Associations Between Cerebral Perfusion Pressure, Hemodynamic Parameters, and Cognitive Test Values in Normal-Tension Glaucoma Patients, Alzheimer’s Disease Patients, and Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- Age under 65 or over 85 years.
- History of ocular trauma or previous ocular surgery, refractive error greater than three diopters.
- Severe and uncompensated systemic conditions that could influence study outcomes (e.g., decompensated cardiovascular disease, acute or chronic respiratory disease, diabetes mellitus, severe renal or hepatic dysfunction, or active cancer).
- Prominent neurological deficit (paresis, ataxia, aphasia, etc.), evident extrapyramidal signs (tremor, rigidity) or psychiatric disorders (severe depression, psychotic type).
- Allergy to local anesthetics.
2.2. Clinical and Cognitive Assessments
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Hemodynamic Differences and Their Implications
4.2. Cognitive Function and Hemodynamic Correlations
4.3. Ocular Perfusion and Glaucoma
4.4. Ocular Perfusion and AD
4.5. Shared Mechanisms and Pathophysiological Overlaps
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and Beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sabel, B.A. Vascular Dysregulation in Glaucoma: Retinal Vasoconstriction and Normal Neurovascular Coupling in Altitudinal Visual Field Defects. EPMA J. 2023, 14, 87–99. [Google Scholar] [CrossRef]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The Impact of Ocular Blood Flow in Glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Park, K.-H. Update on the Prevalence, Etiology, Diagnosis, and Monitoring of Normal-Tension Glaucoma. Asia-Pac. J. Ophthalmol. 2016, 5, 23–31. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative Stress in Glaucomatous Neurodegeneration: Mechanisms and Consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef]
- Flammer, J.; Mozaffarieh, M. What Is the Present Pathogenetic Concept of Glaucomatous Optic Neuropathy? Surv. Ophthalmol. 2007, 52 (Suppl. 2), S162–S173. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Santillo, E.; Balietti, M.; Fabbietti, P.; Antolini, M.S.; Paoloni, C.; Piacenza, F.; Giuli, C. Association between Low Values of Mean Arterial Pressure and Impaired Cognitive Performance in Older Patients with Mild Cognitive Impairment: Cross-Sectional Preliminary Findings from the STRENGTH Project. Aging Clin. Exp. Res. 2024, 36, 9. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. Continuum 2016, 22, 404–418. [Google Scholar] [CrossRef]
- de la Torre, J.C. Alzheimer Disease as a Vascular Disorder: Nosological Evidence. Stroke 2002, 33, 1152–1162. [Google Scholar] [CrossRef]
- Bayer, A.U.; Ferrari, F.; Erb, C. High Occurrence Rate of Glaucoma among Patients with Alzheimer’s Disease. Eur. Neurol. 2002, 47, 165–168. [Google Scholar] [CrossRef]
