Lipoprotein (a) in the Development and Progression of Diabetic Retinopathy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection and Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
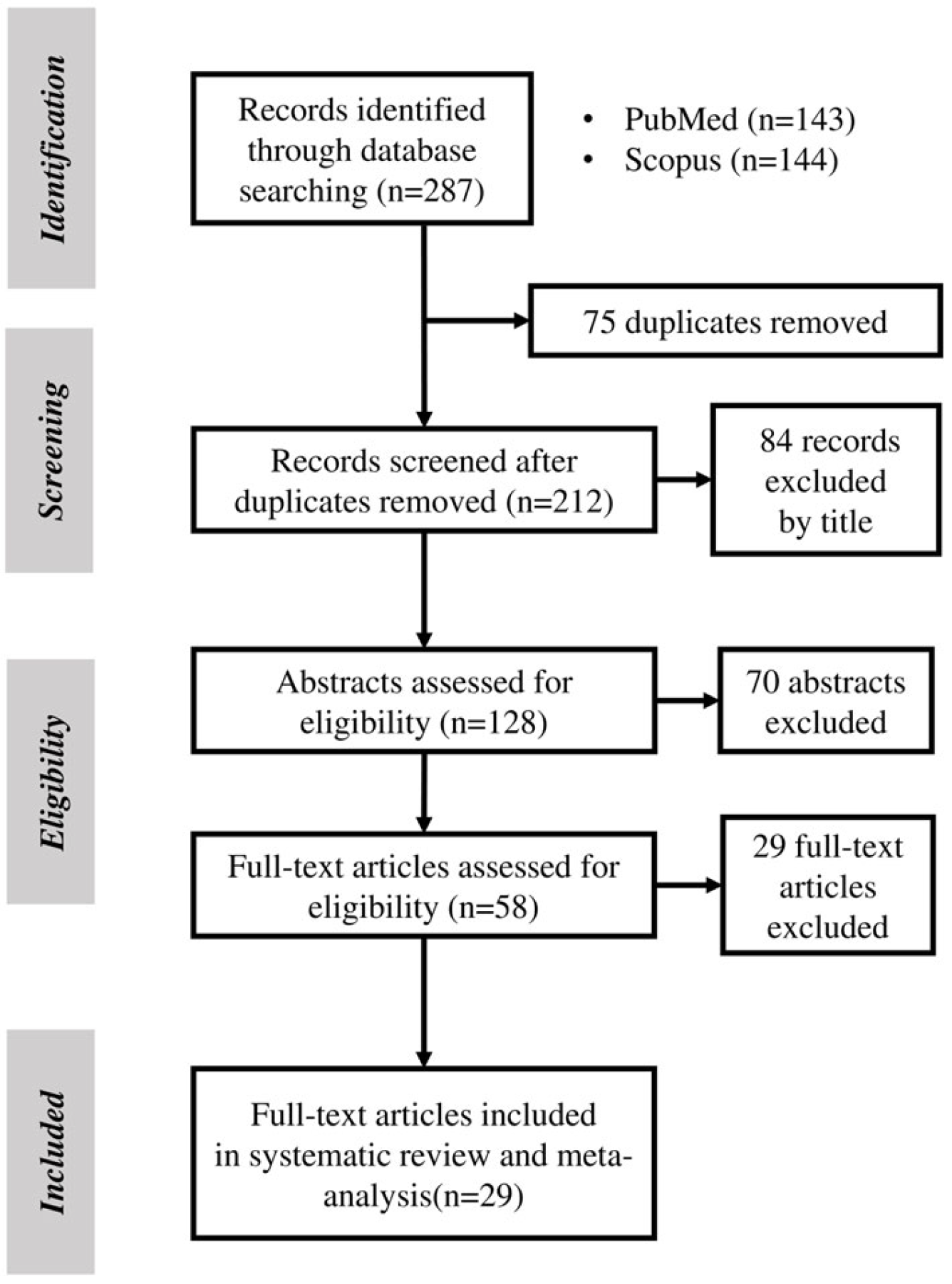
3.1. Search Results
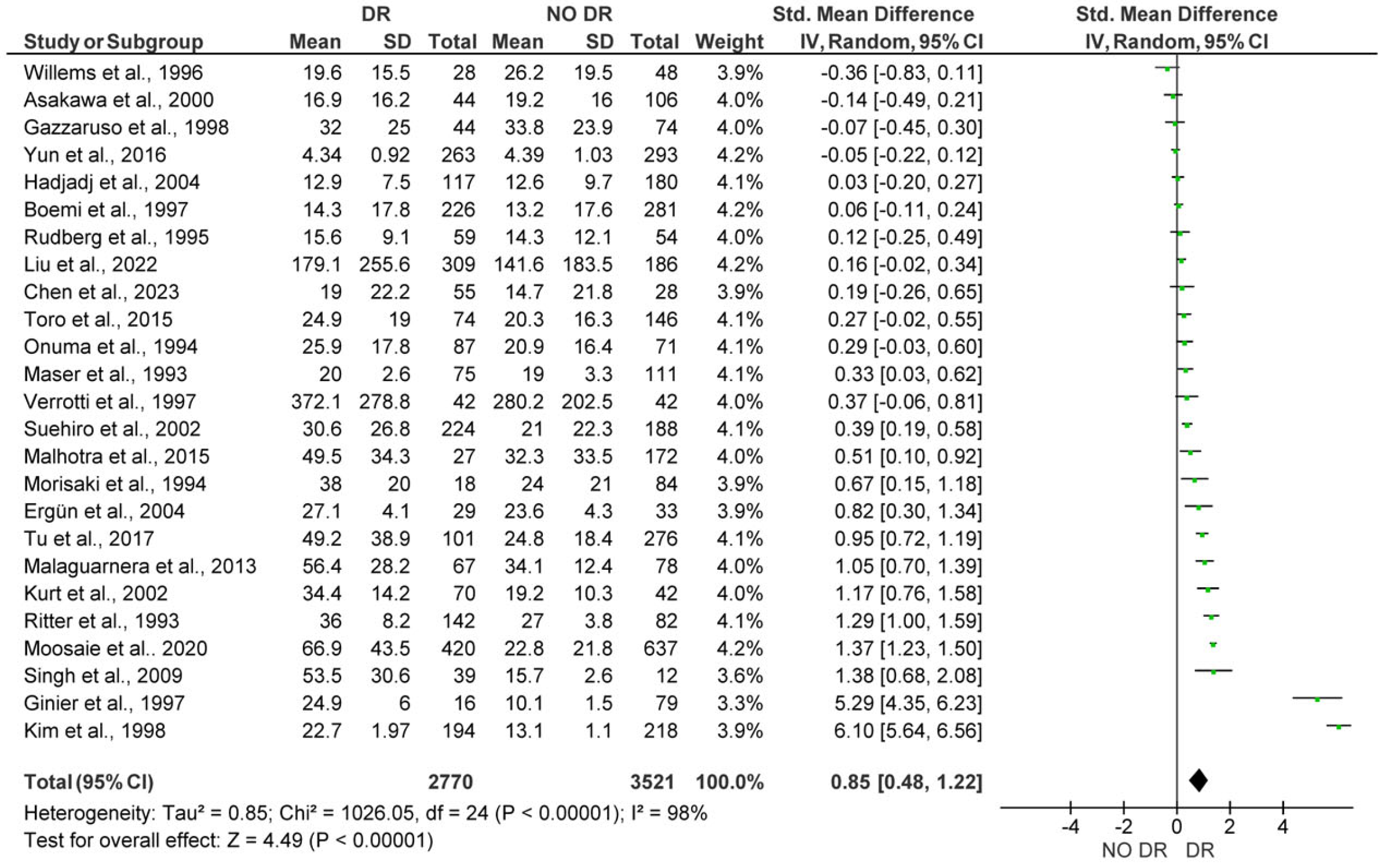
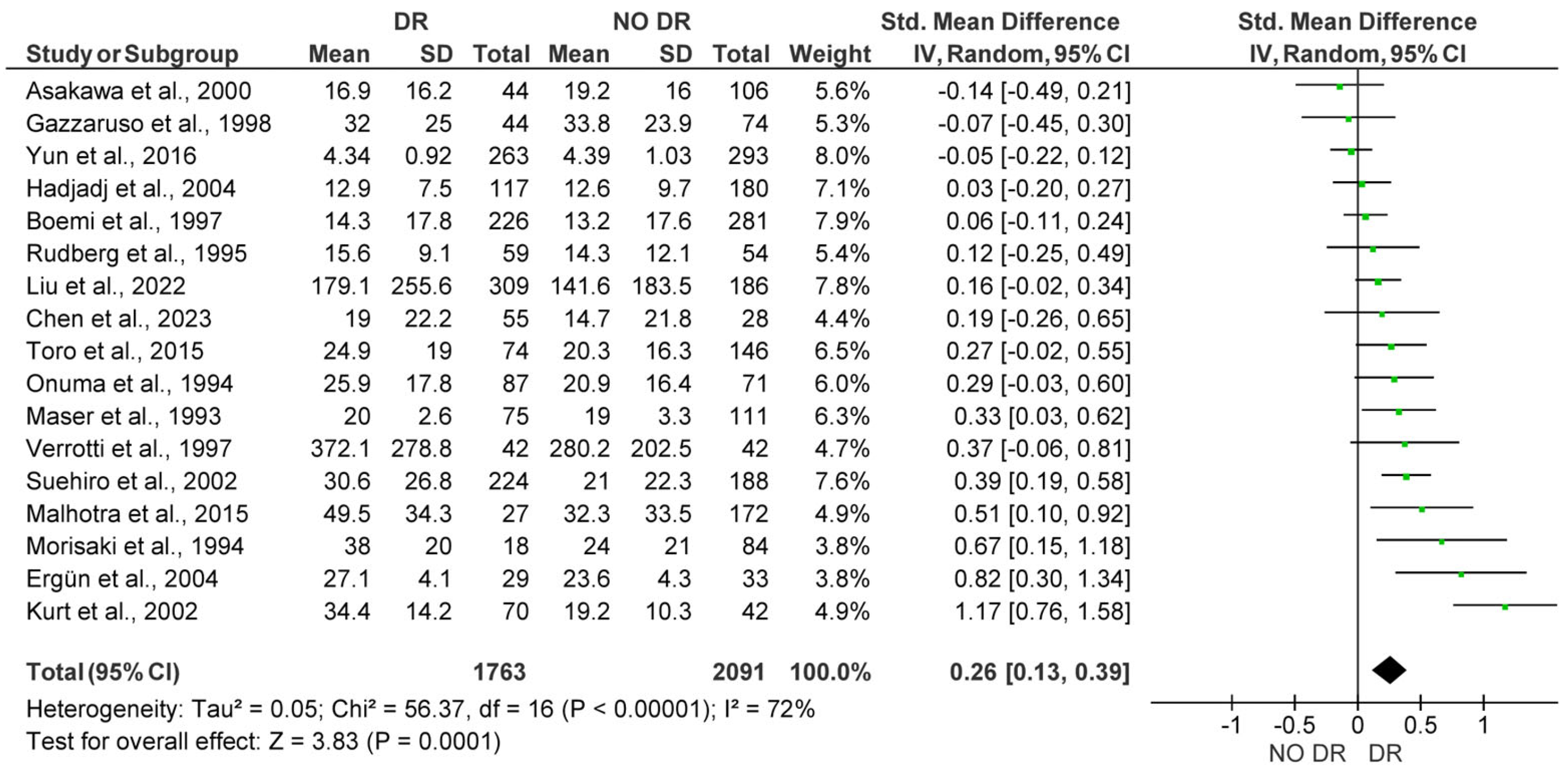
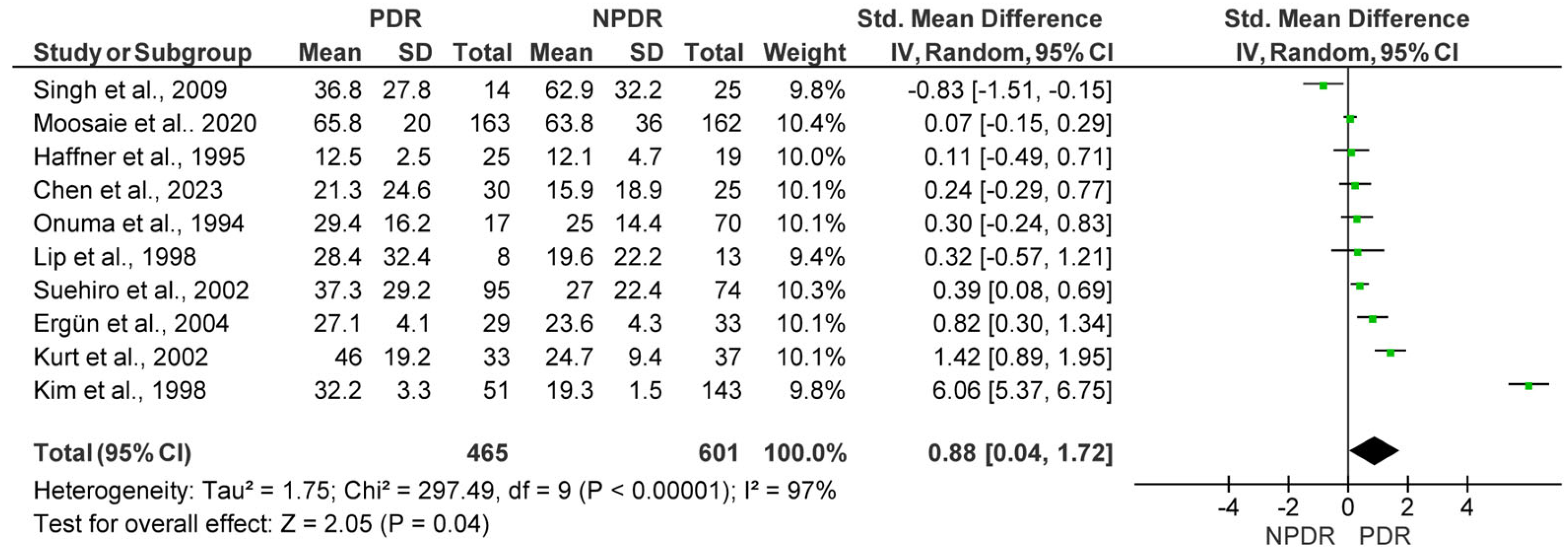
3.2. Quantitative Synthesis
3.2.1. Comparison of Lp(a) Levels Between Subjects with and Without DR
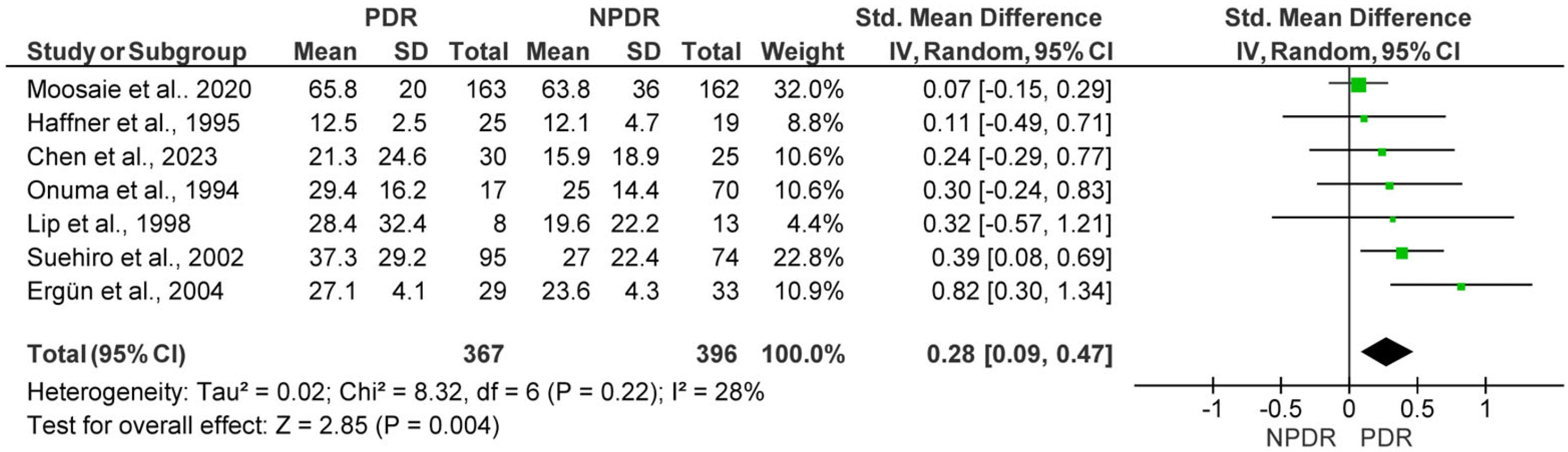
3.2.2. Lp(a) Levels in Subjects with Non-Proliferative Diabetic Retinopathy (NPDR) vs. Proliferative Diabetic Retinopathy (PDR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Lim, C.X.Y.; Wong, T.Y.; Sabanayagam, C. Diabetic Retinopathy in the Asia-Pacific. Asia Pac. J. Ophthalmol. 2018, 7, 3–16. [Google Scholar] [CrossRef]
- Varma, R.; Torres, M.; Pena, F.; Klein, R.; Azen, S.P.; Los Angeles Latino Eye Study, G. Prevalence of diabetic retinopathy in adult Latinos: The Los Angeles Latino eye study. Ophthalmology 2004, 111, 1298–1306. [Google Scholar] [CrossRef]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef] [PubMed]
- Pantelidis, P.; Oikonomou, E.; Lampsas, S.; Zakynthinos, G.E.; Lysandrou, A.; Kalogeras, K.; Katsianos, E.; Theofilis, P.; Siasos, G.; Vavuranakis, M.A.; et al. Lipoprotein(a) and calcific aortic valve disease initiation and progression: A systematic review and meta-analysis. Cardiovasc. Res. 2023, 119, 1641–1655. [Google Scholar] [CrossRef]
- Kamstrup, P.R. Lipoprotein(a) and Cardiovascular Disease. Clin. Chem. 2021, 67, 154–166. [Google Scholar] [CrossRef]
- Pirro, M.; Bianconi, V.; Paciullo, F.; Mannarino, M.R.; Bagaglia, F.; Sahebkar, A. Lipoprotein(a) and inflammation: A dangerous duet leading to endothelial loss of integrity. Pharmacol. Res. 2017, 119, 178–187. [Google Scholar] [CrossRef]
- Simantiris, S.; Antonopoulos, A.S.; Papastamos, C.; Benetos, G.; Koumallos, N.; Tsioufis, K.; Tousoulis, D. Lipoprotein(a) and inflammation- pathophysiological links and clinical implications for cardiovascular disease. J. Clin. Lipidol. 2023, 17, 55–63. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.J.; Kim, K.W.; Yu, Y.S.; Kim, J.H. Oxidized low density lipoprotein-induced senescence of retinal pigment epithelial cells is followed by outer blood-retinal barrier dysfunction. Int. J. Biochem. Cell Biol. 2012, 44, 808–814. [Google Scholar] [CrossRef]
- Willems, D.; Dorchy, H.; Dufrasne, D. Serum lipoprotein (a) in type 1 diabetic children and adolescents: Relationships with HbA1c and subclinical complications. Eur. J. Pediatr. 1996, 155, 175–178. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, H.J.; Park, J.Y.; Hong, S.K.; Yoon, Y.H.; Lee, K.U. High serum lipoprotein(a) levels in Korean type 2 diabetic patients with proliferative diabetic retinopathy. Diabetes Care 1998, 21, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Deraz, H.E.; Ahmed, E.E.S.; El-Shimi, O.S.; Gad, E.A. Relationship between serum level of lipoprotein (a) and central macular thickness in diabetic patients. Delta J. Ophthalmol. 2021, 22, 28–33. [Google Scholar] [CrossRef]
