Clinical and Epidemiological Aspects on Healthcare-Associated Infections with Acinetobacter spp. in a Neurosurgery Hospital in North-East Romania
Abstract
1. Introduction
Aim and Objectives
2. Materials and Methods
2.1. Study Design and Hospital Context
2.2. Information Resources
2.3. Antibiotic Susceptibility Testing
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
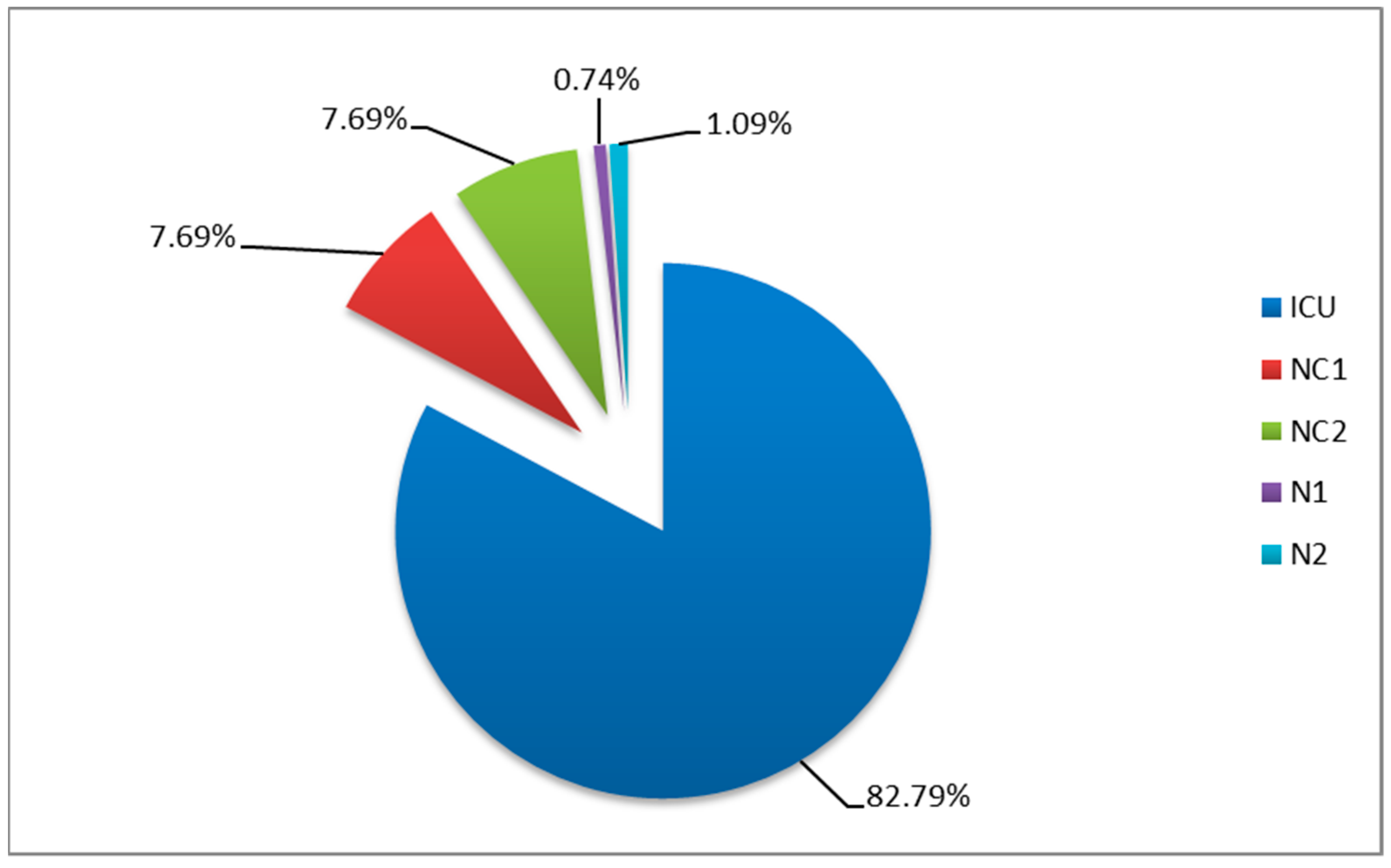
3.1. Distribution by Wards
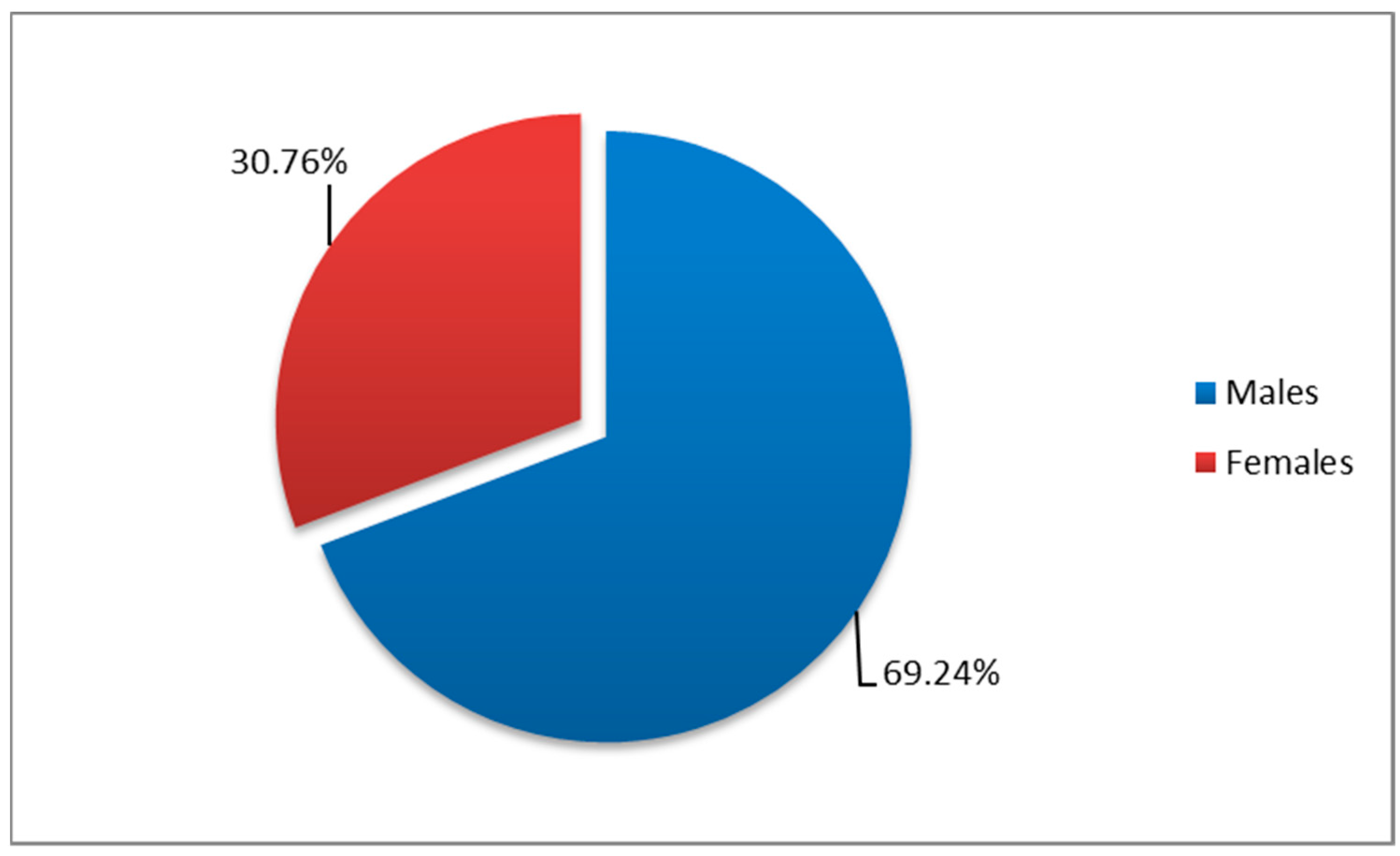
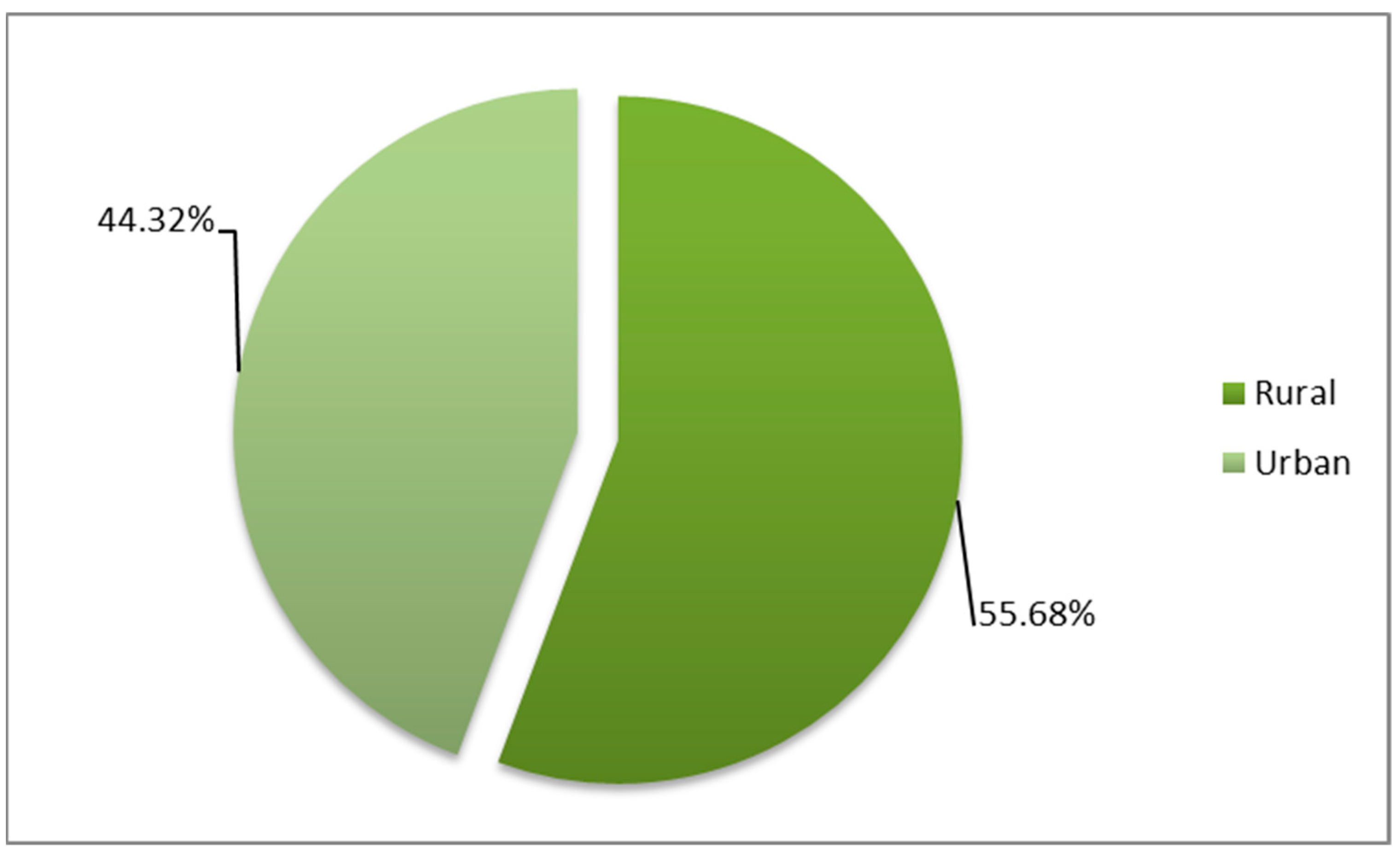
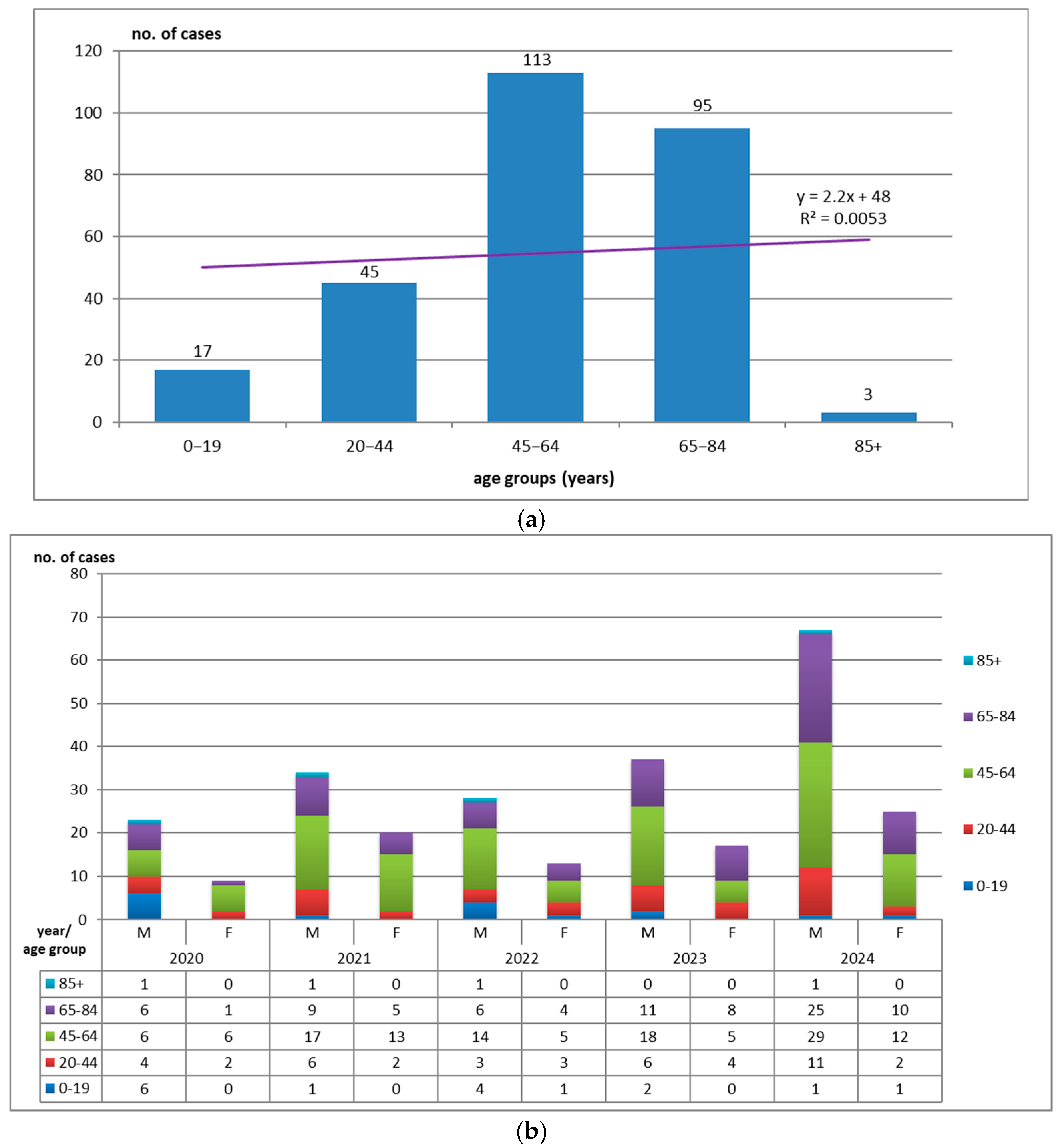
3.2. Distribution by Demographic Characteristics
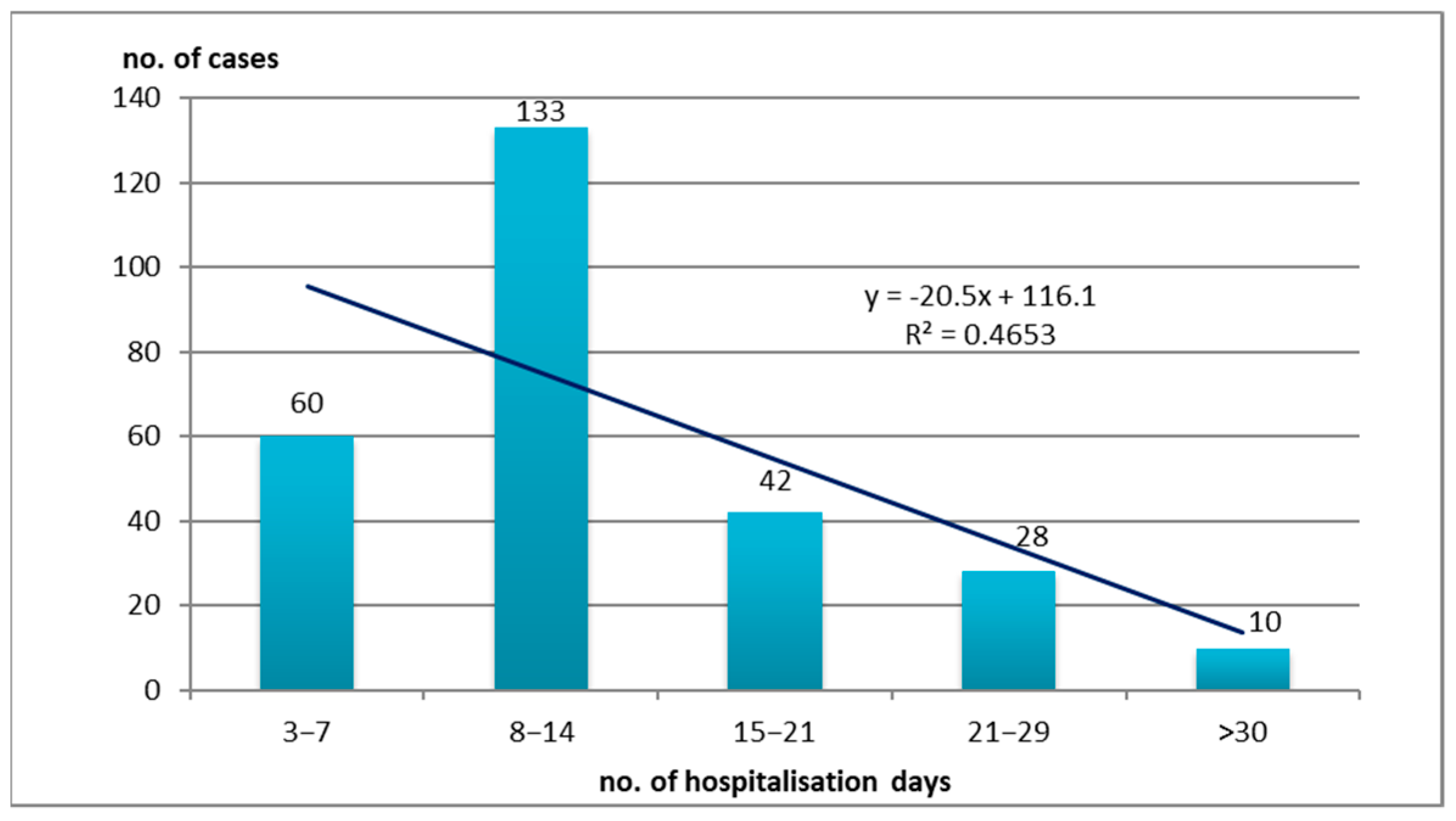
3.3. Hospitalization Days
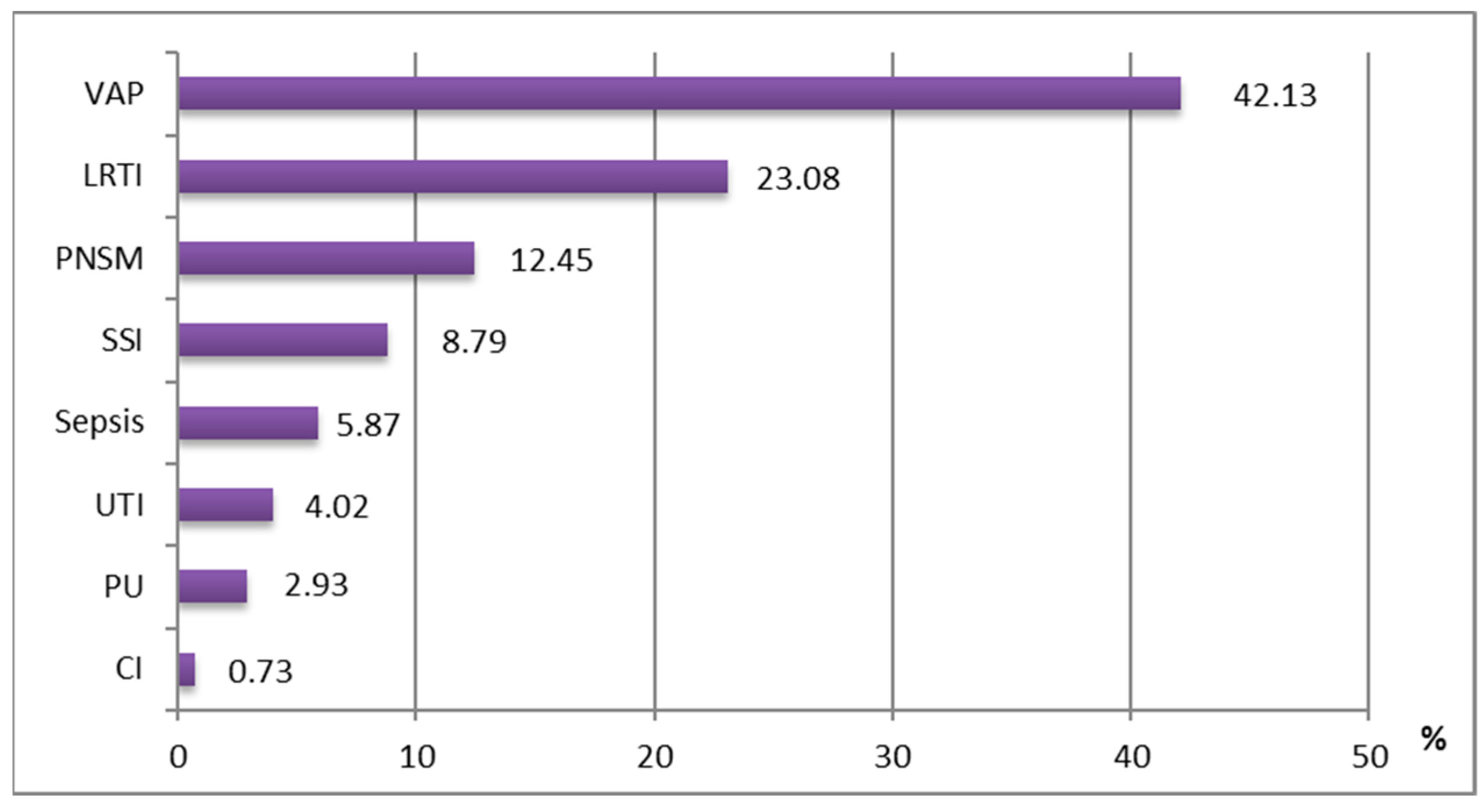
3.4. Clinical Entities
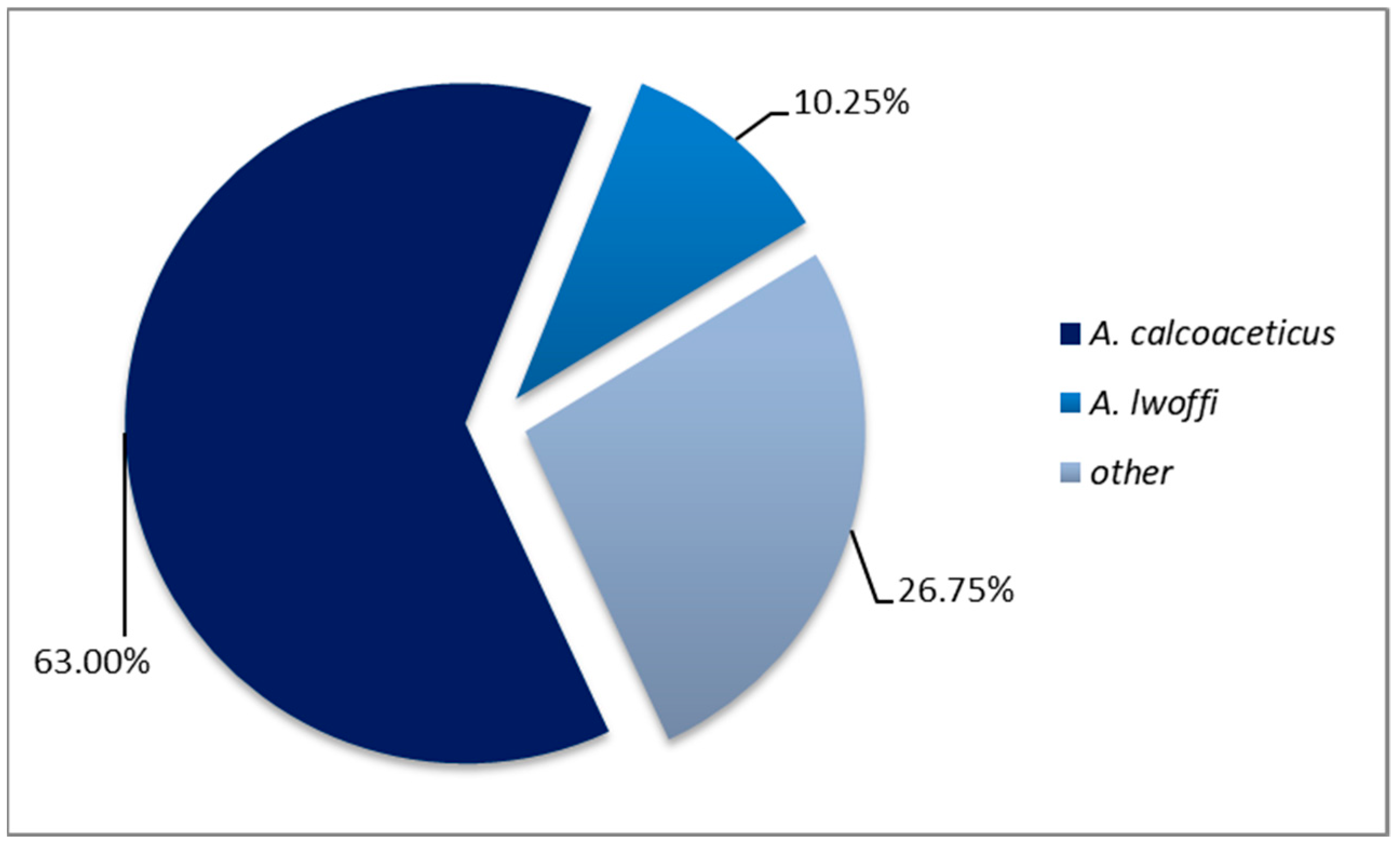
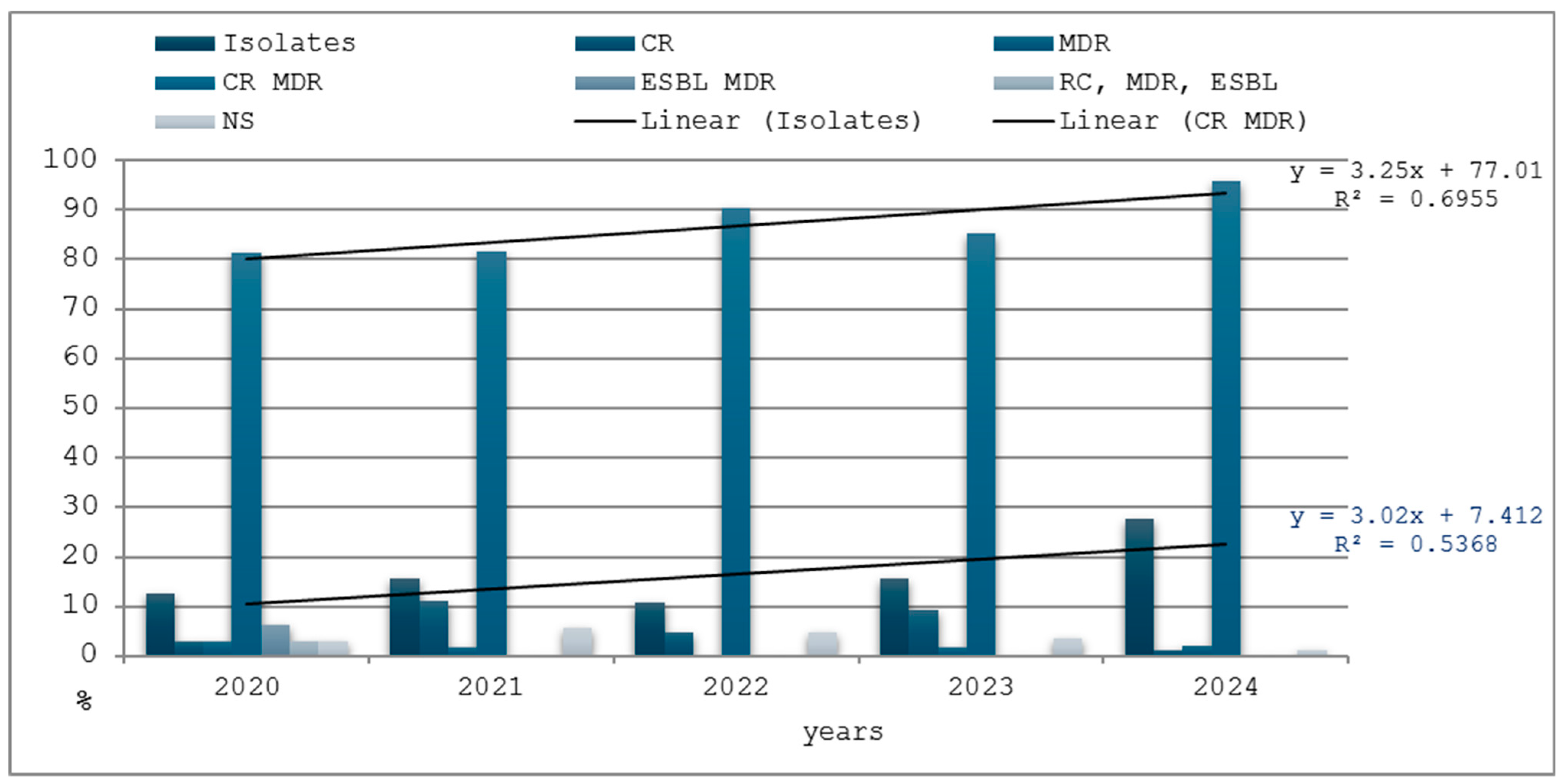
3.5. Antimicrobial Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAI | Healthcare-associated infection |
| CR | Carbapenem resistance |
| MDR | Multidrug resistance |
| ESBL | Extended-spectrum beta-lactamase |
| ICU | Intensive care unit |
References
- Public Health National Institute of Romania. CARMIAAM-ROM, Antibiotic Consumption, Microbial Resistance and Healthcare-Associated Infections in Romania; INSP: Bucharest, Romania, 2019. [Google Scholar]
- World Health Organization. Global Report on Infection Prevention and Control; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An Overview of Healthcare Associated Infections and Their Detection Methods Caused by Pathogen Bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef] [PubMed]