- Keenan, T.D.L.; Goldacre, R.; Goldacre, M.J. Associations between Primary Open Angle Glaucoma, Alzheimer’s Disease and Vascular Dementia: Record Linkage Study. Br. J. Ophthalmol. 2015, 99, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Meiliuniene, I.; Siesky, B.; Harris, A. The Difference in Translaminar Pressure Gradient and Neuroretinal Rim Area in Glaucoma and Healthy Subjects. J. Ophthalmol. 2014, 2014, 937360. [Google Scholar] [CrossRef] [PubMed]
- Rakusa, M.; Jensterle, J.; Mlakar, J. Clock Drawing Test: A Simple Scoring System for the Accurate Screening of Cognitive Impairment in Patients with Mild Cognitive Impairment and Dementia. Dement. Geriatr. Cogn. Disord. 2018, 45, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: A Review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Crawford Downs, J.; Bellezza, A.J.; Francis Suh, J.-K.; Hart, R.T. The Optic Nerve Head as a Biomechanical Structure: A New Paradigm for Understanding the Role of IOP-Related Stress and Strain in the Pathophysiology of Glaucomatous Optic Nerve Head Damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2019. Alzheimer’s Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-Analysis of Modifiable Risk Factors for Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Power, M.C.; Weuve, J.; Gagne, J.J.; McQueen, M.B.; Viswanathan, A.; Blacker, D. The Association between Blood Pressure and Incident Alzheimer Disease: A Systematic Review and Meta-Analysis. Epidemiology 2011, 22, 646–659. [Google Scholar] [CrossRef]
- Mizuhara, R.; Mitaki, S.; Takamura, M.; Abe, S.; Onoda, K.; Yamaguchi, S.; Nagai, A. Pulse Pressure Is Associated with Cognitive Performance in Japanese Non-Demented Population: A Cross-Sectional Study. BMC Neurol. 2022, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-N.; Tan, C.-C.; Shen, X.-N.; Xu, W.; Hou, X.-H.; Dong, Q.; Tan, L.; Yu, J.-T. Blood Pressure and Risks of Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of 209 Prospective Studies. Hypertension 2020, 76, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Winblad, B.; Viitanen, M.; Fratiglioni, L. Pulse Pressure and Risk of Alzheimer Disease in Persons Aged 75 Years and Older: A Community-Based, Longitudinal Study. Stroke 2003, 34, 594–599. [Google Scholar] [CrossRef]
- Gauthier, A.C.; Liu, J. Neurodegeneration and Neuroprotection in Glaucoma. Yale J. Biol. Med. 2016, 89, 73–79. [Google Scholar] [PubMed]
- Siaudvytyte, L.; Januleviciene, I.; Daveckaite, A.; Ragauskas, A.; Bartusis, L.; Kucinoviene, J.; Siesky, B.; Harris, A. Literature Review and Meta-Analysis of Translaminar Pressure Difference in Open-Angle Glaucoma. Eye 2015, 29, 1242–1250. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Fautsch, M.P.; Stinnett, S.S.; Allingham, R.R. Intracranial Pressure in Primary Open Angle Glaucoma, Normal Tension Glaucoma, and Ocular Hypertension: A Case-Control Study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5412–5418. [Google Scholar] [CrossRef]
