Effects of Metformin on Survival and Toxicity in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab
Abstract
1. Introduction
1.1. Background to the Study
1.2. Research Gap
1.3. Objectives
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Demographic and Laboratory Findings
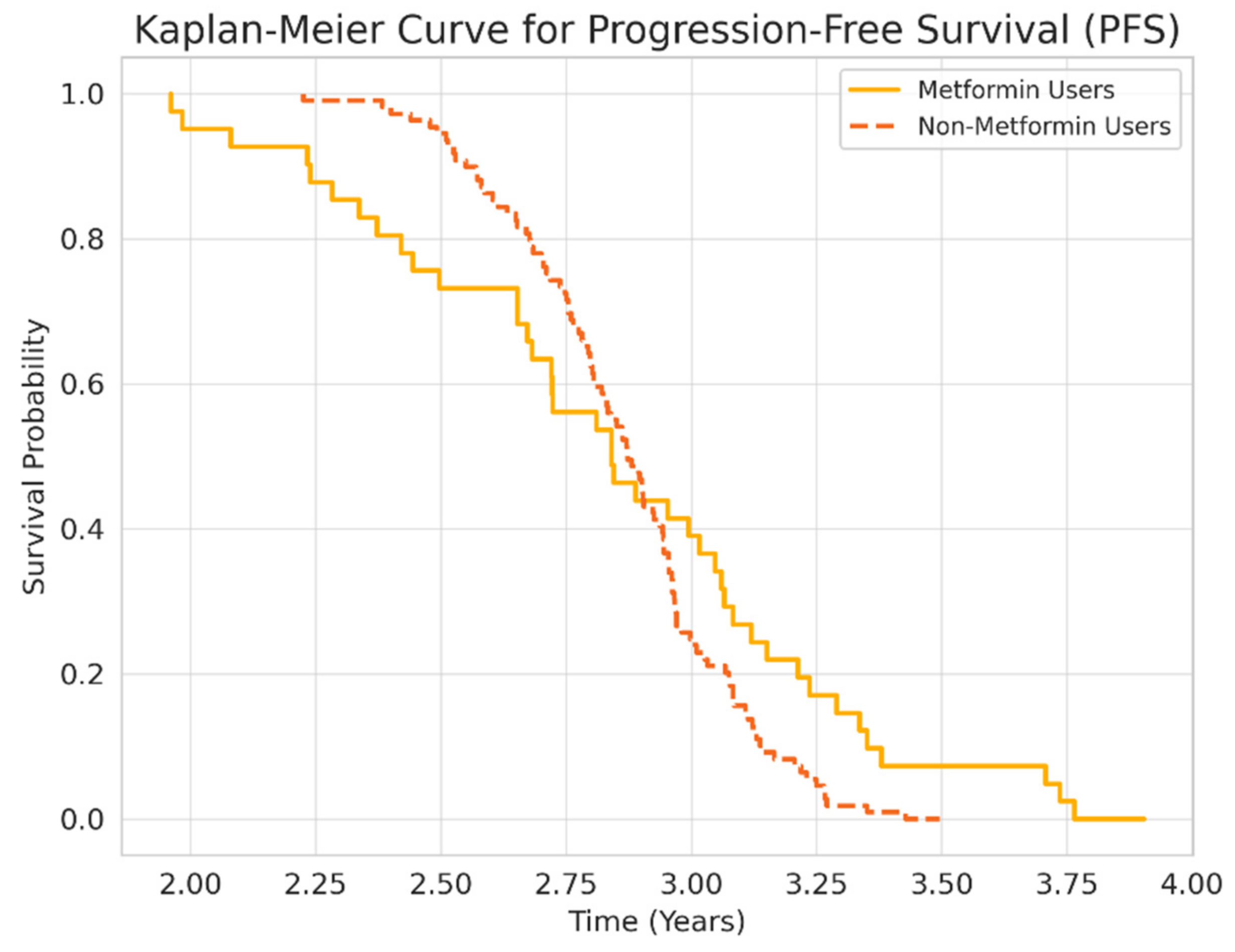
3.2. Survival and Mortality Outcomes
3.3. Treatment-Related Toxicities
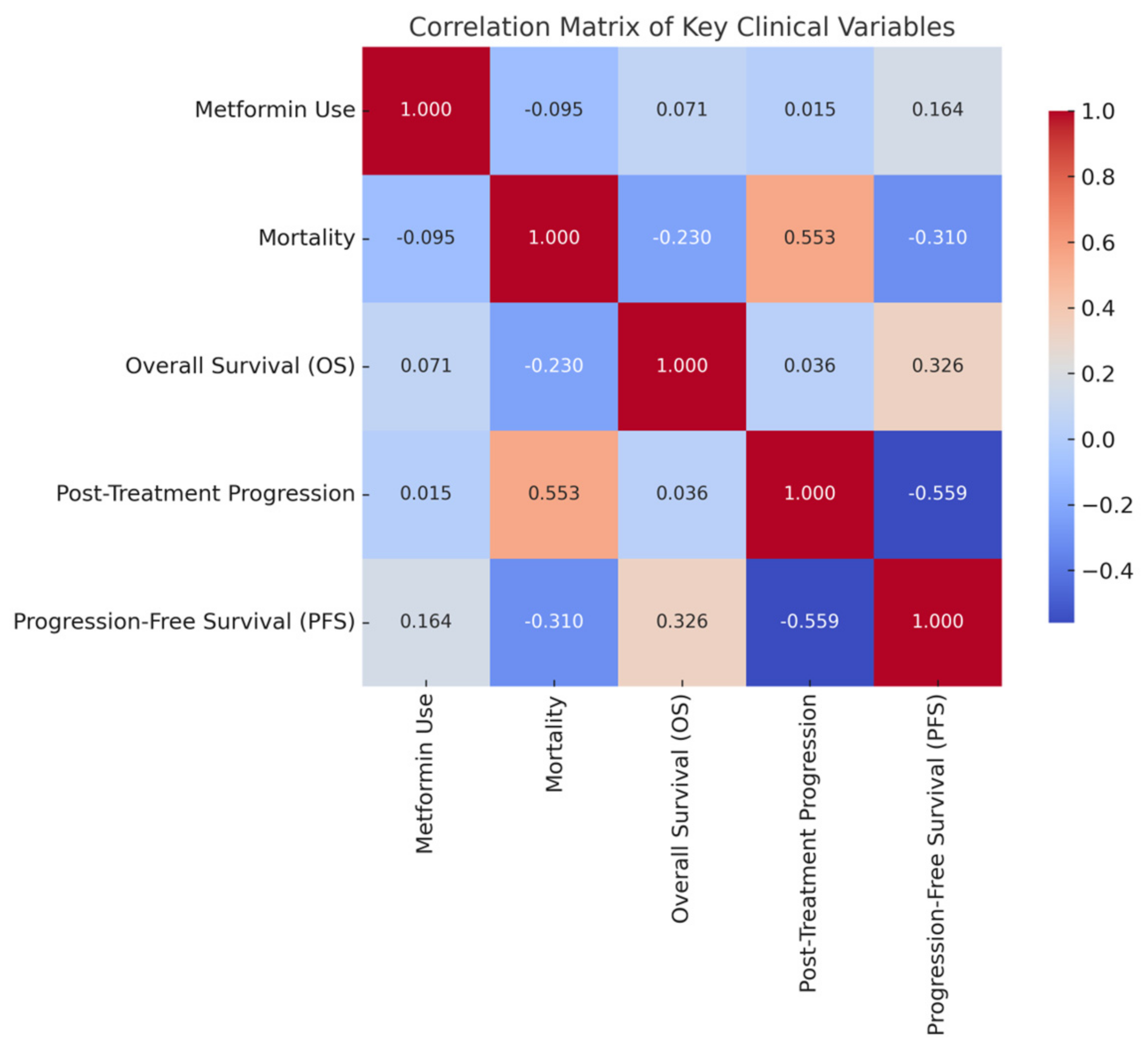
3.4. Correlations and Clinical Variables
4. Discussion
4.1. Interpretation of Key Findings
4.2. Clinical Implications
4.3. Comparison with Existing Studies]
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollak, M.N. Investigating metformin for cancer prevention and treatment: The end of the beginning. Cancer Discov. 2012, 2, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Svaton, M.; Zemanova, M.; Zemanova, P.; Kultan, J.; Fischer, O.; Skrickova, J.; Jakubíková, L.; Cernovska, M.; Hrnciarik, M.; Jirousek, M.; et al. Impact of concomitant medication administered at the time of initiation of nivolumab therapy on outcome in non-small cell lung cancer. Anticancer Res. 2020, 40, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2022, 14, 1355–1375. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.L.; Zevallos, J.P. Repurposing metformin for head and neck cancer: Effects on the tumor microenvironment and immune response. Head Neck. 2021, 43, 3058–3068. [Google Scholar] [CrossRef]
- Gettinger, S.; Borghaei, H.; Brahmer, J.; Chow, L.Q.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar]
- Yang, J.; Kim, S.H.; Jung, E.H. The effect of metformin or dipeptidyl peptidase 4 inhibitors on clinical outcomes in metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac. Cancer 2023, 14, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Chiang, C.H.; Ma, K.S. Effect of metformin on outcomes of patients treated with immune checkpoint inhibitors: A retrospective cohort study. Cancer Immunol. Immunother. 