 2002. Am. Soc. Clin. Oncol. 2019, 32, 172–184. [Google Scholar] [CrossRef]
- Toft, N.; Birgens, H.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Heyman, M.; Klausen, T.W.; et al. Toxicity profile and treatment delays in NOPHO ALL2008—Comparing adults and children with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur. J. Haematol. 2015, 96, 160–169. [Google Scholar] [CrossRef]
- Pui, C.H.; Pei, D.; Raimondi, S.C.; Coustan-Smith, E.; Jeha, S.; Cheng, C.; Bowman, W.P.; Sandlund, J.T.; Ribeiro, R.C.; Rubnitz, J.E.; et al. Clinical Impact of Minimal Residual Disease in Children with Different Subtypes of Acute Lymphoblastic Leukemia Treated with Response-Adapted Therapy. Leukemia 2017, 31, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Veerman, A.J.; Kamps, W.A.; van den Berg, H.; van den Berg, E.; Bökkerink, J.P.; Bruin, M.C.; van den Heuvel-Eibrink, M.M.; Korbijn, C.M.; Korthof, E.T.; van der Pal, K.; et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: Results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004). Lancet Oncol. 2009, 10, 957–966. [Google Scholar] [CrossRef]
- Place, A.E.; Stevenson, K.E.; Vrooman, L.M.; Harris, M.H.; Hunt, S.K.; O’Brien, J.E.; Supko, J.G.; Asselin, B.L.; Athale, U.H.; Clavell, L.A.; et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol. 2015, 16, 1677–1690. [Google Scholar] [CrossRef]
- Stolpa, W.; Zapała, M.; Zwiernik, B.; Mizia-Malarz, A. Relapses Children’s Acute Lymphoblastic Leukemia, Single Center Experience. Children 2022, 9, 1874. [Google Scholar] [CrossRef]
- Wang, N.; Qi, B.Q.; Ruan, M.; Zhang, X.Y.; Zhang, R.R.; Liu, T.F.; Chen, Y.M.; Zou, Y.; Guo, Y.; Yang, W.Y.; et al. Clinical features and long-term prognostic analysis of relapsed pediatric acute lymphoblastic leukemia. Zhonghua Er Ke Za Zhi 2024, 62, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Rheingold, S.R.; Bhojwani, D.; Ji, L.; Xu, X.; Devidas, M.; Kairalla, J.A.; Shago, M.; Heerema, N.A.; Carroll, A.J.; Breidenbach, H.; et al. Determinants of survival after first relapse of acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia 2024, 38, 2382–2394. [Google Scholar] [CrossRef]
- Freyer, D.R.; Devidas, M.; La, M.; Carroll, W.L.; Gaynon, P.S.; Hunger, S.P.; Seibel, N.L. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: A report from the Children’s Oncology Group. Blood J. 2016, 117, 3010–3016. [Google Scholar] [CrossRef]
- Kiem Hao, T.; Nhu Hiep, P.; Kim Hoa, N.T.; Van Ha, C. Causes of Death in Childhood Acute Lymphoblastic Leukemia at Hue Central Hospital for 10 Years (2008–2018). Glob. Pediatr. Health 2020, 7, 2333794X20901930. [Google Scholar] [CrossRef] [PubMed]