- Kurt, E.; Öztürk, F.; Ari, Z.; Yigitoglu, M.R.; Sari, R.A.; Ilker, S.S. Relationship between serum lipoprotein (a) levels and retinopathy in patients with type 2 diabetes. Ann. Ophthalmol. 2002, 34, 198–203. [Google Scholar] [CrossRef]
- Malhotra, M.; Lal, A.K.; Singh, V.P.; Malik, P.K.; Arya, V.; Agarwal, A.K. Prevalence of diabetic retinopathy in type 2 diabetics and its correlation with various clinical and metabolic factors. Int. J. Diabetes Dev. Ctries. 2015, 35, 303–309. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Singh, S.; Singh, T.B.; Singh, S. Lipoprotein (a) and diabetic retinopathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 149–151. [Google Scholar] [CrossRef]
- Rudberg, S.; Persson, B. Association between lipoprotein(a) and insulin-like growth factor I during puberty and the relationship to microalbuminuria in children and adolescents with IDDM. Diabetes Care 1995, 18, 933–939. [Google Scholar] [CrossRef]
- Tu, W.J.; Liu, H.; Liu, Q.; Cao, J.L.; Guo, M. Association Between Serum Lipoprotein(a) and Diabetic Retinopathy in Han Chinese Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Moosaie, F.; Davatgari, R.M.; Firouzabadi, F.D.; Esteghamati, S.; Deravi, N.; Meysamie, A.; Khaloo, P.; Nakhjavani, M.; Esteghamati, A. Lipoprotein(a) and Apolipoproteins as Predictors for Diabetic Retinopathy and Its Severity in Adults with Type 2 Diabetes: A Case-Cohort Study. Can. J. Diabetes 2020, 44, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.S.; Lim, T.S.; Cha, S.A.; Ahn, Y.B.; Song, K.H.; Choi, J.A.; Kwon, J.; Jee, D.; Cho, Y.K.; Park, Y.M.; et al. Lipoprotein(a) predicts the development of diabetic retinopathy in people with type 2 diabetes mellitus. J. Clin. Lipidol. 2016, 10, 426–433. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Gagliano, C.; Bucolo, C.; Vacante, M.; Salomone, S.; Malaguarnera, M.; Leonardi, D.G.; Motta, M.; Drago, F.; Avitabile, T. Lipoprotein(a) serum levels in diabetic patients with retinopathy. Biomed. Res. Int. 2013, 2013, 943505. [Google Scholar] [CrossRef]
- Liu, Z.; Shao, M.; Ren, J.; Qiu, Y.; Li, S.; Cao, W. Association Between Increased Lipid Profiles and Risk of Diabetic Retinopathy in a Population-Based Case-Control Study. J. Inflamm. Res. 2022, 15, 3433–3446. [Google Scholar] [CrossRef]
- Chandni, R.; Ramamoorthy, K.P. Lipoprotein(a) in type 2 diabetic subjects and its relationship to diabetic microvascular complications. World J. Diabetes 2012, 3, 105–109. [Google Scholar] [CrossRef]