2023, 72, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.Y.; Park, C.H. Combination of metformin and immune checkpoint inhibitors in melanoma: A phase II clinical trial. Clin. Cancer Res. 2024, 30, 512–521. [Google Scholar]
- Shen, J.; Ye, X.; Hou, H.; Wang, Y. Clinical evidence for the prognostic impact of metformin in cancer patients treated with immune checkpoint inhibitors. Int. Immunopharmacol. 2024, 134, 112243. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, Y.; Hou, Z.; Zhang, M.; Li, L.; Wei, J. Combination therapy with PD-1 inhibition plus rapamycin and metformin enhances anti-tumor efficacy in triple-negative breast cancer. Exp. Cell Res. 2023, 429, 113647. [Google Scholar] [CrossRef] [PubMed]
- Ranc, K.; Jørgensen, M.E.; Friis, S.; Carstensen, B. Mortality after cancer among patients with diabetes mellitus: Effect of diabetes duration and treatment. Diabetologia 2014, 57, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Buti, S.; Bersanelli, M.; Perrone, F. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: Results from a prospective cohort study. Cancers 2021, 13, 462. [Google Scholar]
- Patel, M.R.; Falchook, G.S.; Bendell, J.C. Phase II trial of nivolumab and metformin in treatment-refractory microsatellite stable metastatic colorectal cancer. J. Immunother. Cancer 2024, 12, e008502. [Google Scholar]

| Variable | Metformin Users (n = 42) | Non-Users (n = 110) | p-Value | Significance |
|---|---|---|---|---|
| Gender | Significant | |||
| Women (%) | 14 (33.3%) | 9 (8.2%) | <0.05 | Yes |
| Men (%) | 28 (66.7%) | 101 (91.8%) | ||
| Age ≥ 65 years (%) | 24 (57.1%) | 42 (38.2%) | 0.054 | No |
| Body Mass Index (BMI) | Not Significant | |||
| Mean (SD) | 26.53 (4.54) | 24.97 (4.72) | 0.065 | No |
