High Insertion Torque—Clinical Implications and Drawbacks: A Scoping Review
Abstract
1. Introduction
Focused Questions
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Research
2.4. Quality Assessment of Included Studies
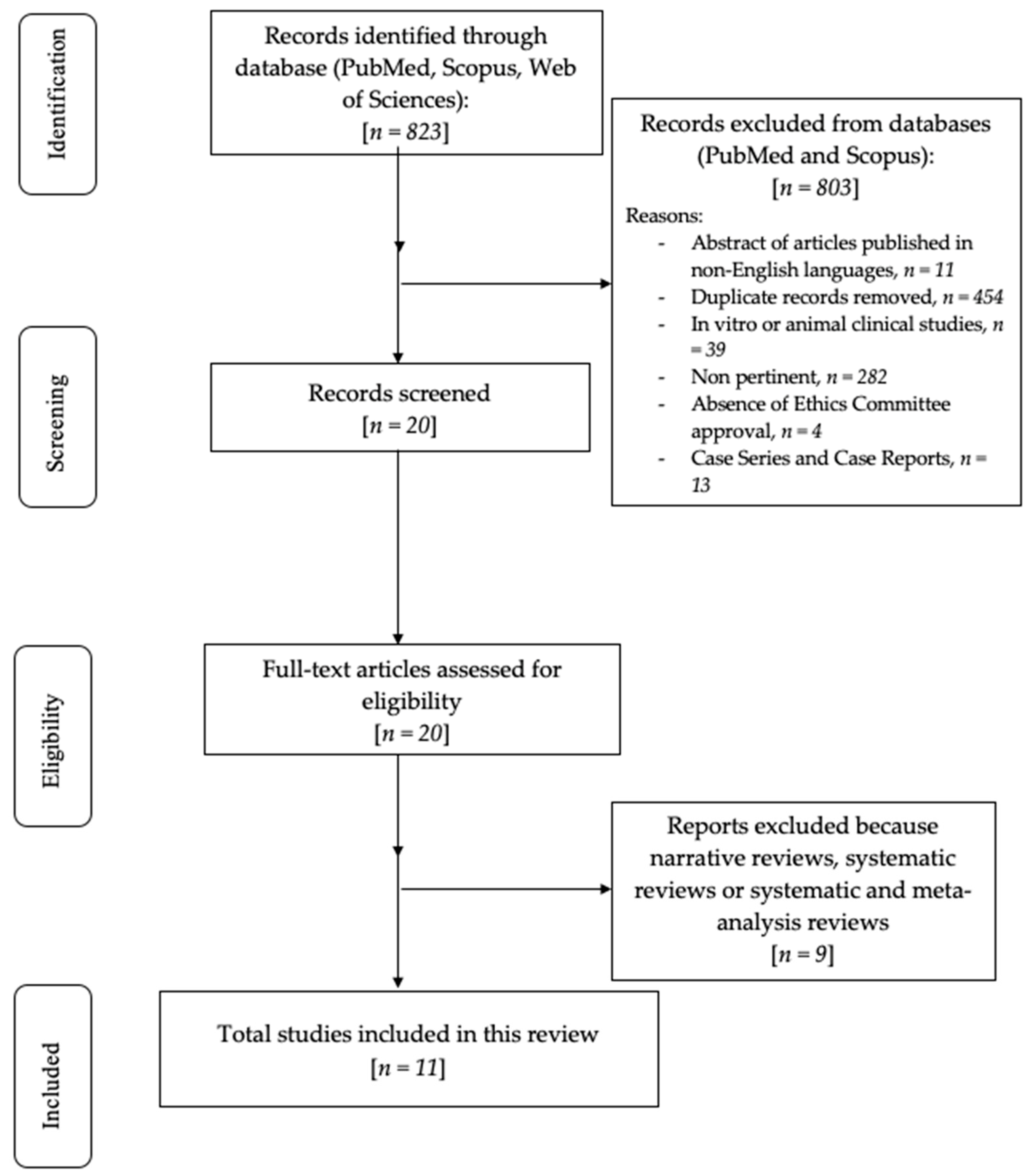
3. Results
Risk of Bias
4. Discussion
4.1. Marginal Bone Loss and Implant Failure
4.2. Insertion Torque and Primary Stability
4.3. Impact of IT on Bone Compression and Necrosis
4.4. Marginal Bone Loss: Comparative Findings
4.5. High Insertion Torque and Soft Tissue Level
4.6. High Insertion Torque and Implant Failure
4.7. High Insertion Torque and Osseointegration
4.8. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Branemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Bavetta, G.; Randazzo, V.; Cavataio, A.; Paderni, C.; Grassia, V.; Dipalma, C.; Isacco, C.G.; Scarano, A.; De Vito, D.; Cantore, S.; et al. A retrospective study on insertion torque and implant stability quotients (ISQ) as stability parameters for immediate loading of implants in fresh extraction sockets. Biomed Res. Int. 2019, 2019, 9720419. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Galli, F.; Capelli, M.; Zuffetti, F.; Testori, T.; Esposito, M. Immediate non-occlusal vs. early loading of dental implants in partially edentulous patients: A multicentre randomized clinical trial. Clin. Oral Implant. Res. 2008, 19, 546–552. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Zheng, Q. Meta-analysis of correlations between marginal bone resorption and high insertion torque of dental implants. Int. J. Oral Maxillofac. Implant. 2015, 30, 767–772. [Google Scholar] [CrossRef][Green Version]
- Greenstein, G.; Cavallaro, J. Implant insertion torque: Its role in achieving primary stability of restorable dental implants. Compend. Contin. Educ. Dent. 2017, 38, 88–95. [Google Scholar]
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C.; Tozum, T. Correlation between insertion torque and implant stability quotient in tapered implants with knife-edge thread design. Biomed Res. Int. 2018, 2018, 7201093. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Pinas, L.; Orive, G. Efficacy of biologically guided implant site preparation to obtain adequate primary implant stability. Ann. Anat. 2015, 199, 9–15. [Google Scholar] [CrossRef]
- Lee, H.C.; Tsai, P.I.; Huang, C.C.; Chen, S.Y.; Chao, C.G.; Tsou, N.T. Numerical method for the design of healing chamber in additive-manufactured dental implants. Biomed Res. Int. 2017, 2017, 1970680. [Google Scholar] [CrossRef]