- Morisaki, N.; Yokote, K.; Tashiro, J.; Inadera, H.; Kobayashi, J.; Kanzaki, T.; Saito, Y.; Yoshida, S. Lipoprotein(a) is a risk factor for diabetic retinopathy in the elderly. J. Am. Geriatr. Soc. 1994, 42, 965–967. [Google Scholar] [CrossRef]
- Haffner, S.M.; Klein, B.E.; Moss, S.E.; Klein, R. Lp(a) is not related to retinopathy in diabetic subjects. Eur. J. Ophthalmol. 1995, 5, 119–123. [Google Scholar] [CrossRef]
- Ergun, U.G.; Oztuzun, S.; Seydaoglu, G. Lipoprotein (A) levels in type 2 diabetic patients with diabetic retinopathy. Med. J. Malays. 2004, 59, 406–410. [Google Scholar]
- Chopra, R.; Saramma, J.G.; Mary, J.; Rebecca, A. Lipoprotein(a) as a risk factor for diabetic retinopathy in patients with type 2 diabetes mellitus. Indian. J. Ophthalmol. 2007, 55, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Onuma, T.; Kikuchi, T.; Shimura, M.; Tsutsui, M.; Matsui, J.; Boku, A.; Takebe, K. Lipoprotein(a) as an independent risk factor for diabetic retinopathy in male patients in non-insulin-dependent diabetes mellitus. Tohoku J. Exp. Med. 1994, 173, 209–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suehiro, M.; Ohkubo, K.; Kato, H.; Kido, Y.; Anzai, K.; Oshima, K.; Ono, J. Analyses of serum lipoprotein(a) and the relation to phenotypes and genotypes of apolipoprotein(a) in type 2 diabetic patients with retinopathy. Exp. Clin. Endocrinol. Diabetes 2002, 110, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Lobefalo, L.; Chiarelli, F.; Mastropasqua, L.; Pallotta, R.; Colangelo, L.; Morgese, G.; Gallenga, P.E. Lipids and lipoproteins in diabetic adolescents and young adults with retinopathy. Eye 1997, 11 Pt 6, 876–881. [Google Scholar] [CrossRef]
- Ritter, M.M.; Loscar, M.; Richter, W.O.; Schwandt, P. Lipoprotein(a) in diabetes mellitus. Clin. Chim. Acta 1993, 214, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Boemi, M.; Sirolla, C.; Amadio, L.; Fumelli, P.; James, R.W. Lipoprotein(a) and retinopathy in IDDM and NIDDM patients. Diabetes Care 1997, 20, 115. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Nie, Y.; Gong, Z.; Sivaprasad, S.; Fung, A.T.; Wang, Q.; Qiu, B.; Xie, R.; Wang, Y. Circulating level of homocysteine contributes to diabetic retinopathy associated with dysregulated lipid profile and impaired kidney function in patients with type 2 diabetes mellitus. Eye 2023, 37, 1383–1389. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Garzaniti, A.; Buscaglia, P.; D’Annunzio, G.; Porta, A.; Vandelli, G.; Lorini, R.; Finardi, G.; Fratino, P.; Geroldi, D. Lipoprotein(a) levels and apolipoprotein(a) polymorphism in type 1 diabetes mellitus: Relationships to microvascular and neurological complications. Acta Diabetol. 1998, 35, 13–18. [Google Scholar] [CrossRef]