| Normal (%) | 16 (38.1%) | 53 (48.2%) | 0.501 | No |
| Obese (%) | 7 (16.7%) | 14 (12.7%) | ||
| Height (cm, Mean ± SD) | 165.9 (7.84) | 169.55 (6.65) | <0.05 | Yes |
| Laboratory Markers | Not Significant | |||
| Lymphocytes (Mean ± SD) | 1830.24 (1059.01) | 1606.82 (739.23) | 0.380 | No |
| Neutrophils (Mean ± SD) | 5183.1 | 4873.27 | 0.275 | No |
| Platelets (Mean ± SD) | 295,704.3 | 275,563.64 | 0.165 | No |
| Monocytes (Mean ± SD) | 604.52 | 785.27 | 0.281 | No |
| Predictor Variable | B (Coefficient) | S.E. (Standard Error) | p-Value | OR (95% CI) (Odds Ratio, OR) | 95% CI for OR (Lower–Upper) |
|---|---|---|---|---|---|
| Age | −0.05 | 0.36 | 0.885 | 0.95 | 0.47–1.90 |
| Gender (Female = 0, Male = 1) | −0.43 | 0.47 | 0.357 | 0.65 | 0.26–1.63 |
| BMI | 0.01 | 0.04 | 0.831 | 1.01 | 0.94–1.08 |
| Histopathology (ADC = Ref.) | |||||
| Squamous cell carcinoma (SDC) | 0.82 | 0.41 | 0.044 | 2.26 | 1.02–5.01 |
| Mixed Type | 0.91 | 0.50 | 0.069 | 2.49 | 0.93–6.65 |
| Other Types | 2.01 | 1.26 | 0.110 | 7.47 | 0.63–88.02 |
| Nivolumab Cycles | −0.45 | 0.08 | 0.000 | 0.64 | 0.54–0.75 |
| Metformin Use (Yes = 1, No = 0) | −0.48 | 0.42 | 0.244 | 0.62 | 0.27–1.39 |
| Adverse Event | Metformin Users (n = 42) | Non-Users (n = 110) | p-Value |
|---|---|---|---|
| Thrombocytopenia | 10 (23.8%) | 8 (7.3%) | <0.05 |
| Anemia | 5 (11.9%) | 12 (10.9%) | >0.05 |
| Neutropenia | 3 (7.1%) | 9 (8.2%) | >0.05 |
| Mucositis | 2 (4.8%) | 4 (3.6%) | >0.05 |
| Nephrotoxicity | 1 (2.4%) | 3 (2.7%) | >0.05 |
| Hepatic toxicity | 2 (4.8%) | 3 (2.7%) | >0.05 |
| Pneumonitis (immune-related) | 1 (2.4%) | 2 (1.8%) | >0.05 |
| Variables | Metformin Use | Mortality Risk | Overall Survival (OS) | Disease Progression | Progression-Free Survival (PFS) |
|---|---|---|---|---|---|
| Metformin Use | 1.000 | −0.095 | 0.071 | 0.015 | 0.164 |
