Abstract
Background and Objectives: Despite advances in immunotherapy, predicting survival outcomes in patients with non-small-cell lung cancer (NSCLC) remains challenging. Inflammatory and nutritional indices such as the Prognostic Nutritional Index (PNI), Geriatric Nutritional Risk Index (GNRI), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Inflammatory Burden Index (IBI) have emerged as promising prognostic markers associated with overall survival (OS) in NSCLC patients. Materials and Methods: We retrospectively analyzed a total of 196 NSCLC patients treated with second-line nivolumab across multiple centers in Turkey. Of these, 101 patients aged ≥ 65 years were included in the elderly subgroup analysis. PNI, GNRI (in patients aged ≥ 65), and inflammation-based indices were calculated using pre-treatment laboratory values. ROC analysis determined optimal cut-off values. The Kaplan–Meier method and Cox proportional hazards models were used for survival analysis. Results: Median overall survival (OS) was 12.9 months in the full cohort and 12.1 months in patients aged ≥ 65. In univariate analysis, ECOG performance status (0–1), lower NLR (<3.3), lower PLR (<196.8), higher PNI (≥45.2), and higher GNRI (≥98.0) were significantly associated with longer OS. However, in the multivariate analysis adjusted for ECOG PS, NLR, PLR, and GNRI, only PNI remained an independent prognostic factor for OS in both the overall cohort [HR: 0.49, 95% CI: 0.26–0.92; p = 0.02] and elderly patients [HR: 0.45, 95% CI: 0.24–0.84; p = 0.01]. PNI is an independent prognostic biomarker for OS in NSCLC patients treated with immune checkpoint inhibitors. Conclusions: These findings support incorporating simple, cost-effective nutritional indices into clinical decision-making, particularly in elderly patients with NSCLC.
1. Introduction
Non-small-cell lung cancer (NSCLC) continues to be one of the most prevalent causes of cancer-related deaths globally, despite significant progress in treatment modalities. Worldwide, lung cancer affects approximately 2.1 million individuals and accounts for an estimated 1.8 million deaths annually [1]. Among recent therapeutic advancements, immune checkpoint inhibitors—particularly those that inhibit the PD-1/PD-L1 pathway, such as nivolumab—have demonstrated improved survival outcomes in patients with advanced NSCLC who have experienced disease progression following platinum-based chemotherapy regimens [2]. Nivolumab was approved by the FDA in 2015 for the treatment of advanced NSCLC in the second-line setting. Nonetheless, the variability in patient responses to immunotherapy underscores the urgent need for practical and reliable prognostic markers that have been validated across diverse populations in order to improve patient selection and guide therapeutic decision-making.
In recent years, systemic inflammatory markers and indicators of nutritional status have gained prominence as prognostic tools in oncology. Easily obtainable from routine blood tests, indices such as the Prognostic Nutritional Index (PNI), Geriatric Nutritional Risk Index (GNRI), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Inflammatory Burden Index (IBI) have been explored for their prognostic relevance across various cancer types, including NSCLC [3,4,5]. These markers are particularly advantageous due to their cost-effectiveness, non-invasiveness, and potential applicability in elderly or comorbidities patient populations.
In this multicenter, retrospective study, we aimed to evaluate the prognostic value of nutrition- and inflammation-based tools in patients with NSCLC treated with second-line nivolumab.
2. Materials and Methods
2.1. Patients and Methods
Patients
We retrospectively collected demographic, clinicopathologic, and laboratory data from 196 NSCLC patients treated with second-line immunotherapy (Nivolumab) between January 2020 and December 2023 in a multicenter study in Turkey. The inclusion criteria were as follows: histologically or cytologically confirmed diagnosis of NSCLC, disease progression after first-line treatment, treatment with single-agent immunotherapy, and the availability of complete medical records including laboratory results, radiologic response, and survival data. The exclusion criteria included patients with incomplete radiological assessment of treatment response, insufficient clinical documentation, the presence of known driver mutations, active infections, autoimmune diseases, concurrent malignancies, or age under 18 years.
All patients included in this study received platinum-based chemotherapy as first-line treatment. None of the patients were treated with first-line immunotherapy as it was not reimbursed in our country during the study period.
