Dynamic Alteration of HALP Score as a Predictor in Patients with Receiving Immunotherapy for Advanced Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HALP | Hemoglobin–albumin–lymphocyte–platelet |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| PFS | Progression-free survival |
| PD-L1 | Programmed death ligand-1 |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| SII | Systemic immune-inflammation index |
| ECOG | Eastern Cooperative Oncology Group |
| HR | Hazard ratios |
| CI | Confidence interval |
| ALI | Advanced lung cancer inflammation index |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2008; pp. 584–594. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Liao, D.; Yu, L.; Shangguan, D.; Zhang, Y.; Xiao, B.; Liu, N.; Yang, N. Recent Advancements of Monotherapy, Combination, and Sequential Treatment of EGFR/ALK-TKIs and ICIs in Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 905947. [Google Scholar] [CrossRef]
- Fang, Q.; Yu, J.; Li, W.; Luo, J.; Deng, Q.; Chen, B.; He, Y.; Zhang, J.; Zhou, C. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin. Exp. Pharmacol. Physiol. 2023, 50, 178–190. [Google Scholar] [CrossRef]
- Chen, M.Y.; Zeng, Y.C. Pseudoprogression in lung cancer patients treated with immunotherapy. Crit. Rev. Oncol. Hematol. 2022, 169, 103531. [Google Scholar] [CrossRef]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Raju, S.; Joseph, R.; Sehgal, S. Review of checkpoint immunotherapy for the management of non-small cell lung cancer. Immunotargets Ther. 2018, 7, 63–75. [Google Scholar] [CrossRef]