- Voidazan, S.; Albu, S.; Toth, R.; Grigorescu, B.; Rachita, A.; Moldovan, I. Healthcare Associated Infections-A New Pathology in Medical Practice? Int. J. Environ. Res. Public Health 2020, 17, 760. [Google Scholar] [CrossRef] [PubMed]
- Ivan, A. (Ed.) Handbook of Communicable Diseases Epidemiology; Polirom Publishing House: Iasi, Romania, 2002. [Google Scholar]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [Google Scholar] [CrossRef] [PubMed]
- Doughari, H.J.; Ndakidemi, P.A.; Human, I.S.; Benade, S. The ecology, biology and pathogenesis of Acinetobacter spp.: An overview. Microbes Environ. 2011, 26, 101–112. [Google Scholar] [CrossRef]
- Towner, A. The genus Acinetobacter. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2006; pp. 746–758. [Google Scholar]
- Kurcik-Trajkovska, B. Acinetobacter spp.—A serious enemy threatening hospitals worldwide. Maced. J. Med. Sci. 2009, 2, 157–162. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Weinstein, R.A. Acinetobacter infection. N. Engl. J. Med. 2008, 358, 1271–1281. [Google Scholar] [CrossRef]
- Whitman, T.J.; Qasba, S.S.; Timpone, J.G.; Babel, B.S.; Kasper, M.R.; English, J.F.; Sanders, J.W.; Hujer, K.M.; Hujer, A.M.; Endimiani, A. Occupational transmission of Acinetobacter baumannii from a United States serviceman wounded in Iraq to a health care worker. Clin. Infect. Dis. 2008, 47, 439–443. [Google Scholar] [CrossRef]
- Luna, C.M.; Aruj, P.K. Nosocomial Acinetobacter pneumonia. Respirology 2007, 12, 787–791. [Google Scholar] [CrossRef]