- Caniza, M.A.; Odio, C.; Mukkada, S.; Gonzalez, M.; Ceppi, F.; Chaisavaneeyakorn, S.; Apiwattanakul, N.; Howard, S.C.; Conter, V.; Bonilla, M. Infectious complications in children with acute lymphoblastic leukemia treated in low-middle-income countries. Expert Rev. Hematol. 2015, 8, 627–645. [Google Scholar] [CrossRef]
- Ehrlich, B.S.; McNeil, M.J.; Pham, L.T.; Chen, Y.; Rivera, J.; Acuna, C.; Sniderman, L.; Sakaan, F.M.; Aceituno, A.M.; Villegas, C.A.; et al. Treatment-related mortality in children with cancer in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Oncol. 2023, 24, 967–977. [Google Scholar] [CrossRef]
- Jeha, S.; Pei, D.; Choi, J.; Cheng, C.; Sandlund, J.T.; Coustan-Smith, E.; Campana, D.; Inaba, H.; Rubnitz, J.E.; Ribeiro, R.C.; et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J. Clin. Oncol. 2019, 37, 3377–3391. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; de Groot-Kruseman, H.; Van der Velden, V.; Fiocco, M.; van den Berg, H.; de Bont, E.; Egeler, R.M.; Hoogerbrugge, P.; Kaspers, G.; Van der Schoot, E.; et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J. Clin. Oncol. 2016, 34, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Horibe, K.; Yumura-Yagi, K.; Kudoh, T.; Nishimura, S.; Oda, M.; Yoshida, M.; Komada, Y.; Hara, J.; Tawa, A.; Usami, I. Long-term results of the risk-adapted treatment for childhood B-cell acute lymphoblastic leukemia: Report from the Japan association of childhood leukemia study ALL-97 trial. J. Pediatr. Hematol. Oncol. 2017, 39, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Xie, L.; McCahan, S.M. Characterization of Pediatric Acute Lymphoblastic Leukemia Survival Patterns by Age at Diagnosis. J. Cancer Epidemiol. 2014, 2014, 865979. [Google Scholar] [CrossRef]
- Rheingold, S.R.; Ji, L.; Xu, X.; Devidas, M.; Brown, P.A.; Gore, L.; Winick, N.J.; Carroll, W.L.; Hunger, S.; Raetz, E.A.; et al. Prognostic factors for survival after relapsed acute lymphoblastic leukemia (ALL): A Children’s Oncology Group (COG) study. J. Clin. Oncol. 2019, 37, 10008. [Google Scholar] [CrossRef]
- Maloney, K.W.; Devidas, M.; Wang, C.; Mattano, L.A.; Friedmann, A.M.; Buckley, P.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; et al. Outcome in Children with Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0331. J. Clin. Oncol. 2019, 38, 602–612. [Google Scholar] [CrossRef]
- Rajeswari, B.; Nair, R.K.S.; Guruprasad, C.S.; Nair, M.; Thankamony, P.; Parukutty, K. Infections during Induction Chemotherapy in Children with Acute Lymphoblastic Leukemia—Profile and Outcomes: Experience from a Cancer Center in South India. Indian J. Med. Paediatr. Oncol. 2021, 39, 188–192. [Google Scholar] [CrossRef]