- Lahme, L.; Esser, E.L.; Mihailovic, N.; Schubert, F.; Lauermann, J.; Johnen, A.; Eter, N.; Duning, T.; Alnawaiseh, M. Evaluation of Ocular Perfusion in Alzheimer’s Disease Using Optical Coherence Tomography Angiography. J. Alzheimer’s Dis. 2018, 66, 1745–1752. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Ho, K.; Bodi, N.E.; Sharma, T.P. Normal-Tension Glaucoma and Potential Clinical Links to Alzheimer’s Disease. J. Clin. Med. 2024, 13, 1948. [Google Scholar] [CrossRef]
- Mroczkowska, S.; Benavente-Perez, A.; Negi, A.; Sung, V.; Patel, S.R.; Gherghel, D. Primary Open-Angle Glaucoma vs Normal-Tension Glaucoma: The Vascular Perspective. JAMA Ophthalmol. 2013, 131, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mroczkowska, S.; Shokr, H.; Benavente-Pérez, A.; Negi, A.; Bentham, P.; Gherghel, D. Retinal Microvascular Dysfunction Occurs Early and Similarly in Mild Alzheimer’s Disease and Primary-Open Angle Glaucoma Patients. J. Clin. Med. 2022, 11, 6702. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Howell, G.R.; MacNicoll, K.H.; Braine, C.E.; Soto, I.; Macalinao, D.G.; Sousa, G.L.; John, S.W.M. Combinatorial Targeting of Early Pathways Profoundly Inhibits Neurodegeneration in a Mouse Model of Glaucoma. Neurobiol. Dis. 2014, 71, 44–52. [Google Scholar] [CrossRef]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. Biomed Res. Int. 2017, 2017, 5123148. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; Killer, H.E. Normal-Tension Glaucoma: A Glymphopathy? Eye Brain 2023, 15, 37–44. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
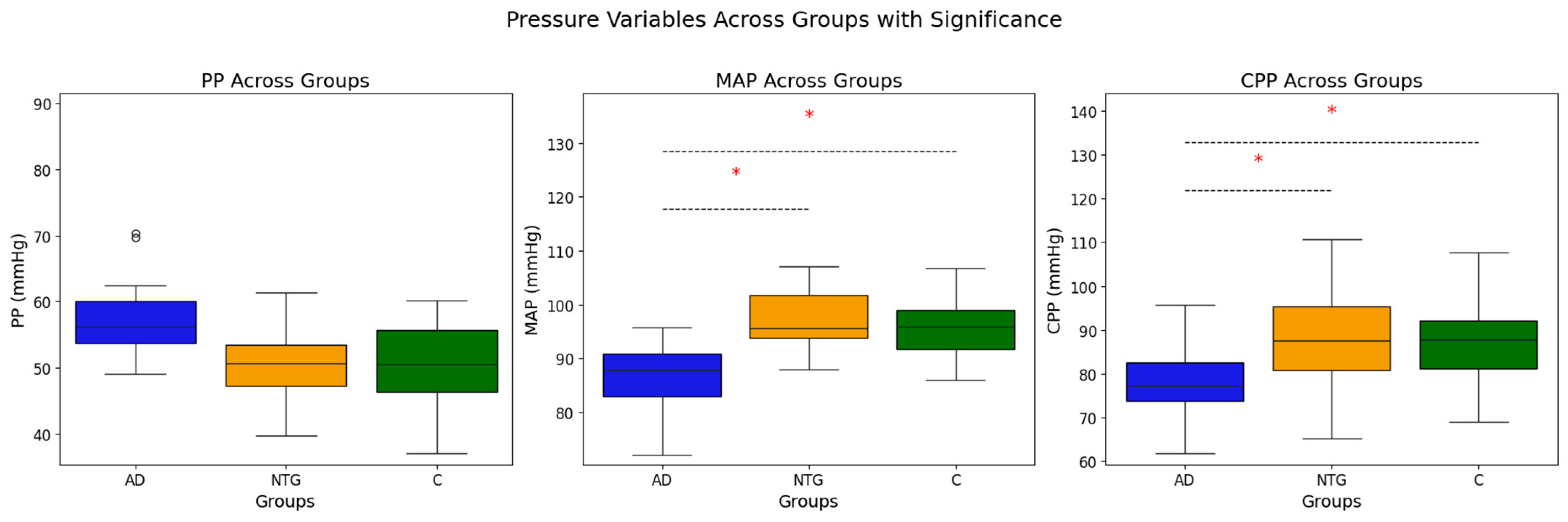

| Characteristic | AD (N = 17) | NTG (N = 35) | Controls (N = 46) | p-Value | Post-Hoc (Bonferroni) |
|---|---|---|---|---|---|
| AGE, YEARS (MEAN ± SD) | 75.12 ± 4.0 | 73.1 ± 4.9 | 72.5 ± 4.9 | 0.169 | - |
| GENDER, MALE (%) | 8 (47.1) | 8 (21.6) | 12 (26.1) | 0.135 (χ2) | - |
| BMI, KG/M2 (MEDIAN [IQR]) | 26.42 [25.76–28.19] | 26.70 [24.61–29.65] | 27.75 [24.61–29.65] | 0.843 (χ2) | - |
| SBP, MMHG (MEDIAN [IQR]) | 126 [118–132] | 132 [124–142] | 130 [125.5–136] | 0.049 (χ2) | AD-NTG: 0.014 (0.043) |
| DBP, MMHG (MEDIAN [IQR]) | 70 [64–75] | 78 [70–82] | 80 [72–82] | <0.001 (χ2) | AD-NTG: <0.001 (0.002), AD-C: <0.001 (0.000) |
| MAP, MMHG (MEDIAN [IQR]) | 87.94 [82.0–94.7] | 96.67 [99.0–102.3] | 95.52 [91.7–100.0] | 0.001 (χ2) | AD-NTG: <0.001 (0.002), AD-C: <0.001 (0.002) |
| PP, MMHG (MEDIAN [IQR]) | 56 [52–62] | 52 [48–63] | 51 [44–60] | 0.204 (χ2) | - |
| ICP, MMHG (MEAN ± SD) | 9.36 ± 2.58 | 8.33 ± 2.72 | 8.74 ± 2.89 | 0.445 (ANOVA) | - |
| CPP, MMHG (MEAN ± SD) | 78.84 ± 6.33 | 88.6 ± 10.58 | 86.9 ± 7.09 | <0.001 (ANOVA) | AD-NTG: <0.001 (0.003), AD-C: <0.001 (<0.001) |