- Eom, T.G.; Kim, H.W.; Jeon, G.R.; Yun, M.J.; Huh, J.B.; Jeong, C.M. Effects of different implant osteotomy preparation sizes on implant stability and bone response in the minipig mandible. Int. J. Oral Maxillofac. Implant. 2016, 31, 997–1006. [Google Scholar] [CrossRef]
- Marin, C.; Bonfante, E.; Granato, R.; Neiva, R.; Gil, L.F.; Marão, H.F.; Suzuki, M.; Coelho, P.G. The effect of osteotomy dimen-sion on implant insertion torque, healing mode, and osseointegration indicators: A study in dogs. Implant Dent. 2016, 25, 739–743. [Google Scholar] [CrossRef]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The effect of implant placement torque on crestal bone remodeling after 1 year of loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotionis related to peak insertion torque and bonedensity. Clin. Oral Implant. Res. 2009, 20, 467. [Google Scholar] [CrossRef]
- Irinakis, T.; Wiebe, C. Initial torque stability of a new bone condensing dental implant. A cohort study of 140 consecutively placed implants. J. Oral Implantol. 2009, 35, 277–282. [Google Scholar] [CrossRef]
- Makary, C.; Rebaudi, A.; Mokbel, N.; Naaman, N. Peak insertion torque correlated to histologically and clinically evaluated bone density. Implant Dent. 2011, 20, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Calandriello, R.; Tomatis, M.; Rangert, B. Immediate functional loading of Brånemark system implants with enhanced initial stability: A prospective 1- to 2-year clinical and radiographic study. Clin. Implant Dent. Relat. Res. 2003, 5, 10–20. [Google Scholar] [CrossRef]
- Beer, A.; Gahleitner, A.; Holm, A.; Tschabitscher, M.; Homolka, P. Correlation of insertion torques with bone mineral density from dental quantitative CT in the mandible. Clin. Oral Implant. Res. 2003, 14, 616–620. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Sennerby, L.; Meredith, N. Measurements com- paring the initial stability of five designs of dental implants: A human cadaver study. Clin. Implant Dent. Relat. Res. 2000, 2, 85–92. [Google Scholar] [CrossRef]
- Nikellis, I.; Levi, A.; Nicolopoulos, C. Immediate loading of 190 endosseous dental implants: A prospective observational study of 40 patients treatments with up to 2-years data. Int. J. Oral Maxillofac. Implant. 2004, 19, 116–123. [Google Scholar]
- Skalak, R.; Zhao, Y. Interaction of force-fitting and surface roughness of implants. Clin. Implant Dent. Relat. Res. 2000, 2, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Mir-Mari, J.; Hämmerle, C.H. Loading protocols for single-implant crowns: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2014, 29, 222–238. [Google Scholar] [CrossRef]