- Killock, D. Frontline nivolumab—CheckMate 026 ends in stalemate. Nat. Rev. Clin. Oncol. 2017, 14, 458. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Olivares-Hernandez, A.; Del Barco Morillo, E.; Parra Perez, C.; Miramontes-Gonzalez, J.P.; Figuero-Perez, L.; Martin-Gomez, T.; Escala-Cornejo, R.; Bellido Hernandez, L.; Gonzalez Sarmiento, R.; Cruz-Hernandez, J.J.; et al. Influence of DNA Mismatch Repair (MMR) System in Survival and Response to Immune Checkpoint Inhibitors (ICIs) in Non-Small Cell Lung Cancer (NSCLC): Retrospective Analysis. Biomedicines 2022, 10, 360. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef]
- Varga, G.; Foell, D. Anti-inflammatory monocytes-interplay of innate and adaptive immunity. Mol. Cell Pediatr. 2018, 5, 5. [Google Scholar] [CrossRef]
- Taylor, M.; Evison, M.; Michael, S.; Obale, E.; Fritsch, N.C.; Abah, U.; Smith, M.; Martin, G.P.; Shackcloth, M.; Granato, F.; et al. Pre-Operative Measures of Systemic Inflammation Predict Survival After Surgery for Primary Lung Cancer. Clin. Lung Cancer 2024, 25, 460–467.e7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H.; Li, A.; Tang, E.; Xu, D.; Chen, Y.; Zhang, Y.; Tang, M.; Zhang, Z.; Deng, X. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget 2016, 7, 72076. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litiere, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C. Evaluation of immune-related response criteria and RECIST v1. 1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016, 34, 1510–1517. [Google Scholar] [CrossRef]
- Ulas, A.; Temel, B.; Kos, F.T. Comparison of Prognostic Values of Seven Immune Indexes in Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab: How Effective Can They Be Regarding Our Treatment Decisions? Medicina 2024, 60, 1792. [Google Scholar] [CrossRef]
- Guc, Z.G.; Alacacioglu, A.; Kalender, M.E.; Oflazoglu, U.; Unal, S.; Yildiz, Y.; Salman, T.; Kucukzeybek, Y.; Tarhan, M.O. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The Izmir oncology group (IZOG) study. Front. Nutr. 2022, 9, 905292. [Google Scholar] [CrossRef]
- Cavdar, E.; Karaboyun, K.; Kara, K. Comprehensive analysis of the prognostic role of laboratory indices in advanced lung cancer patients. Asia Pac. J. Clin. Oncol. 2024. [Google Scholar] [CrossRef]
- Gao, S.; Huang, Q.; Wei, S.; Lv, Y.; Xie, Y.; Hao, Y. Prognostic nomogram based on pre-treatment HALP score for patients with advanced non-small cell lung cancer. Clinics 2024, 79, 100371. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, K.; Ma, X.; Wang, X.; Sun, C.; Qiu, S.; Guo, Y.; Yang, Z.; Liu, Y.; Xu, Y. Imaging findings can’t mean everything in the era of immunotherapy: A case report and literature review. Immunotherapy 2024, 16, 99–106. [Google Scholar] [CrossRef]