| BCVA (MEDIAN [IQR]) | 0.85 [0.70–1.00] | 0.95 [0.90–1.00] | 1.0 [1.0–1.00] | <0.001 (χ2) | AD-C: <0.001 (0.001), NTG-C: 0.011 (0.032) |
| IOP, MMHG (MEDIAN [IQR]) | 14.00 [12.00–15.00] | 14.00 [12.00–15.00] | 14.00 [13.00–15.25] | 0.184 (χ2) | - |
| SE, D (MEDIAN [IQR]) | 0.5 [−0.50–1.89] | 0.38 [0.0–1.19] | 0.88 [−0.19–1.75] | 0.353 (χ2) | - |
| MD, DB (MEDIAN [IQR]) | −4.71 [−5.74 to −2.91] | −2.86 [−5.48 to −1.55] | −0.99 [−1.83 to −0.34] | <0.001 (χ2) | AD-C: <0.001 (0.000), NTG-C: <0.001 (0.000) |
| PSD, DB (MEDIAN [IQR]) | 4.45 [2.73–6.51] | 2.64 [1.58–4.97] | 1.63 [1.37–1.89] | <0.001 (χ2) | AD-C: <0.001 (0.000), NTG-C: <0.001 (0.000) |
| VFI, % (MEDIAN [IQR]) | 92.00 [87.50–94.00] | 95.50 [89.00–98.00] | 99.00 [97.50–99.00] | <0.001 (χ2) | AD-C: <0.001 |
| OPP, MMHG (MEAN ± SD) | 50.28 ± 5.26 | 55.26 ± 6.39 | 54.48 ± 4.77 | 0.008 (ANOVA) | AD-NTG: 0.008, AD-C: 0.025 |
| SPP, MMHG (MEAN ± SD) | 110.5 ± 9.5 | 119.5 ± 12.7 | 116.4 ± 9.1 | 0.02 (ANOVA) | AD-NTG: 0.016 |
| DPP, MMHG (MEDIAN [IQR]) | 59.0 [48.0–63.0] | 65.00 [58.5–69.5] | 64.4 [59.5–68.00] | 0.024 (χ2) | AD-C: 0.008 (0.024) |
| TSG, MMHG (MEAN ± SD) | 4.4 ± 2.5 | 5.58 ± 2.95 | 5.23 ± 2.85 | 0.367 (ANOVA) | - |
| NORMAL BP, N(%) | 5(29.4) | 12(34.3) | 16(34.8) | 0.901 (χ2) | - |
| AH, N(%) | 11(64.7) | 20(57.1) | 28(61.0) | 0.842 (χ2) | - |
| LOW BP, N(%) | 1(5.9) | 3(8.6) | 2(4.4) | 0.439 (χ2) | - |
| BETA-BLOCKERS, N(%) | 7(41.2) | 14(40.0) | 13(28.3) | 0.495 (χ2) | - |
| ACE INHIBITORS, N(%) | 5(29.4) | 8(22.9) | 14(30.0) | 0.707 (χ2) | - |
| CCB, N(%) | 6(35.3) | 4(11.4) | 7(15.2) | 0.094 (χ2) | - |
| LIPID-LOWERING DRUGS, N(%) | 4(23.5) | 5(14.3) | 8(17.4) | 0.712 (χ2) | - |
| DIURETICS, N(%) | 2 (11.8) | 2(6.1) | 4(8.7) | 0.780 (χ2) | - |
| ANTIDEPRESSANTS, N(%) | 5(29.4) | 3(8.6) | 3(6.5) | 0.014 (χ2) | - |
| Cognitive Test | AD (r, p-Value) | NTG (r, p-Value) | Controls (r, p-Value) | |
|---|---|---|---|---|
| Clock Drawing Test (CDT) scores | 0.265, p = 0.303 | 0.283, p = 0.1 | 0.134, p = 0.382 | |
| ACE-R Subtest | Attention and Concentration | 0.025, p = 0.925 | 0.164, p = 0.347 | 0.249, p = 0.1 |
| Memory | 0.338, p = 0.185 | 0.079, p = 0.651 | −0.102, p = 0.507 | |
| Verbal Fluency | −0.024, p = 0.927 | −0.138, p = 0.429 | 0.212, p = 0.163 | |
| Language | 0.032, p = 0.902 | −0.034, p = 0.848 | 0.067, p = 0.663 | |
| Visuospatial Abilities | 0.492, p = 0.045 | 0.094, p = 0.592 | −0.017, p = 0.911 | |
| ACE-R Total Score | 0.223, p = 0.391 | 0.024, p = 0.891 | 0.069, p = 0.650 | |
| MMSE Score | 0.088, p = 0.737 | 0.112, p = 0.522 | 0.127, p = 0.405 | |
| Cognitive Test | AD (r, p-Value) | NTG (r, p-Value) | Controls (r, p-Value) | |
|---|---|---|---|---|
| Clock Drawing Test (CDT) | 0.257, p = 0.320 | 0.378, p = 0.025 | 0.185, p = 0.225 | |
| ACE-R Subtest | Attention and Concentration | −0.085, p = 0.745 | 0.074, p = 0.674 | 0.213, p = 0.159 |
| Memory | 0.350, p = 0.168 | 0.101, p = 0.564 | −0.102, p = 0.507 | |