2.2. Prognostic Nutritional Index (PNI) and Geriatric Nutritional Index (GNRI)
PNI and GNRI were calculated using lymphocyte count, serum albumin levels, and current body weight and height obtained from routine blood tests performed prior to the first dose of nivolumab. GNRI was calculated only for patients aged 65 years and older.
PNI was calculated as follows: [(10 × serum albumin (g/dL)) + (0.005 × total lymphocyte count (103/µL))].
GNRI was calculated as follows: [1.489 × albumin (g/dL)] + [41.7 × (weight/Ideal Body Weight)].
Ideal body weight (IBW) was calculated using the modified Devine formula: 50.0 + 0.91 × (height in cm − 152.4) for males and 45.5 + 0.91 × (height in cm − 152.4) for females [6].
Inflammatory-Based Indices
Neutrophil, lymphocyte, platelet, and monocyte counts, along with CRP levels, were obtained from routine blood tests performed within three days prior to the initiation of nivolumab. These values were used to calculate inflammatory-based indices. The following indices were calculated:
- Neutrophil–Lymphocyte Ratio (NLR): Neutrophil count/lymphocyte count
- Platelet–Lymphocyte Ratio (PLR): Platelet count/lymphocyte count
- Inflammatory Burden Index (IBI): CRP (mg/L) × neutrophil-to-lymphocyte ratio
2.3. Statistical Analysis
Progression-free survival (PFS) was defined as the time from the date of nivolumab initiation to the first radiologically or pathologically confirmed recurrence of disease, or death from any cause, whichever occurred first. Overall survival time (OS) was defined as the time from the date of nivolumab initiation until death for any reason or the last documented clinical follow-up. Survival analysis was performed using the Kaplan–Meier method. Patients who were lost to follow-up were censored at the time of their last documented contact. Prognostic factors were compared using the log-rank test in univariate analyses. Hazard ratios (HRs) with 95% confidence intervals (CIs) were also calculated. All p-values were calculated using two-tailed tests, and a p-value of <0.05 was considered statistically significant. Multivariate analysis was carried out using the Cox proportional hazards model to assess the effects of prognostic factors on OS. To determine the optimal cut-off value of indices for predicting overall survival, receiver operating characteristic (ROC) analysis was performed using SPSS version 25 (IBM Corp., Armonk, NY, USA) with the ROC module. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA)
2.4. Optimal Cut-Off Values for Prognostic Markers for Overall Survival
A significant cut-off value was identified only for NLR. In the ROC analysis, the ideal NLR cut-off value for OS was 3.3 [AUC: 0.66 (0.54–0.78), p = 0.01], with a sensitivity of 73% and a specificity of 57% (Figure 1). Median values for PNI, PLR, and IBI were used as the optimal cut-off values. The cut-off value for GNRI was determined based on previous publications and set at 98 [7]. Patients were categorized based on the determined cut-off values.
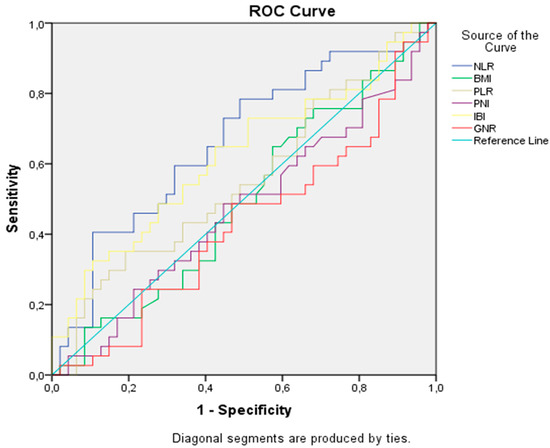
Figure 1.
Receiver operating characteristic analysis of prognostic indicators for overall survival.