- Inaba, H.; Pei, D.; Wolf, J.; Howard, S.C.; Hayden, R.T.; Go, M.; Varechtchouk, O.; Hahn, T.; Buaboonnam, J.; Metzger, M.L.; et al. Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann. Oncol. 2017, 28, 386–392. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kato, M.; Imamura, T.; Imai, C.; Koh, K.; Kawano, Y.; Shimomura, Y.; Watanabe, A.; Kikuta, A.; Saito, A.; et al. In-Hospital Management Might Reduce Induction Deaths in Pediatric Patients with Acute Lymphoblastic Leukemia: Results From a Japanese Cohort. J. Pediatr. Hematol. Oncol. 2021, 43, 39–46. [Google Scholar] [CrossRef]
- Ruijters, V.J.; Oosterom, N.; Wolfs, T.F.; van den Heuvel-Eibrink, M.M.; van Grotel, M. Frequency and Determinants of Invasive Fungal Infections in Children with Solid and Hematologic Malignancies in a Nonallogeneic Stem Cell Transplantation Setting: A Narrative Review. J. Pediatr. Hematol. Oncol. 2019, 41, 345–354. [Google Scholar] [CrossRef]
- Rambaldi, B.; Russo, D.P.L. Letters to the Editor: Defining Invasive Fungal Infection Risk in Hematological Malignancies: A New Tool for Clinical Practice. Mediterr. J. Hematol. Infect. Dis. 2017, 9, 12. [Google Scholar] [CrossRef]
- Hernandez, H.; Martinez, L.R. Relationship of environmental disturbances and the infectious potential of fungi. Microbiology 2018, 164, 233–241. [Google Scholar] [CrossRef]
- Kanamori, H.; Rutala, W.A.; Sickbert-Bennett, E.E.; Weber, D.J. Review of Fungal Outbreaks and Infection Prevention in Healthcare Settings During Construction and Renovation. Helathcare Epidemiol. 2015, 61, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Kanda, J.; Hishizawa, M.; Kitano, T.; Kondo, T.; Yamashita, K.; Takaori-Kondo, A. Effect of laminar air flow and building construction on aspergillosis in acute leukemia patients: A retrospective cohort study. BMC Infect. Dis. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Tuong, P.N.; Kiem Hao, T.; Kim Hoa, N.T. Relapsed Childhood Acute Lymphoblastic Leukemia: A Single-Institution Experience. Cureus 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Abdelmabood, S.; Elsayed, A.; Boujettif, F.; Mansour, A. Treatment outcomes of children with acute lymphoblastic leukemia in a middle-income developing country: High mortalities, early relapses, and poor survival. J. Pediatr. (Rio J.) 2018, 96, 108–116. [Google Scholar] [CrossRef]