- Motbainor, H.; Bereded, F.; Mulu, W. Multidrug resistance of blood stream, urinary tract and surgical site nosocomial infections of Acinetobacter baumannii and Pseudomonas aeruginosa among patients hospitalized at Felegehiwot referral hospital, Northwest Ethiopia: A cross-sectional study. BMC Infect. Dis. 2020, 20, 92. [Google Scholar] [CrossRef]
- Cretu, M.S.; Grigore, A.C.; Maier, A.; Popa, T.O.; Plesea Condratovici, A.; Dorobat, C.; Pavel, L.L.; Chesaru, I.B. Early Stratification of Sepsis Using Presepsine in Emergency Department (North-East of Romania Experience). Mater. Plast. 2017, 54, 190–193. [Google Scholar] [CrossRef]
- Birhanu, A.; Gebre, G.; Getaneh, E.; Yohannes, H.; Baye, N.; Mersha, G.B.; Tigabie, M.; Dagnew, M.; Ferede, G.; Deress, T.; et al. Investigation of methicillin, beta-lactam, carbapenem, and multidrug resistant bacteria from blood cultures of septicemia suspected patients in Northwest Ethiopia. Sci. Rep. 2025, 15, 5769. [Google Scholar] [CrossRef] [PubMed]
- Basri, R.; Zueter, A.R.; Mohamed, Z.; Alam, M.K.; Norsa’adah, B.; Hasan, S.A.; Hasan, H.; Ahmad, F. Burden of bacterial meningitis: A retrospective review on laboratory parameters andfactors associated with death in meningitis, Kelantan Malaysia. Nagoya J. Med. Sci. 2015, 77, 59. [Google Scholar] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 38–582. [Google Scholar] [CrossRef]
- Fournier, P.E.; Richet, H.; Weinstein, R.A. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006, 42, 692–699. [Google Scholar] [CrossRef]
- Busuioc, E. Pneumoniile asociate ventilatiei artificiale a pulmonilor în sectiile de Terapie Intensiva. Analele Stiinfice Ale USMF N. Testemițanu 2012, 2, 35–38. [Google Scholar]