| Mortality Risk | −0.095 | 1.000 | −0.230 | 0.553 | −0.310 |
| Overall Survival (OS) (years) | 0.071 | −0.230 | 1.000 | 0.036 | 0.326 |
| Disease progression Status | 0.015 | 0.553 | 0.036 | 1.000 | −0.559 |
| Progression-Free Survival (PFS) (years) | 0.164 | −0.310 | 0.326 | −0.559 | 1.000 |
| Variable | Metformin Users (n = 42) | Matched Non-Users (n = 42) | p-Value | SMD |
|---|---|---|---|---|
| Age (mean ± SD) | 65.1 ± 7.3 | 64.8 ± 6.9 | 0.81 | 0.04 |
| Female (%) | 33.3% | 31.0% | 0.82 | 0.05 |
| BMI (mean ± SD) | 26.5 ± 4.5 | 26.3 ± 4.4 | 0.74 | 0.03 |
| Histology (ADC %) | 76.2% | 78.6% | 0.79 | 0.06 |
| Nivolumab Cycles (mean) | 7.1 ± 2.3 | 7.0 ± 2.5 | 0.88 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surmeli, H.; Yildirim, S.; Isik, D.; Kinikoglu, O.; Altintas, Y.E.; Ozkerim, U.; Oksuz, S. Effects of Metformin on Survival and Toxicity in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. Medicina 2025, 61, 1161. https://doi.org/10.3390/medicina61071161
Surmeli H, Yildirim S, Isik D, Kinikoglu O, Altintas YE, Ozkerim U, Oksuz S. Effects of Metformin on Survival and Toxicity in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. Medicina. 2025; 61(7):1161. https://doi.org/10.3390/medicina61071161
Chicago/Turabian StyleSurmeli, Heves, Sedat Yildirim, Deniz Isik, Oguzcan Kinikoglu, Yunus Emre Altintas, Ugur Ozkerim, and Sıla Oksuz. 2025. "Effects of Metformin on Survival and Toxicity in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab" Medicina 61, no. 7: 1161. https://doi.org/10.3390/medicina61071161
APA StyleSurmeli, H., Yildirim, S., Isik, D., Kinikoglu, O., Altintas, Y. E., Ozkerim, U., & Oksuz, S. (2025). Effects of Metformin on Survival and Toxicity in Patients with Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. Medicina, 61(7), 1161. https://doi.org/10.3390/medicina61071161