- Kelly, M.E.; Lu, X.; Devidas, M.; Camitta, B.; Abshire, T.; Bernstein, M.L.; Billett, A.; Homans, A.; Sandler, E.; Buchanan, G. Treatment of Relapsed Precursor-B Acute Lymphoblastic Leukemia with Intensive Chemotherapy: POG (Pediatric Oncology Group) Study 9411 (SIMAL 9). J. Pediatr. Hematol. Oncol. 2013, 35, 509–513. [Google Scholar] [CrossRef]
- Möricke, A.; Reiter, A.; Zimmermann, M.; Gadner, H.; Stanulla, M.; Dördelmann, M.; Löning, L.; Beier, R.; Ludwig, W.D.; Ratei, R.; et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008, 111, 4477–4489. [Google Scholar] [CrossRef]
- Biondi, A.; Schrappe, M.; De Lorenzo, P.; Castor, A.; Lucchini, G.; Gandemer, V.; Pieters, R.; Stary, J.; Escherich, G.; Campbell, M.; et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): A randomised, open-label, intergroup study. Lancet Oncol. 2012, 13, 936–945. [Google Scholar] [CrossRef]
- Biondi, A.; Gandemer, V.; De Lorenzo, P.; Cario, G.; Campbell, M.; Castor, A.; Pieters, R.; Baruchel, A.; Vora, A.; Leoni, V.; et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): A prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. 2018, 5, e641–e652. [Google Scholar] [CrossRef]
- van der Sluis, I.M.; de Lorenzo, P.; Kotecha, R.S.; Attarbaschi, A.; Escherich, G.; Nysom, K.; Stary, J.; Ferster, A.; Brethon, B.; Locatelli, F.; et al. Blinatumomab Added to Chemotherapy in Infant Lymphoblastic Leukemia. N. Engl. J. Med. 2023, 388, 1572–1581. [Google Scholar] [CrossRef] [PubMed]

| Number of Patients (%) | 5-Year EFS (%) | 5-Year OS (%) | Number of Relapsed Patients | Percentage of Relapsed Patients | |
|---|---|---|---|---|---|
| In Relation to Overall Number at the Diagnosis (%) | |||||
| Patients | 302 (100%) | 80% | 83% | 41 | 14% |
| Gender | |||||
| Male | 178 (59%) | 77% | 81% | 30 | 17% |
| Female | 124 (41%) | 85% | 86% | 11 | 9% |
| Age groups | |||||
| Infants | 8 (3%) | 50% | 50% | 4 | 50% |
| 1–4 y. | 165 (55%) | 85% | 88% | 16 | 10% |
| 5–9 y. | 66 (22%) | 77% | 83% | 11 | 17% |
| 10–17 y. | 63 (21%) | 73% | 73% | 10 | 16% |
| Immunophenotype | |||||
| common | 181 (60%) | 82% | 86% | 22 | 12% |
| pre B | 63 (21%) | 73% | 78% | 12 | 19% |
| pro B | 5 (1%) | 60% | 60% | 2 | 40% |
| T | 53 (18%) | 83% | 83% | 5 | 10% |
| Risk groups | |||||
| SR | 69 (23%) | 87% | 96% | 4 | 6% |
| IR | 154 (51%) | 85% | 89% | 16 | 10% |
| HR | 79 (26%) | 53% | 63% | 21 | 27% |
| Number | Percentage | Survival | 5-Year OS (%) | |
|---|---|---|---|---|
| Patients | 41 | 100% | 9 | 22% |
| GENDER | ||||
| Male | 30 | 73% | 7 | 23% |
| Female | 11 | 27% | 2 | 18% |
| AGE GROUPS | ||||
| Infants (0 to <12 months) | 4 | 10% | 0 | 0% |
| 1–4 y. | 16 | 39% | 5 | 31% |