- Sotto-Maior, B.S.; Rocha, E.P.; Almeida, E.O.D.; Freitas-Júnior, A.C.; Anchieta, R.B.; Cury, A.A.D.B. Influence of high insertion torque on implant placement—An anisotropic bone stress analysis. Braz. Dent. J. 2010, 21, 508–514. [Google Scholar] [CrossRef]
- Ottoni, J.M.; Oliveira, Z.F.; Mansini, R.; Cabral, A.M. Correlation between placement torque and survival of single-tooth implants. Int. J. Oral Maxillofac. Implant. 2005, 20, 769–776. [Google Scholar]
- Maló, P.; Lopes, A.; De Araújo Nobre, M.; Ferro, A. Immediate function dental implants inserted with less than 30 N·cm of torque in full-arch maxillary rehabilitations using the all-on-4 concept: Retrospective study. Int. J. Oral Maxillofac. Surg. 2018, 47, 1079–1085. [Google Scholar] [CrossRef]
- Verardi, S.; Swoboda, J.; Rebaudi, F.; Rebaudi, A. Osseointegration of tissue-level implants with very low insertion torque in soft bone: A clinical study on SLA surface treatment. Implant Dent. 2018, 27, 5–9. [Google Scholar] [CrossRef]
- Norton, M.R. The influence of low insertion torque on primary stability, implant survival, and maintenance of marginal bone levels: A closed-cohort prospective study. Int. J. Oral Maxillofac. Implant. 2017, 32, 849–857. [Google Scholar] [CrossRef]
- Rizkallah, N.; Fischer, S.; Kraut, R.A. Correlation between insertion torque and survival rates in immediately loaded implants in the maxilla: A retrospective study. Implant Dent. 2013, 22, 250–254. [Google Scholar] [CrossRef]
- Nisapakultorn, K.; Suphanantachat, S.; Silkosessak, O.; Rattanamongkolgul, S. Factors affecting soft tissue level around anterior maxillary single-tooth implants. Clin. Oral Implant. Res. 2010, 21, 662–670. [Google Scholar] [CrossRef]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The effect of insertion torque on the clinical outcome of single implants: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef]
- Alfonsi, F.; Borgia, V.; Barbato, L.; Tonelli, P.; Giammarinaro, E.; Marconcini, S.; Romeggio, S.; Barone, A. The clinical effects of insertion torque for implants placed in healed ridges: A two-year randomized controlled clinical trial. J. Oral Sci. Rehabil. 2016, 2, 62–73. [Google Scholar]
- Hof, M.; Pommer, B.; Strbac, G.D.; Vasak, C.; Agis, H.; Zechner, W. Impact of insertion torque and implant neck design on peri-implant bone level: A randomized split-mouth trial. Clin. Implant Dent. Relat. Res. 2014, 16, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2020. Available online: https://synthesismanual.jbi.global (accessed on 30 April 2025).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tool. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 April 2025).