3. Results
3.1. Demographic and Clinicopathologic Characteristics of Patients
We collected data from 15 oncology centers across Türkiye and included a total of 196 patients in the study. A total of 167 of 196 were male (85.2%) and the median age was 65 (range 23–88). A total of 51.5% of patients were aged 65 years or older, and 26.5% were active smokers. The most common histological subtype was adenocarcinoma (51.8%). At the time of diagnosis, the clinical stage distribution of the patients was as follows: Stage I in 10 patients (5.1%), Stage II in 35 patients (17.9%), Stage III in 52 patients (26.5%), and Stage IV in 99 patients (50.5%). Regarding the initial treatment approach, 40 patients (20.4%) underwent curative surgical resection, 53 patients (27%) received definitive chemoradiotherapy, and 4 patients (2%) were treated with definitive radiotherapy. PD-L1 expression was assessed via immunohistochemistry (IHC) using tumor tissue samples, and 69 patients (35.2%) had a PD-L1 level of ≥1%, with a median PD-L1 level of 35% (range: 1–95%). Median values for PNI and GNRI were 45.2 (range: 24–60) and 103.8 (range: 64.6–139.31), respectively. Median NLR, PLR, and IBI were calculated as 3.7 (range: 0.9–21.5), 196.8 (range: 26.6–1970), and 60.2 (range: 0.17–236.2), respectively. Baseline demographic and clinicopathologic findings are summarized in Table 1.

Table 1.
Baseline demographic and clinicopathologic findings for the whole cohort.
3.2. Survival Outcomes
The data cutoff date for follow-up was 31 May 2024. The median follow-up duration was 5.3 months (range: 1–34.8 months). During the follow-up period, 134 patients (68.4%) experienced disease progression and 74 patients (37.8%) died. In the entire cohort, median PFS was 4.2 months (95% CI: 3.5–4.8) and median OS was 12.4 months (95% CI: 8.4–16.4). The 12- and 24-month OS rates were 53% and 25%, respectively (Figure 2). ECOG PS, NLR, PLR, PNI, and GNRI were significantly associated with OS in univariate analysis. However, in the multivariate analysis (adjusted for age, ECOG PS, NLR, PLR, PNI, and GNRI), only PNI was identified as an independent prognostic factor for OS [HR: 0.49 (95% CI: 0.26–0.92), p = 0.02] (Figure 3). Results of the univariate analysis and OS are presented in Table 2.
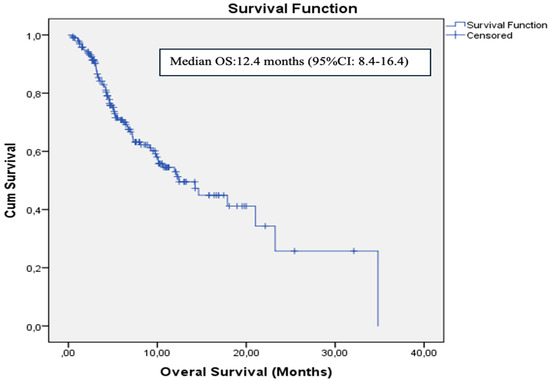
Figure 2.
Kaplan–Meier curve for overall survival in the whole cohort.
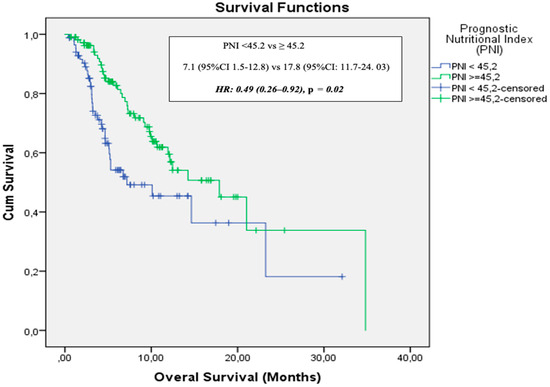
Figure 3.
Overall survival stratified by prognostic nutritional index.

Table 2.
Univariate analysis results for overall survival in the whole cohort.
In the elderly cohort (aged 65 years and older), median follow-up duration was 6.1 months (range: 1–21.04 months). During this period, 42 patients (41.6%) died and 67 patients (70%) experienced disease progression. Median PFS was 4.6 months (95% CI: 2.9–6.3), while median OS was 12.1 months (95% CI: 8.7–15.6). In the univariate analysis, ECOG PS, PNI, and GNRI were significantly associated with OS. Multivariate analysis (adjusted for age, ECOG PS, PNI, and GNRI) identified only PNI as an independent prognostic factor for OS [HR: 0.45 (95% CI: 0.24–0.84), p = 0.01] (Figure 4). Table 3 presents the univariate analysis results and OS data for patients ≥65 years.