- Masse, M.; Chardin, D.; Tricarico, P.; Ferrari, V.; Martin, N.; Otto, J.; Darcourt, J.; Comte, V.; Humbert, O. [(18)F]FDG-PET/CT atypical response patterns to immunotherapy in non-small cell lung cancer patients: Long term prognosis assessment and clinical management proposal. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3696–3708. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Xu, J.; Shi, X.; Yin, T.; Teng, F. Noninvasive radiomic biomarkers for predicting pseudoprogression and hyperprogression in patients with non-small cell lung cancer treated with immune checkpoint inhibition. Oncoimmunology 2024, 13, 2312628. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Li, R.; Xue, J.; Lu, Y. Pseudoprogression and hyperprogression in lung cancer: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2020, 146, 3269–3279. [Google Scholar] [CrossRef]
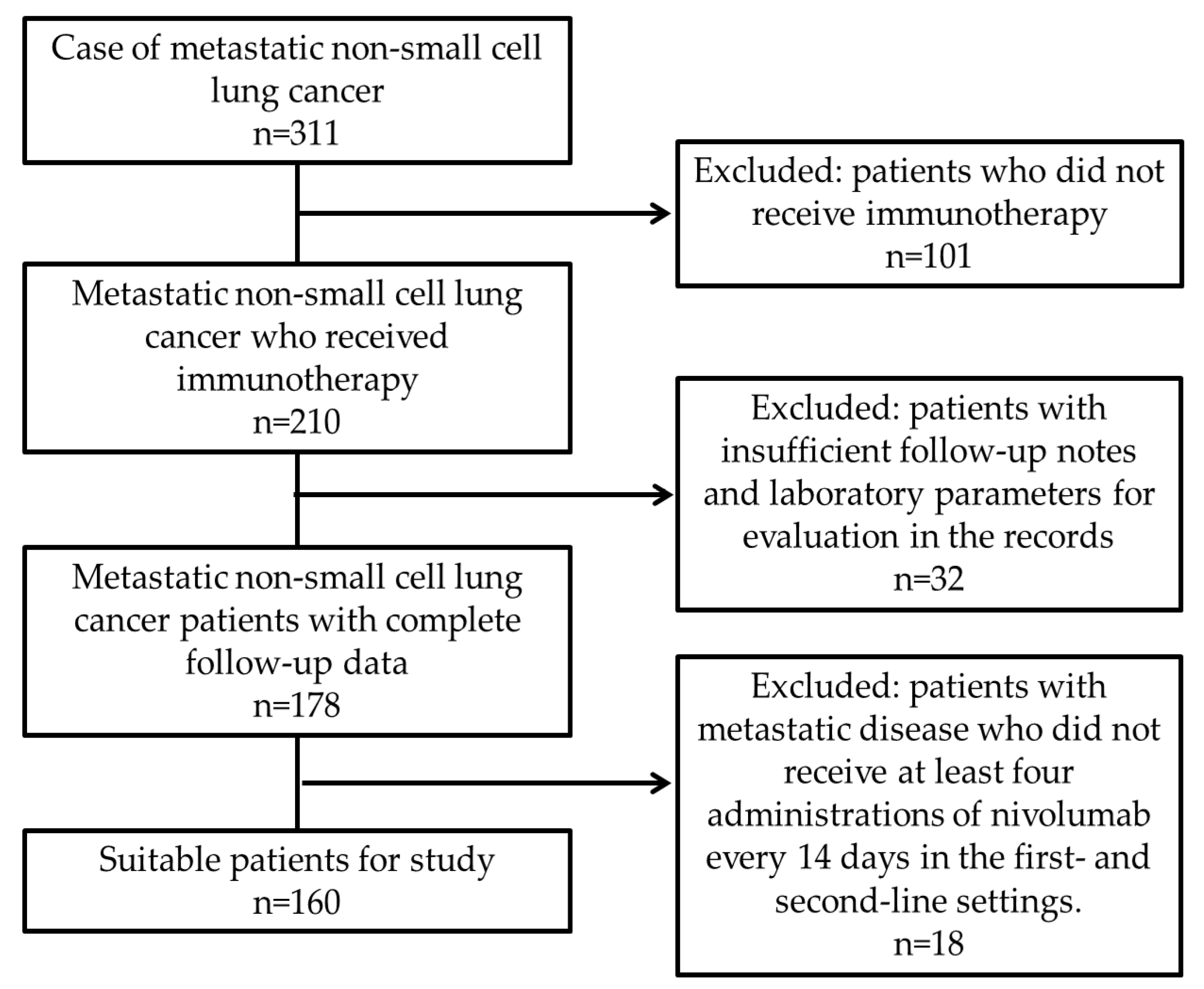
| Features | Frequency n(%) |
|---|---|
| Gender | |
| Female | 26 (16.3) |
| Male | 134 (83.7) |
| Pathology | |
| Adenocarcinoma | 77 (48.1) |
| Squamous cell carcinoma | 69 (43.1) |
| Adenosquamous carcinoma | 2 (1.3) |
| NOS | 12 (7.5) |
| De novo metastasis | |
| No | 82 (50.2) |
| Yes | 78 (48.8) |
| Site of metastasis | |
| Contralateral Lung | 58 (36.3) |
| Bone | 25 (15.6) |
| Liver | 13 (8.1) |
| Brain | 9 (5.6) |
| Pleura/pleural effusion | 30 (18.8) |