- Ginier, P.; Deedwania, P. Lipoprotein(a) in patients who have non-insulin-dependent diabetes with and without coronary artery disease. Endocr. Pract. 1997, 3, 276–280. [Google Scholar] [CrossRef]
- Lip, P.L.; Jones, A.F.; Price, N.; Headon, M.; Beevers, D.G.; Lip, G.Y. Do intraocular angiotensin II levels, plasma prothrombotic factors and endothelial dysfunction contribute to proliferative diabetic retinopathy? Acta Ophthalmol. Scand. 1998, 76, 533–536. [Google Scholar] [CrossRef]
- Hadjadj, S.; Duly-Bouhanick, B.; Bekherraz, A.; BrIdoux, F.; Gallois, Y.; Mauco, G.; Ebran, J.; Marre, M. Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab. 2004, 30, 43–51. [Google Scholar] [CrossRef]
- Asakawa, H.; Tokunaga, K.; Kawakami, F. Elevation of fibrinogen and thrombin-antithrombin III complex levels of type 2 diabetes mellitus patients with retinopathy and nephropathy. J. Diabetes Complications 2000, 14, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Maser, R.E.; Usher, D.; Becker, D.J.; Drash, A.L.; Kuller, L.H.; Orchard, T.J. Lipoprotein(a) concentration shows little relationship to IDDM complications in the Pittsburgh Epidemiology of Diabetes Complications Study cohort. Diabetes Care 1993, 16, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, P.; Tafelmeier, M.; Chittka, D.; Choi, S.H.; Zhang, L.; Byun, Y.S.; Almazan, F.; Yang, X.; Iqbal, N.; Chowdhury, P.; et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J. Lipid Res. 2013, 54, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.A.; Nadolski, M.J.; Liao, X.; Magallon, J.; Nguyen, M.; Feric, N.T.; Koschinsky, M.L.; Harkewicz, R.; Witztum, J.L.; Tsimikas, S.; et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010, 12, 467–482. [Google Scholar] [CrossRef]
- Nurmohamed, N.S.; Gaillard, E.L.; Malkasian, S.; de Groot, R.J.; Ibrahim, S.; Bom, M.J.; Kaiser, Y.; Earls, J.P.; Min, J.K.; Kroon, J.; et al. Lipoprotein(a) and Long-Term Plaque Progression, Low-Density Plaque, and Pericoronary Inflammation. JAMA Cardiol. 2024, 9, 826–834. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Hoogeveen, R.M.; Ali, L.; Prange, K.H.M.; Waissi, F.; van Weeghel, M.; Bachmann, J.C.; Versloot, M.; Borrelli, M.J.; Yeang, C.; et al. Atherogenic Lipoprotein(a) Increases Vascular Glycolysis, Thereby Facilitating Inflammation and Leukocyte Extravasation. Circ. Res. 2020, 126, 1346–1359. [Google Scholar] [CrossRef]
- Shariatzadeh, M.; Nagtzaam, N.M.A.; van Vark-van der Zee, L.; van Holten-Neelen, C.; Verhoeven, A.J.M.; Dehairs, J.; Swinnen, J.V.; Leijten, F.; Ten Berge, J.C.; Ciriano, J.P.M.; et al. Altered Functionality of Lipoprotein(a) Impacts on Angiogenesis in Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2023, 64, 8. [Google Scholar] [CrossRef]