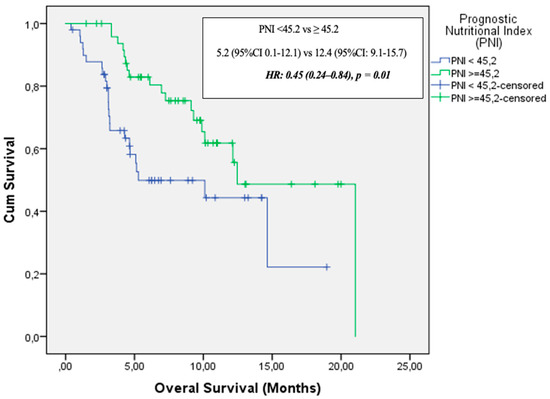
Figure 4.
Overall survival by PNI in elderly patients (≥65 Years).

Table 3.
Univariate analysis results for overall survival in patients aged 65 years and older.
4. Discussion
In our study, PNI was identified as an independent prognostic factor for OS in the entire NSCLC cohort, as well as in patients aged 65 years and older. Patients with a high PNI demonstrated significantly improved OS. Although GNI was associated with better OS, it was not an independent prognostic factor.
PNI is an inflammation-based prognostic scoring system derived from two inflammatory markers: lymphocyte count and serum albumin. Initially developed to evaluate the prognostic impact of nutritional and immunological status in malnourished cancer patients undergoing gastrointestinal surgery by Onedera et al., PNI has since been recognized as an independent and valuable prognostic tool for predicting survival in various malignancies, including colorectal and genitourinary cancers and glial tumors [8,9,10,11,12].
Malnutrition and muscle loss are highly prevalent among patients with NSCLC, affecting approximately 70% of cases, which underscores the critical importance of nutritional assessment during anti-cancer treatment [13]. Pretreatment prognostic nutritional indices have been associated with the therapeutic efficacy of various treatment modalities, including targeted therapy, chemotherapy, and radiotherapy in lung cancer patients [14,15]. With respect to immune checkpoint inhibitor (ICI)-based immunotherapy, evidence from a small cohort study of NSCLC patients (n = 24) treated with anti-PD-L1 indicated that a higher PNI was linked to a longer time to treatment failure and improved OS [16]. Fang et al. reported that pretreatment PNI served as an independent prognostic marker for OS in a cohort of 223 advanced NSCLC patients receiving anti-PD-1 therapy [17]. In a meta-analysis conducted by Xuebing Yan et al., a total of 1260 patients from 14 studies were evaluated. High PNI level was significantly associated with improved OS (HR = 2.56, 95% CI: 1.86–3.54) and PFS (HR = 1.91, 95% CI: 1.53–2.40) in patients with lung cancer. Subgroup analyses supported these findings, except for PFS in patients receiving anti-PD-1 therapy, where the association was not statistically significant (HR = 1.51, 95% CI: 0.86–2.65; p = 0.15). When stratified by age, high PNI was also significantly associated with better OS in patients aged 65 years and over (HR = 2.23, 95% CI: 1.71–2.89; p <0.001) [18]. Similarly, in our study, a high PNI level was found to be associated with better OS both in the entire cohort and in patients aged 65 years and over [HR = 0.49 (95% CI: 0.26–0.92), p = 0.02 and HR = 0.45 (95% CI: 0.24–0.84), p = 0.01, respectively]. The current literature evaluating the relationship between the Prognostic Nutritional Index and survival outcomes in lung cancer patients treated with immune checkpoint inhibitors is summarized in Figure 5 [19,20,21,22,23,24].
