Carcinosarcoma of the Endometrium—Pathology, Molecular Landscape and Novel Therapeutic Approaches
Abstract
1. Introduction
2. Epidemiology
3. Risk Factors
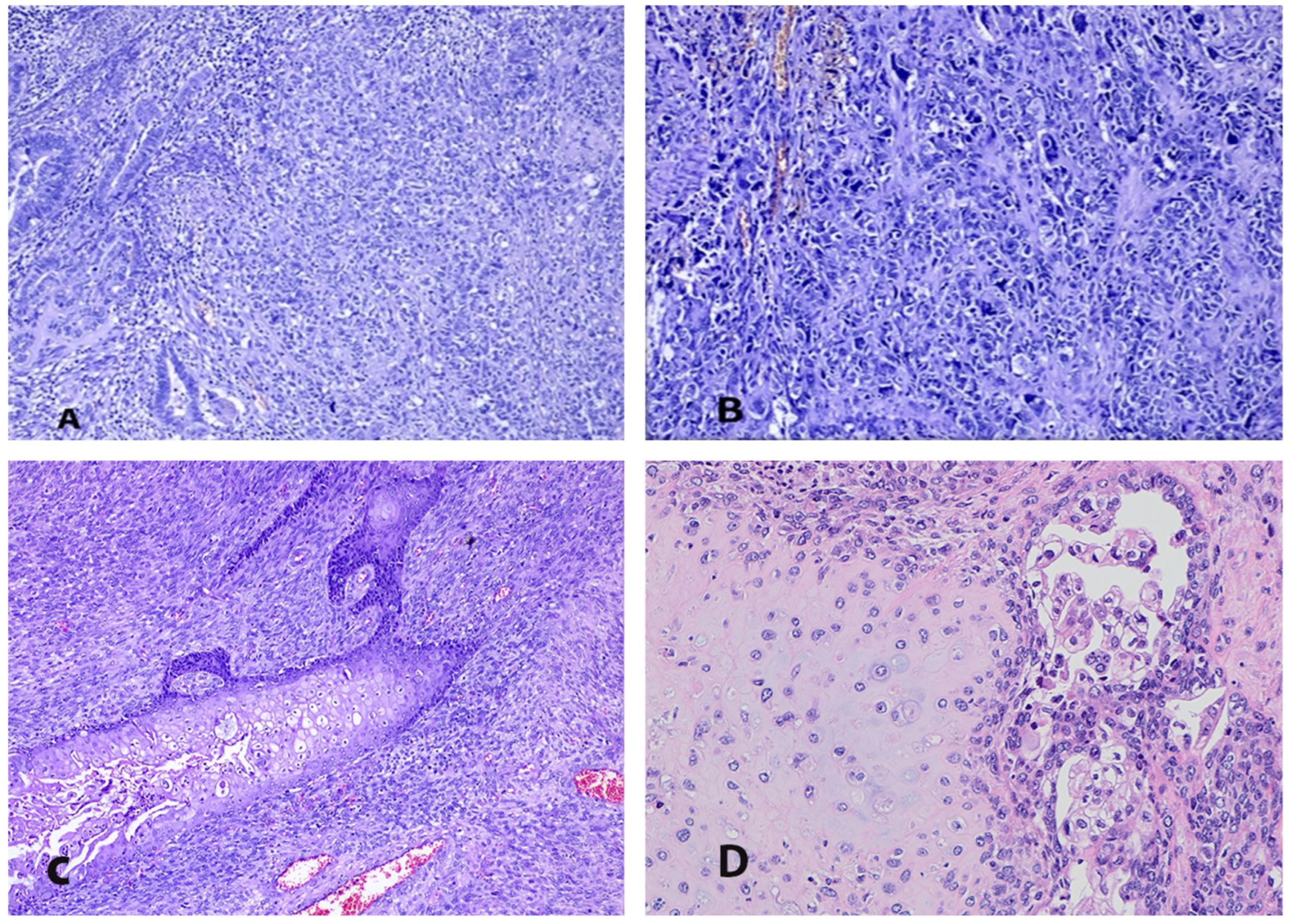
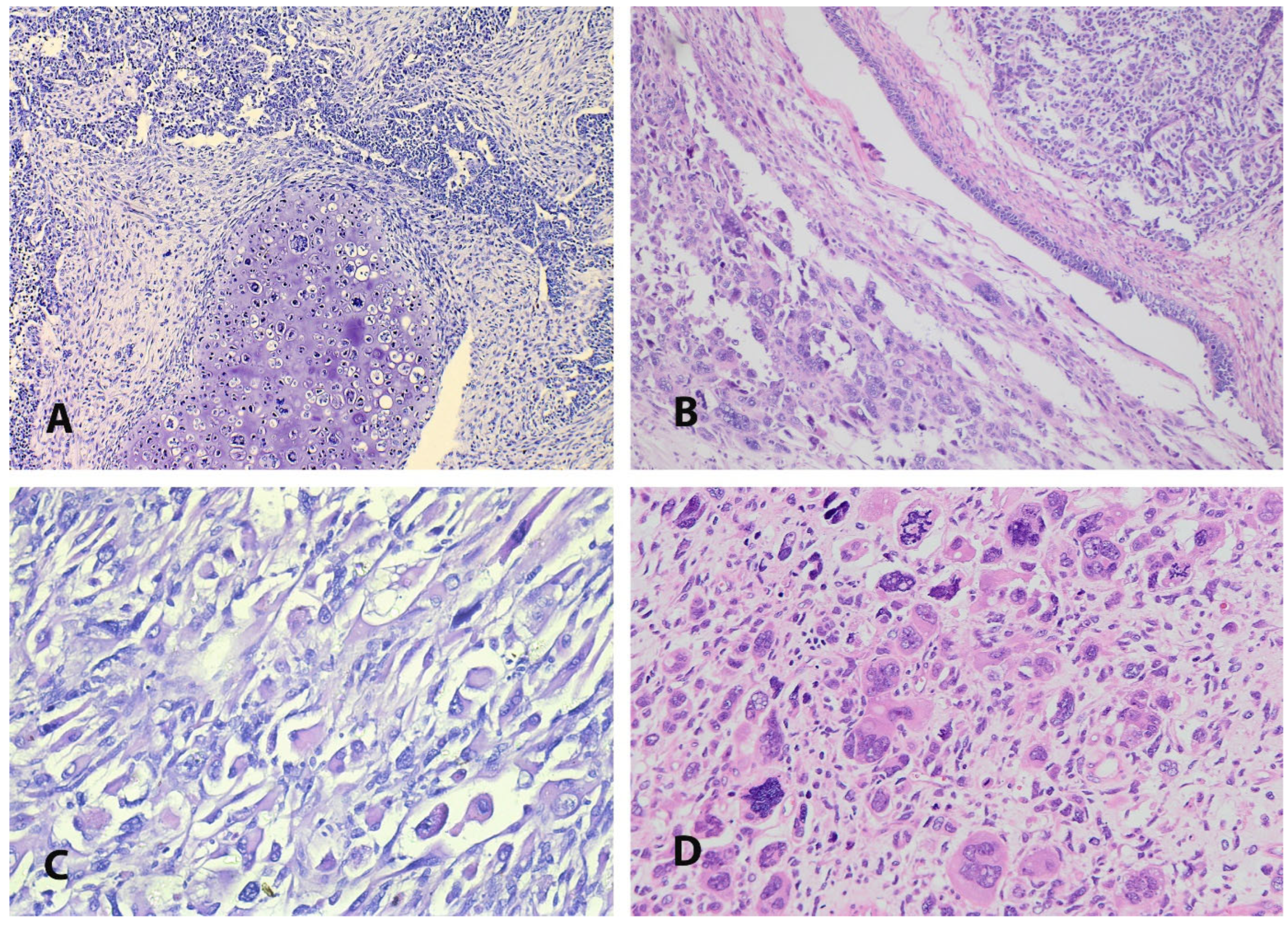
4. Pathogenesis and Pathology
5. The Molecular Landscape
6. Clinical Manifestations
7. Diagnosis
8. Prognostic Factors
9. Treatment
9.1. Surgery
9.2. Chemotherapy
9.3. Radiotherapy
9.4. Combined Treatment
9.5. Recurrent Disease
9.6. Hormone Therapy
10. Novel Therapeutic Options
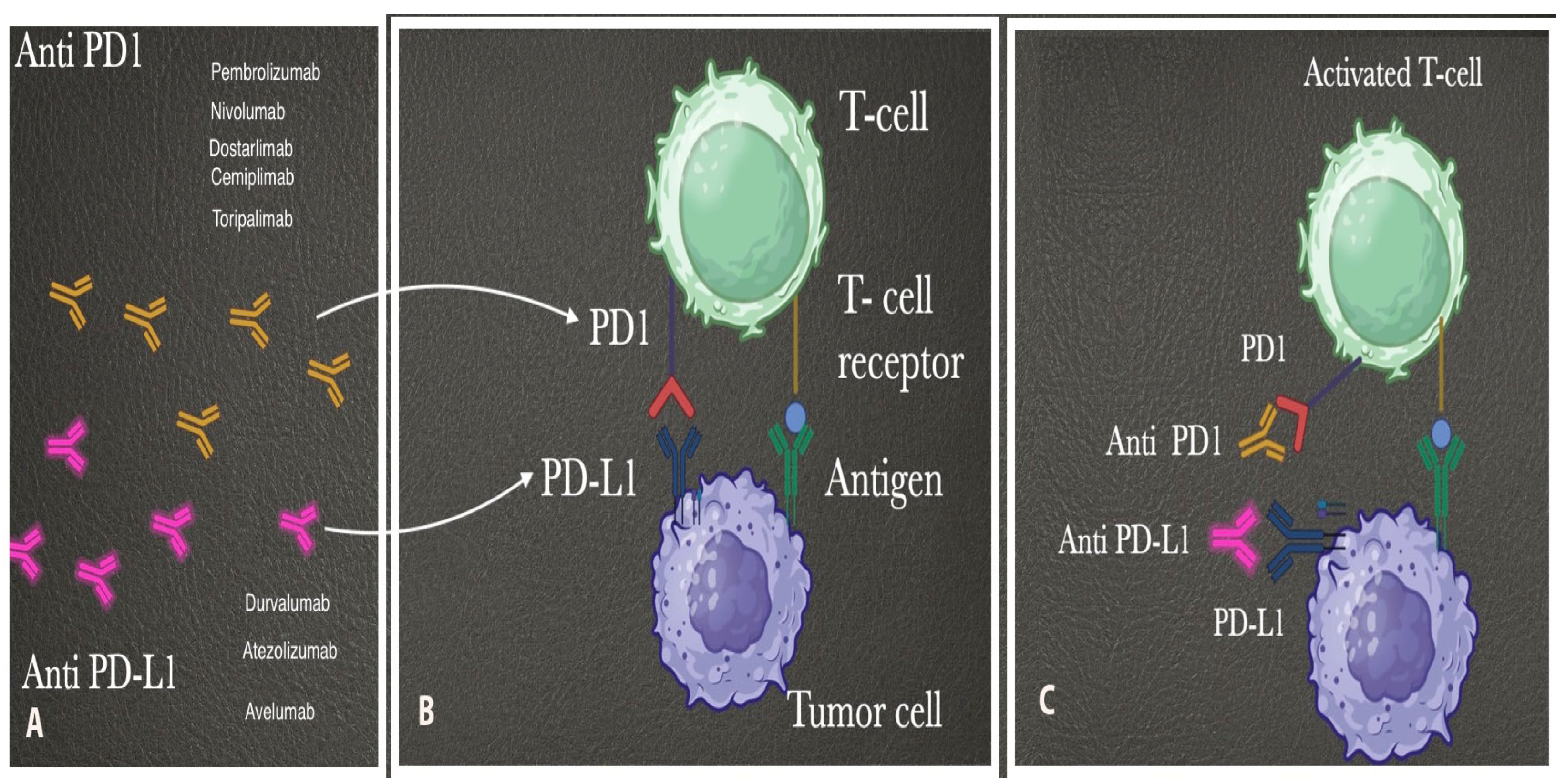
10.1. Immunotherapy
10.1.1. Immune Checkpoint Inhibitors
10.1.2. Immune Checkpoint Inhibitors with Multi-Targeted Tyrosine Kinase Inhibitors
10.1.3. PARP Inhibitors
10.1.4. HER2-Targeting Agents
10.1.5. Trophoblast Cell Surface Antigen 2 (TROP2)-Targeting Agents
10.1.6. RAF/MEK Inhibitors Combined with FAK Inhibitors
10.1.7. Epithelial–Mesenchymal Transition Target Therapies
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pezzicoli, G.; Moscaritolo, F.; Silvestris, E.; Silvestris, F.; Cormio, G.; Porta, C.; D’Oronzo, S. Uterine carcinosarcoma: An overview. Crit. Rev. Oncol. Hematol. 2021, 163, 103369. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, S.A.; Mariani, A. Uterine Carcinosarcoma. Clin. Obstet. Gynecol. 2011, 54, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Mccluggage, W.G. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int. J. Gynecol. Cancer 2002, 12, 687–690. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Klar, M.; Matsuzaki, S.; Roman, L.D.; Sood, A.K.; Matsuo, K. Uterine carcinosarcoma: Contemporary clinical summary, molecular updates, and future research opportunity. Gynecol. Oncol. 2020, 160, 586–601. [Google Scholar] [CrossRef]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Caruso, G.; Enomoto, T.; Takehara, K.; Hannelore Denys, H.; Lorusso, D.; et al. Endometrial carcinosarcoma. Int. J. Gynecol. Cancer 2023, 33, 147–174. [Google Scholar] [CrossRef]
- Dixon, C.F.; Dockerty, M.B. Carcinosarcomatodes of the uterus. Am. J. Obstet. Gynecol. 1940, 39, 128–132. [Google Scholar] [CrossRef]
- Cantrell, L.A.; Blank, S.V.; Duska, L.R. Uterine carcinosarcoma: A review of the literature. Gynecol. Oncol. 2015, 137, 581–588. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Hoang, L.N.; Chui, M.H.; Senz, J.; Yang, W.; Rozenberg, N.; Mackenzie, R.; McAlpine, J.N.; Huntsman, D.G.; Clarke, B.A.; et al. In-depth molecular profiling of the biphasic components of uterine carcinosarcomas. J. Path: Clin. Res. 2015, 1, 173–185. [Google Scholar] [CrossRef]
- Cherniack, A.D.; Shen, H.; Walter, V.; Stewart, C.; Murray, B.A.; Bowlby, R.; Hu, X.; Ling, S.; Soslow, R.A.; Broaddus, R.R. Integrated Molecular Characterization of Uterine Carcinosarcoma. Cancer Cell 2017, 31, 411–423. [Google Scholar] [CrossRef]
- Huvila, J.; Orte, K.; Vainio, P.; Mettälä, T.; Joutsiniemi, T.; Hietanen, S. Molecular subtype diagnosis of endometrial carcinoma: Comparison of the next-generation sequencing panel and Proactive Molecular Risk Classifier for Endometrial Cancer classifier. Hum. Pathol. 2021, 111, 98–109. [Google Scholar] [CrossRef]
- Brooks, S.E.; Zhan, M.; Cote, T.; Baquet, C.R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol. Oncol. 2004, 93, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Boll, D.; Verhoeven, R.H.A.; van der Aa, M.A.; Pauwels, P.; Karim-Kos, H.E.; Coebergh, J.W.W.; van Doorn, H.C. Incidence and Survival Trends of Uncommon Corpus Uteri Malignancies in the Netherlands, 1989–2008. Int. J. Gynecol. Cancer 2012, 22, 599–606. [Google Scholar] [CrossRef]
- Toboni, M.D.; Crane, E.K.; Brown, J.; Shushkevich, A.; Chiang, S.; Slomovitz, B.M.; Slomovitz, B.M.; Levine, D.A.; Dowdy, S.C.; Klopp, A.; et al. Uterine carcinosarcomas: From pathology to practice. Gynecol. Oncol. 2021, 162, 235–241. [Google Scholar] [CrossRef]
- Bansal, N.; Herzog, T.J.; Seshan, V.E.; Schiff, P.B.; Burke, W.M.; Cohen, C.J.; Wright, J.D. Uterine carcinosarcomas and grade 3 endometrioid cancers: Evidence for distinct tumor behavior. Obstet. Gynecol. 2008, 112, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.; Morice, P.; Enomoto, T.; Takehara, K.; Denys, H.; Nout, R.A.; Lorusso, D.; et al. Uterine serous carcinoma. Gynecol. Oncol. 2021, 162, 226–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pappa, C.; Le Thanh, V.; Smyth, S.L.; Zouridis, A.; Kashif, A.; Sadeghi, N.; Sattar, A.; Damato, S.; Abdalla, M.; Laganà, A.S.; et al. Mixed Endometrial Epithelial Carcinoma: Epidemiology, Treatment and Survival Rates—A 10-Year Retrospective Cohort Study from a Single Institution. J. Clin. Med. 2023, 12, 6373. [Google Scholar] [CrossRef]
- Kostov, S.; Kornovski, Y.; Ivanova, V.; Dzhenkov, D.; Metodiev, D.; Watrowski, R.; Ivanova, Y.; Slavchev, S.; Mitev, D.; Yordanov, A. New Aspects of Sarcomas of Uterine Corpus—A Brief Narrative Review. Clin. Pract. 2021, 11, 878–900. [Google Scholar] [CrossRef]
- Tang, L.S.; Zhou, Y.W.; Wang, J.L.; Zhang, G.X.; Xu, C.H.; Liu, J.Y.; Qiu, M. Epidemiology, site-specific characteristics and survival of carcinosarcoma: A retrospective study based on SEER database. BMJ Open 2023, 13, e077974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuo, K.; Ross, M.S.; Machida, H.; Blake, E.A.; Roman, L.D. Trends of uterine carcinosarcoma in the United States. J. Gynecol. Oncol. 2018, 29, e22. [Google Scholar] [CrossRef] [PubMed]