- Handa, J.T.; Tagami, M.; Ebrahimi, K.; Leibundgut, G.; Janiak, A.; Witztum, J.L.; Tsimikas, S. Lipoprotein(A) with An Intact Lysine Binding Site Protects the Retina From an Age-Related Macular Degeneration Phenotype in Mice (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2015, 113, T5. [Google Scholar]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic Supplementation with a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Gologorsky, D.; Thanos, A.; Vavvas, D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediators Inflamm. 2012, 2012, 629452. [Google Scholar] [CrossRef]
- Pellegrino, M.; Furmaniak-Kazmierczak, E.; LeBlanc, J.C.; Cho, T.; Cao, K.; Marcovina, S.M.; Boffa, M.B.; Cote, G.P.; Koschinsky, M.L. The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell-cell contact in cultured endothelial cells. J. Biol. Chem. 2004, 279, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Ramharack, R.; Barkalow, D.; Spahr, M.A. Dominant negative effect of TGF-beta1 and TNF-alpha on basal and IL-6-induced lipoprotein(a) and apolipoprotein(a) mRNA expression in primary monkey hepatocyte cultures. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Canning, P.; Kenny, B.A.; Prise, V.; Glenn, J.; Sarker, M.H.; Hudson, N.; Brandt, M.; Lopez, F.J.; Gale, D.; Luthert, P.J.; et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 7213–7218. [Google Scholar] [CrossRef]
- Chang, N.C.; Yeh, C.T.; Lin, Y.K.; Kuo, K.T.; Fong, I.H.; Kounis, N.G.; Hu, P.; Hung, M.Y. Garcinol Attenuates Lipoprotein(a)-Induced Oxidative Stress and Inflammatory Cytokine Production in Ventricular Cardiomyocyte through alpha7-Nicotinic Acetylcholine Receptor-Mediated Inhibition of the p38 MAPK and NF-kappaB Signaling Pathways. Antioxidants 2021, 10, 461. [Google Scholar] [CrossRef]
- Hrovat, K.; Rehberger Likozar, A.; Zupan, J.; Sebestjen, M. Gene Expression Profiling of Markers of Inflammation, Angiogenesis, Coagulation and Fibrinolysis in Patients with Coronary Artery Disease with Very High Lipoprotein(a) Levels Treated with PCSK9 Inhibitors. J. Cardiovasc. Dev. Dis. 2022, 9, 211. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef]
- Benkhalifa, M.; Zidi, W.; Bahri, H.; Mahjoub, S.; Boudhraa, K.; Sanhaji, H.; Khorsi-Cauet, H.; Feki, M.; Benkhalifa, M.; Allal-Elasmi, M. Circulating MMP-7 and VEGF as potential predictive biomarkers for recurrent implantation failures. Zygote 2021, 29, 365–371. [Google Scholar] [CrossRef]