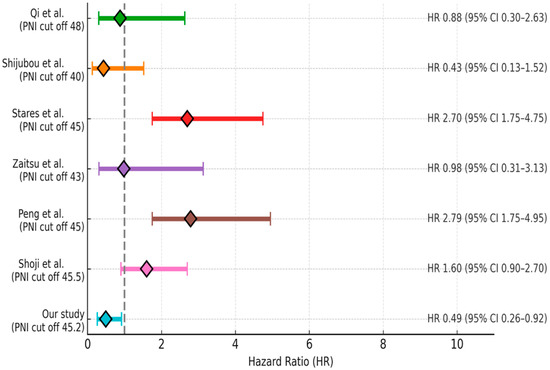
Figure 5.
Summary of the current literature on the association between PNI and survival outcomes in lung cancer patients treated with immune checkpoint inhibitors [19,20,21,22,23,24].
GNRI was originally developed to assess the nutrition-related risk of mortality in elderly, non-cancer inpatients. GNRI is calculated using serum albumin levels and ideal body weight. High GNRI has been reported to be associated with favorable survival outcomes in various solid tumors, including non-small cell lung cancer [25,26,27]. In the era of immunotherapy, nutritional status may influence immunological competence and the effectiveness of treatment. As immune checkpoint inhibitors become established as a cornerstone in the management of metastatic NSCLC, the identification of reliable and non-invasive prognostic markers such as GNRI is becoming increasingly important.
Several studies have evaluated the prognostic value of GNRI in patients with metastatic non-small-cell lung cancer receiving immune checkpoint inhibitors. In a retrospective cohort study involving 85 patients with advanced NSCLC treated with PD-1 inhibitors, Sonehara et al. demonstrated that a higher GNRI ≥ 89.5 was significantly associated with improved clinical outcomes compared to low GNRI, including prolonged PFS (3.7 vs. 2.4 months; p = 0.041) and OS (14.2 vs. 6.1 months; p = 0.008). Multivariate analysis further identified high GNRI (≥89.5), ECOG performance status of 0–1, and age of ≥ 70 years as independent prognostic factors for favorable overall survival. Moreover, patients with a high GNRI (≥89.5) were significantly more likely to receive subsequent lines of systemic therapy following immune checkpoint inhibitor treatment compared to those with a low GNRI (<89.5) [(68.6% vs. 33.3%; p = 0.008)], highlighting the potential role of nutritional status in sustaining treatment eligibility and improving long-term outcomes in this population [28]. Similarly, Jiang et al. evaluated the prognostic significance of the GNRI in a cohort of 148 patients with NSCLC treated with immune checkpoint inhibitors. Using ROC curve analysis, the optimal GNRI cutoff was identified as 108.15 [area under the curve (AUC) = 0.575, p = 0.048]. Multivariate Cox regression analysis demonstrated that a high GNRI was independently associated with improved OS [hazard ratio (HR): 0.536, p = 0.03]. Notably, subgroup analyses revealed that the prognostic utility of GNRI was particularly evident in patients with tumor–node–metastasis (TNM) stage II–III disease and those aged ≥ 65 years, highlighting its potential role as a clinically relevant biomarker in selected populations undergoing immunotherapy [29].
A study by Karayama et al. indicated that among 158 patients with NSCLC treated with nivolumab, a low GNRI was correlated with a significantly shorter PFS [median: 1.9 months; 95% CI: 0.6–3.3 months] compared to moderate (median: 4.0 months; 95% CI: 2.3–5.8 months; p = 0.017) and high (median: 3.0 months; 95% CI: 1.9–7.2 months; p = 0.014) GNRI. A low GNRI was associated with a significantly shorter OS (median: 7.8 months; 95% CI: 2.6–12.0 months) compared to moderate (median: 13.0 months; 95% CI: 9.6–15.2 months; p = 0.006) and high (median: 20.6 months; 95% CI: 15.6 months reached; p < 0.001) GNRI [30]. Two additional studies also demonstrated that a high GNRI is associated with prolonged OS [31,32]. In the present study, a high GNRI level was associated with improved OS in patients aged 65 years and over [HR = 0.40; 95% CI: 0.24–0.80; p = 0.01]. However, GNRI was not identified as an independent prognostic factor in the multivariate analysis.