| Adrenal gland | 19 (11.9) |
| Distant lymph node | 19 (11.9) |
| Age group | |
| ≤62 | 80 (50) |
| >62 | 80 (50) |
| PDL-1 groups | |
| PDL-1 < 1% | 65 (40.6) |
| PDL-1 1–10% | 20 (12.5) |
| PDL-1 10–50% | 21 (23.1) |
| PDL-1 > 50% | 20 (12.5) |
| Missing | 34 (21.3) |
| Pseudoprogression | |
| No | 135 (84.4) |
| Yes | 25 (15.6) |
| Hyperprogression | |
| No | 121 (75.6) |
| Yes | 39 (24.4) |
| Line of immunotherapy | |
| First-line | 49 (30.6) |
| Second-line | 111 (69.4) |
| Immun-related adverse events | |
| Grade 1 | 17 (10.6) |
| Grade 2 | 13 (8.1) |
| Grade 3 | 3 (1.9) |
| Features | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Median OS (Months, 95% CI) | p Value | HR (95% CI) | p Value | |
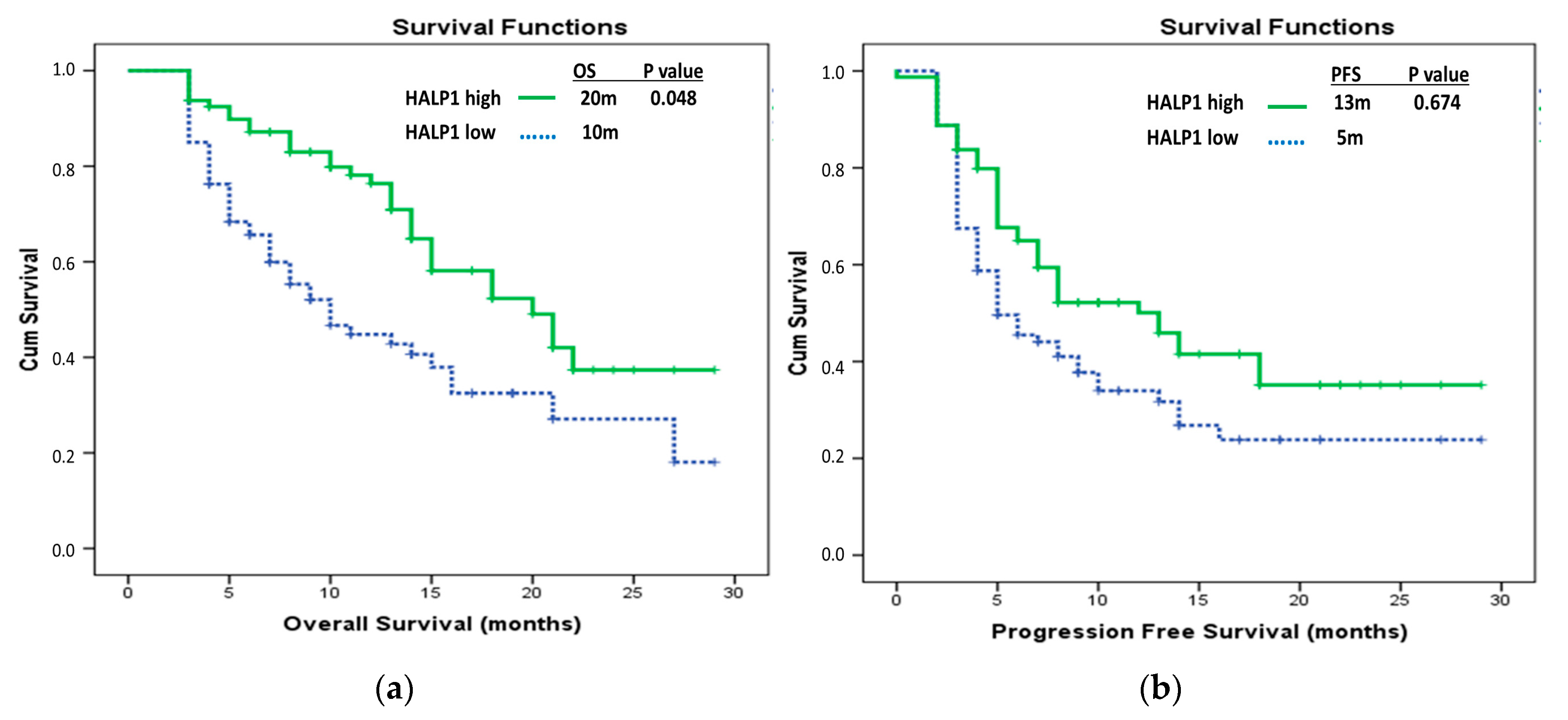
| HALP1 groups | ||||
| HALP1 low | 10 (6.62–13.38) | 0.001 * | Ref 0.58 (0.34–1.00) | 0.048 * |
| HALP1 high | 20 (14.61–25.40) | |||
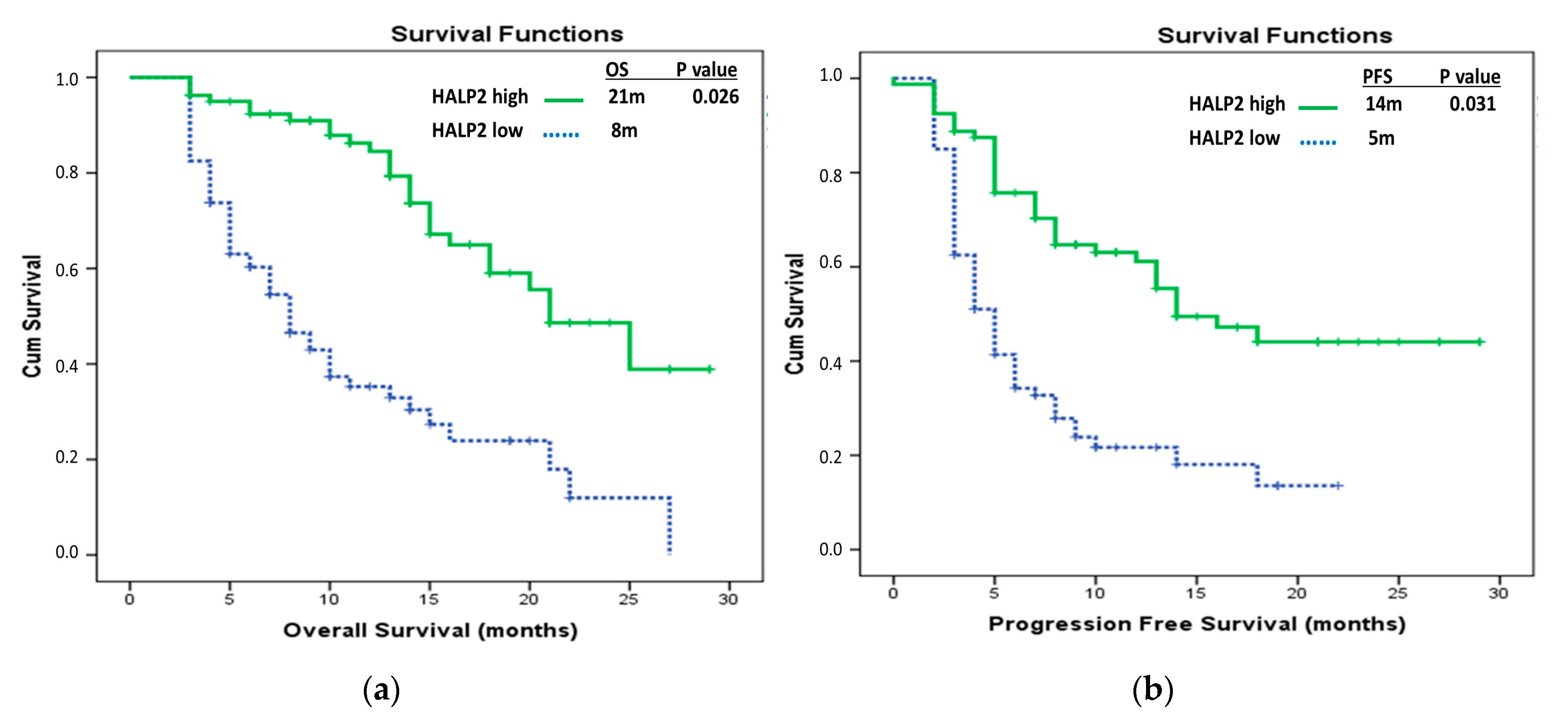
| HALP2 groups | ||||
| HALP2 low | 8 (5.99–10.00) | 0.000 * | Ref 0.472 (0.24–0.91) | 0.026 * |
| HALP2 high | 21 (16.31–25.69) | |||
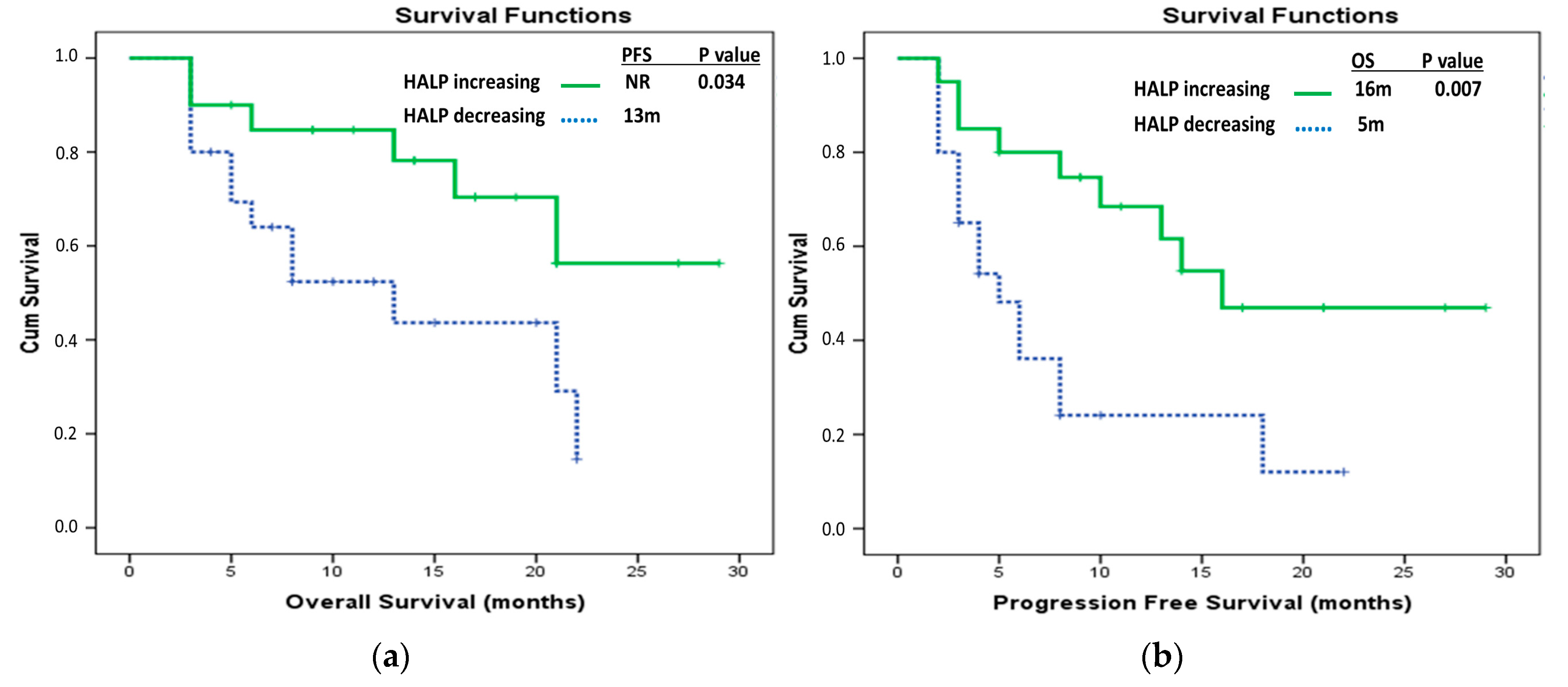
| Delta HALP groups | ||||
| Delta HALP low | 10 (3.41–16.59) | 0.005 * | Ref 0.94 (0.52–1.70) | 0.839 |
| Delta HALP high | 18 (13.52–22.48) | |||
| Age groups | ||||
| ≤62 | 15 (8.80–21.20) | 0.782 | ||
| >62 | 16 (13.38–18.62) | |||
| Line of IO | ||||
| First-line | 27 (14.28–39.72) | 0.003 * | Ref 2.86 (1.70–4.82) | 0.252 |