- Pavlyha, M.; Li, Y.; Crook, S.; Anderson, B.R.; Reyes-Soffer, G. Race/ethnicity and socioeconomic status affect the assessment of lipoprotein(a) levels in clinical practice. J. Clin. Lipidol. 2024, 18, e720–e728. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Koschinsky, M.L.; Bajaj, A.; Boffa, M.B.; Dixon, D.L.; Ferdinand, K.C.; Gidding, S.S.; Gill, E.A.; Jacobson, T.A.; Michos, E.D.; Safarova, M.S.; et al. A focused update to the 2019 NLA scientific statement on use of lipoprotein(a) in clinical practice. J. Clin. Lipidol. 2024, 18, e308–e319. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soffer, G. The impact of race and ethnicity on lipoprotein(a) levels and cardiovascular risk. Curr. Opin. Lipidol. 2021, 32, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Katsiki, N.; Pirro, M.; Banach, M.; Rasadi, K.A.; Sahebkar, A. Dietary natural products as emerging lipoprotein(a)-lowering agents. J. Cell Physiol. 2019, 234, 12581–12594. [Google Scholar] [CrossRef] [PubMed]
- Scanu, A.M.; Bamba, R. Niacin and lipoprotein(a): Facts, uncertainties, and clinical considerations. Am. J. Cardiol. 2008, 101, 44B–47B. [Google Scholar] [CrossRef]
- Tsaban, G. Statins and lipoprotein(a); facing the residual risk. Eur. J. Prev. Cardiol. 2022, 29, 777–778. [Google Scholar] [CrossRef]
- Gaudet, D.; Watts, G.F.; Robinson, J.G.; Minini, P.; Sasiela, W.J.; Edelberg, J.; Louie, M.J.; Raal, F.J. Effect of Alirocumab on Lipoprotein(a) Over >/=1.5 Years (from the Phase 3 ODYSSEY Program). Am. J. Cardiol. 2017, 119, 40–46. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Ceska, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Tsimikas, S.; Viney, N.J.; Hughes, S.G.; Singleton, W.; Graham, M.J.; Baker, B.F.; Burkey, J.L.; Yang, Q.; Marcovina, S.M.; Geary, R.S.; et al. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015, 386, 1472–1483. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; JA, G.L.; Knusel, B.; Gencer, B.; Wang, H.; Wu, Y.; Kassahun, H.; Sabatine, M.S. Study design and rationale for the Olpasiran trials of Cardiovascular Events And lipoproteiN(a) reduction-DOSE finding study (OCEAN(a)-DOSE). Am. Heart J. 2022, 251, 61–69. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K.; Watts, G.F.; Koren, M.J.; Fok, H.; Nicholls, S.J.; Rider, D.A.; Cho, L.; Romano, S.; Melgaard, C.; et al. Single Ascending and Multiple-Dose Trial of Zerlasiran, a Short Interfering RNA Targeting Lipoprotein(a): A Randomized Clinical Trial. JAMA 2024, 331, 1534–1543. [Google Scholar] [CrossRef]
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA 2023, 330, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ni, W.; Rhodes, G.M.; Nissen, S.E.; Navar, A.M.; Michael, L.F.; Haupt, A.; Krege, J.H. Oral Muvalaplin for Lowering of Lipoprotein(a): A Randomized Clinical Trial. JAMA 2025, 333, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Karwatowska-Prokopczuk, E.; Su, F.; Dinh, B.; Xia, S.; Witztum, J.L.; Tsimikas, S. Effect of Pelacarsen on Lipoprotein(a) Cholesterol and Corrected Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2022, 79, 1035–1046. [Google Scholar] [CrossRef]