- Beggs, C.; Kerr, K.; Snelling, A.; Sleigh, P. Acinetobacter spp. and the clinical environment. Indoor Built Environ. 2006, 15, 19–24. [Google Scholar] [CrossRef]
- Sobouti, B.; Mirshekar, M.; Fallah, S.; Tabaei, A.; Fallah Mehrabadi, J.; Darbandi, A. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections among burnt children. Med. J. Islam. Repub. Iran 2020, 34, 24. [Google Scholar]
- Zhang, Y.; Xu, G.; Miao, F.; Huang, W.; Wang, H.; Wang, X. Insights into the epidemiology, risk factors, and clinical outcomes of carbapenem-resistant Acinetobacter baumannii infections in critically ill children. Front. Public Health 2023, 11, 1282413. [Google Scholar] [CrossRef]
- Plesea-Condratovici, A.; Plesea-Condratovici, C.; Rosoga, N.; Nedelcu, N. Efficacity and tolerability of a novel food supplement (Turbofer) containing microencapsulatyed iron in liposomal form, in female iron deficiency anaemia. Prog. Nutr. 2015, 17, 214–219. [Google Scholar]
- Modrigan, M.; Drăgănescu, M.; Condratovici, C.P.; Pavel, L.L.; Condratovici, A.P. Clinical Personality Patterns in Young Adults with HIV Nosocomial Infection from the Region of Southeast Romania. Mater. Plast. 2017, 54, 175–179. [Google Scholar] [CrossRef]
- Duceac, L.D.; Banu, E.A.; Baciu, G.; Lupu, V.V.; Ciomaga, I.M.; Tarca, E.; Mitrea, G.; Ichim, D.L.; Damir, D.; Marcu, C.; et al. Assessment of Bacteria Resistance According to Antibiotic Chemical Structure. Rev. Chim. 2019, 70, 906–908. [Google Scholar] [CrossRef]
- Olaru, I.; Stefanache, A.; Gutu, C.; Lungu, I.I.; Mihai, C.; Grierosu, C.; Calin, G.; Marcu, C.; Ciuhodaru, T. Combating Bacterial Resistance by Polymers and Antibiotic Composites. Polymers 2024, 16, 3247. [Google Scholar] [CrossRef] [PubMed]