- Hosh, M.; Antar, S.; Nazzal, A.; Warda, M.; Gibreel, A.; Refky, B. Uterine Sarcoma: Analysis of 13,089 Cases Based on Surveillance, Epidemiology, and End Results Database. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2016, 26, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Review literature on uterine carcinosarcoma. J. Cancer Res. Ther. 2014, 10, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.E.; Devesa, S.S. Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer 2003, 98, 176–186. [Google Scholar] [CrossRef]
- Erickson, B.K.; Doo, D.W.; Zhang, B.; Huh, W.K.; Leath, C.A. Black race independently predicts worse survival in uterine carcinosarcoma. Gynecol. Oncol. 2014, 133, 238–241. [Google Scholar] [CrossRef]
- Varela-Duran, J.; Nochomovitz, L.E.; Prem, K.A.; Dehner, L.P. Postirradiation mixed müllerian tumors of the uterus: A comparative clinicopathologic study. Cancer 1980, 45, 1625–1631. [Google Scholar] [CrossRef]
- Pothuri, B.; Ramondetta, L.; Eifel, P.; Deavers, M.T.; Wilton, A.; Alektiar, K.; Barakat, R.; Soslow, R.A. Radiation-associated endometrial cancers are prognostically unfavorable tumors: A clinicopathologic comparison with 527 sporadic endometrial cancers. Gynecol. Oncol. 2006, 103, 948–951. [Google Scholar] [CrossRef]
- Shen, L.; Hong, L.; Zhou, S.; Zhang, G.; Mai, R. Mutational landscape implicates epithelial-mesenchymal transition gene TGF-β2 mutations for uterine carcinosarcoma after adjuvant tamoxifen therapy for breast carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 996–1002. [Google Scholar] [PubMed] [PubMed Central]
- Fisher, B.; Costantino, J.P.; Redmond, C.K.; Fisher, E.R.; Wickerham, D.L.; Cronin, W.M. Endometrial cancer in tamoxifen-treated breast cancer patients: Findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J. Natl. Cancer Inst. 1994, 86, 527–537. [Google Scholar] [CrossRef]
- Wysowski, D.K.; Honig, S.F.; Beitz, J. Uterine Sarcoma Associated with Tamoxifen Use. N. Engl. J. Med. 2002, 346, 1832–1833. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Nguyen, V.T. Evaluating Clinical Features in Intracavitary Uterine Pathologies among Vietnamese Women Presenting with Peri-and Postmenopausal Bleeding: A Bicentric Observational Descriptive Analysis. J. Midlife Health 2022, 13, 225–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- South, S.A.; Hutton, M.; Farrell, C.; Mhawech-Fauceglia, P.; Rodabaugh, K.J. Uterine carcinosarcoma associated with hereditary nonpolyposis colorectal cancer. Obstet. Gynecol. 2007, 110 Pt 2, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Kim, H.R.; Kim, M.K.; Lee, E.J. A novel germline mutation in hMLH1 in three Korean women with endometrial cancer in a family of Lynch syndrome: Case report and literature review. Hered. Cancer Clin. Pract. 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kostov, S.; Watrowski, R.; Kornovski, Y.; Dzhenkov, D.; Slavchev, S.; Ivanova, Y.; Yordanov, A. Hereditary Gynecologic Cancer Syndromes—A Narrative Review. Onco Targets Ther. 2022, 15, 381–405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ueki, A.; Hirasawa, A. Molecular Features and Clinical Management of Hereditary Gynecological Cancers. Int. J. Mol. Sci. 2020, 21, 9504. [Google Scholar] [CrossRef]
- WHO. Female Genital Tumors. In World Health Organization Classification of Tumours, 5th ed.; WHO of Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 1980. [Google Scholar]
- Higuchi, D.; Matsuura, T.; Takamine, E.; Hosokawa, M.; Kobori, K.; Ikeda, S.; Harada, S.; Honma, K.; Tajima, A.; Yoshida, M.; et al. Gan to kagaku ryoho. Cancer Chemother. 2022, 49, 783–787. [Google Scholar]
- Wilson, B.T.; Cordell, H.J. Uterine carcinosarcoma/malignant mixed Müllerian tumor incidence is increased in women with breast cancer, but independent of hormone therapy. J. Gynecol. Oncol. 2015, 26, 249–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Mc Farland, J. Dysontogenic and mixed tumours of the urogenital region. Surg. Gynecol. Obstet. 1935, 61, 42–47. [Google Scholar]
- Ober, W.B. Uterine sarcomas: Histogensis and taxonomy. Ann. N. Y Acad. Sci. 1959, 75, 568–585. [Google Scholar] [CrossRef]
- Matsuo, K.; Takazawa, Y.; Ross, M.S.; Elishaev, E.; Yunokawa, M.; Sheridan, T.B.; Bush, S.H.; Klobocista, M.M.; Blake, E.A.; Takano, T.; et al. Characterizing sarcoma dominance pattern in uterine carcinosarcoma: Homologous versus heterologous element. Surg. Oncol. 2018, 27, 433–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kernochan, L.E.; Garcia, R.L. Carcinosarcomas (malignant mixed Mullerian tumor) of the uterus: Advances in elucidation of biologic and clinical characteristics. J. Natl. Compr. Cancer Netw. 2009, 7, 550–556, (quiz 557). [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Khoo, U.S.; Cheung, A. Collision of endometrioid carcinoma and stromal sarcoma of the uterus: A report of two cases. Int. J. Gynecol. Pathol. Jan. 1999, 18, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, V.; Marinova, L.; Gabrovski, I. Carcinosarcomas and epithelial to mesenchymal transition (EMT). Med. Clin. Res. 2023, 8, 1–9. [Google Scholar]
- Hay, E.D. The Fine Structure of Blastema Cells and Differentiating Cartilage Cells in Regenerating Limbs of Amblystoma Larvae. J. Cell Biol. 1958, 4, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Abdulfatah, E.; Lordello, L.; Khurram, M.; Van de Vijver, K.; Alosh, B.; Bandyopadhyay, S.; Oliva, E.; Ali-Fehmi, R. Predictive Histologic Factors in Carcinosarcomas of the Uterus: A Multi-institutional Study. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2019, 38, 205–215. [Google Scholar] [CrossRef]