- Kronenberg, F. Lipoprotein(a) measurement issues: Are we making a mountain out of a molehill? Atherosclerosis 2022, 349, 123–135. [Google Scholar] [CrossRef]
| Study | Study Design | Sample Size | Age (Yrs) | Sex (Male) | Compared Groups | Key Findings |
|---|---|---|---|---|---|---|
| Deraz et al., 2021 [16] | Case–control | 40 | 52.7 ± 5.2 | 47.5% | 1. NO DM and NO-DR 2. DR |
|
| Kurt et al., 2002 [17] | Cross-sectional | 112 | 61.7 ± 6.4 | 50.9% | 1. NO DM and NO DR 2. DM and NO DR 3. NPDR 4. PDR |
|
| Malhotra et al., 2014 [18] | Cross-sectional | 199 | 61 ± 9 | 51.% | 1. DM and NO DR 2. DR |
|
| Singh et al., 2009 [19] | Cross-sectional | 51 | N/A | 100% | 1. DM and NO DR 2. NPDR 3. PDR |
|
| Rudberg et al., 1995 [20] | Cross-sectional | 133 | 15.7 ± 1.6 | 46.6% | 1. DM and NO DR 2. DR |
|
| Tu et al., 2017 [21] | Cross-sectional | 377 | 58 ± 12.4 | 52.7% | 1. DM and NO DR 2. DR |
|
| Moosaie et al., 2020 [22] | Case–control | 1057 | 56.8 ± 9.7 | 52.9% | 1. DM and NO DR 2. DR 3. NPDR 4. PDR |
|
| Yun et al., 2016 [23] | Cohort | 556 | 54.2 ± 10 | 42.4% | 1. DM and NO DR 2. DR |
|
| Malaguarnera et al., 2013 [24] | Cross-sectional | 145 | 66.8 ± 12.4 | 43.4% | 1. DM and NO DR 2. DR |
|
| Liu et al., 2022 [25] | Case–control | 667 | 56.3 ± 10.2 | 50.7% | 1. NO DM and NO DR 2. DM and NO DR 3. DR |
|
| Chandni et al., 2012 [26] | Cross-sectional | 144 | 53.9 ± 10.7 | 56.9% | 1. NO DM and NO DR 2. DR |
|
| Morisaki et al., 1994 [27] | Cross-sectional | 104 | 66 ± 10 | 34% | 1. DM and NO DR 2. DR |
|
| Haffner et al., 1995 [28] | Cross-sectional | 70 | 61.4 ± 2.1 | 58% | 1. DM and NO DR 2. MILD NPDR 3. MODERATE NPDR 4. PDR |
|
| Ergün et al., 2004 [29] | Cross-sectional | 100 | 57.5 ± 3.1 | 33% | 1. DM and NO DR 2. DR 3. PDR |
|
| Chopra et al., 2007 [30] | Cross-sectional | 200 | 55.1 (N/A) | 41.5% | 1. DM and NO DR 2. PDR |
|
| Onuma et al., 1994 [31] | Cross-sectional | 158 | 58.6 ± 11.4 | 52.5% | 1. DM+ NO DR 2. NPDR 3. PDR |
|
| Willems et al., 1996 [10] | Cross-sectional | 106 | N/A | 61.3% | 1. DM and NO DR 2. DR |
|
| Suehiro et al., 2002 [32] | Cross-sectional | 412 | 57.4 ± 12.5 | 56.8% | 1. NO DM and NO DR 2. DM and NO DR 3. NPDR 4. SEVERE NPDR 5. PDR |
|
| Verrotti et al., 1997 [33] | Cross-sectional | 126 | 20 ± 5.5 | 50% | 1. NO DM and NO DR 2. DM and NO DR 3. NPDR 4. PDR |
|
| Kim et al., 1998 [11] | Cross-sectional | 412 | 56.8 ± 0.8 | N/A | 1. DM and NO DR 2. NPDR 3. PDR |
|
| Ritter et al., 1993 [34] | Cross-sectional | 224 | 49.8 (N/A) | 56.2% | 1. DM and NO DR 2. DR |
|
| Boemi et al., 1997 [35] | Cross-sectional | 507 | 54.6 ± 16.6 | 48.9% | 1. DM and NO DR 2. DR |
|
| Chen et al., 2022 [36] | Case–control | 113 | 53.9 ± 9.4 | 81.9% | 1. NO DM and NO DR 2. DM and NO DR 3. NPDR 4. PDR |
|
| Gazzaruso et al., 1998 [37] | Cross-sectional | 245 | 21.9 ± 4.0 | 55.1% | 1. DM and NO DR 2. DR |
|