- Marconcini, S.; Giammarinaro, E.; Toti, P.; Alfonsi, F.; Covani, U.; Barone, A. Longitudinal analysis on the effect of insertion torque on delayed single implants: A 3-year randomized clinical study. Clin. Implant Dent. Relat. Res. 2018, 20, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Bidgoli, M.; Soheilifar, S.; Faradmal, J.; Soheilifar, S. High insertion torque and peri-implant bone loss: Is there a relationship? J. Long. Term. Eff. Med. Implant. 2015, 25, 209–213. [Google Scholar] [CrossRef]
- Khayat, P.G.; Arnal, H.M.; Tourbah, B.I.; Sennerby, L. Clinical outcome of dental implants placed with high insertion torques (up to 176 Ncm). Clin. Implant Dent. Relat. Res. 2013, 15, 227–233. [Google Scholar] [CrossRef]
- Oskouei, A.B.; Golkar, M.; Badkoobeh, A.; Jahri, M.; Sadeghi, H.M.M.; Mohammadikhah, M.; Abbasi, K.; Tabrizi, R.; Alam, M. Investigating the effect of insertion torque on marginal bone loss around dental implants. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101523. [Google Scholar] [CrossRef]
- Grandi, T.; Guazzi, P.; Samarani, R.; Grandi, G. Clinical outcome and bone healing of implants placed with high insertion torque: 12-month results from a multicenter controlled cohort study. Int. J. Oral Maxillofac. Surg. 2013, 42, 516–520. [Google Scholar] [CrossRef]
- Bailey, E.; Kashbour, W.; Shah, N.; Worthington, H.V.; Renton, T.F.; Coulthard, P. Surgical techniques for the removal of mandibular wisdom teeth. Cochrane Database Syst. Rev. 2020, 7, CD004345. [Google Scholar] [PubMed]
- Meredith, N.; Book, K.; Friberg, B.; Jemt, T.; Sennerby, L. Resonance frequency measurements of implant stability in vivo. Clin. Oral Implant. Res. 1997, 8, 226–233. [Google Scholar] [CrossRef]
- McArdle, L.W.; Andiappan, M.; Khan, I.; Jones, J.; McDonald, F. Diseases associated with mandibular third molar teeth. Br. Dent. J. 2018, 224, 434–440. [Google Scholar] [CrossRef]
- Camps-Font, O.; Martín-Fatás, P.; Clé-Ovejero, A.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Postoperative infections after dental implant placement: Variables associated with increased risk of failure. J. Periodontol. 2018, 89, 1165–1173. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; López-López, P.J.; Pérez-Albacete Martínez, C.; Javed, F.; Granero-Marín, J.M.; Maté Sánchez de Val, J.E.; Ramírez Fernández, M.P. Peri-implant bone loss clinical and radiographic evaluation around rough neck and microthread implants: A 5-year study. Clin. Oral Implant. Res. 2018, 29, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wennerberg, A.; Albrektsson, T. Reasons for marginal bone loss around oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 792–807. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Lekholm, U.; Wennström, J.L. Tissue alterations at implant-supported single-tooth replacements: A 1-year prospective clinical study. Clin. Oral Implant. Res. 2006, 17, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Iannello, G.; Peñarocha, M.; Garcia, B. Impact of implant diameter on bone level changes around platform switched implants: Preliminary results of 18 months follow-up a prospective randomized match-paired controlled trial. Clin. Oral Implant. Res. 2012, 23, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Fauri, M.; Avila-Ortiz, G.; Fernández-Barbero, J.E.; Cabrera-León, A.; Sánchez-Fernández, E. Influence of alcohol and tobacco habits on peri-implant marginal bone loss: A prospective study. Clin. Oral Implant. Res. 2005, 16, 579–586. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Fernández-Jiménez, A.; Avila-Ortiz, G.; Silvestre, F.J.; Hernández-Cortés, P.; Wang, H.L. Marginal bone loss around implants placed in maxillary native bone or grafted sinuses: A retrospective cohort study. Clin. Oral Implant. Res. 2014, 25, 378–384. [Google Scholar] [CrossRef]