- Barbosa, Y.E.S.; Lopes, A.C.X.; Chaves, C.D.; Conrado, R.P.; Costa, M.L.V.; Leal, R.M.L.V. Epithelial-mesenchymal transition in uterine carcinosarcoma from a dedifferentiated papillary serous carcinoma to a sarcoma: Case report. Braz. J. Oncol. 2023, 19, e20230403. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial–mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef]
- Cuevas, I.C.; Sahoo, S.S.; Kumar, A.; Zhang, H.; Westcott, J.; Aguilar, M.; Cortez, J.D.; Sullivan, S.A.; Xing, C.; Hayes, D.N.; et al. Fbxw7 is a driver of uterine carcinosarcoma by promoting epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2019, 116, 25880–25890. [Google Scholar] [CrossRef]
- Osakabe, M.; Fukagawa, D.; Sato, C.; Sugimoto, R.; Uesugi, N.; Ishida, K.; Itamochi, H.; Sugiyama, T.; Sugai, T. Immunohistochemical analysis of the epithelial to mesenchymal transition in uterine carcinosarcoma. Int. J. Gynecol. Cancer 2019, 29, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Enomoto, T.; Fujita, M.; Yoshino, K.; Nakashima, R.; Kurachi, H.; Haba, T.; Wakasa, K.; Shroyer, K.R.; Tsujimoto, M.; et al. Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors. Cancer Res. 1997, 57, 5379–5385. [Google Scholar]
- Leskela, S.; Pérez-Mies, B.; Rosa-Rosa, J.M.; Cristobal, E.; Biscuola, M.; Palacios-Berraquero, M.L.; Ong, S.; Matias-Guiu Guia, X.; Palacios, J. Molecular Basis of Tumor Heterogeneity in Endometrial Carcinosarcoma. Cancers 2019, 11, 964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCluggage, W.G. Malignant biphasic uterine tumours: Carcinosarcomas or metaplastic carcinomas? J. Clin. Pathol. 2002, 55, 321–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.; Vasdev, N.; Pandey, D. Uterine collision tumor of endometrial stromal sarcoma and endometrioid adenocarcinoma: A rare case report and review of literature. Indian J. Pathol. Microbiol. 2021, 64, 802–805. [Google Scholar] [CrossRef]
- Matsuo, K.; Takazawa, Y.; Ross, M.S.; Elishaev, E.; Yunokawa, M.; Sheridan, T.B.; Bush, S.H.; Klobocista, M.M.; Blake, E.A.; Takano, T.; et al. Significance of Lymphovascular Space Invasion by the Sarcomatous Component in Uterine Carcinosarcoma. Ann. Surg. Oncol. 2018, 25, 2756–2766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gotoh, O.; Sugiyama, Y.; Takazawa, Y.; Kato, K.; Tanaka, N.; Omatsu, K.; Takeshima, N.; Nomura, H.; Hasegawa, K.; Fujiwara, K.; et al. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat. Commun. 2019, 10, 4965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428, Erratum in J. Clin. Investig. 2010, 120, 1786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stockhammer, P.; Okumus, Ö.; Hegedus, L.; Rittler, D.; Ploenes, T.; Herold, T.; Kalbourtzis, S.; Bankfalvi, A.; Sucker, A.; Kimmig, R.; et al. HDAC Inhibition Induces Cell Cycle Arrest and Mesenchymal-Epithelial Transition in a Novel Pleural-Effusion Derived Uterine Carcinosarcoma Cell Line. Pathol. Oncol. Res. 2021, 27, 636088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez-Garcia, M.A.; Palacios, J. Pathologic and molecular features of uterine carcinosarcomas. Semin. Diagn. Pathol. 2010, 27, 274–286. [Google Scholar] [CrossRef]
- McCluggage, W.G. A practical approach to the diagnosis of mixed epithelial and mesenchymal tumours of the uterus. Mod. Pathol. 2016, 29 (Suppl. S1), S78–S91. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Vargiu, V.; Certelli, C.; Arcieri, M.; Vizza, E.; Legge, F.; Cosentino, F.; Ferrandina, G.; Fanfani, F.; Scambia, G.; et al. Is the sarcomatous component (homologous vs heterologous) the prognostic “driving force” in early-stage uterine carcinosarcomas? A retrospective multicenter study. J. Cancer Res. Clin. Oncol. 2023, 149, 6479–6488. [Google Scholar] [CrossRef]
- Clearman, T.; Cimic, A.; Ellenson, L.H.; Gupta, D. Clinically aggressive “low-grade” uterine carcinosarcoma: A case report. Gynecol. Oncol. Rep. 2015, 14, 9–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferguson, S.E.; Tornos, C.; Hummer, A.; Barakat, R.R.; Soslow, R.A. Prognostic features of surgical stage I uterine carcinosarcoma. Am. J. Surg. Pathol. 2007, 31, 1653–1661. [Google Scholar] [CrossRef]
- Djordjevic, B.; Gien, L.T.; Covens, A.; Malpica, A.; Khalifa, M.A. Polypoid or non-polypoid? A novel dichotomous approach to uterine carcinosarcoma. Gynecol. Oncol. 2009, 115, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.M.; Wang, L.M.; Yao, J.M. Unexpected rare uterine carcinosarcoma with neuroendocrine differentiation: Reflections on clinical diagnosis and treatment of a case report. Medicine 2024, 103, e38800. [Google Scholar] [CrossRef]
- Naito, M.; Terasaki, M.; Ouchi, N.; Toyoshima, M. Uterine carcinosarcoma showing immature teratoid-like differentiation. BMJ Case Rep. CP 2023, 16, e257228. [Google Scholar] [CrossRef]