- Duceac, L.D.; Marcu, C.; Ichim, D.L.; Ciomaga, I.M.; Tarca, E.; Iordache, A.C.; Ciuhodaru, M.I.; Florescu, L.; Tutunaru, D.; Luca, A.C.; et al. Antibiotic Molecules Involved in Increasing Microbial Resistance. Rev. Chim. 2019, 70, 2622–2626. [Google Scholar] [CrossRef]
- Oda, Y.; Shapiro, M.M.; Lewis, N.M.; Zhong, X.; Huse, H.K.; Zhong, W.; Bruce, J.E.; Manoil, C.; Harwood, C.S. CsrA-Controlled Proteins Reveal New Dimensions of Acinetobacter baumannii Desiccation Tolerance. J. Bacteriol. 2022, 204, e00479-21. [Google Scholar] [CrossRef]
- Rafa, E.; Kołpa, M.; Wałaszek, M.Z.; Domański, A.; Wałaszek, M.J.; Różańska, A.; Wójkowska-Mach, J. Healthcare-Acquired Infection Surveillance in Neurosurgery Patients, Incidence and Microbiology, Five Years of Experience in Two Polish Units. Int. J. Environ. Res. Public Health 2022, 19, 7544. [Google Scholar] [CrossRef]
- Karageorgopoulos, D.E.; Falagas, M.E. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect. Dis. 2008, 8, 751–762. [Google Scholar] [CrossRef]
- Chiereghin, A.; Felici, S.; Gibertoni, D.; Foschi, C.; Turello, G.; Piccirilli, G.; Gabrielli, L.; Clerici, P.; Landini, M.P.; Lazzarotto, T. Microbial Contamination of Medical Staff Clothing During Patient Care Activities: Performance of Decontamination of Domestic Versus Industrial Laundering Procedures. Curr. Microbiol. 2020, 77, 1159–1166. [Google Scholar] [CrossRef]
- Seifert, H.; Dijkshoorn, L.; Gerner-Smidt, P.N.; Tjernberg, I.; Vaneechoutte, M. Distribution of Acinetobacter species on human skin: Comparison of phenotypic and genotypic identification methods. J. Clin. Microbiol. 1997, 35, 2819–2825. [Google Scholar] [CrossRef]