- Ueda, M.; Matsuki, M.; Jacobsson, M.; Tjellström, A. The relationship between insertion torque and removal torque analysed in fresh temporal bone. Int. J. Oral Maxillofac. Implant. 1991, 6, 442–447. [Google Scholar]
- Batth, R.K. What is the Difference Between Implant Success and Survival and How Will It Change the Future Use of Implants as a Permanent Solution to Tooth Loss? Ph.D. Thesis, Boston University Libraries, Boston, MA, USA, 2014. [Google Scholar]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Rebaudi, A.; Trisi, P.; Cella, R.; Cecchini, G. Pre-operative evaluation of bone quality and bone density using a novel CT/microCT-based hard-normal-soft classification system. Int. J. Oral Maxillofac. Implant. 2010, 25, 75–85. [Google Scholar]
- Rodoni, L.R.; Glauser, R.; Feloutzis, A.; Hämmerle, C.H. Implants in the posterior maxilla: A comparative clinical and radiologic study. Int. J. Oral Maxillofac. Implant. 2005, 20, 231–237. [Google Scholar]
- Peñarrocha-Diago, M.A.; Flichy-Fernández, A.J.; Alonso-González, R.; Peñarrocha-Oltra, D.; Balaguer-Martínez, J.; Peñarrocha-Diago, M. Influence of implant neck design and implant-abutment connection type on peri-implant health: Radiological study. Clin. Oral Implant. Res. 2013, 24, 1192–1200. [Google Scholar] [CrossRef]
- Monje, A.; Suarez, F.; Galindo-Moreno, P.; García-Nogales, A.; Fu, J.H.; Wang, H.L. A systematic review on marginal bone loss around short dental implants (<10 mm) for implant-supported fixed prostheses. Clin. Oral Implant. Res. 2014, 25, 1119–1124. [Google Scholar]
- Karl, T.G.; Karl, M.; Steiner, C. Insertion torque/time integral as a measure of primary implant stability. Biomed. Eng. Biomed. Tech. 2020, 65, 729–733. [Google Scholar] [CrossRef]
- Duyck, J.; Roesems, R.; Cardoso, M.V.; Ogawa, T.; De Villa Camargos, G.; Vandamme, K. Effect of insertion torque on titanium implant osseointegration: An animal experimental study. Clin. Oral Implant. Res. 2015, 26, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Checa, S.; Prendergast, P.J. Effect of cell seeding and mechanical loading on vascularization and tissue formation inside a scaffold: A mechano-biological model using a lattice approach to simulate cell activity. Biomech. Model. Mechanobiol. 2010, 5, 961–968. [Google Scholar] [CrossRef]
- Friberg, B.; Sennerby, L.; Meredith, N.; Lekholm, U. A comparison between cutting torque and resonance frequency measurements of maxillary implants: A 20-month clinical study. Int. J. Oral Maxillofac. Surg. 1999, 28, 297–303. [Google Scholar] [CrossRef]
- Brancacci, E.; García González, S.; Galve-Huertas, A.; Bennani, A.; Hernández Alfaro, F.; Aboul-Hosn Centenero, S. Influence of insertion torque on the surface integrity in different dental implants: An ex vivo descriptive study. Materials 2023, 16, 2330. [Google Scholar] [CrossRef]
- Perren, S.M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Do, T.A.; Le, H.S.; Shen, Y.-W.; Huang, H.-L.; Fuh, L.-J. Risk factors related to late failure of dental implant—A systematic review of recent studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef]
- Campos, F.E.; Gomes, J.B.; Marin, C.; Teixeira, H.S.; Suzuki, M.; Witek, L.; Zanetta-Barbosa, D.; Coelho, P.G. Effect of drilling dimension on implant placement torque and early osseointegration stages: An experimental study in dogs. J. Oral Maxillofac. Surg. 2012, 70, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Çankaya, A.B.; Akçay, Ç.; Kahraman, N.; Köseoğlu, B.G. Oral surgical procedures under local anaesthesia in day surgery. BMC Oral Health 2018, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Cortellari, G.C.; de Oliveira Fernandes, G.V.; Scarano, A.; Martins, R.G.; Cançado, R.M.; Mesquita, A.M.M. Randomized clinical trial comparing insertion torque and implant stability of two different implant macrogeometries in the initial periods of osseointegration. Medicina 2023, 59, 168. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.A.; Verri, F.R.; De Oliveira Neto, O.B.; Cruz, R.S.; Luna Gomes, J.M.; Da Silva Casado, B.G.; Pellizzer, E.P. Clinical effect of the high insertion torque on dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 126, 490–496. [Google Scholar] [CrossRef]