- Euscher, E.D.; Deavers, M.T.; Lopez-Terrada, D.; Lazar, A.J.; Silva, E.G.; Malpica, A. Uterine tumors with neuroectodermal differentiation: A series of 17 cases and review of the literature. Am. J. Surg. Pathol. 2008, 32, 219–228. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Davel, G.H.; De Vos, R.; Vergote, I.; Lindeque, B.G.; de Jonge, E. Uterine carcinosarcoma with melanocytic differentiation. Int. J. Gynecol. Pathol. 2001, 20, 186–190. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Tjota, M.Y.; Choi, D.N.; Chapel, D.B.; Kolin, D.L.; Euscher, E.D.; Barroeta, J.E.; Numan, T.A.; Xing, D.; Afkhami, M.; et al. Clinicopathologic and Molecular Characterization of Gynecologic Carcinosarcomas with a Mesonephric-Like Carcinomatous Component. Am. J. Surg. Pathol. 2025, 49, 439–447. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, G.H.; Kim, H. Prognostic significance of heterologous component in carcinosarcoma of the gynecologic organs: A systematic review and meta-analysis. J. Gynecol. Oncol. 2023, 34, e73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanthan, R.; Senger, J.L. Uterine carcinosarcomas (malignant mixed müllerian tumours): A review with special emphasis on the controversies in management. Obstet. Gynecol. Int. 2011, 2011, 470795. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Takazawa, Y.; Ross, M.S.; Elishaev, E.; Podzielinski, I.; Yunokawa, M.; Sheridan, T.B.; Bush, S.H.; Klobocista, M.M.; Blake, E.A.; et al. Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Sreenan, J.J.; Hart, W.R. Carcinosarcomas of the female genital tract. A pathologic study of 29 metastatic tumors: Further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am. J. Surg. Pathol. 1995, 19, 666–674. [Google Scholar] [CrossRef]
- Amin, S.E.; Elzamly, S.; Ahmed, A.A.; Tandon, N. Uterine Carcinosarcoma Presenting as Metastatic Osteosarcoma in the Lung: A Case Report and Literature Review. Ann. Clin. Lab. Sci. 2023, 53, 969–973. [Google Scholar]
- Mayall, F.; Rutty, K.; Campbell, F.; Goddard, H. p53 immunostaining suggests that uterine carcinosarcomas are monoclonal. Histopathology 1994, 24, 211–214. [Google Scholar] [CrossRef]
- de Jong, R.; Nijman, H.; Wijbrandi, T.; Reyners, A.K.; Boezen, H.M.; Hollema , H. Molecular markers and clinical behavior of uterine carcinosarcomas: Focus on the epithelial tumor component. Mod. Pathol. 2011, 24, 1368–1379. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73, Erratum in Nature 2013, 500, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Electronic address: Clinicalguidelines@esmo.org Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Gencarelli, A.; Mollo, A.; Guida, M.; Insabato, L.; Santoro, A.; Zannoni, G.F.; Zullo, F. TCGA Classification of Endometrial Cancer: The Place of Carcinosarcoma. Pathol. Oncol. Res. 2020, 26, 2067–2073. [Google Scholar] [CrossRef]
- Borrego, S.N.; Lengyel, E.; Kurnit, K.C. Molecular Characterizations of Gynecologic Carcinosarcomas: A Focus on the Immune Microenvironment. Cancers 2022, 14, 4465. [Google Scholar] [CrossRef]
- Galant, N.; Krawczyk, P.; Monist, M.; Obara, A.; Gajek, Ł.; Grenda, A.; Nicoś, M.; Kalinka, E.; Milanowski, J. Molecular Classification of Endometrial Cancer and Its Impact on Therapy Selection. Int. J. Mol. Sci. 2024, 25, 5893. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.T.; Wang, T.L.; Fader, A.N.; Shih, I.M.; Gaillard, S. Molecular Classification and Emerging Targeted Therapy in Endometrial Cancer. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2020, 39, 26–35. [Google Scholar] [CrossRef]
- Le Gallo, M.; Rudd, M.L.; Urick, M.E.; Hansen, N.F.; Zhang, S.; NISC Comparative Sequencing Program; Lozy, F.; Sgroi, D.C.; Vidal Bel, A.; Matias-Guiu, X.; et al. Somatic mutation profiles of clear cell endometrial tumors revealed by whole exome and targeted gene sequencing. Cancer 2017, 123, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Choi, M.; Overton, J.D.; Bellone, S.; Roque, D.M.; Cocco, E.; Guzzo, F.; English, D.P.; Varughese, J.; Gasparrini, S.; et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Santin, A.D. Genetic landscape of clear cell endometrial cancer and the era of precision medicine. Cancer 2017, 123, 3216–3218. [Google Scholar] [CrossRef]
- Momeni-Boroujeni, A.; Nguyen, B.; Vanderbilt, C.M.; Ladanyi, M.; Abu-Rustum, N.R.; Aghajanian, C.; Ellenson, L.H.; Weigelt, B.; Soslow, R.A. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod. Pathol. 2022, 35, 1269–1278. [Google Scholar] [CrossRef]
- Devereaux, K.A.; Weiel, J.J.; Pors, J.; Steiner, D.F.; Ho, C.; Charu, V.; Suarez, C.J.; Renz, M.; Diver, E.; Karam, A.; et al. Prospective molecular classification of endometrial carcinomas: Institutional implementation, practice, and clinical experience. Mod. Pathol. 2022, 35, 688–696. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Mascolo, M.; Guida, M.; Insabato, L.; Zannoni, G.F.; Zullo, F. Clear cell endometrial carcinoma and the TCGA classification. Histopathology 2020, 76, 336–338. [Google Scholar] [CrossRef]
- DeLair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The genetic landscape of endometrial clear cell carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Irshaid, L.; Costigan, D.C.; Dong, F.; Matulonis, U.A.; Nucci, M.R.; Kolin, D.L. Molecular Landscape of Mullerian Clear Cell Carcinomas Identifies The Cancer Genome Atlas-like Prognostic Subgroups. Mod. Pathol 2023, 36, 100123. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakayama, N.; Ishikawa, M.; Miyazaki, K. Endometrial serous carcinoma: Its molecular characteristics and histology-specific treatment strategies. Cancers 2012, 4, 799–807. [Google Scholar] [CrossRef]