- Cisneros, J.; Rodriguez-Bano, J. Nosocomial bacteremia due to Acinetobacter baumannii: Epidemiology, clinical features and treatment. Clin. Microbiol. Infect. 2002, 8, 687–693. [Google Scholar] [CrossRef]
- Chang, Y.; Jeon, K.; Lee, S.M.; Cho, Y.J.; Kim, Y.S.; Chong, Y.P.; Hong, S.B. The Distribution of Multidrug-resistant Microorganisms and Treatment Status of Hospital-acquired Pneumonia/Ventilator-associated Pneumonia in Adult Intensive Care Units: A Prospective Cohort Observational Study. J. Korean Med. Sci. 2021, 36, e251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luchian, N.; Eva, L.; Dabija, M.G.; Druguș, D.; Duceac, M.; Mitrea, G.; Marcu, C.; Popescu, M.R.; Duceac, L.D. Health—Associated infections in hospital in the North-East region of Romania—A multidisciplinary approach. Rom. J. Oral Rehabil. 2023, 14, 219–229. [Google Scholar]
- Duceac, L.D.; Lupu, V.V.; Luchian, N.; Lupu, A.; Popa, M.; Stărcea, M.; Elkan, E.M.; Fotea, S. Clinical Epidemiological study on healthcare associated infections in a regional emergency hospital from Northeastern Romania. Rom. J. Oral Rehabil. 2023, 14, 32–39. [Google Scholar]
- Luchian, N.; Dabija, M.G.; Eva, L.; Marcu, C.; Popescu, M.R.; Scutariu, M.M.; Goroftei Bogdan, E.R.; Guțu, C.; Cretu, O.C.; Popa, F.; et al. Antimicrobial resistance to Klebsiella pneumoniae in a North Eastern Romanian hospital: A descriptive study. Rom. J. Oral Rehabil. 2024, 1, 16–19. [Google Scholar] [CrossRef]