| Second-line | 14 (11.43–16.58) | |||
| De novo metastasis | ||||
| Yes | 11 (7.67–14.33) | 0.000 * | Ref 0.59 (0.35–1.01) | 0.55 |
| No | 22 (14.27–29.74) | |||
| Gender | ||||
| Female | 15 (8.59–21.41) | 0.568 | ||
| Male | 15 (11.64–18.36) | |||
| Pseudoprogression | ||||
| No | 14 (11.70–16.30) | 0.017 * | Ref 0.93 (0.45–1.91) | 0.844 |
| Yes | NR | |||
| Hyperprogression | ||||
| No | 21 (16.56–25.45) | 0.000 * | Ref 2.86 (1.70–4.82) | <0.001 * |
| Yes | 5 (3.80–6.20) | |||
| Features | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Median OS (Months, 95% CI) | p Value | HR (95% CI) | p Value | |
| HALP1 groups | ||||
| HALP1 low | 5 (2.75–7.25) | 0.027 * | Ref 1.11 (0.69–1.79) | 0.674 |
| HALP1 high | 13 (8.29–17.71) | |||
| HALP2 groups | ||||
| HALP2 low | 5 (4.00–6.04) | <0.001 * | Ref 0.506 (0.27–0.94) | 0.031 * |
| HALP2 high | 14 (8.53–19.47) | |||
| DeltaHALP groups | ||||
| DeltaHALP low | 5 (3.56–6.44) | 0.002 * | Ref 1.16 (0.67–1.99) | 0.6 |
| DeltaHALP high | 13 (9.49–16.51) | |||
| Age groups | ||||
| ≤62 | 7 (2.67–11.33) | 0.791 | ||
| >62 | 8 (5.81–10.19) | |||
| Line of IO | ||||
| First-line | NR | <0.001 * | Ref 1.78 (1.01–3.15) | 0.046 * |
| Second-line | 5 (3.65–6.35) | |||
| De novo metastasis | ||||
| Yes | 5 (3.84–6.16) | <0.001 * | Ref 0.82 (0.52–1.30) | 0.405 |
| No | 14 (6.87–21.14) | |||
| Gender | ||||
| Female | 6 (3.67–8.34) | 0.034 * | Ref 0.66 (0.39–1.13) | 0.13 |