- Cai, Y.; Han, Q.; Guo, H. Identifying clinical features and molecular characteristics of the endometrial clear cell carcinoma. Front. Oncol. 2023, 13, 1286176. [Google Scholar] [CrossRef] [PubMed]
- Huvila, J.M.; Jamieson, A.M.; Pors, J.; Hoang, L.; Mirkovic, J.; Cochrane, D.; McAlpine, J.N.; Gilks, C.B. Endometrial Carcinosarcomas are Almost Exclusively of p53abn Molecular Subtype After Exclusion of Mimics. Int. J. Gynecol. Pathol. 2024, 43, 506–514. [Google Scholar] [CrossRef]
- Zhao, S.; Bellone, S.; Lopez, S.; Thakral, D.; Schwab, C.; English, D.P.; Black, J.; Cocco, E.; Choi, J.; Zammataro, L.; et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2016, 113, 12238–12243. [Google Scholar] [CrossRef]
- Crane, E.; Naumann, W.; Tait, D.; Higgins, R.; Herzog, T.; Brown, J. Molecular variations in uterine carcinosarcomas identify therapeutic opportunities. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 480–484. [Google Scholar] [CrossRef]
- Mori, S.; Gotoh, O.; Kiyotani, K.; Low, S.K. Genomic alterations in gynecological malignancies: Histotype-associated driver mutations, molecular subtyping schemes, and tumorigenic mechanisms. J. Hum. Genet. 2021, 66, 853–868. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Raimondo, D.; Maletta, M.; De Vivo, V.; Visiello, U.; Casadio, P.; Seracchioli, R.; Zullo, F.; Insabato, L.; et al. Uterine carcinosarcoma vs endometrial serous and clear cell carcinoma: A systematic review and meta-analysis of survival. Int. J. Gynaecol. Obstet. 2022, 158, 520–527. [Google Scholar] [CrossRef]
- Jones, N.L.; Xiu, J.; Chatterjee-Paer, S.; Buckley de Meritens, A.; Burke, W.M.; Tergas, A.I.; Wright, J.D.; Hou, J.Y. Distinct molecular landscapes between endometrioid and nonendometrioid uterine carcinomas. Int. J. Cancer 2017, 140, 1396–1404. [Google Scholar] [CrossRef]
- Sağnıç, S.; Tuncer, S.F.; Iltar, E.; Güner, F.C.; Tuncer, H.A.; Doğan, S.; Şimşek, T. Uterine Carcinosarcoma: Adaptation to New FIGO 2023 Staging System Through Clinical Profile and Oncologic Outcomes. J. Clin. Med. 2025, 14, 2299. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee. FIGO Women’s Cancer Committee FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Pham, E.; Berg, C.V.D.; Es, R.V.; Groenendijk, F.; Doorn, H.C.; Van Beekhuizen, H. Low accuracy of preoperative sampling for diagnosing uterine carcinosarcoma. Int. J. Gynecol. Cancer 2023, 33, A52–A53. [Google Scholar]
- Pandita, P.; Wang, X.; Jones, D.E.; Collins, K.; Hawkins, S.M. Unique Molecular Features in High-Risk Histology Endometrial Cancers. Cancers 2019, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Q.; Chen, X.; Zheng, W.; Kang, Y.; Cao, D. Clinical and multiparametric MRI features for differentiating uterine carcinosarcoma from endometrioid adenocarcinoma. BMC Med. Imaging 2024, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.N.; Nguyen, V.T. Endometrial thickness and uterine artery Doppler parameters as soft markers for prediction of endometrial cancer in postmenopausal bleeding women: A cross-sectional study at tertiary referral hospitals from Vietnam. Obstet. Gynecol. Sci. 2022, 65, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Chiu, L.G.; Gebb, J.S.; Gunter, M.J.; Sukumvanich, P.; Goldberg, G.L.; Einstein, M.H. Serum CA125 predicts extrauterine disease and survival in uterine carcinosarcoma. Gynecol. Oncol. 2007, 107, 513–517. [Google Scholar] [CrossRef]
- Liu, T.; Tu, H.; Li, Y.; Liu, Z.; Liu, G.; Gu, H. Impact of Radical Hysterectomy Versus Simple Hysterectomy on Survival of Patients with Stage 2 Endometrial Cancer: A Meta-analysis. Ann. Surg. Oncol. 2019, 26, 2933–2942. [Google Scholar] [CrossRef]
- Smyth, S.L.; Ripullone, K.; Zouridis, A.; Pappa, C.; Spain, G.; Gkorila, A.; McCulloch, A.; Tupper, P.; Bibi, F.; Sadeghi, N.; et al. Uterine Carcinosarcoma—A Retrospective Cohort Analysis from a Tertiary Centre on Epidemiology, Management Approach, Outcomes and Survival Patterns. Cancers 2025, 17, 635. [Google Scholar] [CrossRef]
- Holub, K.; Biete, A. New pre-treatment eosinophil-related ratios as prognostic biomarkers for survival outcomes in endometrial cancer. BMC Cancer 2018, 18, 1280. [Google Scholar] [CrossRef]
- Ross, M.S.; Elishaev, E.; Berger, J.L.; Kelley, J.L.; Taylor, S.E. Prognostic Significance of omental Disease and the Role of Omental Sampling in Patients with Uterine Carcinosarcoma. Int. J. Gynecol. Cancer 2018, 28, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zammarrelli, W.A., 3rd; Greenman, M.; Rios-Doria, E.; Miller, K.; Broach, V.; Mueller, J.J.; Aviki, E.; Alektiar, K.M.; Soslow, R.A.; Ellenson, L.H.; et al. Sentinel lymph node biopsy alone compared to systematic lymphadenectomy in patients with uterine carcinosarcoma. Gynecol. Oncol. 2022, 165, 287–292. [Google Scholar] [CrossRef]
- Matsuo, K.; Johnson, M.S.; Im, D.D.; Ross, M.S.; Bush, S.H.; Yunokawa, M.; Blake, E.A.; Takano, T.; Klobocista, M.M.; Hasegawa, K.; et al. Survival outcome of women with stage IV uterine carcinosarcoma who received neoadjuvant chemotherapy followed by surgery. J. Surg. Oncol. 2018, 117, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.A.; Filiaci, V.L.; Hensley, M.L.; Huang, H.Q.; Moore, K.N.; Tewari, K.S.; Copeland, L.J.; Secord, A.A.; Mutch, D.G.; Santin, A.; et al. Randomized Phase III Trial of Paclitaxel and Carboplatin Versus Paclitaxel and Ifosfamide in Patients with Carcinosarcoma of the Uterus or Ovary: An NRG Oncology Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 968–977. [Google Scholar] [CrossRef]