This study has several limitations that warrant consideration. First, its retrospective, multicenter, single-arm design may have introduced selection bias, limiting the external validity of the findings. Second, data regarding the nutritional status of patients at the time of lung cancer diagnosis were not available, as such information was not routinely documented during the initial diagnostic process. Therefore, our analysis focused on nutritional and inflammatory indices measured at the initiation of second-line nivolumab therapy. Third, the sample size, particularly in certain subgroups such as patients with PD-L1 ≥ 1%, may have reduced the statistical power to detect statistically significant associations. Additionally, the predominance of male patients in the study population (85.2%) likely reflects regional smoking patterns and sex-related epidemiological differences in NSCLC [33]. However, this imbalance may limit the generalizability of the findings to female patients. Lastly, the use of multiple inflammatory and nutritional biomarkers, each with variable cut-off values across studies, introduces a degree of heterogeneity that may affect the reproducibility and clinical interpretation of prognostic associations.
5. Conclusions
This study highlighted the prognostic significance of the PNI in patients with NSCLC treated with immune checkpoint inhibitors, with a PNI cut-off of ≥ 45.2 emerging as an independent predictor of overall survival, particularly in elderly patients. These findings support the integration of simple, cost-effective nutritional biomarkers into routine clinical assessment for better risk stratification; notably, the PNI could also guide nutritional interventions prior to the initiation of immunotherapy. Prospective studies are warranted to validate the prognostic value of the PNI in larger and more diverse patient cohorts.
Author Contributions
Conceptualization, O.A., A.M.T. and P.F.Y.; methodology, O.A., T.A.T.; software, O.A. and A.M.T.; validation, O.A., A.M.T. and P.F.Y.; formal analysis, O.A.; investigation, O.A.; resources, T.A.T., S.A., S.I., E.Ç., K.K., A.M.C., F.E., A.P.E., M.B., M.M.E., M.K.E., T.G.K., G.G.D., T.S., E.B., A.I., F.A.K., İ.H. and F.S.; data curation, S.A., S.I., E.Ç., K.K., A.M.C., F.E., A.P.E., M.B., M.M.E., M.K.E., T.G.K., G.G.D., T.S., E.B., A.I., F.A.K., İ.H. and F.S.; writing—original draft preparation, O.A., A.M.T. and P.F.Y.; writing—review and editing, O.A., A.M.T. and P.F.Y.; visualization, P.F.Y.; supervision, P.F.Y.; project administration, O.A.; funding acquisition, O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the local ethics committee for clinical research (approval date: 29 December 2021; approval number: 2021/514/216/9). Due to the retrospective nature of this study, the requirement for informed consent was waived.
Informed Consent Statement
Patient consent was waived due to the retrospective design of this study, which posed no additional risk to participants and did not require direct patient interaction, in accordance with the approval of the local ethics committee.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body Mass Index. |
| CRP | C-Reactive Protein. |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status. |
| GNRI | Geriatric Nutritional Risk Index. |
| HR | Hazard Ratio. |
| IBI | Inflammatory Burden Index. |
| ICIs | Immune Checkpoint Inhibitors. |
| NLR | Neutrophil-to-Lymphocyte Ratio. |
| NOS | Not Otherwise Specified |
| NSCLC | Non-Small-Cell Lung Cancer. |
| OS | Overall Survival. |
| PD-1 | Programmed Death-1. |
| PD-L1 | Programmed Death Ligand-1. |
| PFS | Progression-Free Survival. |
| PLR | Platelet-to-Lymphocyte Ratio. |
| PNI | Prognostic Nutritional Index. |
| ROC | Receiver Operating Characteristic. |