| Male | 8 (4.03–11.97) | |||
| Pseudoprogression | ||||
| No | 6 (4.73–7.27) | 0.001 * | Ref 0.58 (0.29–1.19) | 0.137 |
| Yes | (NR) | |||
| Hyperprogression | ||||
| No | 14 (10.55–17.46) | <0.001 * | Ref 10.90 (5.91–20.10) | <0.001 * |
| Yes | 3 (2.74–3.76) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koçanoğlu, A.; Karakaya, S.; Zeynelgil, E.; Düzköprü, Y.; Doğan, Ö. Dynamic Alteration of HALP Score as a Predictor in Patients with Receiving Immunotherapy for Advanced Non-Small Cell Lung Cancer. Medicina 2025, 61, 989. https://doi.org/10.3390/medicina61060989
Koçanoğlu A, Karakaya S, Zeynelgil E, Düzköprü Y, Doğan Ö. Dynamic Alteration of HALP Score as a Predictor in Patients with Receiving Immunotherapy for Advanced Non-Small Cell Lung Cancer. Medicina. 2025; 61(6):989. https://doi.org/10.3390/medicina61060989
Chicago/Turabian StyleKoçanoğlu, Abdülkadir, Serdar Karakaya, Esra Zeynelgil, Yakup Düzköprü, and Özlem Doğan. 2025. "Dynamic Alteration of HALP Score as a Predictor in Patients with Receiving Immunotherapy for Advanced Non-Small Cell Lung Cancer" Medicina 61, no. 6: 989. https://doi.org/10.3390/medicina61060989
APA StyleKoçanoğlu, A., Karakaya, S., Zeynelgil, E., Düzköprü, Y., & Doğan, Ö. (2025). Dynamic Alteration of HALP Score as a Predictor in Patients with Receiving Immunotherapy for Advanced Non-Small Cell Lung Cancer. Medicina, 61(6), 989. https://doi.org/10.3390/medicina61060989