- Dahl, M.J.; Lofgren, K.A.; Haugen, C.; Harmon, G.E.; Hughes, S.P.; Dahl, K.D.C. Gemcitabine combination therapies induce apoptosis in uterine carcinosarcoma patient-derived organoids. Front. Oncol. 2024, 14, 1368592. [Google Scholar] [CrossRef]
- Reed, N.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.; Coens, C.; et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Hapsari, K.; Bhugwandass, C.; van Rijn, G.W.J.; van der Wurff, A.A.M.; van ‘t Veer, M.; Boll, D.; Vos, M.C.; Pijlman, B.; Kok, A.; Piek, J.M.J. Treatment and Outcome of Patients with Uterine Carcinosarcoma in a Comprehensive Cancer Network. Indian J. Gynecol. Oncol. 2020, 18, 17. [Google Scholar] [CrossRef]
- Chow, S.; Chan, J.K.; Kapp, D.S.; Mann, A.; Liou, W.S.; Liao, C.I. Does chemotherapy or radiation benefit surgical stage I uterine carcinosarcoma patients? Am. J. Obstet. Gynecol. 2020, 222, 383–384. [Google Scholar] [CrossRef]
- Yurttas, C.; Ladurner, R.; Mihaljević, A.L.; Strohäker, J. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Sarcomatosis. Cancers 2024, 16, 3034. [Google Scholar] [CrossRef]
- Psilopatis, I.; Damaskos, C.; Garmpis, N.; Vrettou, K.; Garmpi, A.; Sarantis, P.; Koustas, E.; Antoniou, E.A.; Kouraklis, G.; Chionis, A.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Uterine Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 12353. [Google Scholar] [CrossRef]
- Navarro-Barrios, Á.; Gil-Martínez, J.; Ramos-Bernardo, I.; Barrios, P.; Muñoz-Casares, C.; Torres-Melero, J.; Pereira, F.; Manzanedo, I.; Arjona, Á.; Martínez-Regueira, F.; et al. Intraperitoneal hyperthermic chemotherapy after cytoreduction in patients with peritoneal metastases from endometrial cancer. The next frontier? Surg. Oncol. 2020, 33, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.A.; Boisen, M.M.; Comerci, J.T.; Kim, H.; Houser, C.J.; Sukumvanich, P.; Olawaiye, A.B.; Kelley, J.L.; Edwards, R.P.; Huang, M.; et al. Neoadjuvant radiotherapy with or without chemotherapy followed by extrafascial hysterectomy for locally advanced endometrial cancer clinically extending to the cervix or parametria. Gynecol. Oncol. 2014, 135, 190–195. [Google Scholar] [CrossRef]
- Makker, V.; Abu-Rustum, N.R.; Alektiar, K.M.; Aghajanian, C.A.; Zhou, Q.; Iasonos, A.; Hensley, M.L. A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I-IV uterine carcinosarcoma. Gynecol. Oncol. 2008, 111, 249–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maione, H.; Sienna, J.; Schnarr, K.L.; Donovan, E.K. Non-Metastatic Uterine Carcinosarcoma: A Tailored Approach or One Size Fits All? Radiation 2024, 4, 183–191. [Google Scholar] [CrossRef]
- Seagle, B.L.; Kanis, M.; Kocherginsky, M.; Strauss, J.B.; Shahabi, S. Stage I uterine carcinosarcoma: Matched cohort analyses for lymphadenectomy, chemotherapy, and brachytherapy. Gynecol. Oncol. 2017, 145, 71–77. [Google Scholar] [CrossRef]
- McEachron, J.; Heyman, T.; Shanahan, L.; Tran, V.; Friedman, M.; Gorelick, C.; Economos, K.; Singhal, P.K.; Lee, Y.C.; Kanis, M.J. Multimodality adjuvant therapy and survival outcomes in stage I-IV uterine carcinosarcoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 1012–1017. [Google Scholar] [CrossRef]
- Tung, H.-J.; Chiang, C.-Y.; Chang, W.-Y.; Wu, R.-C.; Huang, H.-J.; Yang, L.-Y.; Lin, C.-Y.; Wang, C.-C.; Chao, A.; Lai, C.-H. Management and Prognosis of Patients with Recurrent or Persistent/Progressive Uterine Carcinosarcoma. Curr. Oncol. 2022, 29, 7607–7623. [Google Scholar] [CrossRef] [PubMed]
- Bellone, S.; McNamara, B.; Mutlu, L.; Demirkiran, C.; Hartwich, T.M.P.; Harold, J.; Yang-Hartwich, Y.; Siegel, E.R.; Santin, A.D. Monitoring Treatment Response, Early Recurrence, and Survival in Uterine Serous Carcinoma and Carcinosarcoma Patients Using Personalized Circulating Tumor DNA Biomarkers. Int. J. Mol. Sci. 2023, 24, 8873. [Google Scholar] [CrossRef] [PubMed]
- Soiffer, J.L.; Fife, A.J.; Gadad, S.S.; Laurini, J.A.; Elvin, J.A.; Isani, S.S.; Lin, K.Y. Durable partial response to pembrolizumab, lenvatinib, and letrozole in a case of recurrent uterine carcinosarcoma with ESR1 gene amplification. Gynecol. Oncol. Rep. 2024, 54, 101426. [Google Scholar] [CrossRef]
- Liang, A.L.; Katebi Kashi, P.; Hopkins, M.; Beavis, A.; Gaillard, S.; Shih, I.M.; Fader, A.N. Progestin and aromatase inhibitor therapy in recurrent, estrogen/progestin receptor positive uterine carcinosarcoma: A case report. Gynecol. Oncol. Rep. 2021, 38, 100877. [Google Scholar] [CrossRef]
- Edmondson, R.J.; O’Connell, R.; Banerjee, S.; Mileshkin, L.; Sykes, P.; Beale, P.; Fisher, A.; Bonaventura, A.; Millan, D.; Nottley, S.; et al. Phase 2 study of anastrozole in rare cohorts of patients with estrogen receptor/progesterone receptor positive leiomyosarcomas and carcinosarcomas of the uterine corpus: The PARAGON trial (ANZGOG 0903). Gynecol. Oncol. 2021, 163, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romano, P.; Jurado, M.; Idoate, M.A.; Arbea, L.; Hernandez-Lizoain, J.L.; Cano, D.; Paramo, J.A.; Martin-Algarra, S. Durable complete remission with aromatase inhibitor therapy in a patient with metastatic uterine carcinosarcoma with poor performance status and coagulation disorders: A case report. J. Med. Case Reports 2017, 11, 115. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Available online: https://app.biorender.com (accessed on 11 May 2025).