| 5–9 y. | 11 | 27% | 4 | 36% |
| 10–17 y. | 10 | 24% | 0 | 0% |
| IMMUNOPHENOTYPE | ||||
| common | 22 | 54% | 6 | 27% |
| pre B | 12 | 29% | 3 | 25% |
| pro B | 2 | 5% | 0 | 0% |
| T | 5 | 12% | 0 | 0% |
| RISK GROUPS | ||||
| SR | 4 | 10% | 1 | 25% |
| IR | 16 | 39% | 7 | 44% |
| HR | 21 | 51% | 1 | 5% |
| TIME OF RELAPSE | ||||
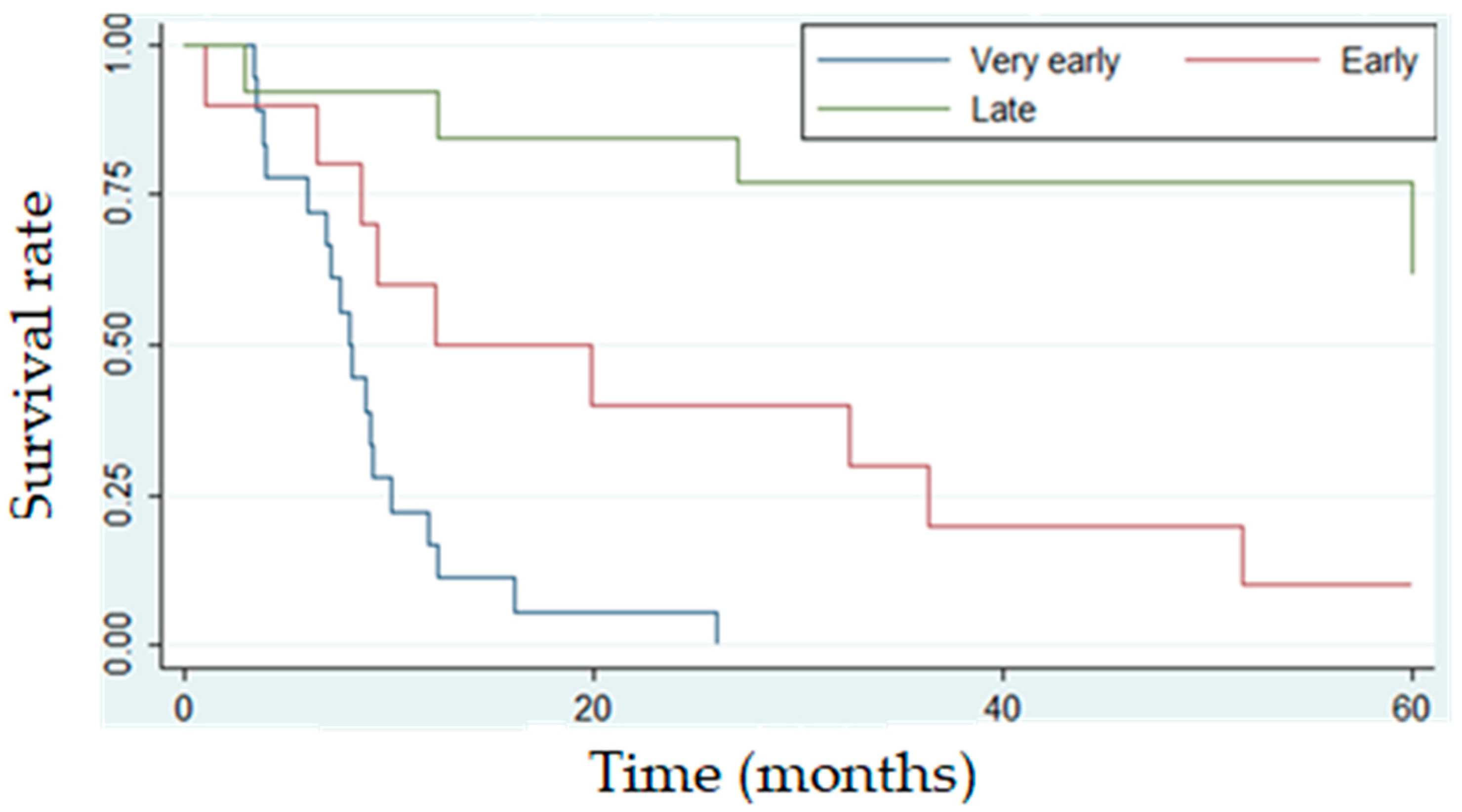
| Very early | 18 | 44% | 0 | 0% |
| Early | 10 | 24% | 1 | 10% |
| Late | 13 | 32% | 8 | 62% |
| SITE OF RELAPSE | ||||
| Bone marrow | 27 | 66% | 6 | 22% |
| Isolated CNS | 1 | 2% | 1 | 100% |
| Bone marrow + CNS | 13 | 32% | 2 | 15% |
| TREATMENT | ||||
| Chemotherapy | 22 | 54% | 3 | 14% |
| Chemotherapy + HSCT | 19 | 46% | 6 | 32% |
| SITE OF RELAPSE | ||||
| Bone marrow | 27 | 66% | 6 | 22% |
| Isolated CNS | 1 | 2% | 1 | 100% |
| Bone marrow + CNS | 13 | 32% | 2 | 15% |
| TREATMENT | ||||
| Chemotherapy | 22 | 54% | 3 | 14% |
| Chemotherapy + HSCT | 19 | 46% | 6 | 32% |
| Analysis | Univariate HR | p | Multivariate HR | p |
|---|---|---|---|---|
| INITIAL DIAGNOSIS | ||||
| Relapse | ||||
| No | 1.00 | Reference | 1.00 | Reference |
| Yes | 15.78 (8.76–28.43) | <0.05 | 13.24 (6.92–25.37) | <0.05 |
| Sex | ||||
| Male | 1.00 | Reference | 1.00 | Reference |
| Female | 0.66 (0.37–1.2) | 0.17 | 1.02 (0.54–1.91) | 0.96 |
| Age groups | ||||
| Infants | 1.00 | Reference | 1.00 | Reference |
| 1–4 y. | 0.19 (0.06–0.55) | 0.002 | 1.07 (0.33–3.54) | 0.9 |
| 5–9 y. | 0.24 (0.08–0.77) | 0.016 | 0.66 (0.19–2.3) | 0.52 |
| 10–17 y. | 0.43 (0.14–1.28) | 0.128 | 1.03 (0.33–3.27) | 0.95 |
| Immunophenotype | ||||
| common | 1.00 | Reference | 1.00 | Reference |
| pre B | 1.72 (0.89–3.31) | 0.104 | 1.41 (0.72–2.77) | 0.32 |
| pro B | 3.6 (0.85–15.22) | 0.082 | 1.48 (0.32–6.73) | 0.61 |
| T ALL | 1.3 (0.61–2.78) | 0.502 | 1.75 (0.77–3.98) | 0.18 |
| Risk group | ||||
| SR | 1.00 | Reference | 1.00 | Reference |
| IR | 2.51 (0.73–8.64) | 0.142 | 2.18 (0.61–7.75) | 0.23 |
| HR | 11.76 (3.59–38.5) | <0.05 | 6.85 (1.87–24.9) | <0.05 |
| Analysis | Univariate HR | p | Multivariate HR | p |
|---|---|---|---|---|
| RELAPSED PATIENTS | ||||
| Sex | ||||
| male | 1.00 | Reference | 1.00 | Reference |
| female | 1.02 (0.47–2.2) | 0.95 | 1.06 (0.44–2.57) | 0.897 |
| Age groups | ||||
| infants | 1.00 | Reference | 1.00 | Reference |
| 1–4 y. | 0.14 (0.04–0.5) | <0.05 | 0.36 (0.05–2.78) | 0.33 |
| 5–9 y. | 0.14 (0.03–0.52) | <0.05 | 0.81 (0.14–4.69) | 0.81 |
| 10–17 y. | 0.29 (0.08–1.03) | 0.055 | 0.72 (0.14–3.59) | 0.69 |
| Immunophenotype | ||||
| common | 1.00 | Reference | 1.00 | Reference |