- Radi, I.A.; Samy, M.A.E.Y. Limited evidence suggests no correlation between implant insertion torque and implant survival and marginal bone loss. J. Evid. Based Dent. Pract. 2023, 23, 101839. [Google Scholar] [CrossRef]
| References (Authors, Year of Publication) | Random Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting |
|---|---|---|---|---|---|
| Barone et al., 2015 RCT [29] |  |  |  |  |  |
| Alfonsi et al., 2016 RCT [30] |  |  |  |  |  |
| Hof et al., 2014 RCT [31] |  |  |  |  |  |
| Marconcini et al., 2018 RCT [35] |  |  |  |  |  |
| References (Authors, Year of Publication, and Study Design) | N° of Patients and % Women | Mean Age (Years), Mean (SD or Range) | Inclusion and Exclusion Criteria | Torque Value |
|---|---|---|---|---|
| Aldahlawi et al., 2018 Retrospective study [12] | 66 46.9% Fe | 56.31 ±16.20 (range 28–84) | Inclusion criteria: patients who received at least one implant that had been in function for an average post-loading period of 12 months and availability of post-loading radiographic and clinical follow-up, partially edentulous. | Regular-IT group: 37.9 ± 12.62 Ncm High-IT group: 67.35 ± 4.0 Ncm |
| Makary et al., 2011 XX [15] | 18 61.1% Fe | 48.8 ± 13.8 (range 30–74) | Inclusion criteria: good health, with no systemic disorders, and able to sign an informed consent form. | IT within the range: 15–150 Ncm (mean value 78.30 Ncm) |
| Rizkallah et al., 2013 Retrospective study [27] | 145 57.2% Fe | 57.5 | Inclusion criteria: N.R. Exclusion criteria: N.R. | Mean IT 72.0 Ncm (range 23.8–178 Ncm) |
| Barone et al., 2015 RCT [29] | 116 66.4% Fe | 51.4 ± 8.1 |
Inclusion criteria: able to sign an informed consent form, partially edentulous, required at least one single implant, older than 18 years Exclusion criteria: history of systemic diseases that would contraindicate oral surgical treatment, long-term non-steroidal anti-inflammatory drug therapy, lack of opposite occluding dentition in the area intended for implant supported restoration, extraction sites with less than 3 months of healing, presence of severe untreated periodontal disease, bone augmentation required at the time of implant placement, poor oral hygiene and compliance (presence of stain, calculus and plaque before dental implant surgery), pregnancy or nursing, unwillingness to return for the follow-up examination, use of more than 10 cigarettes per day (subject smoking <10 cigarettes per day were required to stop smoking before and after surgery). | Regular-IT group: 30.3 ± 7.5 Ncm High-IT group: 68.8 ± 9.0 Ncm |
| Alfonsi et al., 2016 RCT [30] | 116 66.4% Fe | 51.4 ± 8.1 |
Including criteria: able to sign an informed consent form, partially edentulous, required to have at least one single implant, and older than 18 years. Exclusion criteria: history of systemic diseases that would contraindicate oral surgical treatment, long-term non-steroidal anti-inflammatory drug therapy, lack of opposite occluding dentition in the area intended for implant supported restoration, extraction sites with less than 3 months of healing, presence of severe untreated periodontal disease, poor oral hygiene and compliance (presence of stain, calculus and plaque before dental implant surgery), pregnancy or nursing, unwillingness to return for the follow-up examination, use of more than 10 cigarettes per day (subject smoking <10 cigarettes per day were required to stop smoking before and after surgery). | Regular-IT group: 30.3 ± 7.5 Ncm High-IT group: 68.8 ± 9.0 Ncm |