| Verbal Fluency | 0.128, p = 0.623 | −0.103, p = 0.555 | 0.119, p = 0.437 | |
| Language | 0.055, p = 0.835 | −0.119, p = 0.496 | 0.031, p = 0.837 | |
| Visuospatial Abilities | 0.365, p = 0.150 | 0.119, p = 0.252 | −0.027, p = 0.862 | |
| ACE-R Total Score | 0.232, p = 0.371 | 0.022, p = 0.902 | 0.017, p = 0.912 | |
| MMSE Score | 0.027, p = 0.919 | 0.125, p = 0.476 | 0.016, p = 0.915 | |
| Cognitive Test | AD (r, p-Value) | NTG (r, p-Value) | Controls (r, p-Value) | |
|---|---|---|---|---|
| Clock Drawing Test (CDT) | −0.527, p = 0.016 | 0.136, p = 0.435 | 0.074, p = 0.628 | |
| ACE-R Subtest | Attention and Concentration | −0.364, p = 0.151 | −0.024, p = 0.890 | −0.139, p = 0.364 |
| Memory | −0.018, p = 0.467 | −0.197, p = 0.303 | −0.232, p = 0.125 | |
| Verbal Fluency | −0.416, p = 0.097 | −0.193, p = 0.268 | −0.440, p = 0.002 | |
| Language | −0.332, p = 0.193 | −0.053, p = 0.762 | −0.028, p = 0.853 | |
| Visuospatial Abilities | −0.373, p = 0.14 | −0.018, p = 0.920 | −0.063, p = 0.679 | |
| ACE-R Total Score | −0.395, p = 0.117 | −0.174, p = 0.316 | −0.348, p = 0.019 | |
| MMSE Score | −0.168, p = 0.52 | 0.055, p = 0.756 | −0.210, p = 0.167 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoskuviene, A.; Chaleckas, E.; Grusauskiene, E.; Bartusis, L.; Celikkaya, G.; Januleviciene, I.; Vaitkus, A.; Ragauskas, A.; Hamarat, Y. Associations Between Cerebral Perfusion Pressure, Hemodynamic Parameters, and Cognitive Test Values in Normal-Tension Glaucoma Patients, Alzheimer’s Disease Patients, and Healthy Controls. Medicina 2025, 61, 972. https://doi.org/10.3390/medicina61060972
Stoskuviene A, Chaleckas E, Grusauskiene E, Bartusis L, Celikkaya G, Januleviciene I, Vaitkus A, Ragauskas A, Hamarat Y. Associations Between Cerebral Perfusion Pressure, Hemodynamic Parameters, and Cognitive Test Values in Normal-Tension Glaucoma Patients, Alzheimer’s Disease Patients, and Healthy Controls. Medicina. 2025; 61(6):972. https://doi.org/10.3390/medicina61060972
Chicago/Turabian StyleStoskuviene, Akvile, Edvinas Chaleckas, Evelina Grusauskiene, Laimonas Bartusis, Guven Celikkaya, Ingrida Januleviciene, Antanas Vaitkus, Arminas Ragauskas, and Yasin Hamarat. 2025. "Associations Between Cerebral Perfusion Pressure, Hemodynamic Parameters, and Cognitive Test Values in Normal-Tension Glaucoma Patients, Alzheimer’s Disease Patients, and Healthy Controls" Medicina 61, no. 6: 972. https://doi.org/10.3390/medicina61060972
APA StyleStoskuviene, A., Chaleckas, E., Grusauskiene, E., Bartusis, L., Celikkaya, G., Januleviciene, I., Vaitkus, A., Ragauskas, A., & Hamarat, Y. (2025). Associations Between Cerebral Perfusion Pressure, Hemodynamic Parameters, and Cognitive Test Values in Normal-Tension Glaucoma Patients, Alzheimer’s Disease Patients, and Healthy Controls. Medicina, 61(6), 972. https://doi.org/10.3390/medicina61060972