- Engerud, H.; Berg, H.F.; Myrvold, M.; Halle, M.K.; Bjorge, L.; Haldorsen, I.S.; Hoivik, E.A.; Trovik, J.; Krakstad, C. High degree of heterogeneity of PD-L1 and PD-1 from primary to metastatic endometrial cancer. Gynecol. Oncol. 2020, 157, 260–267. [Google Scholar] [CrossRef]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; de Braud, F.; Arkenau, H.T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients with Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef]
- Van Gorp, T.; Cibula, D.; Lv, W.; Backes, F.; Ortaç, F.; Hasegawa, K.; Lindemann, K.; Savarese, A.; Laenen, A.; Kim, Y.M.; et al. ENGOT-en11/GOG-3053/KEYNOTE-B21: A randomised, double-blind, phase III study of pembrolizumab or placebo plus adjuvant chemotherapy with or without radiotherapy in patients with newly diagnosed, high-risk endometrial cancer. Ann. Oncol. 2024, 35, 968–980. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; de Albuquerque, L.Z.; Rodrigues, F.R.; de Mesquita, G.G.; Chaves, C.B.P.; Bonamino, M.H.; de Melo, A.C. The prevalence and prognostic impact of tumor-infiltrating lymphocytes in uterine carcinosarcoma. BMC Cancer 2021, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef]
- Makker, V.; Aghajanian, C.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; Messing, M.; Dutta, L.; Dutcus, C.E.; Huang, J.; et al. A Phase Ib/II Study of Lenvatinib and Pembrolizumab in Advanced Endometrial Carcinoma (Study 111/KEYNOTE-146): Long-Term Efficacy and Safety Update. J. Clin. Oncol. 2023, 41, 974–979. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Monk, B.J.; Mackay, H.; Santin, A.D.; Miller, D.S.; Moore, R.G.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J. Clin. Oncol. 2023, 41, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Imhoff, S.; Fogel, L.; Alkhatatneh, H.; Rastogi, N.; Foster, A.; Janosky, M. Combining lenvatinib and pembrolizumab for the managementofendometrialcarcinosarcoma: Aretrospectivecase series. Eur. J. Gynaecol. Oncol. 2024, 45, 174–178. [Google Scholar] [CrossRef]
- Ozawa, R.; Nishikawa, T.; Yamamoto, K.; Shimoi, T.; Ishikawa, M.; Kato, T.; Yonemori, K. The efficacy and safety of lenvatinib plus pembrolizumab therapy in patients with uterine carcinosarcoma. Gynecol. Oncol. Rep. 2024, 55, 101479. [Google Scholar] [CrossRef]
- Garmezy, B.; Gheeya, J.; Lin, H.Y.; Huang, Y.; Kim, T.; Jiang, X.; Thein, K.Z.; Pilié, P.G.; Zeineddine, F.; Wang, W.; et al. Clinical and Molecular Characterization of POLE Mutations as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100267. [Google Scholar] [CrossRef]
- Hammer, P.M.; Momeni-Boroujeni, A.; Kolin, D.L.; Kingsley, L.; Folkins, A.; Geisick, R.L.P.; Ho, C.; Suarez, C.J.; Howitt, B.E. POLE-mutated uterine carcinosarcomas: A clinicopathologic and molecular study of 11 cases. Mod. Pathol. 2024, 38, 100676. [Google Scholar] [CrossRef]
- Hunt, J.T.; Chambers, L.M.; Yao, M.; Joehlin-Price, A.; Debernardo, R.; Rose, P.G. Lenvatinib plus pembrolizumab in patients with advanced or recurrent uterine carcinosarcoma. Gynecol. Oncol. Rep. 2021, 37, 100840. [Google Scholar] [CrossRef]
- Laurent, J.D.S.; Abel, M.K.; Liu, J.; Quade, B.J.; Davis, M.R. Successful treatment of stage IVB ovarian carcinosarcoma with PARP Inhibitor: A case report. Gynecol. Oncol. Rep. 2024, 51, 101322. [Google Scholar] [CrossRef]
- Tymon-Rosario, J.R.; Manara, P.; Manavella, D.D.; Bellone, S.; Hartwich, T.M.P.; Harold, J.; Yang-Hartwich, Y.; Zipponi, M.; Choi, J.; Jeong, K.; et al. Homologous recombination deficiency (HRD) signature-3 in ovarian and uterine carcinosarcomas correlates with preclinical sensitivity to Olaparib, a poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor. Gynecol. Oncol. 2022, 166, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Post, C.C.B.; Westermann, A.M.; Boere, I.A.; Witteveen, P.O.; Ottevanger, P.B.; Sonke, G.S.; Lalisang, R.I.; Putter, H.; Meershoek-Klein Kranenbarg, E.; Braak, J.P.B.M.; et al. Efficacy and safety of durvalumab with olaparib in metastatic or recurrent endometrial cancer (phase II DOMEC trial). Gynecol. Oncol. 2022, 165, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, D.; Snir, O.L.; Wu, X.; Wong, S.; Hui, P.; Santin, A.D.; Buza, N. HER2 testing of gynecologic carcinosarcomas: Tumor stratification for potential targeted therapy. Mod. Pathol. 2020, 33, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Gysler, S.; Latif, N.; Cory, L.; Giuntoli, R.L., II; Kim, S.H.; Simpkins, F.; Martin, L.; Ko, E.M. Molecular landscape of ERBB2/HER2 gene amplification among patients with gynecologic malignancies; clinical implications and future directions. Gynecol. Oncol. 2024, 180, 1–5. [Google Scholar] [CrossRef]
- Halle, M.K.; Tangen, I.L.; Berg, H.F.; Hoivik, E.A.; Mauland, K.K.; Kusonmano, K.; Berg, A.; Hurtado, A.; Kalland, K.H.; Øyan, A.M.; et al. HER2 expression patterns in paired primary and metastatic endometrial cancer lesions. Br. J. Cancer 2018, 118, 378–387. [Google Scholar] [CrossRef]
- Yoshida, H.; Mizoguchi, C.; Saito, A.; Kitadai, R.; Yamamoto, K.; Nishikawa, T.; Kato, T.; Yonemori, K. Discordances in expression of human epidermal growth factor receptor 2 between primary and metastatic uterine carcinosarcoma: A proposal for HER2-targeted therapy specimen selection. Ann. Diagn. Pathol. 2023, 65, 152150. [Google Scholar] [CrossRef]
- Lu, T.; Lou, S.; Shih, Y.; Chen, Y.; Fan, C.T.; Wang, S.; Hsu, S.; Liu, C.; Hwang, S.; Lu, C. Adding trastuzumab to carboplatin and paclitaxel improves overall survival in advanced-stage HER2/NEU overexpressing uterine serous carcinoma or carcinosarcoma. Int. J. Gynecol. Cancer 2024, 34, A45–A46. [Google Scholar]