| TNM | Tumor Node Metastasis. |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, M.L.; Fitzgerald, B.G.; Paz-Ares, L.; Cappuzzo, F.; Jänne, P.A.; Peters, S.; Hirsch, F.R. New promises and challenges in the treatment of advanced non-small-cell lung cancer. Lancet 2024, 404, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, C.; Yang, R.; Jin, J.; Liu, D.; Li, W. Prognostic Value of the Geriatric Nutritional Risk Index in Non-Small Cell Lung Cancer Patients: A Systematic Review and Meta-Analysis. Front Oncol. 2022, 11, 794862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Chen, Y.; Guo, C.; Li, S.; Huang, C. Systemic immune-inflammation index as a predictor of survival in non-small cell lung cancer patients undergoing immune checkpoint inhibition: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2025, 210, 104669. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chen, S.W.; Chiang, Y.S. Prognostic role of geriatric nutritional risk index (GNRI) and controlling nutritional status (CONUT) on outcomes in patients with head and neck cancer: A systematic review and meta-analysis. BMC Cancer 2025, 25, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peterson, C.M.; Thomas, D.M.; Blackburn, G.L.; Heymsfield, S.B. Universal equation for estimating ideal body weight and body weight at any BMI. Am. J. Clin. Nutr. 2016, 103, 1197–1203, Erratum in Am. J. Clin. Nutr. 2017, 105, 772. https://doi.org/10.3945/ajcn.116.151985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Li, M.; Lian, S.; Hou, X.; Ling, Y. Geriatric nutritional risk index as a predictor for postoperative complications in patients with solid cancers: A meta-analysis. Front. Oncol. 2024, 14, 1266291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Chen, J.-H.; Iskandar, E.A.; Cai, S.I.; Chen, C.-Q.; Wu, H.; Xu, J.-B.; He, Y.-L. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: A large cohort study in a single Chinese institution. Tumour. Biol. 2016, 37, 3277–3283. [Google Scholar] [CrossRef]
- Ida, N.; Nakamura, K.; Saijo, M.; Kusumoto, T.; Masuyama, H. Prognostic nutritional index as a predictor of survival in patients with recurrent cervical cancer. Mol. Clin. Oncol. 2018, 8, 257–263. [Google Scholar] [CrossRef]
- Demir, A.; Alan, O.; Koca, S.; Surmeli, H. The relationship between the prognostic nutritional index and overall survival in elderly patients with epithelial ovarian cancer. ejmi 2020, 4, 269–273. [Google Scholar] [CrossRef]
- Alan, O.; Telli, T.A.; Basoğlu, T.; Arikan, R.; Demircan, N.C.; Ercelep, O.; Sakar, M.; Bozkurt, S.; Atasoy, B.M.; Dane, F.; et al. Impact of prognostic nutritional index on survival in recurrent glioblastoma. Neurocir. (Engl. Ed.) 2022, 33, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Curtis, A. Current insights in nutrition assessment and intervention for malnutrition or muscle loss in people with lung cancer: A narrative review. Adv. Nutr. 2022, 13, 2420–2432. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, J.; Zhu, Z.Y.; Jin, M.X. Prognostic nutritional index as a prognostic factor in lung cancer patients receiving chemotherapy: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5636–5652. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Yang, P.; Xu, L.; Liu, S.; Zhang, S.; Weng, X. Correlation of the controlling nutritional status score and the prognostic nutritional index with the prognosis of patients treated with radiotherapy for small-cell lung cancer. Ann. Palliat. Med. 2021, 10, 11635–11642. [Google Scholar] [CrossRef]
- Matsubara, T.; Takamori, S.; Haratake, N.; Toyozawa, R.; Miura, N.; Shimokawa, M.; Yamaguchi, M.; Seto, T.; Takenoyama, M. The impact of immune-inflammation-nutritional parameters on the prognosis of non-small cell lung cancer patients treated with atezolizumab. J. Thorac. Dis. 2020, 12, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Yu, J.; Li, W.; Luo, J.; Deng, Q.; Chen, B.; He, Y.; Zhang, J.; Zhou, C. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin. Exp. Pharmacol. Physiol. 2023, 50, 178–190. [Google Scholar] [CrossRef]