- Iancu, D.; Moldovan, I.; Țilea, B.; Voidăzan, S. Evaluating Healthcare-Associated Infections in Public Hospitals: A Cross-Sectional Study. Antibiotics 2023, 12, 1693. [Google Scholar] [CrossRef]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef]
- Shenkutie, A.M.; Yao, M.Z.; Siu, G.K.; Wong, B.K.C.; Leung, P.H. Biofilm-Induced Antibiotic Resistance in Clinical Acinetobacter baumannii Isolates. Antibiotics 2020, 9, 817. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Narimisa, N.; Mirshekar, M.; Dadgar-Zankbar, L.; Taki, E.; Navidifar, T.; Darban-Sarokhalil, D. Prevalence of colistin resistance in clinical isolates of Acinetobacter baumannii: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2024, 13, 24. [Google Scholar] [CrossRef]
- Duceac, L.D.; Mitrea, G.; Banu, E.A.; Ciuhodaru, M.I.; Ciomaga, I.M.; Ichim, D.L.; Constantin, M.; Luca, A.C. Synthesis and Characterization of Carbapenem Based Nanohybrids as Antimicrobial Agents for Multidrug Resistant Bacteria. Mater. Plast. 2019, 56, 388–391. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bagheri Nejad, S.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of Endemic Health-Care-Associated Infection in Developing Countries: Systematic Review and Meta-Analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Surveillance Report. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2011–2012; Publications Office of the European Union: Stockholm, Sweden, 2013; ISBN 978-92-9193-485-0. [Google Scholar]
- National Statistics Institute of Romania. Point Prevalence Study of Healthcare-Associated Infections and Antimicrobial Consumption in Romanian Hospitals; National Statistics Institute of Romania: Bucharest, Romania, 2023.