| Ginier et al., 1997 [38] | Cross-sectional | 95 | 61.7 ± 1.4 | 100% | 1. DM and NO DR 2. DR |
|
| Lip et al., 1998 [39] | Cross-sectional | 21 | 67 ± 10.6 | 57.1% | 1. NO DM + NO DR 2. NPDR 3. PDR |
|
| Hadjadj et al., 2004 [40] | Cohort | 297 | 33.8 ± 11.2 | 58.5% | 1. DM and NO DR 2. NPDR 3. SEVERE NPDR 4. PDR |
|
| Asakawa et al., 2000 [41] | Cross-sectional | 150 | 60.3 ± 11.0 | 52.7% | 1. DM and NO DR 2. DR |
|
| Maser et al., 1993 [42] | Cross-sectional | 186 | 34 ± 8 | N/A | 1. DM and NO DR 2. PDR |
|
| Pathogenetic Mechanisms | Details |
|---|---|
| Endothelial Dysfunction | Lp(a) reduces nitric oxide bioavailability, increases ROS quantities, and damages endothelial cells, disrupting the blood–retinal barrier. |
| Oxidative Stress | Induces ROS production in endothelial and retinal cells, damages mitochondria, and disrupts cellular metabolism. |
| Inflammation | Stimulates IL-6, IL-1β, TNF-α, ICAM-1, and VCAM-1 expression; promotes leukostasis and chronic inflammation. |
| Prothrombotic and Vasoconstrictive Effects | Mimics plasminogen, leading to prothrombotic activity and vasoconstriction, worsening retinal ischemia. |
| VEGF Upregulation and Neovascularization | Increases VEGF expression due to oxidative stress, driving pathological angiogenesis and retinal hemorrhages. |
| Extracellular Matrix Remodeling and MMP Activation | Enhances deposition of ECM proteins and activates MMPs, promoting angiogenesis and vascular remodeling. |
| Retinal Vascular Permeability and Pericyte Loss | Increases retinal vascular permeability and pericyte dropout, contributing to structural retinal damage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampsas, S.; Lambadiari, V.; Agapitou, C.; Lampsa, A.; Oikonomou, E.; Siasos, G.; Chatziralli, I. Lipoprotein (a) in the Development and Progression of Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1137. https://doi.org/10.3390/medicina61071137
Lampsas S, Lambadiari V, Agapitou C, Lampsa A, Oikonomou E, Siasos G, Chatziralli I. Lipoprotein (a) in the Development and Progression of Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(7):1137. https://doi.org/10.3390/medicina61071137
Chicago/Turabian StyleLampsas, Stamatios, Vaia Lambadiari, Chrysa Agapitou, Aikaterini Lampsa, Evangelos Oikonomou, Gerasimos Siasos, and Irini Chatziralli. 2025. "Lipoprotein (a) in the Development and Progression of Diabetic Retinopathy: A Systematic Review and Meta-Analysis" Medicina 61, no. 7: 1137. https://doi.org/10.3390/medicina61071137
APA StyleLampsas, S., Lambadiari, V., Agapitou, C., Lampsa, A., Oikonomou, E., Siasos, G., & Chatziralli, I. (2025). Lipoprotein (a) in the Development and Progression of Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Medicina, 61(7), 1137. https://doi.org/10.3390/medicina61071137