| Hof et al., 2014 RCT [31] | 21 61.9% Fe | 67.4 (range 45–86) | Inclusion criteria: edentulous mandibles, teeth extracted for at least 6 months, sufficient bone volume in height and width to allow implant placement, without any augmentation procedure. Exclusion criteria: any medical or psychiatric contraindication to implant surgery. | Low-IT group: ≤20 Ncm High-IT group: >50 Ncm |
| Marconcini et al., 2018 RCT [35] | 116 66.4% Fe | 51.4 ± 8.1 |
Inclusion criteria: able to sign an informed consent form, partially edentulous, required at least one single implant, older than 18 years. Exclusion criteria: history of systemic diseases that would contraindicate oral surgical treatment, long-term non-steroidal anti-inflammatory drug therapy, lack of opposite occluding dentition in the area intended for implant-supported restoration, recent (<10 years) or ongoing intravenous/oral bisphosphonate therapy, extraction sites with less than 3 months of healing, presence of severe untreated periodontal disease, bone augmentation required at the time of implant placement, poor oral hygiene and motivation, pregnancy or nursing, unwillingness to return for the follow-up examination, use of more than 10 cigarettes per day (subject smoking <10 cigarettes per day were required to stop smoking before and after surgery). | Regular-IT group: 20–50 Ncm High-IT group: 50–100 Ncm |
| Bidgoli et al., 2015 Retrospective cohort study [36] | 136 57.4% Fe | Age within the range 21–69 | Exclusion criteria: systemic disease or conditions such as controlled diabetes, osteoporosis and history of radiotherapy or chemotherapy, pregnancy or nursing, consuming corticosteroids 4 months before surgery, smoking, substance abuse, consumption of alcoholic beverages, performing bone augmentation or sinus elevation protocol during implant insertion, periodontal disease, complication in healing after implant surgery. | Low-IT group: 20–30 Ncm High-IT group: 45–70 Ncm |
| Khayat et al., 2011 Prospective study [37] | 38 60.5% Fe | Regular-IT group: 63 (range 34–75) High-IT group: 64 (range 32–84) | Inclusion criteria: exhibited adequate oral hygiene and expressed a firm commitment to follow-up visits, >18 years, 1 or more missing teeth in either jaw, ability and willingness to comply with all study requirements, available for clinical follow-up, sufficient bone volume with or without localized bone grafting to accommodate implants at least 10 mm in length, absence of clinical or systemic conditions that would contraindicate surgery, implant placement, and/or implant survival. Accessibility for insertion torque measurement device. Exclusion criteria: heavy smoking (>20 cigarettes daily), alcohol or drug abuse, infection, endodontic or periodontal problems in teeth adjacent to the implant site, extraction sites with less than 6 months of healing, general pathologies or contraindications for implant treatment or surgery. | Regular-IT group: 30–50 Ncm High-IT group: 50–70 Ncm |
| Oskouei et al., 2023 Prospective cohort study [38] | 37 45.9% | 49.16 ± 9.42 (range 35–70) | Inclusion criteria: did not have a specific systemic problem interfering with the surgical process and osseointegration, did not have a history of taking bisphosphonates, volume and quality of the bone sufficient for placing the implant, no need for bone grafting at the implant placement site, had a missing tooth area in the posterior region of the mandible that had been extracted for at least 6 months, had the ability and willingness to participate in the 1-year follow-up. Exclusion criteria: had a systemic problem interfering with the surgical process and osseointegration, had a history of taking bisphosphonates or was currently taking it, had a history of head and neck radiotherapy, volume and quality of the bone were not enough to place the implant, bone augmentation was required at the time of implant placement, had parafunctional habits such as bruxism and clenching, there were infection and endodontal and periodontal problems around the implant placement, there was no natural dentition in the opposite jaw. | 20–40 Ncm (mean value 28.32 Ncm) |