- Lăzureanu, V.; Poroșnicu, M.; Gândac, C.; Moisil, T.; Bădițoiu, L.; Laza, R.; Musta, V.; Crișan, A.; Marinescu, A.-R. Infection with Acinetobacter baumannii in an intensive care unit in the Western part of Romania. BMC Infect. Dis. 2016, 16 (Suppl. S1), 95. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.F.; Yelamanchili, S.; Thati, S. A Comparative Study of Acinetobacter Infections in COVID and Non-COVID Patients. J. Infect. Dis. Epidemiol. 2022, 8, 250. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falagas, M.E.; Karveli, E.A. The changing global epidemiology of Acinetobacter baumannii infections: A development with major public health implications. Clin. Microbiol. Infect. 2007, 13, 117–119. [Google Scholar] [CrossRef]
- Rodríguez, C.; Nastro, M.; Flores, S.; Rodriguez, M.; Spinozzi, M.; Bruni, G.L.; López, A.; David, V.; Aiassa, M.; Marqués, I.R.; et al. Molecular epidemiology of carbapenem-resistant isolates of Acinetobacter baumannii in Argentina. Rev. Argent. Microbiol. 2019, 51, 247–250. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Li, S.; Yang, N.; Zhang, S.; Hu, D.; Li, Q.; Lu, M. Post-neurosurgical meningitis caused by Acinetobacter baumannii: Case series and review of the literature. Int. J. Clin. Exp. Med. 2015, 8, 21833–21838. [Google Scholar] [PubMed] [PubMed Central]
- Hussein, K.; Bitterman, R.; Shofty, B.; Paul, M.; Neuberger, A. Management of post-neurosurgical meningitis: Narrative review. Clin. Microbiol. Infect. 2017, 23, 621–628. [Google Scholar] [CrossRef]
- Zhao, L.; Li, P.; Xu, Z.; Ji, X.; Guan, L.; Wang, X.; Luo, J.; Cheng, H.; Ye, L. Diagnosis of post-neurosurgical bacterial meningitis in patients with aneurysmal subarachnoid hemorrhage based on the immunity-related proteomics signature of the cerebrospinal fluid. Front. Neurol. 2023, 14, 1166598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Years | No. of Patients | HAIs Cases | HAIs with Acinetobacter spp. | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | Incidence * (‰) | ||
| 2020 | 5786 | 255 | 4.41 | 32 | 12.55 | 5.53 |
| 2021 | 6632 | 347 | 5.23 | 54 | 15.56 | 8.14 |
| 2022 | 8467 | 375 | 4.43 | 41 | 10.93 | 4.84 |
| 2023 | 10,343 | 342 | 3.31 | 54 | 15.78 | 5.22 |
| 2024 | 11,623 | 334 | 2.37 | 92 | 27.54 | 7.92 |
| Total | 42,851 | 1653 | 3.85 | 273 | 16.51 | 6.37 |
| Years | Isolates | CR | MDR | CR MDR | ESBL MDR | CR, MDR, ESBL | NS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | Nr. | % | No. | % | |
| 2020 | 32 | 12.55 | 1 | 3.12 | 1 | 3.12 | 26 | 81.25 | 2 | 6.25 | 1 | 3.12 | 1 | 3.12 |
| 2021 | 54 | 15.56 | 6 | 11.11 | 1 | 1.85 | 44 | 81.48 | 0 | 0.00 | 0 | 0.00 | 3 | 5.55 |
| 2022 | 41 | 10.93 | 2 | 4.87 | 0 | 0.00 | 37 | 90.24 | 0 | 0.00 | 0 | 0.00 | 2 | 4.87 |
| 2023 | 54 | 15.78 | 5 | 9.25 | 1 | 1.85 | 46 | 85.18 | 0 | 0.00 | 0 | 0.00 | 2 | 3.70 |
| 2024 | 92 | 27.54 | 1 | 1.08 | 2 | 2.17 | 88 | 95.65 | 0 | 0.00 | 0 | 0.00 | 1 | 1.08 |
| Total | 273 | 16.51 | 15 | 5.49 | 5 | 1.83 | 241 | 88.27 | 2 | 0.73 | 1 | 0.36 | 9 | 3.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchian, N.; Olaru, I.; Pleșea-Condratovici, A.; Duceac, M.; Mătăsaru, M.; Dabija, M.G.; Elkan, E.M.; Dabija, V.A.; Eva, L.; Duceac, L.D. Clinical and Epidemiological Aspects on Healthcare-Associated Infections with Acinetobacter spp. in a Neurosurgery Hospital in North-East Romania. Medicina 2025, 61, 990. https://doi.org/10.3390/medicina61060990
Luchian N, Olaru I, Pleșea-Condratovici A, Duceac M, Mătăsaru M, Dabija MG, Elkan EM, Dabija VA, Eva L, Duceac LD. Clinical and Epidemiological Aspects on Healthcare-Associated Infections with Acinetobacter spp. in a Neurosurgery Hospital in North-East Romania. Medicina. 2025; 61(6):990. https://doi.org/10.3390/medicina61060990
Chicago/Turabian StyleLuchian, Nicoleta, Iulia Olaru, Alina Pleșea-Condratovici, Mădălina Duceac (Covrig), Mirela Mătăsaru, Marius Gabriel Dabija, Eva Maria Elkan, Vlad Andrei Dabija, Lucian Eva, and Letitia Doina Duceac. 2025. "Clinical and Epidemiological Aspects on Healthcare-Associated Infections with Acinetobacter spp. in a Neurosurgery Hospital in North-East Romania" Medicina 61, no. 6: 990. https://doi.org/10.3390/medicina61060990
APA StyleLuchian, N., Olaru, I., Pleșea-Condratovici, A., Duceac, M., Mătăsaru, M., Dabija, M. G., Elkan, E. M., Dabija, V. A., Eva, L., & Duceac, L. D. (2025). Clinical and Epidemiological Aspects on Healthcare-Associated Infections with Acinetobacter spp. in a Neurosurgery Hospital in North-East Romania. Medicina, 61(6), 990. https://doi.org/10.3390/medicina61060990