- Nishikawa, T.; Hasegawa, K.; Matsumoto, K.; Mori, M.; Hirashima, Y.; Takehara, K.; Ariyoshi, K.; Kato, T.; Yagishita, S.; Hamada, A.; et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. J. Clin. Oncol. 2023, 41, 2789–2799. [Google Scholar] [CrossRef]
- Moufarrij, S.; Chui, M.H.; Aghajanian, C.; Ellenson, L.; Green, H.; Paula, A.D.C.; Zammarrelli, W.; Abu-Rustum, N.; Wu, M.; Brown, D.; et al. TROP2 as a novel target in uterine carcinosarcoma organoid models. Gynecol. Oncol. 2024, 190, S204. [Google Scholar] [CrossRef]
- Santin, A.; McNamara, B.; Siegel, E.R.; Harold, J.; Mutlu, L.; Altwerger, G.; Huang, G.S.; Andikyan, V.; Clark, M.B.; Ratner, E.; et al. Preliminary results of a phase II trial with sacituzumab govitecan-hziy in patients with recurrent endometrial carcinoma overexpressing Trop-2. J. Clin. Oncol. 2023, 41, 5599. [Google Scholar] [CrossRef]
- Bignotti, E.; Ravaggi, A.; Romani, C.; Falchetti, M.; Lonardi, S.; Facchetti, F.; Pecorelli, S.; Varughese, J.; Cocco, E.; Bellone, S.; et al. Trop-2 overexpression in poorly differentiated endometrial endometrioid carcinoma: Implications for immunotherapy with hRS7, a humanized anti-trop-2 monoclonal antibody. Int. J. Gynecol. Cancer. 2011, 21, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Demirkiran, C.; Greenman, M.; Bellone, S.; McNamara, B.; Hartwich, T.M.P.; Manavella, D.; Mutlu, L.; Zipponi, M.; Yang-Hartwich, Y.; Yang, K.; et al. Preclinical in vitro and in vivo activity of the RAF/MEK clamp avutometinib in combination with FAK inhibition in uterine carcinosarcomas. Gynecol. Oncol. 2024, 187, 12–20. [Google Scholar] [CrossRef]
- Pillai, M.S.G.; Shaw, P.; Bhowmik, A.D.; Bhattacharya, R.; Rao, G.; Dwivedi, S.K.D. Uterine carcinosarcoma: Unraveling the role of epithelial-to-mesenchymal transition in progression and therapeutic potential. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e70132. [Google Scholar] [CrossRef]

| Endometrial Endometrioid Cancer | Endometrial Carcinosarcoma | Serous Endometrial Carcinoma | Clear Cell Endometrial Carcinoma | |
|---|---|---|---|---|
| Molecular subtypes | ||||
| POLE-ultramutated | 7–9% | 1.8–5.3% | 2–7% | 3.8–10.5% |
| MSI-H | 15–28% | 3.6–7.3% | 2–11.8% | 9.8–15.8% |
| TP53 abnormal | 15–26% | 73.9–91% | 71–94% | 42.5–57.9% |
| NSMP | 39–55% | 3.6–13.5% | 0–5% | 15.8–46% |
| Gene Mutation | Endometrial Endometrioid Cancer | Endometrial Carcinosarcoma | Serous Endometrial Carcinoma | Clear Cell Endometrial Carcinoma |
|---|---|---|---|---|
| p53-abn | 7.5–21% | 62–91% | 71.1–88.4% | 39.7–46% |
| ARID1A | 32.5–54% | 10–27% | 6.7–9.3% | 15.9–24% |
| PTEN | 40–84% | 18–41% | 10–11% | 7–21% |
| PPP2R1A | 5–11% | 10–28% | 38–41% | 15.9–36% |
| PIK3CA | 10% | 11–41% | 22–35.6% | 23.8–33% |
| FBXW7 | 17% | 19–39% | 24% | 7.9–25% |
| SPOP | 9.3% | 3–18% | 7–8% | 14.3% |
| KRAS | 24% | 9–27%% | 3% | 7–14% |
| TAF1 | 17.1% | 4–8% | 4.7–13.5% | 9.5% |
| PIK3R1 | 36–45% | 11–23% | 4.7–11% | 15.9–18% |
| CTNNB1 | 34% | 2–12% | 1% | 0% |
| ERBB2 (ampl.) | 8% | 9% | 19% | 6–11% |
| CHD4 | 9% | 11–17% | 18% | Not reported |
| CTCF | 31% | 7–17% | 2% | Not reported |
| Prognostic Factors | Favorable | Unfavorable |
|---|---|---|
| ||
| ||
| <5 cm | >5 cm | |
| ||
| (−) Peritoneal cytology | (+) Peritoneal cytology | |
| Safe uterine removal | Uterine perforation or rupture | |
| Adequate staging (infracolic omentectomy, lymph node dissection, or sentinel node mapping) | Inadequate staging | |
| Ovariectomy | Ovarian preservation | |
| No fertility sparing | Fertility-sparing procedures | |
| ||
| (−) LVSI | (+) LVSI | |
| (−) LVSI within the sarcomatous component | (+) LVSI within the sarcomatous component | |
| Myometrial invasion < 50% | Myometrial invasion > 50% | |
| No invasion into the cervix | Cervical stromal involvement | |
| Endometrioid carcinoma component | Non-endometrioid carcinoma component (mainly serous-like) | |
| Homologous sarcomatous component | Heterologous sarcomatous component | |
| (−) Rhabdomyoblastic sarcomatous differentiation | (+) Rhabdomyoblastic sarcomatous differentiation | |
| (−) Sarcomatous dominance | (+) Sarcomatous dominance | |
| Low-grade carcinoma/low-grade sarcoma components | High-grade carcinoma/high-grade sarcoma components | |
| ||
| Pole mutation, MSI | TP53 mutation, NSMP | |
| ||
| Normal | Elevated | |
| ||
| Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Kornovski, Y.; Ivanova, V.; Dzhenkov, D.; Metodiev, D.; Wafa, M.; Ivanova, Y.; Slavchev, S.; Tsoneva, E.; Yordanov, A. Carcinosarcoma of the Endometrium—Pathology, Molecular Landscape and Novel Therapeutic Approaches. Medicina 2025, 61, 1156. https://doi.org/10.3390/medicina61071156
Kostov S, Kornovski Y, Ivanova V, Dzhenkov D, Metodiev D, Wafa M, Ivanova Y, Slavchev S, Tsoneva E, Yordanov A. Carcinosarcoma of the Endometrium—Pathology, Molecular Landscape and Novel Therapeutic Approaches. Medicina. 2025; 61(7):1156. https://doi.org/10.3390/medicina61071156
Chicago/Turabian StyleKostov, Stoyan, Yavor Kornovski, Vesela Ivanova, Deyan Dzhenkov, Dimitar Metodiev, Mohamed Wafa, Yonka Ivanova, Stanislav Slavchev, Eva Tsoneva, and Angel Yordanov. 2025. "Carcinosarcoma of the Endometrium—Pathology, Molecular Landscape and Novel Therapeutic Approaches" Medicina 61, no. 7: 1156. https://doi.org/10.3390/medicina61071156
APA StyleKostov, S., Kornovski, Y., Ivanova, V., Dzhenkov, D., Metodiev, D., Wafa, M., Ivanova, Y., Slavchev, S., Tsoneva, E., & Yordanov, A. (2025). Carcinosarcoma of the Endometrium—Pathology, Molecular Landscape and Novel Therapeutic Approaches. Medicina, 61(7), 1156. https://doi.org/10.3390/medicina61071156