| pre B | 1.96 (0.87–4.4) | 0.102 | 2.16 (0.66–7.1) | 0.21 |
| pro B | 23.56 (3.74–148.2) | <0.05 | 7.17 (0.76–67.76) | 0.086 |
| T ALL | 9.1 (2.77–29.9) | <0.05 | 2.87 (0.49–16.84) | 0.24 |
| Risk group | ||||
| Standard risk | 1.00 | Reference | 1.00 | Reference |
| Intermediate risk | 0.95 (0.26–3.5) | 0.94 | 3.08 (0.44–21.69) | 0.26 |
| High risk | 3.57 (1.04–12.27) | 0.043 | 14.18 (1.2–167.4) | 0.035 |
| Time of relapse | ||||
| very early | 1.00 | Reference | 1.00 | Reference |
| early | 0.1 (0.03–0.32) | <0.05 | 0.22 (0.03–1.55) | 0.129 |
| late | 0.02 (0.004–0.08) | <0.05 | 0.008 (0.001–0.06) | <0.05 |
| Site of relapse | ||||
| Bone marrow | 1.00 | Reference | 1.00 | Reference |
| Isolated CNS | / | / | / | / |
| Bone marrow + CNS | 1.05 (0.5–2.2) | 0.9 | 2.27 (0.6–8.4) | 0.22 |
| Chemotherapy/HSCT | ||||
| Chemotherapy + HSCT | 1.00 | Reference | 1.00 | Reference |
| Chemotherapy | 1.01 (0.5–2.02) | 0.982 | 1.54 (0.64–3.74) | 0.34 |
| Number of Patients (%) | 5-Year OS (%) | Number of Relapsed Patients | Percentage of Relapsed Patients | |
|---|---|---|---|---|
| Interfant 99/Interfant 06 | 8 (2.6%) | 50.0% | 4 | 50.0% |
| ALL-BFM 95 | 54 (17.9%) | 81.5% | 9 | 16.7% |
| ALL IC-BFM 2002 | 134 (44.3%) | 85.8% | 17 | 12.7% |
| ALL IC-BFM 2009 | 103 (34.1%) | 83.5% | 10 | 9.7% |
| EsPhALL2004/EsPhALL2010 2010 | 3 (1.0%) | 66.7% | 1 | 33.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelić, M.; Pavlović, M.; Mucavac, L.; Dejanović Bekić, S.; Šalek, Z.; Matić, T.; Turudić, D.; Lovrenčić, L.; Roganović, J.; Bilić, E. Causes of Death in Childhood Acute Lymphoblastic Leukemia: A Single-Center Experience. Medicina 2025, 61, 1193. https://doi.org/10.3390/medicina61071193
Jelić M, Pavlović M, Mucavac L, Dejanović Bekić S, Šalek Z, Matić T, Turudić D, Lovrenčić L, Roganović J, Bilić E. Causes of Death in Childhood Acute Lymphoblastic Leukemia: A Single-Center Experience. Medicina. 2025; 61(7):1193. https://doi.org/10.3390/medicina61071193
Chicago/Turabian StyleJelić, Matej, Maja Pavlović, Lucija Mucavac, Sara Dejanović Bekić, Zrinko Šalek, Toni Matić, Daniel Turudić, Luka Lovrenčić, Jelena Roganović, and Ernest Bilić. 2025. "Causes of Death in Childhood Acute Lymphoblastic Leukemia: A Single-Center Experience" Medicina 61, no. 7: 1193. https://doi.org/10.3390/medicina61071193
APA StyleJelić, M., Pavlović, M., Mucavac, L., Dejanović Bekić, S., Šalek, Z., Matić, T., Turudić, D., Lovrenčić, L., Roganović, J., & Bilić, E. (2025). Causes of Death in Childhood Acute Lymphoblastic Leukemia: A Single-Center Experience. Medicina, 61(7), 1193. https://doi.org/10.3390/medicina61071193