- Yan, X.; Wang, J.; Mao, J.; Wang, Y.; Wang, X.; Yang, M.; Qiao, H. Identification of prognostic nutritional index as a reliable prognostic indicator for advanced lung cancer patients receiving immune checkpoint inhibitors. Front. Nutr. 2023, 10, 1213255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, W.X.; Xiang, Y.; Zhao, S.; Chen, J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with firstline chemotherapy and atezolizumab. Cancer Immunol. Immunother. 2021, 70, 3199–3206. [Google Scholar] [CrossRef]
- Shijubou, N.; Sumi, T.; Yamada, Y.; Nakata, H.; Mori, Y.; Chiba, H. Immunological and nutritional predictive factors in patients receiving pembrolizumab for the first-line treatment of non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 1893–1901. [Google Scholar] [CrossRef]
- Stares, M.; Ding, T.E.; Stratton, C.; Thomson, F.; Baxter, M.; Cagney, H.; Cumming, K.; Swan, A.; Ross, F.; Barrie, C.; et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 2022, 7, 100445. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, J.; Yamashita, Y.; Ishikawa, A.; Saito, A.; Kagimoto, A.; Mimura, T.; Hirakawa, T.; Mito, M.; Fukuhara, K.; Senoo, T.; et al. Systemic inflammatory score predicts response and prognosis in patients with lung cancer treated with immunotherapy. Anticancer Res. 2021, 41, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef]
- Shoji, F.; Takeoka, H.; Kozuma, Y.; Toyokawa, G.; Yamazaki, K.; Ichiki, M.; Takeo, S. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer 2019, 136, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Li, G.; Li, B.; Li, C.; Xiao, L.; Zhou, J. Geriatric Nutritional Risk Index as a prognostic factor of patients with non-small cell lung cancer: A meta-analysis. Horm. Metab. Res. 2022, 54, 604–612. [Google Scholar] [CrossRef]
- Karayama, M.; Inoue, Y.; Yasui, H.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; Nakamura, Y.; Inui, N.; et al. Association of the Geriatric Nutritional Risk Index with the survival of patients with non-small-cell lung cancer after platinum-based chemotherapy. BMC Pulm. Med. 2021, 21, 409. [Google Scholar] [CrossRef]
- Sonehara, K.; Tateishi, K.; Araki, T.; Komatsu, M.; Yamamoto, H.; Hanaoka, M. Prognostic value of the geriatric nutritional risk index among patients with previously treated advanced non-small cell lung cancer who subsequently underwent immunotherapy. Thorac. Cancer 2021, 12, 1366–1372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, S.; Yang, A.; Yang, F.; Zhu, X.; Chen, X.; Li, Z.; Yao, Y.; Xu, S.; Yang, Z.; Mo, N.; et al. The Geriatric Nutritional Risk Index as a prognostic factor in patients treated with immune checkpoint inhibitors with non-small-cell lung cancer. J. Thorac. Dis. 2024, 16, 5222–5237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karayama, M.; Inoue, Y.; Yoshimura, K.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; Nakamura, Y.; Inui, N.; et al. Association of the Geriatric Nutritional Risk Index with the Survival of Patients with Non-Small Cell Lung Cancer After Nivolumab Therapy. J. Immunother. 2022, 45, 125–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimizu, A.; Fukasawa, M.; Mitani, K.; Goto, K.; Wakamoto, A.; Hatsuyama, T.; Hoshi, T.; Hasegawa, I.; Sato, H. Association of Geriatric Nutritional Risk Index with Immune Checkpoint Inhibitor Treatment Duration and Adverse Events in Lung Cancer. In Vivo 2024, 38, 418–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirabayashi, T.; Sonehara, K.; Ozawa, R.; Hachiya, T.; Nozawa, S.; Agatsuma, T.; Yamamoto, H.; Kato, A.; Matsuo, A.; Araki, T.; et al. Prognostic Value of the Geriatric Nutritional Risk Index in Previously Untreated Patients with Advanced Non-Small Cell Lung Cancer Treated with a Combination Therapy of Anti-PD-1/-PD-L1 Antibodies and Platinum-Based Chemotherapy: A Multicenter Retrospective Study. Oncology 2024, 102, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Karadoğan, D.; Telatar, T.G.; Kaya, İ.; Atlı, S.; Kabil, N.K.; Marım, F.; Şenel, M.Y.; Yüksel, A.; Yalçın, B.; Gültekin, Ö.; et al. Immediately scheduled for an appointment to smoking cessation clinics: Key to quitting smoking in chronic airway disease—A multicenter randomized study. Tob. Induc. Dis. 2025, 23, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).