| Grandi et al., 2012 Cohort study [39] | 102 62.7% Fe | Regular-IT group: 55.3 (range 43–67) High-IT group: 51.8 (range 39–65) | Inclusion criteria: at least 18 years, sufficient bone volume for placement of implants at least 8 mm in length and 3.7 mm in diameter, healed bone sites (at least 4 months post-extraction), adequate oral hygiene, plaque index ≤2. Exclusion criteria: systemic disease that could compromise osseointegration, previous irradiation in the head and neck area, treatment or under treatment with intravenous amino-bisphosphonates, uncontrolled diabetes, substance abuse, heavy smoking (>20 cigarettes daily), acute or chronic infection/inflammation in the area intended for implant placement. | Regular-IT group: 30–45 Ncm High-IT group: 50–80 Ncm |
| Author | MBL | FSTL | Implant Failure |
|---|---|---|---|
| Aldahlawi et al., 2018 [12] | Regular-IT group: 0.18 ± 0.68 High-IT group: 0.95 ± 1.60 | Survival rate: 94.6% | |
| Makary et al., 2011 [15] | |||
| Rizkallah et al., 2013 [27] | |||
| Barone et al., 2016 [29] | Regular-IT group: Maxilla: −0.55 ± 0.37 mm Mandible: −0.36 ± 0.31 mm High-IT group: Maxilla: −0.88 ± 0.43 mm Mandible: −1.23 ± 0.36 mm | Regular-IT group: Maxilla: −0.07 ± 0.38 mm Mandible: −0.57 ± 0.50 mm High-IT group: Maxilla: −0.13 ± 0.34 mm Mandible: −0.90 ± 0.48 mm | Survival rate: 97.4% |
| Alfonsi et al., 2016 [30] | Regular-IT group: Maxilla: −0.67 ± 0.43 mm Mandible: −0.75 ± 0.28 mm High-IT group: Maxilla: −0.93 ± 0.57 mm Mandible: −1.31± 0.33 mm | Regular-IT group: Maxilla: −0.10 ± 0.49 mm Mandible: −0.13 ± 0.34 mm High-IT group: Maxilla: −0.62 ± 0.57 mm Mandible: −1.13 ± 0.50 mm | Regular-IT group survival rate: 98.2% High-IT group survival rate: 94.8% |
| Hof et al., 2014 [31] | Low-IT group: 0.69 mm High-IT group: 0.68 mm | ||
| Marconcini et al., 2018 [35] | Regular-IT group: Maxilla: 0.96 ± 0.46 mm Mandible: 1.03± 0.12 mm High-IT group: Maxilla: 1.16 ± 0.61 mm Mandible: 1.53 ± 0.29 mm | Regular-IT group: Maxilla: 0.03 mm Mandible: −0.26 mm High-IT group: Maxilla: −0.54 mm Mandible: −1.40 mm | Survival rate: 96.5% |
| Bidgoli et al., 2015 [36] | |||
| Khayat et al., 2011 [37] | |||
| Oskouei et al., 2023 [38] | 1.01 ± 0.15 mm | ||
| Grandi et al., 2012 [39] | Regular-IT group: 0.45± 0.25 High-IT group: 0.41 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredini, M.; Ghizzoni, M.; Cusaro, B.; Beretta, M.; Maiorana, C.; Souza, F.Á.; Poli, P.P. High Insertion Torque—Clinical Implications and Drawbacks: A Scoping Review. Medicina 2025, 61, 1187. https://doi.org/10.3390/medicina61071187
Manfredini M, Ghizzoni M, Cusaro B, Beretta M, Maiorana C, Souza FÁ, Poli PP. High Insertion Torque—Clinical Implications and Drawbacks: A Scoping Review. Medicina. 2025; 61(7):1187. https://doi.org/10.3390/medicina61071187
Chicago/Turabian StyleManfredini, Mattia, Martina Ghizzoni, Beatrice Cusaro, Mario Beretta, Carlo Maiorana, Francisley Ávila Souza, and Pier Paolo Poli. 2025. "High Insertion Torque—Clinical Implications and Drawbacks: A Scoping Review" Medicina 61, no. 7: 1187. https://doi.org/10.3390/medicina61071187
APA StyleManfredini, M., Ghizzoni, M., Cusaro, B., Beretta, M., Maiorana, C., Souza, F. Á., & Poli, P. P. (2025). High Insertion Torque—Clinical Implications and Drawbacks: A Scoping Review. Medicina, 61(7), 1187. https://doi.org/10.3390/medicina61071187









